Abstract
Wetting characteristics of a hydrocarbon reservoir are generally quantified for cost-effective field development. The wetting process of rock by oil is a complex process involving reactions among compounds (rock, oil, and brine), the impact of environmental conditions (temperature, pressure, etc.), and treatment history (coring, transportation, etc.). There has not been much attention given to understanding the mechanisms causing different rock wetting states to quantify rock’s wettability. This work aims to provide an in-depth insight into rock wettability influencing factors including CO2 & H2. In addition, advanced computational approaches such as molecular dynamics simulation, computational fluid dynamics, and machine learning for wettability have also been reviewed to govern the undiscovered interactions and mechanisms of this complex process. The key observation is that the polarity of organic components (asphaltenes and long-chain acids) determines the oil wetness in crude oil. In addition, acidic polar organics dominate oil-wetting in carbonate rocks; basic polar organics are key in sandstone. Also, environmental factors such as water films, brine salinity, and pH influence wettability significantly.
1. Introduction
Wettability has been a subject of debate and investigation, especially regarding the factors that lead to water-wet or oil-wet conditions.1−3 It has been observed that the adsorption of organic matter on rock surfaces is the source of oil-wetness.4,5 This understanding is supported by many reported observations, including the formation of an organic layer of tens of nanometers thick on the rock surface after crude oil adsorption,6 the presence of oblate hemispheric organic adsorbate (diameter 120–360 nm, height 30–90 nm),7 and the adsorption of asphaltenes.8 Many factors determine the adsorption of organic materials at the solid–liquid interface, including the electrostatic attraction between the adsorbate and the surface, the fit of the adsorbate in the surface lattice pattern, the solubility of the adsorbate, the distribution of attachment sites on the adsorbate, and so on.9 The reactions between functional groups in the oil and the rock surface are under the influence of the polarity of the oil and the surface charge density of the rock,10 facilitating the adsorption of other organic materials. In the oil-wetting process of rock, alongside other interactions including ligand binding, van der Waals forces, electrical double layer (EDL) repulsion, hydration force, hydroxyl adsorption, etc., four main interactions11 in the oil/brine/rock system are (i) polar interactions (in the absence of a water film between oil and solid), (ii) surface precipitation (concerning the asphaltenes), (iii) acid/base interactions (control surface charge at oil/water and solid/water interfaces), and (iv) ion binding (between charged sites and higher valency ions).12,13
In oil-wet formation, waterflooding typically sweeps larger pores, forming an uneven displacement front, causing a pore scale maneuver resembling overtaking that breaks the oil continuity and traps oil blobs.14 The oil recovery rate can be enhanced by wettability alteration,15,16 which promotes the spontaneous imbibition of brine and thus the displacement of oil.17−19 It also enhances the relative permeability of oil15 and assists in mobilizing the trapped oil in the dead-ends.20 On the other hand, oil-wetness is found to reduce polymer adsorption in chemical flooding;21 conflicting observations were also recorded.22 It is believed in the case of initially water-wet rock that changing the wettability toward oil-wet conditions can enhance oil recovery.23,24 To do this, there are different types of wettability modifiers, including commonly used materials such as polymers,25 surfactants,26 alkalis,27 low salinity water,28 and novel materials (nanofluids,29 ionic liquids,30 CO2 saturated brine31). To determine the most applicable material, it is of critical importance to know the specific mechanism for oil-wetness.
In practice, there are various techniques to determine the wettability: by contact angle,32 Amott-Harvey test,33 USBM (U.S. Bureau of Mines) test,34 flotation test,35,36 and relative permeability measurement.37 Novel techniques include MRI (magnetic resonance imaging),38 solution-state NMR (nuclear magnetic resonance spectroscopy) index39 and solid-state NMR,40 in situ contact angle by Micro-CT,41 QCM-D (quartz crystal microbalance with dissipation),42 and IGC (inverse gas chromatography).43,44 There are other techniques usually used to validate or explain the wettability evaluations, including SEM (scanning electron microscope) for surface adsorption visualization,45,46 FTIR (Fourier transform infrared) for functional groups in the adsorbate,47 TGA (thermogravimetric analysis) for mass and composition of the adsorbate,48 AFM (atomic force microscopy) for surface morphology change before and after adsorption,49 QCM (quartz crystal microbalance) for adsorption studies,50 oil-film-peeling test for wettability alteration,51 and zeta potential for surface charge study.52
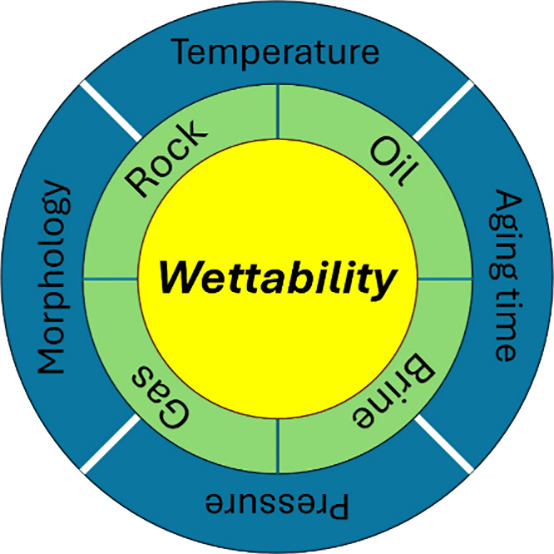
The wettability of rock cannot be described without specifying the conditions (oil composition, brine properties, gas composition, rock mineralogy and morphology, pH, temperature, pressure, and aging time) of the oil/water/rock system.53 Previous review work mostly focuses on wettability alteration of oil-wet rock to water-wet conditions for enhanced oil recovery (EOR) purposes,3,54,55 except for the work of Anderson.56 This review’s key objective is to provide in-depth insight into numerous influencing factors (oil composition, brine composition, CO2, H2, rock types of wettability, pressure, temperature, and aging time) on the oil-wetting process. Furthermore, advancements in studying the electrokinetics of the oil/water/rock system are also highlighted using numerical simulations based on thermodynamics57 or molecular dynamics,25,58 and Artificial Intelligence (AI) tools. The information and insights provided in the paper can be helpful for researchers interested in preparing oil-wet rock surfaces or in assessing the wettability alteration performance of different materials. Besides, it also emphasizes the need to study the impact of new materials (such as CO2 and H2) on rock wettability while studying their oil recovery implications.
2. Rock Wettability Influencing Factors
2.1. Impact of Oil Type
As mixtures of thousands of compounds, crude oils are generally divided into four categories: saturates, aromatics, resins, and asphaltenes. Crude oil used in the laboratory is usually dead oil without dissolved gas. The impact of light phases, such as methane, light alkanes, N2, CO2, etc., which remain in live crude oil, should be considered. To simulate formation conditions, it is preferable to use live oil at deoxygenated reservoir conditions59 instead of dead oil in an open environment.
In oil-bearing formations, kerogen organic matter contributes strongly to the oil-wetness of the formation. Experiments showed that a thin layer of kerogen (0.08% TOC or 6 μg-acid/g-dry-sediment) made carbonate rock oil-wet.60 The higher the kerogen maturity, the stronger the oil-wetness.61 Organic acids bonded to kerogen are believed to promote the adsorption of kerogen on rock surfaces.60 Among the crude oil components, heavy components (residue after distillation at 400 °C) significantly changed rock samples to oil-wet conditions.62,63 They have nitrogen-, sulfur-, or oxygen-containing functional groups (usually aromatic, sulfurized, and oxidized structures64), increasing the polarity.62 Crude oil deprived of heavy components (asphaltenes and acidic components) can fail to alter rock wettability significantly.65 The less heavy (or “intermediate”, weakly polar) components are least effective in altering rock wettability.1,66
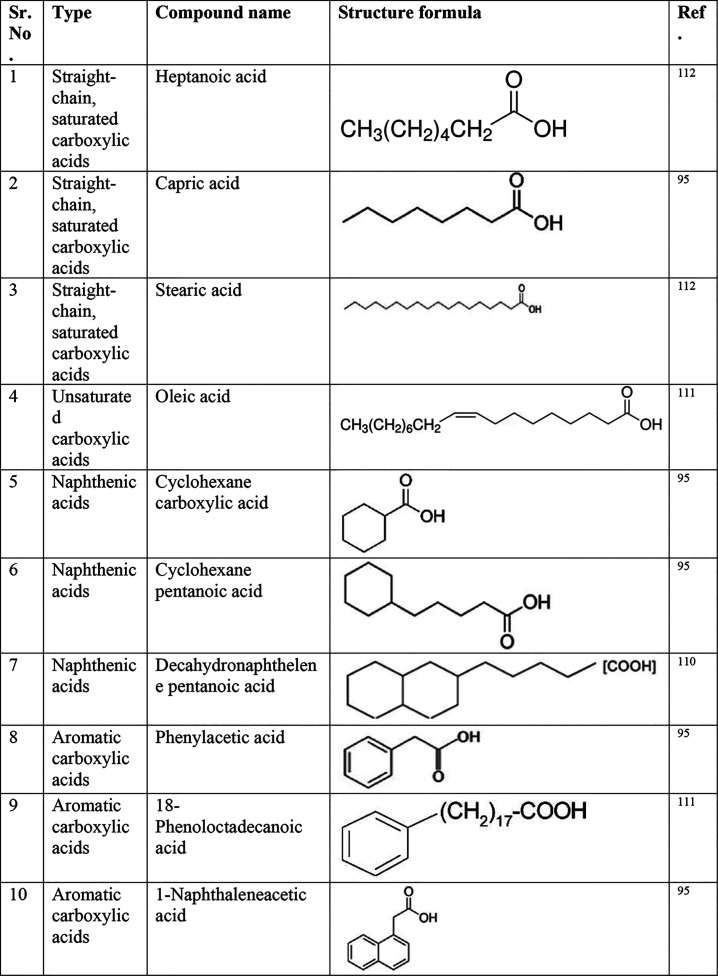
Depending on the specific conditions, the oil-wetness contribution of the polar components varies (several examples of polar compounds are shown in Figure 1). For example, in a crude oil flooding study using sandstone, about 30% base and 10% acid contents were retained. Acid adsorption was found to be lower than basic adsorption in general.67 In a coreflooding study, the results indicated the contribution of water-soluble molecules in the early rapid wettability change.68 Wettability alteration is not solely dependent on the organic adsorption;69 the strength of adsorption is also an important factor.70 The strength of the adsorption is largely influenced by the oil composition. For example, after aging mica surfaces in centrifuged crude oil, and rinsing the surfaces with n-hexane, part of the organic materials were removed while asphaltenes remained on the surfaces.53
Figure 1.
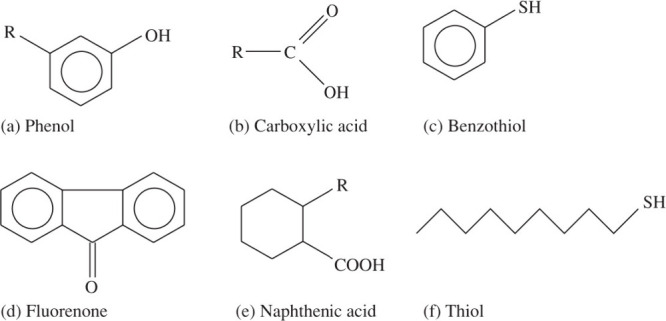
Molecular structure of some common polar compounds. Reprinted with permission from ref (71). Copyright 2009 John Wiley and Sons.
Components in crude oil are affected by each other. For example, resins reduce the aggregation of asphaltenes, thereby enhancing their solubility.72 Stearic acid and asphaltenes exhibit a synergic effect on oil-wetting calcite mineral.73 Asphaltenes also interact with maltenes, very likely saturates, to form macro-accumulations at a later stage of the aging process.40 The adsorption of asphaltenes on rock is found to be largely influenced by the oil composition.7 However, some studies suggest that minimal interactions among components exist, implying that the overall activity of crude oil is merely the sum of the activity of its individual components.74
Four parameters, API gravity, viscosity, TAN (total acid number), and TBN (total base number), are usually used to characterize a crude oil sample. API gravity is calculated based on the density of the oil. Lower API crude oils are usually good solvents for asphaltenes75 and are more likely to render rock surfaces oil-wet.61,75 Viscosity is typically measured by using a rheometer. TAN and TBN can be obtained by titration.76 There exists a strong correlation between TAN, TBN, and the amount of polar components.77 In a study, the AN/BN ratio is claimed to be a more representative parameter than TAN or TBN alone.78 The effect of AN/BN on the oil-wetting of rock, however, is not yet clear. Buckley et al.79 found a higher AN/BN to be roughly associated with the stronger oil-wetting potential of oil on sandstone,12 consistent with findings from a study on chalk,79 yet Skauge et al.77 proposed the opposite observation when studying quartz wettability.77 For crude oil with a low AN/BN ratio, the wetting potential is significantly influenced by brine composition, whereas for oil with a high AN/BN ratio, brine composition showed negligible influence.80 The AN/BN ratio also affects ion concentrations in the formation brine.12 A study involving 20 crude oil samples showed a strong correlation between asphaltene content and TBN,76 as described in eq 1.
| 1 |
2.1.1. Oil Component Analysis Techniques
There are different categorizations of oil components that can sometimes overlap. Conventionally used names such as paraffin and bitumen, overlap with the asphaltenes category in the widely adopted SARA categorization.81,82Table 1 provides examples of SARA analysis results for the crude oil.
Table 1. SARA Analysis Results of Multiple Crude Oil Samples from the Literature.
Various techniques are available for analyzing the oil components. Distillation is usually used to separate crude oils into fractions of different boiling points, which roughly correspond to different molecular weights.62 TLC-FID (thin-layer chromatography–flame ionization detection) using iatroscan is also widely employed for group separation of organic compounds.85 XRD (X-ray diffraction) can be used to analyze functional groups, such as benzene rings, in powdered samples.86 Infrared,87 near-infrared,88 and fluorescence spectroscopy are other techniques for identifying functional groups. The SCME (sulfuric acid-methanol extraction) can be used to extract nitrogenous components from crude oils. The sulfuric acid neutralizes the basic nitrogenous compounds and forms salts that are soluble in methanol.62 SAC (solid adsorbent chromatography) can be used to separate polar components in crude oils, as more polar compounds displace those adsorbed on the solid adsorbent.62 GC-MS (gas chromatography-mass spectrometry) is one of the commonly used conventional geochemical methods for surface-active component identification. It works well with low-molecular-weight (50–330 Da) heteroatomic compounds but is limited for high-molecular-weight compounds.89 FT-ICR-MS (Fourier transform-ion cyclotron resonance-mass spectrometry) and ESI (electrospray ionization) are novel techniques that extend the analytical window up to 800 Da.89 Molecular structures of oil components can be analyzed using elemental analysis methods including 13CNMR and 1HNMR.90
2.1.2. Asphaltenes
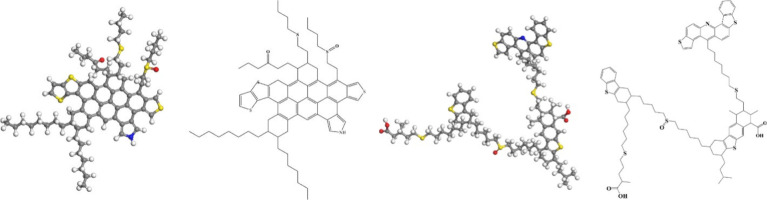
Generally, asphaltenes are the residue after dissolving crude oil in a large volume of low-molecular-weight hydrocarbon solvent (typically 40 volumes of pentane).75,91 They are known as large, heterocyclic, aromatic compounds with nitrogen, oxygen, and sulfur atoms throughout the ring structure,92 as well as trace amounts of metal elements such as iron, nickel, and vanadium.84 They contain the heaviest and most polar components (represented by O-containing carboxylic acids and N-containing aromatic compounds,93 charged species such as salts and zwitterionic complexes could also exist in asphaltenes94) in the crude oil.75 They are believed to adsorb horizontally and make the surface oil-wet.95 The adsorption of asphaltenes happens much stronger, more continuous,7 and faster6 than any other components; sometimes the adsorbed asphaltenes can be easily removed.96 They are found to be one of the major contributors of rock oil-wetness.91,97 For example, the wettability alteration of a mica sample treated with oil changed rapidly only after the onset of asphaltenes flocculation.98 Nevertheless, it should be noted that the wetting potential of oil is not directly related to the amount of asphaltenes.99Figure 2 illustrates two possible asphaltene molecular structures.
Figure 2.
Proposed molecular structures (3D and 2D) for two asphaltenes. Adapted from ref (90). Copyright 2022 Elsevier.
It is difficult to quantify the properties of asphaltenes, which is a complex group of large organic molecular materials associating among themselves and with other compounds.84 Asphaltenes extracted from crude oil from different sources can exhibit significant behaviors in oil-wetting rock due to compositional differences.6,98 Interestingly, asphaltenes from the same crude oil can also vary depending on the isolation methods applied.75 Determining the molecular weight of asphaltenes is difficult, as it can range significantly from 700 to about 10,000 amu.100 The measured molecular weight can vary substantially even for the same sample, depending on the measurement methods, including cryoscopy, viscosimetry, tonometry, ebullioscopy, and ultracentrifugation.84 The calculation of the asphaltenes adsorption amount is not a straightforward task101 by spectrophotometric due to a lack of a distinct absorbance peak at any wavelength.6 One way to improve the accuracy is to also monitor the concentration of asphaltenes from the effluent by techniques like measuring ultraviolet (UV) absorbance.6
Besides, the classical CMC (critical micellization concentration) concept does not directly apply to asphaltenes. The term “aggregate” is more appropriate than “micelle”,86 though the asphaltenes aggregation shares similarities with the micellization process of surfactants, and micellization of asphaltenes in solution has been observed, by experiments102 and molecular simulations.103 The aggregation process of asphaltenes (mainly due to the hydrogen bond, and the π–π bond) can be divided into four steps: molecule, micelle, elementary aggregate, and high-size aggregate (which ultimately leads to flocculation).84 Asphaltenes affect the oil/water IFT, a property leveraged to measure the so-called “CMC” of asphaltenes and to detect the onset of flocculation.104 The mixture refractive index is often used to monitor the stability of asphaltenes in crude oil.75,96 The “CMC” of asphaltenes (in a stepwise manner) in a pure solvent can also be obtained by calorimetric measurements.102
The adsorption of asphaltenes on rock surfaces can occur by different mechanisms. Buckley suggested polar interaction (without brine), ionic interaction (with brine), and colloidal interaction (near the onset of asphaltene precipitation), as the three mechanisms.75 Jaber et al. suggested that there are three factors determining the eventual wettability: strength of the electrostatic interaction, the capacity of calcite surface to adsorb asphaltene particles, and the potential of asphaltene to change the wettability (which is mainly related to the hydrogen bonding).52 Asphaltenes precipitation is a major source of oil-wetness, which is strongly associated with the aggregation and flocculation processes of asphaltenes. Various models have been developed to describe and predict asphaltene aggregation, among them the Yen-Mullins model is widely accepted.40,105 The onset of asphaltene flocculation can be determined through accurate measurements of the viscosity of crude oil upon titration with a precipitating agent.106 Elementary studies have shown the critical importance of heteroatoms including sulfur, nitrogen, and oxygen atoms, in making asphaltenes as strong oil-wetting materials.52 The relative importance of them, however, needs further clarity.52 Metal elements, such as vanadium and nickel, also have an impact on the aggregation of asphaltenes, which leads to solid deposition.107,108
Specific techniques are applied to extract, categorize, and remove asphaltenes. Asphaltenes can be extracted from crude oil by mixing with a solvent, stirring, filtering, and repeated washing.6 The subfractions of asphaltenes can be obtained using heptane-toluene mixtures with increasing toluene content.86 Though decane is the most commonly used base solvent to form the oil phase in experiments,73 asphaltenes do not dissolve in decane, which represents typical alkanes. Instead, toluene is often used for asphaltenes.10,96 The most commonly used solvents to remove asphaltene deposits in the formation include toluene, xylene, and BTX (the mixture of benzene, toluene, and xylene).84
2.1.3. Organic Acids
Polar organic components (POC) have a strong potential to alter rock wetting characteristics to oil-wet conditions.109 While asphaltenes are the most oil-wetting components in crude oil, they are not the most common polar materials present. Weak organic acids, such as phenols and indoles, and weak bases with strengths comparable to those of amides are the most prevalent polar components. Strong acids (e.g., carboxylic) and strong bases (e.g., pyridine) constitute 10–20%.94 Organic acids in crude oils are usually narrowed to carboxylic acids, as shown in Table 2. Organic acids exhibit higher polarity than most crude oil components. An organic layer is formed upon the adsorption of the polar group in the organic acid’s molecule onto the rock surface. The nonpolar part of the molecule then dominates the surface, resulting in an oil-wet condition.
Table 2. Examples of Common Organic Acids from Crude Oilsa.
Carboxylic acids adsorb, chemically97 and physically,95 in vertical and horizontal111 orientation toward the calcite surface. Physical adsorption, which is found to be reversible due to the weak bonds between adsorbate and adsorbent, may partially contribute to wettability alteration.95 When a water film covers the rock surface, the oil-wetting process by organic acids is controlled by acid dissociation in water, surface activity of acid, and water solubility.95
The structure or type of acid may be more important than its concentrations in affecting the oil-wetting of rock.113 Oils containing phenolic materials and alkyl acids or cyclopentane/cyclohexane acids exhibit stronger oil-wetting effects than oils with high-molecular-weight ring-structured acids.113 The adsorption of naphthenic acids on calcite from n-decane follows the order: cyclohexanepropionic acid > cyclohexanebutyric acid > cyclohexanepentanoic acid.114 Organic acid that has a longer alkyl chain has lower adsorption.114 Within long-chain fatty acids, the one with the saturated aromatic ring (least efficient in oil-wetting) seems to be oriented completely parallel. In contrast, those with an unsaturated aromatic ring (most efficient in oil-wetting), fully saturated straight chain (more efficient in oil-wetting), or unsaturated straight chain (less efficient in oil-wetting) tend to orient perpendicular on the calcite surface. For mica, the orientation was probably parallel for all fatty acids.111 The reported results stated that long-chain fatty acids, and long-chain naphthenic acids have a main contribution to oil-wetness while unsaturated acids have minor effects.95 Oleic acid is found to have higher water affinity than heptanoic acid, stearic acid, 18-phenoloctadecanoic acid, and 18-cyclohexyloctadecanoic acid.115 There is a study showing long-chain acids and short-chain acids having opposite wetting impacts on sandstone and carbonate with a decreasing salinity.36
Several studies on specific functional groups suggest that increasing the alkyl chain length in carboxylic acids enhances wettability alteration potential.95,112 Naphthenic acid containing two rings (decahydro- naphthelen-pentanoic acid) are more effective at altering wettability than naphthenic acid containing only one ring (cyclohexane-pentanoic acid).110 Oleic acid with a double bond in its alkyl chain exhibits adsorption on calcite lower than that of oleic acid without a double bond. The presence of a double bond makes the molecule more polar, reactive, and less hydrophobic, leading to nonlinear adsorption and a reduced adsorption amount.95 An increase in the benzene ring number in 1-naphalenacetic acids decreases their oil-wetting potential. The addition of benzene rings increases the reactivity and polarity of organic acids. However, it may affect the orientation of molecules on the surface and reduce the amount of adsorption.95
2.2. Impact of Rock Minerals
Rock mineralogy has a fundamental impact on wettability, partially because of the surface charge difference between different minerals. This effect is minimal when the oil lacks polar components.81 Techniques like QEMSCAN (quantitative evaluation of materials by scanning electron microscopy)116 and XRD117 are convenient for quantifying rock mineral composition.
When treated with the same oil components, sandstone and carbonate vary in the resulting wetting conditions.62 Studies have used specific materials to restore oil-wetness in different rock types: organic acids for carbonate and organochlorosilane for sandstone.97 Carbonate has a basic surface. Sandstone has an acidic surface. This fundamental difference contributes to the organic adsorption difference.62 Calcite is positively charged at pH < ∼9, while sandstone is generally negatively charged at pH > ∼2.95,109 The main oil-wetting materials for carbonate rocks are found to be the negatively charged acidic polar organic components (POC), i.e., carboxylate,95 which adsorb as anions at pH above 4.5–5.118 For sandstone rock, basic POC components play a larger role.109 The adsorption of both types occurs rapidly, within the first pore volume of oil injection during crude oil flooding tests.109
Compared to calcite, dolomite appears more hydrophobic based on contact angle measurements on calcite and dolomite samples (∼70°difference)119 and mixed dolomite/calcite systems (22.1°difference).120 Aromatics and resins have a higher affinity to quartz than calcite.81 Clay contents affect the rock wettability significantly, possibly due to its predominant contribution to the rock surface.116 Clay content on carbonate121 and sandstone116 rock surfaces can increase organic material adsorption. When sandstone outcrops contain a limited amount of clay, they have a low organic adsorption capacity and no apparent adsorption affinity for acidic or basic components. When it contains a larger amount of clay minerals, however, the adsorption capacity is larger, and there is a clear affinity for basic components.93
2.3. Impact of Brine
Water in the form of water film75 (with a simulated thickness of around 0.5 nm122) adsorbed on rock surface can largely inhibit the adsorption of organic materials,69,70,96 though a difference between the adsorption of asphaltenes and organic acids is noted when water is present.123 The stability of the water film can be described using the DLVO (Derjaguin and Landau’s and Verwey and Overbeek’s) theory124,125 of colloidal stability and its extensions.11 The rapture of water film is considered necessary for significant wettability alteration of rock by crude oil.75 The presence of water is found to enhance fatty acid adsorption on calcite,123 whereas it prevents the adsorption on mica.111 In the absence of water, crude oils with asphaltenes can change the wettability of dry glass in less than a day, with minimal impact from the temperature and time. With water, the wettability alteration is much slower.75 When treated with asphaltenes, Berea sandstone samples of higher initial water saturation showed a weaker oil-wetness.6
Three main parameters of the brine affect the oil-wetting process: salinity, ion type, and pH value. Salinity is an important factor, though results are sometimes conflicting for both sandstone36,66 and carbonate rocks. In one study, substrates treated with seawater or formation brine (230 kppm, 50–132.5 °C, 200–2000 psi), in the crude oil/brine/carbonate systems, became oil-wet, while those treated with deionized water became water-wet.126 This indicates that brine with a higher salinity has a weaker resistance to wettability alteration caused by crude oil. However, in another study, decreasing salinity (2.87–287 kppm, 60 °C, 2000 psi) increased oil-wetness.127 There are many common conditions in these two studies, yet they gave conflicting results, probably due to differences in the following. (1) Rock sample mineralogy: the reported mineralogy of pink desert limestone128,129 and Silurian dolomite130,131 features no quartz content, unlike the calcite (98.1% calcite, 0.7% quartz, and 1.2% ankerite) used in the later study. (2) Crude oil density: The latter study used oil with higher density, which means a larger proportion of polar heavy components, increasing oil-wetting potential. (3) AN/BN: the AN/BN value of crude oil in the latter study was around 0.5. Since base components more easily alter sandstone wettability, this may explain the discrepancy. (4) Brine composition: sometimes the impact of brine composition is more significant than that of salinity.80 There is a significant difference in the portion of divalent ions in the two brines.
In another experimental report, natural surfactants and other amphiphilic macromolecules, instead of asphaltenes, became the main oil-wetness contributors at high pH and high salinity case.53 When model oils of organic acids and decane were used, lowered salinity increased oil-wetness in long-chain acid cases and decreased it in short-chain acid cases,36 possibly due to an optimal salinity for maximum wettability alteration, which is usually much lower than formation brine and seawater salinities.132 A uniform shrinkage of the brine film was observed with an increasing brine salinity in sandstone/crude oil/brine systems,133 which could be due to the stronger polarity of the aqueous phase promoting aromatic and resin adsorption,81 or greater attraction between oil/brine and brine/mineral interfaces.133 Zeta potential experiments find that when salinity is decreased, the negative charges on both the oil/brine and the brine/mineral interfaces increase,134 which leads to a stronger repulsion between the two interfaces, and thus a more water-wet condition.
The type of ion also plays a significant role in oil-wetting, sometimes more so than oil composition. A study showed that stearic acid adsorption was more affected by Ca2+ in brine than by the POC.135 Mg2+ may induce ion binding and increase the oil-wetness.136 After treating with asphaltenes, the rock wettability varies from oil-wet to water-wet, depending on the brine composition.75 Researchers observed the impact of water in the lab that when rock samples were pretreated with a small amount of brine, the properties of deposited oil films were largely influenced by the brine composition.137 Iron ions can be a particularly powerful mordant to link organic molecules to the rock surface.138 The removal of iron ions from the core sample was inferred as the main reason that the sample remained water-wet after the oil-aging process.59 Cations may slow down the adsorption of positively charged organic molecules on quartz surfaces due to competitive adsorption.139
A higher divalent cation ratio can strengthen the attraction between the oil/brine and the brine/mineral interfaces, resulting in a more oil-wet condition.133 Multivalent ions are found to promote the adsorption of asphaltenes6 and organic acids.135 The higher the cation valency, the greater the adsorption and oil-wetness.6 A lot of studies have been done to see the impact of common salts or ions. For clay minerals, kaolinite, montmorillonite, Illite, and chlorite, the presence of salts in brine enhances the organic material adsorption in a sequence of NaHCO3 < Na2SO4 < NaCl < MgCl2 < CaCl2.121
pH controls surface chemistries at oil/brine and brine/rock interfaces140 and is a dominant factor in crude oil adhesion. The charges of the organic bases and acids are dependent on water pH (see eqs 2 and 3).93 The number of oil surface chemical groups also depends strongly on the pH, as shown in Figure 3. With an increasing pH in the range of 1–12, the zeta potential of crude oils reduces from +50 mV to −150 mV.141
| 2 |
| 3 |
Figure 3.
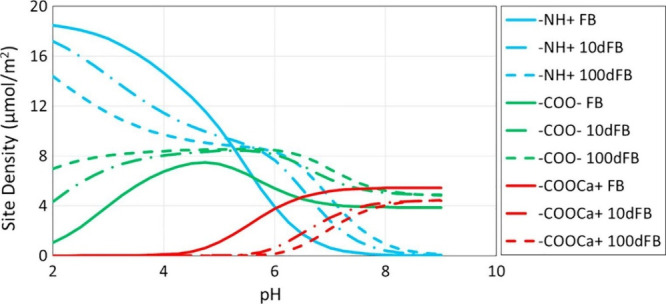
Number of oil surface chemical groups versus pH and dilution. Reprinted from ref (127). Copyright 2018 Elsevier.
Twenty out of twenty-two crude oil samples changed from adhesion to nonadhesion behavior when the pH value increased over a narrow range of about 6.5 ± 2.91 H+ adsorption at the oil–brine and brine–carbonate interfaces has been reported to result in a strong water-wetness. At low pH (i.e., pH < 5), this effect dominates the wettability alteration, making the effect of salinity, ionic strength, and ion type, less important.134,140 However, other studies have found that a lower pH can lead to stronger oil-wetness, especially when the oil has a high TBN.127 A higher pH (i.e., pH = 12) can deprotonate the stearic acid and reduce its concentration at the oil/brine interface, thus resulting in a weaker oil-wetness.135 When pH increases, the adhesion force between the nonpolar oil group (−CH3) and calcite decreases.142 The zeta potential of both the nonpolar group (−CO3)-brine and calcite-brine interfaces becomes more negative,142 leading to a stronger repulsion between the two interfaces.
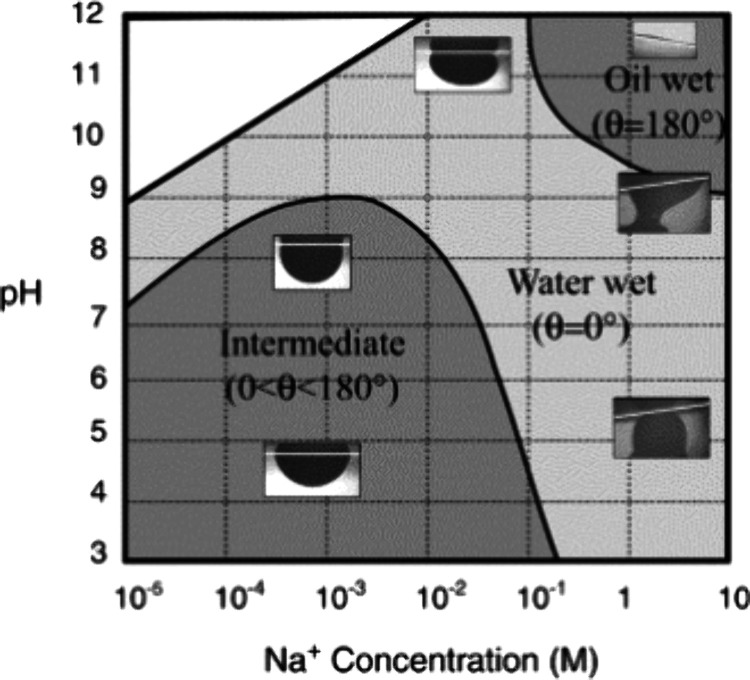
A 2D wettability map, obtained by aging mica with live crude oil under different salinity and pH conditions, demonstrates the impact of pH on the oil-wetting process53 in a straightforward way, as shown in Figure 4. With a pH of 9–12, and Na+ concentration between 10–1∼10 M, the rock was oil-wet. For pH between 3–9 and Na+ concentration between 10–5∼10–1, the rock was mostly intermediate-wet with partial rupture of the water film. Other conditions tested showed water-wetness. The thin water film was found to be stable, likely due to enhanced electrostatic repulsion between surfaces by high pH and high salinity.53
Figure 4.
Wettability map based on static contact angle measurements for live crude oil on mica in aqueous NaNO3 and NaCl solutions. Reprinted from ref (53). Copyright 2002 Elsevier.
Indeed, the salinity, ion type, and pH interact with each other. For instance, the impact of certain salts is dependent on pH. One study relates Mg2+ to more water-wetness at pH values below and above 7. However, it shows SO42– increases water-wetness at pH < 7, while increasing oil-wetness at pH > 7.110 On the other hand, the impact of pH value is dependent on the ion type. In the NaCl solution, an increased pH led to less oil-wetness. In the CaCl2 solution, however, this phenomenon was not observed.143 At a moderate pH value (i.e., 8), the impact of the salinity and ion type can play a major role in the oil-wetting process. At a low pH, however, the impact of salinity and ion type becomes insignificant.
With the recent development of the LSW (low salinity water) technique,144 the wettability alteration potential of brine has been intensively studied. Brine ions are found to change rock wettability.145 Several possible mechanisms have been proposed for the effects of LSW,146 which may depend on timing, including the formation of microdispersions (water particles wrapped by organic materials) with polar agents enriched with nitrogen, sulfur, and aromatic rings in the crude oils,82 the change of zeta potential on the rock surface147 and thus the removal of organic materials,146,148 and so on. Ions can affect the surface potential, thus affecting the adsorption of organic materials. Potential determining ions (PDI) for carbonate (Mg2+, Ca2+,149 and SO42–)112,148,150,151 are proven to have promising efficiency in the removal of organic materials from carbonate surface,147,149,152 among which sulfate might be the most efficient one.78,149 Mg2+ and Ca2+ are found to adsorb more on clay than on silica, relating the efficiency of LSW with clay content.153 There are also studies reporting negative results brought by some ions.73 Besides, other ions, including K+,147 Mn2+,152 and so on, have also been studied. Aside from their impact individually, the interactions among ions can result in different wettability alteration extents.154
Apart from the main parameters, there are other properties of brine, such as the oxidation of brine,143 also affecting the oil-wetting process. Brine properties not only directly affect the oil-wetting process of the rock but also significantly impact the effect of temperature.155 In one study on the calcite/crude oil/brine system, the temperature effect was observed to be strongly dependent on NaCl concentration, while a weak dependence was observed in the MgCl2 case.155
2.4. Impact of CO2 and H2
Considering the CO2 EOR and CO2 sequestration,156−158 it is crucial to understand the impact of CO2 on rock wettability.
CO2 exists as a supercritical phase above its critical temperature (31 °C) and critical pressure (7.38 MPa). Applications of CO2 are typically either in supercritical condition159 or as emulsion.160 It can largely increase the salinity of brine to the extent of triggering salt precipitation near sc-CO2/brine interfaces by the dry-out effect.161 It can also reduce the brine pH to around 5,162 resulting in water-wet conditions.140 The effect of CO2 in increasing the water-wetness of the rock is supported by much research163−165 and is found to be enhanced at lower temperatures or higher pressures, due to the increased solubility of CO2 under these conditions.166 Besides, CO2 can change the properties167,168 and the composition169 of crude oil by dissolving into crude oil and by extracting oil components. Adding CO2 destabilizes asphaltenes in the crude oil,98,170,171 resulting in a more oil-wet condition. The wettability impact of CO2 is largely dependent on the oil composition.
Apart from CO2, H2 storage has also gained a lot of attention in the recent decade.172 Although H2 storage in depleted oil reservoirs is a viable option, some research considers combining H2 storage and oil recovery.173 H2 itself does not directly affect rock wettability,174 but its solubility in crude oil172 can modify oil properties and thereby affect the oil-wetness. In addition, the temperature and pressure conditions during H2 injection also influence rock wettability.
2.5. Impact of Temperature
The temperature impacts the oil components’ stability and the interfacial behavior of the oil/water/rock system, thus significantly affecting rock wettability. With an increasing temperature, the oil/water IFT in a glass cell decreases, while the oil/water IFT in a high-pressure cell increases.175 This phenomenon may be explained by a combination of oxidation (reducing IFT) and temperature increase (reducing surfactant concentration at the interface).175
An increase in temperature alters rock more water-wet as tested in Berea sandstone during waterflooding in the range of 22–75 °C,66 in quartz by contact angle measurements in the range of 25–150 °C,97 in calcite in the range of 25–130 °C176 and 25–65 °C.155 The underlying mechanism is that adsorbed organic materials dissolve at higher temperatures, thus reducing the oil-wetness.97,118 Increased temperature accelerates the adsorption and rearrangement of crude oil components.177 During oil-aging, an increase in temperature has been reported to enhance the oil-wetness of silicate, sandstone, and carbonate rocks.36,178 Higher temperature can also destabilize wettability modifiers in the oil/brine/rock system, leading to an enhanced oil-wetness.30 Temperature increase is found to enhance the impact of organic acids on rock wettability for Berea, chalk, dolomite, and limestone.36
There are other aspects to consider: (i) increased temperature enhances mineral dissolution,118,179 altering formation brine composition and affecting the rock wettability; (ii) temperature also affects the partition coefficient of organic acids in the oil/water system. Organic acids tend to have higher partition coefficients at lower temperatures. The partition coefficient of saturated organic acids are more affected by temperature than that of unsaturated ones;176 (iii) below the bubble point of crude oil, temperature increase decreases asphaltenes solubility. Above the bubble point, the temperature increase enhances asphaltenes solubility. As a result, the rock wettability can have drastic changes with changing temperature;180 and (iv) responses of different minerals to the temperature change can differ. For example, contact angle changes with a changing temperature are found to be different in calcite and quartz cases,181 calcite turns more water-wet with an increasing temperature, while dolomite may turn more oil-wet.119
2.6. Impact of Pressure
Pressure has a less pronounced effect on rock properties than temperature. For model oil systems, pressure changes between 0.1 and 50 MPa had little impact on the wettability of n-decane/water/mica or n-decane + stearic acid/water/calcite systems.123 Similar findings were reported for stock tank oil/brine/quartz or calcite mineral systems at 66 °C, 600 psi,182 and crude oil/brine/calcite systems at 50 °C, 15 MPa.119 However, pressure changes can impact rock wettability by altering the oil composition. For instance, when pressure drops, some oils may have asphaltene precipitation.98 An experiment showed that with a pressure reduction from 2500 to 500 psi, the oil/water contact angle on the pure dolomite surface increased from 111.2° to 135.5°.120
2.7. Impact of Surface Roughness and Aging Time
The impact of roughness on surface wettability and wettability measurement is well-recognized183 but not yet fully understood.184 Greater rock roughness may limit the contact between rock and the introduced fluid, reducing or delaying the wettability alteration.183 Increased convexity of the rock surface can rupture the water film covering the rock surface, exposing it to oil and promoting oil-wetting.4 An AFM study showed that organic materials tend to adsorb along the convex edges on calcite surface.7
Aging time can be understood as the reaction time of the oil/water/rock system. Oil-wetness increases, either rapidly or gradually, until reaching a plateau, after which further aging does not increase oil-wetness.185 Depending on the specific condition, the relationship between aging time and rock oil-wetness can vary greatly.186 For example, dry rock generally requires a much shorter time than rock with connate water to reach the oil-wet status. Surface wetting is faster and easier than the wetting of the internal.99 Saputra et al. compiled the data from 44 shale EOR publications and obtained a wide aging time range of 0 days ∼1 year.81Table 3 summarizes studies involving the oil-aging process under different conditions.
Table 3. Collection of Oil-Wetting Results from Experiments under Varying Conditions.
| Sr. no. | Rock | Organic materials | Water saturation | Salinity | pH | Temperature (°C) | Pressure | Aging time | Testing method | Aging results | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Berea sandstone | Crude oil | 0.15–0.261 (Swi) | 22.2 g/L | NA | 88 | Ambient | 10 days | Amott test | Oil-wetness increased with time gradually | (187) |
| 2 | Berea sandstone | Asphaltenes | ∼0.25 (Swi) | 0.09–0.51 M | 3.3–11.2 | Ambient | Ambient | 10 days | Amott test | Oil-wetness increased rapidly in the first 12 h | (6) |
| 3 | Silica and calcite crystals | Crude oil | 0.155–0.408 (connate water) | Synthetic formation brine | NA | 100 | NA | 2255 h | Contact angle and relative permeability | Some reservoir rock became oil-wet after 300 h; some reservoir rock remained water-wet after 2255 h | (185) |
| 4 | Berea sandstone | Moutray crude oil | 0–0.5 | 2.5–3.0% | 6.80, 6.85 | Ambient | Ambient | 20 days | Amott test | Initially, strongly water-wet rock became weakly water-wet | (188) |
| 5 | Berea sandstone | Crude oil | 0.192–0.393 | 22.2–38.6 g/L | NA | Ambient ∼75 | NA | 75 days | USBM test | Water-wet changed to mixed-wet conditions; aging time beyond 10 days does not have a significant impact | (46) |
| 6 | Berea sandstone | Loudon crude oil | 0.2–0.3 (Swi) and ∼0.75 | 21507 g/cm3 | 6.6–6.89 | 24.5 | NA | 54 days | USBM test | Strongly water-wet to mixed-wet | (59) |
| 7 | Berea sandstone (treated to a reduced state) | Loudon crude oil | 0.2–0.3 (Swi) and ∼0.75 | 21507 g/cm3 | 6.6–6.89 | 24.5 | NA | 54 days, 196 days | USBM test | Remained water-wet after 54 days, but changed to mixed-wet after 196 days | (59) |
| 8 | Iceland-spar calcite | n-decane + organic acids | 0 (mineral plate) | NA | NA | 25–130 | NA | 1 day | Contact angle | When the temperature was higher, calcite was more water-wet | (176) |
| 9 | Calcite | Crude oil | 0.295–0.348 (connate) | NA | NA | 65 | NA | 3 days | Relative permeability and contact angle | Intermediate-wet sample became weakly oil-wet | (52) |
| 10 | Calcite | 1 wt % stearic acid + toluene | 0 | 206911 | NA | 90 | NA | 5 days | Contact angle | Intermediate-wet | (112) |
| 11 | Dolomite | Crude oil | 0 (dry) | 100072.5 ppm | NA | 75 | NA | 40 days | Contact angle | Rock became strongly oil-wet | (147) |
| 12 | Shale | Crude oils | 0 | 1676–307213 ppm | NA | 76.7 | NA | 49 days | Contact angle | A minimum of 35 days is required to reach a stable wettability condition | (81) |
| 13 | Pure chalk, sandstone, and silica-containing chalk | Crude oil with <1% of asphaltenes | 0.1 | 62830, 50000 | 6.7, 5.8 | 50 | 20 bar, 10 bar | 3 days | Spontaneous imbibition | Water-wetness for all cases was significantly reduced | (109) |
| 14 | Chalk | Crude oil | 0 | 44.94 g/L | 8.0 | 50 | Ambient | 4 days | Spontaneous imbibition | Chalk plugs were unevenly aged, with the outer layer oil-wet and the center water-wet | (99) |
3. Advanced Computational Approaches for the Wettability Study
Given the complex factors affecting rock oil-wetness and uncertainties in restoring the rock surface condition as well as inherent rock heterogeneity, performing experiments that isolate a single factor while controlling others is extremely challenging. Numerical simulation can address these limitations by avoiding the multifactor complications inherent in experiments, allowing the characterization of the influence of different factors on wettability.189 Numerical simulation has shown its potential in various aspects related to wettability, such as the dynamics of droplet motion on wettability-graded surfaces190−192 and in microchannels,193 the relationship between the charges on the oil/brine, as well as the brine/rock, interfaces and rock wettability,194 the impact of impurities, pH, and PDI concentration on rock wettability,57 using methods including the Finite Element Method (FEM),195 Lattice Boltzmann Method (LBM),196 etc. Computational Fluid Dynamics (CFD),197 numerical simulations emphasizing fluid flow in porous media, is widely used to study the impact of wettability and temperature on oil recovery198 and the effectiveness of surfactants,199,200 nanofluids,197 carbonated water,201 and so on, as EOR materials.
Molecular Dynamics Simulation (MDS) is very helpful in understanding the structure, dynamics, and thermodynamics of surface physiochemistry, encompassing aspects such as surface chemistry, energy, and contact angles. MDS provides a detailed atomistic view, exploring the static and dynamic aspects of such phenomena on an atomic scale. Various MDS scenarios have been explored to understand interactions and determine wettability, using contact angle experiments and MDS calculations on different surfaces.202 It has been applied in studying the impact of CO2 on the shale,203 kerogen,204 graphene wettability,205 clay,206,207 the impact of increasing salinity on reducing the water-wetness of sandstone,189 the impact of increasing temperature on promoting the adsorption of asphaltenes on quartz,208 the impact of surfactants on changing rock wettability,209 the interactions between particles,210 the adsorption of organic molecules on the mineral surface,211,212 clay,213 and so on.214−216
Machine learning enables the discovery of relationships and dependencies among wettability parameters, in addition to predicting outcomes, resulting in the design of optimized systems.217 Various ML techniques, including artificial neural network (ANN), linear regression (LR), nonlinear regression (NLR), random forests (RF), function networks (FN), adaptive neuro-fuzzy inference systems (ANFIS), and support vector machines (SVM), are capable of forecasting certain parameters using easily accessible data without incurring additional expenses.218−220 One common technique in this paradigm is artificial neural networks (ANN). ANN is nowadays extensively used in wettability predictions221−226 without complex extensive programming.227
In conclusion, numerical simulations, MDS, and ML offer faster, more cost-effective, and powerful alternatives to complex experimental analyses. Table 4 summarizes previous studies that have used MDS, CFD, and ML for wettability evaluations, outlining the primary objectives, types of simulations used, simulation tools and software applied, and the key findings of each study.
Table 4. Comparative Analysis of Wettability Measurement Techniques Using MDS, CFD, and ML Approaches.
| Type | Objective | Algorithm | Key finding | Ref |
|---|---|---|---|---|
| MDS | Studying nanoscale wettability of different rock surfaces for ore flotation and oil recovery | Condensed-phase Optimized Molecular Potentials for Atomistic Simulation Studies (COMPASS) force-field, Andersen thermostat, and Ewald summation methods | Rock wettability ranks as gypsum > calcite > halite > silica > graphite | (58) |
| Silica’s water contact angles change with oil polarity, ranging from 58° in hexane to 118° in toluene | ||||
| To utilize MDS for simulating some phenomena at a molecular level (oil/brine/rock system) and develop a model that correlates free energies with contact angles | Forcite module in Materials Studio, incorporating the COMPASS force field and Nosé–Hoover thermostat | The model accurately predicts experimental results (R2 ∼ 0.98) | (228) | |
| MDS effectively and economically assesses sandstone wettability changes | ||||
| Analyzing shale organic matter’s wettability under reservoir conditions | COMPASS force field | Water becomes more wettable with rising temperature under certain pressures | (203) | |
| Water wettability drops with higher salinity; Mg2+ and Ca2+ ions affect wetting more than Na+ | ||||
| Studying how interface wettability affects Lennard-Jones fluid flow in nanochannels via molecular dynamic simulations | Non-Equilibrium Molecular Dynamics (NEMD) simulation with a modified Lennard-Jones potential | Wettability greatly affects interfacial hydrodynamic resistance | (229) | |
| Near solid walls, temperature and pressure profiles vary due to wettability, with higher interfacial friction increasing temperatures | ||||
| Assessing wetting properties and adsorption capacities of surfactants (ALES, SLES, TD, SDS) on coal dust, by using MD simulations and quantum calculations | Amorphous cell module | Wetting performance is mainly influenced by surfactants’ molecular structure, EO groups, and hydrolytic cations | (230) | |
| NH4+ in ALES and the combined hydrophilicity of EO groups and SO4– in ALES and SLES greatly improve their wetting and adsorption on coal dust | ||||
| To simulate nano water droplets’ wetting behavior on flat and pillar surfaces using molecular dynamics | Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) with Lennard-Jones potential and SPC/E water model | Higher surface energy on flat surfaces reduces the contact angle, indicating better wettability | (231) | |
| Nanostructured pillar surfaces are more hydrophobic than flat ones, with sparser pillars increasing the contact angle | ||||
| To assess how reservoir temperature and kerogen structure affect shale kerogen wettability | LAMMPS with Lennard-Jones potential and SPC/E water model | Kerogen contact angles vary by type and maturity, with Type II being more water-wet and Type III the least | (232) | |
| Rising reservoir temperatures decrease contactangles by 62%, increasing water-wettability | ||||
| CFD | To study the dynamic wetting and wrapping process of coal dust by spray droplets | A numerical simulation study focusing on the dynamic wetting and wrapping process of coal dust particles | The study found that a particle size ratio (θ) over 2 between droplets and dust allows droplets to fully cover and wet coal dust, while a ratio under 1 results in ineffective wetting | (233) |
| To determine the optimal conditions for effectively wetting and settling respirable dust in mining environments | Droplet velocity is key to wetting efficiency; lower velocities cause poor coverage, but at around 20 m/s, wetting becomes faster and more effective | |||
| To evaluate how surfactant-based nanofluids change wettability and their impact on enhancing oil recovery | multicomponent multiphase, compositional model | Surfactant-enhanced nanofluids can shift rock wettability in tight oil reservoirs from oil-wet to water-wet; this change improves capillary pressure and permeability, aiding in increased oil extraction | (234) | |
| To understand how self-propelled droplets on superhydrophobic surfaces with varying wettability control their jumping direction | The interFoam solver in OpenFOAM which is based on the VOF model | Surface energy gradients, influenced by wettability, direct droplet movement toward smaller contact angles | (235) | |
| To examine how reservoir wettability, pore geometry, and fluid viscosity ratios impact relative permeability | LBM for simulating two-fluid flow in porous media | Increasing reservoir rock wettability from neutral to strong reduces the wetting fluid’s relative permeability | (236) | |
| This effect on the wetting fluid’s permeability diminishes at higher viscosity ratios, while the nonwetting fluid’s permeability is significantly higher in strongly wet conditions compared to neutral wettability | ||||
| To assess how silicon oxide nanoparticles, affect wettability in glass micromodels to boost oil recovery | CFD (with method performed using Ansys Fluent software) | Initial wettability significantly affects oil saturation and recovery | (197) | |
| Nanofluid-induced wettability alteration is time-dependent | ||||
| Silica nanoparticles form a nanotexture coating on solid surfaces, changing the wettability from oil-wet to intermediate-wet, enhancing oil recovery | ||||
| To explore microtexture lubrication mechanisms through the lens of interface wettability | CFD | Isotropic textures with low skewness and high kurtosis improve wettability and spreading | (237) | |
| Groove-shaped textures cause anisotropic spreading through continuous triple contact lines, improving wettability | ||||
| To evaluate the wettability of silicon microstructures made | CFD with VOF model | The VOF model accurately simulates macroscopic contact-angle characteristics on complex surfaces | (238) | |
| To analyze and understand discrepancies between experimental and theoretical contact angles of these surfaces complex surfaces | A 2D CFD model with an equivalent triangular waveform precisely predicts contact angles | |||
| To examine how surface wettability, inclination, liquid properties, and impact velocity affect drop impact and spreading dynamics | VOF model | The Static Contact Angle (SCA) model is precise for less wettable surfaces (SCA > 90°), matching experimental results | (239) | |
| For more wettable surfaces (SCA < 90°), the dynamic contact angle (DCA) is higher initially, requiring the DCA model for accurate predictions | ||||
| ML | To employ ML tools to efficiently determine the contact angle, is crucial for assessing shale wettability in CO2-enhanced oil recovery, CO2 sequestration in saline aquifers, and hydraulic fracturing | LR and RF | The operating pressure, temperature, total organic content (TOC), and mineral matter significantly affect shale wettability | (240) |
| The RF model proved reliable for predicting contact angles, offering an alternative to complex experimental methods in CO2 sequestration applications | ||||
| To improve carbon capture, utilization, and storage by leveraging ANN and ANFIS for estimating the contact angle in coal-water-CO2 systems. | ANN and ANFIS | The ANN and ANFIS models demonstrated high accuracy in predicting contact angles in coal formations | (222) | |
| A new empirical equation derived from the ANN model showed high accuracy, confirming the reliability of machine learning in accurately predicting contact angles | ||||
| To estimate shale wettability, a key factor in CO2-EOR and CO2 storage in shale formations | Decision tree, RF, function networks, and gradient boosting regressor | The operating pressure, temperature, rock mineralogy, and TOC as significant factors affecting shale wettability | (241) | |
| Machine learning models, especially the gradient boosting regressor, were effective in predicting contact angles | ||||
| To explore how rock mineral composition and properties of oil-based drilling fluids affect the wettability of tight gas sandstone formations | General Regression Neural Network (GRNN) | The mineral compositions of quartz, feldspar, carbonate, and clay significantly affect the wettability of tight sandstone | (242) | |
| The GRNN model accurately predicted wettability | ||||
| To evaluate shale wettability based on formation pressure, temperature, salinity, TOC, and theta zero | Multilayer perceptron (MLP) and radial basis function neural networks (RBFNN) | The Radial Basis Function Neural Network-Many-Objective Optimization (RBFNN-MVO) emerged as the most accurate | (243) | |
| Sensitivity analysis identified theta zero, TOC, pressure, temperature, and salinity as key factors influencing shale wettability | ||||
| To assess shale wettability by analyzing critical factors such as formation pressure, temperature, salinity, TOC, and theta zero | MLP and RBFNN | The RBFNN-MVO model was the most accurate among tested ML models for predicting shale wettability | (225) | |
| The RBFNN-MVO model was validated as an efficient and cost-effective tool for shale wettability prediction | ||||
| To assess the wettability of CO2/brine/rock minerals (quartz and mica) in geological formations | Fully connected feedforward neural networks, extreme gradient boosting, k-nearest neighbors, decision trees, adaptive boosting, and RF | FCFNN model was more effective than other machine learning techniques in predicting the wettability of the mineral/CO2/brine system, achieving an R2 above 0.98 and an error below 3% | (224) |
4. Research Gaps
On the basis of the review, the following research gaps are listed. (1) Quantifying the relationship between the polarity of organic components and their wetting potential: Polarity dominates the wetting potential of an organic component. Polar organic molecules, such as asphaltenes and organic acids, are the main oil-wetness contributors in crude oil in most cases. However, no work has quantified the polarity of organic components and relate it to the oil-wetting potential. (2) Quantifying the impact of mineralogy: Rock minerals show different surface charges and other properties in the same condition. As a result, the adsorption of acidic polar organic components presents as the main contributor to oil-wetting carbonate rocks, while the basic polar organic components are more important for sandstone rocks. However, relating the mineralogy, including the portion and distribution of all minerals, to the wettability remains a problem. (3) Formation Water and Temperature Effects: The impact of formation water varies significantly depending on the oil composition. The presence of salts in brine seems to promote the oil-wetting process. However, the impact of salinity is not clear. Among all common ions, the potential determining ions (PDI) usually play the main role. It has been noticed that a higher temperature can fasten the adsorption of organic acids (which increases oil-wetness), while on the other hand dissolving the precipitated asphaltenes on the rock surface (which reduces oil-wetness). (4) CO2 and H2 Injection: CO2 injection increases the salinity in the vicinity of the CO2/brine interface. It also reduces the pH value of the brine, possibly destabilizes asphaltenes, and induces other changes that can affect wettability in different directions. H2 is reported not to affect rock wettability, though its injection can cause changes in temperature and pressure and indirectly change wettability to a limited extent. To date, reports focusing on the wettability implication of CO2 and H2 injection are still limited in number. (5) Aging Time Standardization: Reported aging time varies from 1 day to 1 year, depending on the specific rock, brine, oil, and environmental conditions. There is a need to study this aspect in future studies to set a standard time in couple with influencing factors. (6) Properties of Asphaltenes: More insights are needed regarding the properties, structure, flocculation, and adsorption mechanisms of asphaltenes. Given the interdependence of all factors (oil components, brine, rock minerals, temperature, pressure), numerical simulations and AI tools are essential for understanding these complex systems.
5. Summary
This paper summarizes the impact of materials, including oil, rock minerals, brine, and CO2, H2, as well as environmental factors, including temperature, pressure, and aging time, on the wetting properties. The potential of numerical simulation and Artificial Intelligence tools in the study of wettability mechanisms, factors, and predictions is also emphasized. Although the impact of a specific factor can be very different, sometimes opposite, in different situations, some general understandings about the impact of factors can be given. (i) Polarity of organic components (asphaltenes and long-chain acids) determines oil-wetness in crude oil. Though asphaltenes can render rock oil-wetness by adsorption, asphaltenes’ flocculation is usually a sign of strong oil-wetness. For organic acids, the molecular structure is more important than the concentration. Long-chain acids are significantly stronger than short-chain acids. (ii) A lower pH value (pH < 5) usually relates to water-wet conditions and mitigates the effect of salinity and ion type. When pH is higher, its impact is more dependent on the oil composition. Brine injection in low-salinity-waterflooding is reported to make oil-wet rock more water-wet. (iii) Acidic polar organics dominate oil-wetting in carbonate rocks; basic polar organics are key in sandstone. (iv) Environmental factors like water films, brine salinity, and pH influence wettability significantly.
The information and insights provided in this article can help researchers prepare oil-wet rock surfaces, especially when multiple factors are required. Besides, with the increasing interest in CCUS (carbon capture, utilization, and storage) and hydrogen storage, wettability change induced by these gases requires a deeper understanding. This work organized information presented in the literature to briefly explain the main mechanism of the wettability change caused by CO2 and H2. Last but not least, considering the complexity of rock surface wettability as a parameter impacted by many factors, as well as the difficulty in conducting a control study by experiments, this paper emphasizes the need and potential of applying computational approaches for wettability study.
Acknowledgments
The authors would like to thank College of Petroleum Engineering & Geoscience (CPG) at King Fahd University of Petroleum & Minerals for providing resources to complete this work.
Glossary
Abbreviations
- 2D
Two-dimensional
- AFM
Atomic force microscopy
- AI
Artificial intelligence
- AN/BN
Acid number/base number
- ANFIS
Adaptive neuro-fuzzy inference systems
- ANN
Artificial neural network
- API
American Petroleum Institute
- BTX
Benzene, toluene, and xylene
- CCUS
Carbon capture, utilization, and storage
- CMC
Critical micelle concentration
- CFD
Computational fluid dynamics
- DCA
Dynamic contact angle
- DLVO
Derjaguin and Landau’s and Verwey and Overbeek’s
- EDL
Electrical double layer
- EO
Ethoxylate
- EOR
Enhanced oil recovery
- ESI
Electrospray ionization
- FEM
Finite element method
- FCFNN
Fully connected feedforward neural network
- FN
Function networks
- FT-ICR-MS
Fourier transform-ion cyclotron resonance-mass spectrometry
- FTIR
Fourier transform infrared
- GC-MS
Gas chromatography-mass spectrometry
- GRNN
General regression neural network
- IFT
Interfacial tension
- IGC
Inverse gas chromatography
- LAMMPS
Large-scale atomic/molecular massively parallel simulator
- LBM
Lattice Boltzmann method
- LR
Linear regression
- LSW
Low salinity water
- MDS
Molecular dynamics simulation
- ML
Machine learning
- Micro-CT
Microcomputed tomography
- MRI
Magnetic resonance imaging
- MVO
Many-objective optimization
- NEMD
Nonequilibrium molecular dynamics
- NLR
Nonlinear regression
- NMR
Nuclear magnetic resonance spectroscopy
- PDI
Potential determining ions
- POC
Polar organic components
- QCM
Quartz crystal microbalance
- QCM-D
Quartz crystal microbalance with dissipation
- QEMSCAN
Quantitative evaluation of materials by scanning electron microscopy
- RBFNN
Radial basis function neural networks
- RF
Random forests
- SAC
Solid adsorbent chromatography
- SARA
Saturates, aromatics, resins, asphaltenes
- SCME
Sulfuric acid-methanol extraction
- SCA
Static contact angle
- SEM
Scanning electron microscope
- SVM
Support vector machines
- TAN
Total acid number
- TBN
Total base number
- TGA
Thermogravimetric analysis
- TLC-FID
Thin-layer chromatography–flame ionization detection
- TOC
Total organic carbon
- USBM
U.S. Bureau of Mines
- UV
Ultraviolet
- VOF
Volume-of-fluid
- XRD
X-ray diffraction
Author Contributions
X.D.: Conceptualization and writing – original draft. A.B.: Writing – original draft. M.S.K.: Supervision. A.R.: Methodology. S.P.: Supervision. X.Z.: Methodology. M.M.: Supervision. S.M.S.H.: Supervision.
This research adheres to the highest ethical standards in accordance with established guidelines and principles. Informed consent was obtained from all participants involved in the study, ensuring their voluntary participation and understanding of the research objectives. Any potential conflicts of interest were disclosed and managed appropriately to uphold the credibility and integrity of the research findings. This ethics statement serves as a testament to our commitment to upholding ethical standards and safeguarding the rights and well-being of all individuals involved in this research endeavor.
The authors declare no competing financial interest.
References
- Cuiec L.Rock-Crude-Oil Interactions and Wettability: an Attempt To Understand Their Interrelation. 59th Annual Society of Petroleum Engineers of AIME Technical Conference, Houston, TX, Sep 16, 1984; Vol. SPE13211, 10.2118/13211-ms. [DOI]
- Mitchell A. G., Hazell L. B.; Webb K. J.. Wettability determination. Pore surface analysis. in Proceedings - SPE Annual Technical Conference and Exhibition; OnePetro, 1990; pp 351–360.
- Deng X.; Kamal M. S.; Patil S.; Hussain S. M. S.; Zhou X. A Review on Wettability Alteration in Carbonate Rocks: Wettability Modifiers. Energy Fuels 2020, 34, 31–54. 10.1021/acs.energyfuels.9b03409. [DOI] [Google Scholar]
- Hirasaki G. J. Wettability: fundamentals and surface forces. SPE Form. Eval. 1991, 6, 217–226. 10.2118/17367-PA. [DOI] [Google Scholar]
- Yan J. N.; Menezes J. L.; Sharma M. M. Wettability alteration caused by oil-based muds and mud components. SPE Drill. Complet. 1993, 8, 35–44. 10.2118/18162-PA. [DOI] [Google Scholar]
- Yan J.; Plancher H.; Morrow N. R. Wettability changes induced by adsorption of asphaltenes. SPE Prod. Facil. 1997, 12, 259–266. 10.2118/37232-PA. [DOI] [Google Scholar]
- Marcano M. C.; Kim S.; Becker U. Surface interaction of crude oil, maltenes, and asphaltenes with calcite: An atomic force microscopy perspective of incipient wettability change. Appl. Geochem. 2020, 113, 104501. 10.1016/j.apgeochem.2019.104501. [DOI] [Google Scholar]
- Zhang Y.; et al. Pore-scale imaging of asphaltene deposition with permeability reduction and wettability alteration. Fuel 2022, 316, 123202. 10.1016/j.fuel.2022.123202. [DOI] [Google Scholar]
- Thomas M. M.; Clouse J. A.; Longo J. M. Adsorption of organic compounds on carbonate minerals. 1. Model compounds and their influence on mineral wettability. Chem. Geol. 1993, 109, 201–213. 10.1016/0009-2541(93)90070-Y. [DOI] [Google Scholar]
- Marcano M. C.; Kim S.; Taylor S. D.; Becker U. Exploring wettability by imaging the adsorption of crude oil, re-dissolved asphaltene, and phenol solutions onto calcite. Implications to sorption mechanisms and molecular structure of surface-active compounds in crude oil. Chem. Geol. 2019, 525, 462–478. 10.1016/j.chemgeo.2019.07.030. [DOI] [Google Scholar]
- Tian H.; Wang M. Electrokinetic mechanism of wettability alternation at oil-water-rock interface. Surf. Sci. Rep. 2017, 72, 369–391. 10.1016/j.surfrep.2018.01.001. [DOI] [Google Scholar]
- Buckley J. S.; Liu Y.; Monsterleet S. Mechanisms of Wetting Alteration by Crude Oils. SPE J. 1998, 3, 54–61. 10.2118/37230-PA. [DOI] [Google Scholar]
- Buckley J. S.; Liu Y. Some mechanisms of crude oil/brine/solid interactions. J. Pet. Sci. Eng. 1998, 20, 155–160. 10.1016/S0920-4105(98)00015-1. [DOI] [Google Scholar]
- Setiawan A.; Suekane T.; Deguchi Y.; Kusano K. Three-Dimensional Imaging of Pore-Scale Water Flooding Phenomena in Water-Wet and Oil-Wet Porous Media. J. Flow Control. Meas. & Vis. 2014, 02, 25–31. 10.4236/jfcmv.2014.22005. [DOI] [Google Scholar]
- Adibhatla B.; Mohanty K. K. Oil recovery from fractured carbonates by surfactant-aided gravity drainage: Laboratory experiments and mechanistic simulations. SPE Reserv. Eval. Eng. 2008, 11, 119–130. 10.2118/99773-PA. [DOI] [Google Scholar]
- Aghajanzadeh M. R.; Ahmadi P.; Sharifi M.; Riazi M. Wettability modification of oil-wet carbonate reservoirs using silica-based nanofluid: An experimental approach. J. Pet. Sci. Eng. 2019, 178, 700–710. 10.1016/j.petrol.2019.03.059. [DOI] [Google Scholar]
- Nazari Moghaddam R.; Bahramian A.; Fakhroueian Z.; Karimi A.; Arya S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels 2015, 29, 2111–2119. 10.1021/ef5024719. [DOI] [Google Scholar]
- Robin S.; Mohanty Kishore K.. Foams with wettability-altering capabilities for oil-wet carbonates: A synergistic approach. In SPE Journal; Society of Petroleum Engineers, 2016; Vol. 21, pp 1126–1139. [Google Scholar]
- Liu J.; Sheng J. J.; Wang X.; Ge H.; Yao E. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. 10.1016/j.petrol.2019.01.003. [DOI] [Google Scholar]
- Yekeen N.; Padmanabhan E.; Syed A. H.; Sevoo T.; Kanesen K. Synergistic influence of nanoparticles and surfactants on interfacial tension reduction, wettability alteration and stabilization of oil-in-water emulsion. J. Pet. Sci. Eng. 2020, 186, 106779. 10.1016/j.petrol.2019.106779. [DOI] [Google Scholar]
- Chiappa L.; Mennella A.; Lockhart T. P.; Burrafato G. Polymer adsorption at the brine/rock interface: The role of electrostatic interactions and wettability. J. Pet. Sci. Eng. 1999, 24, 113–122. 10.1016/S0920-4105(99)00035-2. [DOI] [Google Scholar]
- Broseta D.; Medjahed F.; Lecourtier J.; Robin M. Polymer adsorption/retention in porous media: effects of core wettability and residual oil. SPE Adv. Technol. Ser. 1995, 3, 103–112. 10.2118/24149-PA. [DOI] [Google Scholar]
- Kowalewski E.; Rueslåtten I.; Steen K. H.; Bødtker G.; Torsæter O. Microbial improved oil recovery-bacterial induced wettability and interfacial tension effects on oil production. J. Pet. Sci. Eng. 2006, 52, 275–286. 10.1016/j.petrol.2006.03.011. [DOI] [Google Scholar]
- Ayirala S. C.; Rao D. N.. Multiphase flow and wettability effects of surfactants in porous media. In Colloids and Surfaces A: Physicochemical and Engineering Aspects; Elsevier, 2004; Vol. 241, pp 313–322. [Google Scholar]
- Ahsani T.; Tamsilian Y.; Rezaei A. Molecular dynamic simulation and experimental study of wettability alteration by hydrolyzed polyacrylamide for enhanced oil recovery: A new finding for polymer flooding process. J. Pet. Sci. Eng. 2021, 196, 108029. 10.1016/j.petrol.2020.108029. [DOI] [Google Scholar]
- Standnes D. C.; Austad T. Wettability alteration in chalk 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J. Pet. Sci. Eng. 2000, 28, 123–143. 10.1016/S0920-4105(00)00084-X. [DOI] [Google Scholar]
- Ehrlich R.; Hasiba H. H.; Raimondi P. Alkaline Waterflooding for Wettability Alteration-Evaluating a Potential Field Application. JPT, J. Pet. Technol. 1974, 26, 1335–1343. 10.2118/4905-PA. [DOI] [Google Scholar]
- Ding H.; Rahman S. Experimental and theoretical study of wettability alteration during low salinity water flooding-an state of the art review. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 622–639. 10.1016/j.colsurfa.2017.02.006. [DOI] [Google Scholar]
- Minakov A. V.; Pryazhnikov M. I.; Suleymana Y. N.; Meshkova V. D.; Guzei D. V. Experimental study of nanoparticle size and material effect on the oil wettability characteristics of various rock types. J. Mol. Liq. 2021, 327, 114906. 10.1016/j.molliq.2020.114906. [DOI] [Google Scholar]
- Cao N.; Almojtaba Mohammed M.; Babadagli T. Wettability alteration of heavy-oil-bitumen-containing carbonates by use of solvents, high-pH solutions, and nano/ionic liquids. SPE Reserv. Eval. Eng. 2017, 20, 363–371. 10.2118/183646-PA. [DOI] [Google Scholar]
- Drexler S.; Silveira T. M. G.; De Belli G.; Couto P. Experimental study of the effect of carbonated brine on wettability and oil displacement for EOR application in the Brazilian Pre-Salt reservoirs. Energy Sources, Part A Recover. Util. Environ. Eff. 2021, 43, 3282–3296. 10.1080/15567036.2019.1604877. [DOI] [Google Scholar]
- Gu H.; et al. Investigation on contact angle measurement methods and wettability transition of porous surfaces. Surf. Coat. Technol. 2016, 292, 72–77. 10.1016/j.surfcoat.2016.03.014. [DOI] [Google Scholar]
- Graue A.; Viksund B. G.; Eilertsen T.; Moe R. Systematic wettability alteration by aging sandstone and carbonate rock in crude oil. Journal of Petroleum Science and Engineering 1999, 24, 85–97. [Google Scholar]
- Yan J.; Sharma M. M. Wettability alteration and restoration for cores contaminated with oil-based muds. J. Pet. Sci. Eng. 1989, 2, 63–76. 10.1016/0920-4105(89)90051-X. [DOI] [Google Scholar]
- Mwangi P.; Thyne G.; Rao D.. Extensive experimental wettability study in sandstone and carbonate-oil-brine systems: Part 1-screening tool development. 2013 International Symposium of the Society of Core Analysts, Napa Valley, California, Sept 16–19, 2013; Vol.SCA2013-084.
- Mwangi P.; Brady P. V.; Radonjic M.; Thyne G. The effect of organic acids on wettability of sandstone and carbonate rocks. J. Pet. Sci. Eng. 2018, 165, 428–435. 10.1016/j.petrol.2018.01.033. [DOI] [Google Scholar]
- Anderson W. G. Wettability Literature Survey - Part 5: the Effects of Wettability on Relative Permeability. JPT, J. Pet. Technol. 1987, 39, 1453–1468. 10.2118/16323-PA. [DOI] [Google Scholar]
- Aspenes E.; Graue A.; Ramsdal J. In situ wettability distribution and wetting stability in outcrop chalk aged in crude oil. J. Pet. Sci. Eng. 2003, 39, 337–350. 10.1016/S0920-4105(03)00073-1. [DOI] [Google Scholar]
- Elsayed M.; et al. A review on the applications of nuclear magnetic resonance (NMR) in the oil and gas industry: laboratory and field-scale measurements. Journal of Petroleum Exploration and Production Technology 2022, 12, 2747–2784. 10.1007/s13202-022-01476-3. [DOI] [Google Scholar]
- Shikhov I.; et al. Application of low-field, 1H/13C high-field solution and solid state NMR for characterisation of oil fractions responsible for wettability change in sandstones. Magn. Reson. Imaging 2019, 56, 77–85. 10.1016/j.mri.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Andrew M.; Bijeljic B.; Blunt M. J. Pore-scale contact angle measurements at reservoir conditions using X-ray microtomography. Adv. Water Resour. 2014, 68, 24–31. 10.1016/j.advwatres.2014.02.014. [DOI] [Google Scholar]
- Erzuah S.; Fjelde I.; Omekeh A. V. Challenges associated with Quartz Crystal Microbalance with Dissipation (QCM-D) as a wettability screening tool. Oil Gas Sci. Technol. 2018, 73, 58. 10.2516/ogst/2018069. [DOI] [Google Scholar]
- Bendada K.; Hamdi B.; Boudriche L.; Balard H.; Calvet R. Surface characterization of reservoir rocks by inverse gas chromatography: Effect of a surfactant. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 504, 75–85. 10.1016/j.colsurfa.2016.05.047. [DOI] [Google Scholar]
- Arsalan N.; Buiting J. J.; Nguyen Q. P. Surface energy and wetting behavior of reservoir rocks. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 467, 107–112. 10.1016/j.colsurfa.2014.11.024. [DOI] [Google Scholar]
- Hazra B.; et al. FTIR, XRF, XRD and SEM characteristics of Permian shales, India. J. Nat. Gas Sci. Eng. 2016, 32, 239–255. 10.1016/j.jngse.2016.03.098. [DOI] [Google Scholar]
- Kowalewski E.; Boassen T.; Torsaeter O. Wettability alterations due to aging in crude oil; wettability and Cryo-ESEM analyses. J. Pet. Sci. Eng. 2003, 39, 377–388. 10.1016/S0920-4105(03)00076-7. [DOI] [Google Scholar]
- Khorasani M. T.; Mirzadeh H.; Kermani Z. Wettability of porous polydimethylsiloxane surface: Morphology study. Appl. Surf. Sci. 2005, 242, 339–345. 10.1016/j.apsusc.2004.08.035. [DOI] [Google Scholar]
- Jarrahian K.; Seiedi O.; Sheykhan M.; Sefti M. V.; Ayatollahi S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 410, 1–10. 10.1016/j.colsurfa.2012.06.007. [DOI] [Google Scholar]
- Matthiesen J.; Hassenkam T.; Bovet N.; Dalby K. N.; Stipp S. L. S. Adsorbed organic material and its control on wettability. Energy Fuels 2017, 31, 55–64. 10.1021/acs.energyfuels.6b00627. [DOI] [Google Scholar]
- Joonaki E.; Buckman J.; Burgass R.; Tohidi B. Water versus Asphaltenes; Liquid-Liquid and Solid-Liquid Molecular Interactions Unravel the Mechanisms behind an Improved Oil Recovery Methodology. Sci. Rep. 2019, 9, 1–13. 10.1038/s41598-019-47782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; et al. Novel polyhydroxy anionic surfactants with excellent water-solid interfacial wettability control capability for enhanced oil recovery. J. Mol. Liq. 2021, 343, 116973. 10.1016/j.molliq.2021.116973. [DOI] [Google Scholar]
- Taheri-Shakib J.; et al. Wettability alteration by surface adsorption of asphaltene molecular in carbonate porous media. J. Mol. Liq. 2022, 345, 118128. 10.1016/j.molliq.2021.118128. [DOI] [Google Scholar]
- Drummond C.; Israelachvili J. Surface forces and wettability. J. Pet. Sci. Eng. 2002, 33, 123–133. 10.1016/S0920-4105(01)00180-2. [DOI] [Google Scholar]
- Purswani P.; Tawfik M. S.; Karpyn Z. T. Factors and Mechanisms Governing Wettability Alteration by Chemically Tuned Waterflooding: A Review. Energy Fuels 2017, 31, 7734–7745. 10.1021/acs.energyfuels.7b01067. [DOI] [Google Scholar]
- Ding F.; Gao M. Pore wettability for enhanced oil recovery, contaminant adsorption and oil/water separation: A review. Adv. Colloid Interface Sci. 2021, 289, 102377. 10.1016/j.cis.2021.102377. [DOI] [PubMed] [Google Scholar]
- Anderson W. G. Wettability Literature Survey- Part 1: Rock/Brine lnteractionsand the Effects of Core Handling on Wettability. JPT, J. Pet. Technol. 1986, 38, 1125–1144. 10.2118/13932-PA. [DOI] [Google Scholar]
- Hao X., Abu-Al-Saud M., Ayirala S.; Elakneswaran Y.. Evaluating the Effect of Carbonate Impurities on Wettability Alteration Using a Geochemical Model. in Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2022; Vol. 2022. [Google Scholar]
- Chang X.; et al. Inherent wettability of different rock surfaces at nanoscale: a theoretical study. Appl. Surf. Sci. 2018, 434, 73–81. 10.1016/j.apsusc.2017.10.173. [DOI] [Google Scholar]
- Wang F. H. L.; Guidry L. J. Effect of oxidation-reduction condition on wettability alteration. SPE Form. Eval. 1994, 9, 140–148. 10.2118/20504-PA. [DOI] [Google Scholar]
- Frye G. C.; Thomas M. M. Adsorption of organic compounds on carbonate minerals. 2. Extraction of carboxylic acids from recent and ancient carbonates. Chem. Geol. 1993, 109, 215–226. 10.1016/0009-2541(93)90071-P. [DOI] [Google Scholar]
- Yassin M. R.; Begum M.; Dehghanpour H. Organic shale wettability and its relationship to other petrophysical properties: A Duvernay case study. Int. J. Coal Geol. 2017, 169, 74–91. 10.1016/j.coal.2016.11.015. [DOI] [Google Scholar]
- Denekas M. O.; Mattax C. C.; Davis G. T. Effects of Crude Oil Components on Rock Wettability. Trans. AIME 1959, 216, 330–333. 10.2118/1276-G. [DOI] [Google Scholar]
- Mousavi S. P.; et al. Toward mechanistic understanding of wettability alteration in calcite and dolomite rocks: The effects of resin, asphaltene, anionic surfactant, and hydrophilic nano particles. J. Mol. Liq. 2021, 321, 114672. 10.1016/j.molliq.2020.114672. [DOI] [Google Scholar]
- Mikhailov N. N.; Ermilov O. M.; Sechina L. S. Influence of the Component Composition of Adsorbed Oil on the Microstructural Wettability of Hydrocarbon Reservoirs. Dokl. Earth Sci. 2021, 496, 107–111. 10.1134/S1028334X21020124. [DOI] [Google Scholar]
- Hadia N. J.; Hansen T.; Tweheyo M. T.; Torsæter O. Influence of crude oil components on recovery by high and low salinity waterflooding. Energy Fuels 2012, 26, 4328–4335. 10.1021/ef3003119. [DOI] [Google Scholar]
- Tang G. Q.; Morrow N. R.. Salinity, Temperature, Oil Composition, and Oil Recovery by Waterflooding. In SPE Reservoir Engineering (Society of Petroleum Engineers); OnePetro, 1997; Vol. 12, pp 269–276. [Google Scholar]
- Mamonov A.; Kvandal O. A.; Strand S.; Puntervold T. Adsorption of polar organic components onto sandstone rock minerals and its effect on wettability and enhanced oil recovery potential by smart water. Energy Fuels 2019, 33, 5954–5960. 10.1021/acs.energyfuels.9b00101. [DOI] [Google Scholar]
- Bennett B.; Buckman J. O.; Bowler B. F. J.; Larter S. R. Wettability alteration in petroleum systems: The role of polar non-hydrocarbons. Pet. Geosci. 2004, 10, 271–277. 10.1144/1354-079303-606. [DOI] [Google Scholar]
- Wolcott J. M.; Groves F. R.; Lee H. G.. Investigation of crude-oil/mineral interactions: Influence of oil chemistry on wettability alteration. In Proceedings of the 1993 SPE International Symposium on Oilfield Chemistry; OnePetro, 1993, pp 435–440, 10.2523/25194-ms. [DOI] [Google Scholar]
- Buckley J. S.; Lord D. L. Wettability and morphology of mica surfaces after exposure to crude oil. J. Pet. Sci. Eng. 2003, 39, 261–273. 10.1016/S0920-4105(03)00067-6. [DOI] [Google Scholar]
- Barclay S. A.; Worden R. H.. Effects of Reservoir Wettability on Quartz Cementation in Oil Fields. Quartz Cem. Sandstones; Wiley, 2000; pp 103–117, 10.1002/9781444304237.ch8. [DOI] [Google Scholar]
- Leontaritis K. J.; Mansoori G. A.. Asphaltene Flocculation During Oil Production and Processing: A Thermodynamic Colloidal Model. In Society of Petroleum Engineers of AIME, (Paper) SPE; OnePetro, 1987; pp 149–158, 10.2118/16258-ms. [DOI] [Google Scholar]
- Chukwudeme E. A.; Hamouda A. A. Oil recovery from polar components (asphaltene and SA) treated chalk rocks by low salinity water and water containing SO42- and Mg2+ at different temperatures. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 336, 174–182. 10.1016/j.colsurfa.2008.11.051. [DOI] [Google Scholar]
- Nenningsland A. L.; Simon S.; Sjöblom J. Surface properties of basic components extracted from petroleum crude oil. Energy Fuels 2010, 24, 6501–6505. 10.1021/ef101094p. [DOI] [Google Scholar]
- Buckley J. S. Wetting alteration of solid surfaces by crude oils and their asphaltenes. Rev. Inst. Fr. Pet. 1998, 53, 303–312. 10.2516/ogst:1998026. [DOI] [Google Scholar]
- Barth T.; et al. Relationship between the content of asphaltenes and bases in some crude oils. Energy Fuels 2005, 19, 1624–1630. 10.1021/ef049750a. [DOI] [Google Scholar]
- Skauge A., Standal S., Boe S. O., Skauge T.; Blokhus A. M.. Effects of organic acids and bases, and oil composition on wettability. In Proceedings - SPE Annual Technical Conference and Exhibition; OnePetro, 1999; Vol. 2, pp 103–111. [Google Scholar]
- Puntervold T.; Strand S.; Austad T. New method to prepare outcrop chalk cores for wettability and oil recovery studies at low initial water saturation. Energy Fuels 2007, 21, 3425–3430. 10.1021/ef700323c. [DOI] [Google Scholar]
- Puntervold T.; Strand S.; Austad T. Water flooding of carbonate reservoirs: Effects of a model base and natural crude oil bases on chalk wettability. Energy Fuels 2007, 21, 1606–1616. 10.1021/ef060624b. [DOI] [Google Scholar]
- Fjelde I.; Omekeh A. V.; Sokama-Neuyam Y. A.. Low salinity water flooding: Effect of crude oil composition. 2014 SPE Symposium on Improved Oil Recovery, Tulsa, Oklahoma, 2014; Vol. 2, pp 864–875.
- Saputra I. W. R.; et al. The Influence of Oil Composition, Rock Mineralogy, Aging Time, and Brine Pre-soak on Shale Wettability. ACS Omega 2022, 7, 85–100. 10.1021/acsomega.1c03940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahzari P.; Sohrabi M.; Cooke A. J.; Carnegie A. Direct pore-scale visualization of interactions between different crude oils and low salinity brine. J. Pet. Sci. Eng. 2018, 166, 73–84. 10.1016/j.petrol.2018.02.051. [DOI] [Google Scholar]
- Greaves M.; Ayatollahi S.; Moshfeghian M.; Alboudwarej H.; Yarranton H.W. Estimation of SARA fraction properties with the SRK EOS. J. Can. Pet. Technol. 2004, 43, 31–39. 10.2118/04-09-02. [DOI] [Google Scholar]
- Amroun H.; Tiab D.. Alteration of reservoir wettability due to asphaltene deposition in Rhourd-Nouss Sud Est field, Algeria. In Society of Petroleum Engineers - SPE Rocky Mountain Petroleum Technology Conference 2001, RMPTC 2001; OnePetro, 2001; 10.2118/71060-ms. [DOI] [Google Scholar]
- Anyakudo F.; Adams E.; Van Schepdael A. Thin-Layer Chromatography-Flame Ionization Detection. Chromatographia 2020, 83, 149–157. 10.1007/s10337-019-03849-z. [DOI] [Google Scholar]
- Andersen S. I.; Jensen J. O.; Speight J. G. X-ray diffraction of subfractions of petroleum asphaltenes. Energy Fuels 2005, 19, 2371–2377. 10.1021/ef050039v. [DOI] [Google Scholar]
- Rogel E.; Miao T.; Vien J.; Roye M. Comparing asphaltenes: Deposit versus crude oil. Fuel 2015, 147, 155–160. 10.1016/j.fuel.2015.01.045. [DOI] [Google Scholar]
- Aspenes G.; Høiland S.; Borgund A. E.; Barth T. Wettability of petroleum pipelines: Influence of crude oil and pipeline material in relation to hydrate deposition. Energy Fuels 2010, 24, 483–491. 10.1021/ef900809r. [DOI] [Google Scholar]
- Yan G.; et al. Molecular Characterization of Polar Compounds in Crude Oil Affecting Sandstone Wettability Revealed by Fourier Transform-Ion Cyclotron Resonance-Mass Spectrometry. SPE J. 2022, 27, 1782–1795. 10.2118/209218-PA. [DOI] [Google Scholar]
- Tazikeh S.; Sayyad Amin J.; Zendehboudi S.; Shafiei A. Effects of asphaltene structure and polythiophene-coated magnetite nanoparticles on surface topography and wettability alteration of silica surface. J. Mol. Liq. 2022, 349, 118470. 10.1016/j.molliq.2022.118470. [DOI] [Google Scholar]
- Morrow N. R. Wettability and its effect on oil recovery. JPT, J. Pet. Technol. 1990, 42, 1476–1484. 10.2118/21621-PA. [DOI] [Google Scholar]
- Speight J. G.; Moschopedis S. E.. On the Molecular Nature of Petroleum Asphaltenes. In Chemistry of Asphaltenes; Advances in Chemistry; American Chemical Society: Washington, D.C., 1982; pp 1–15, 10.1021/ba-1981-0195.ch001. [DOI] [Google Scholar]
- Mamonov A.; Aslanidis P.; Fazilani N.; Puntervold T.; Strand S. Influence of Sandstone Mineralogy on the Adsorption of Polar Crude Oil Components and Its Effect on Wettability. Energy Fuels 2022, 36, 10785–10793. 10.1021/acs.energyfuels.2c01540. [DOI] [Google Scholar]
- Dutta P. K.; Holland R. J. Acid-base characteristics of petroleum asphaltenes as studied by non-aqueous potentiometric titrations. Fuel 1984, 63, 197–201. 10.1016/0016-2361(84)90036-X. [DOI] [Google Scholar]
- Al-Busaidi I. K.; Al-Maamari R. S.; Karimi M.; Naser J. Effect of different polar organic compounds on wettability of calcite surfaces. J. Pet. Sci. Eng. 2019, 180, 569–583. 10.1016/j.petrol.2019.05.080. [DOI] [Google Scholar]
- Al-Maamari R. S. H.; Buckley J. S.. Asphaltene Precipitation and Alteration of Wetting: Can Wettability Change during Oil Production? In SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, April 2000; OnePetro, 2000; 10.2118/59292-ms. [DOI]
- Anderson W. G. Wettability Literature Survey- Part 1: Rock/Oil/Brine Interactions Part 1: Rock/Oil/Brine Interactions and the Effects of Core Handling on Wettability. JPT, J. Pet. Technol. 1986, 38, 1125–1144. 10.2118/13932-PA. [DOI] [Google Scholar]
- Al-Maamari R. S. H.; Buckley J. S. Asphaltene precipitation and alteration of wetting: The potential for wettability changes during oil production. SPE Reserv. Eval. Eng. 2003, 6, 210–214. 10.2118/84938-PA. [DOI] [Google Scholar]
- Standnes D. C.; Austad T. Wettability alteration in chalk 1. Preparation of core material and oil properties. J. Pet. Sci. Eng. 2000, 28, 111–121. 10.1016/S0920-4105(00)00083-8. [DOI] [Google Scholar]
- Parra-Barraza H.; et al. The zeta potential and surface properties of asphaltenes obtained with different crude oil/n-heptane proportions. Fuel 2003, 82, 869–874. 10.1016/S0016-2361(03)00002-4. [DOI] [Google Scholar]
- Kaminsky R.; Radke C. J. Asphaltenes, water films, and wettability reversal. SPE J. 1997, 2, 485–491. 10.2118/39087-PA. [DOI] [Google Scholar]
- Andersen S. I.; Christensen S. D. Critical micelle concentration of asphaltenes As measured by calorimetry. Energy Fuels 2000, 14, 38–42. 10.1021/ef990122g. [DOI] [Google Scholar]
- Headen T. F.; Boek E. S.; Skipper N. T.. Evidence for asphaltene nanoaggregation in toluene and heptane from molecular dynamics simulations. In Energy and Fuels; American Chemical Society, 2009; Vol. 23, pp 1220–1229. [Google Scholar]
- Kim S. T.; Boudh-Hir M. E.; Mansoori G. A.. Role of asphaltene in wettability reversal. Proceedings of the 65th SPE Ann. Tech. Conf. Exhib., New Orleans, LA, Sept 23–26, 1990; pp 799–809.
- Mullins O. C.The modified yen model. In Energy and Fuels; American Chemical Society, 2010; Vol. 24, pp 2179–2207. [Google Scholar]
- Escobedo J.; Mansoori G. A. Viscometric determination of the onset of asphaltene flocculation: A novel method. SPE Prod. Facil. 1995, 10, 115–118. 10.2118/28018-PA. [DOI] [Google Scholar]
- Chacón-Patiño M. L.; et al. Vanadium and nickel distributions in selective-separated n-heptane asphaltenes of heavy crude oils. Fuel 2022, 312, 122939. 10.1016/j.fuel.2021.122939. [DOI] [Google Scholar]
- Chacón-Patiño M. L.; et al. Vanadium and nickel distributions in Pentane, In-between C5-C7 Asphaltenes, and heptane asphaltenes of heavy crude oils. Fuel 2021, 292, 120259. 10.1016/j.fuel.2021.120259. [DOI] [Google Scholar]
- Puntervold T.; Mamonov A.; Piñerez Torrijos I. D.; Strand S. Adsorption of Crude Oil Components onto Carbonate and Sandstone Outcrop Rocks and Its Effect on Wettability. Energy Fuels 2021, 35, 5738–5747. 10.1021/acs.energyfuels.0c03003. [DOI] [Google Scholar]
- Rezaei Gomari K. A.; Hamouda A. A. Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J. Pet. Sci. Eng. 2006, 50, 140–150. 10.1016/j.petrol.2005.10.007. [DOI] [Google Scholar]
- Rezaei Gomari K. A.; Denoyel R.; Hamouda A. A. Wettability of calcite and mica modified by different long-chain fatty acids (C18 acids). J. Colloid Interface Sci. 2006, 297, 470–479. 10.1016/j.jcis.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Jabbar M. Y., Al-Hashim H. S.; Abdallah W.. Effect of brine composition on wettability alteration of carbonate rocks in the presence of polar compounds. In Society of Petroleum Engineers - SPE Saudi Arabia Section Technical Symposium and Exhibition 2013, Al-Khobar, Saudi Arabia, May 19–22, 2013; OnePetro, 2013; pp 131–144, 10.2118/168067-ms. [DOI] [Google Scholar]
- Hoeiland S.; Barth T.; Blokhus A. M.; Skauge A. The effect of crude oil acid fractions on wettability as studied by interfacial tension and contact angles. J. Pet. Sci. Eng. 2001, 30, 91–103. 10.1016/S0920-4105(01)00106-1. [DOI] [Google Scholar]
- Wu Y.; Shuler P. J.; Blanco M.; Tang Y.; Goddard W. A. An experimental study of wetting behavior and surfactant EOR in carbonates with model compounds. SPE J. 2008, 13, 26–34. 10.2118/99612-PA. [DOI] [Google Scholar]
- Hamouda A. A.; Gomari K. A. R.. Influence of temperature on wettability alteration of carbonate reservoirs. in Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2006; Vol. 2, pp 848–859. [Google Scholar]
- Zhang Y.; Lin C.; Wu Y.; Ren L.; An S. Effects of Wettability and Minerals on Residual Oil Distributions Based on Digital Rock and Machine Learning. Lithosphere 2022, 2022, 1029304. 10.2113/2022/1029304. [DOI] [Google Scholar]
- Zhou D.; et al. Changes in microstructure and mechanical properties of shales exposed to supercritical CO2 and brine. Int. J. Rock Mech. Min. Sci. 2022, 160, 105228. 10.1016/j.ijrmms.2022.105228. [DOI] [Google Scholar]
- Thomas M. M.; Clouse J. A.; Longo J. M. Adsorption of organic compounds on carbonate minerals. 3. Influence on dissolution rates. Chem. Geol. 1993, 109, 227–237. 10.1016/0009-2541(93)90072-Q. [DOI] [Google Scholar]
- Arif M.; Abu-Khamsin S. A.; Zhang Y.; Iglauer S. Experimental investigation of carbonate wettability as a function of mineralogical and thermo-physical conditions. Fuel 2020, 264, 116846. 10.1016/j.fuel.2019.116846. [DOI] [Google Scholar]
- Alqam M. H.; Abu-Khamsin S. A.; Sultan A. S.; Okasha T. M.; Yildiz H. O. Effect of Rock Mineralogy and Oil Composition on Wettability Alteration and Interfacial Tension by Brine and Carbonated Water. Energy Fuels 2019, 33, 1983–1989. 10.1021/acs.energyfuels.8b04143. [DOI] [Google Scholar]
- Mohammed I.; Al Shehri D.; Mahmoud M.; Kamal M. S.; Alade O. S. A Surface Charge Approach to Investigating the Influence of Oil Contacting Clay Minerals on Wettability Alteration. ACS Omega 2021, 6, 12841–12852. 10.1021/acsomega.1c01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Ángeles F.; Firoozabadi A. Tunable Substrate Wettability by Thin Water Layer. J. Phys. Chem. C 2016, 120, 24688–24696. 10.1021/acs.jpcc.6b06054. [DOI] [Google Scholar]
- Hansen G.; Hamouda A. A.; Denoyel R. The effect of pressure on contact angles and wettability in the mica/water/n-decane system and the calcite+stearic acid/water/n-decane system. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 172, 7–16. 10.1016/S0927-7757(99)00498-7. [DOI] [Google Scholar]
- Hirasakl G. J. Wettability: fundamentals and surface forces. SPE Form. Eval. 1991, 6, 217–226. 10.2118/17367-PA. [DOI] [Google Scholar]
- Tokunaga T. K. DLVO-based estimates of adsorbed water film thicknesses in geologic CO2 reservoirs. Langmuir 2012, 28, 8001–8009. 10.1021/la2044587. [DOI] [PubMed] [Google Scholar]
- Alotaibi M. B., Nasralla R. A.; Nasr-El-Din H. A.. Wettability challenges in carbonate reservoirs. In Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2010; Vol. 2, pp 1420–1439. [Google Scholar]
- Chen Y.; Xie Q.; Sari A.; Brady P. V.; Saeedi A. Oil/water/rock wettability: Influencing factors and implications for low salinity water flooding in carbonate reservoirs. Fuel 2018, 215, 171–177. 10.1016/j.fuel.2017.10.031. [DOI] [Google Scholar]
- Eliebid M.; Mahmoud M.; Shawabkeh R.; Elkatatny S.; Hussein I. A. Effect of CO2 adsorption on enhanced natural gas recovery and sequestration in carbonate reservoirs. J. Nat. Gas Sci. Eng. 2018, 55, 575–584. 10.1016/j.jngse.2017.04.019. [DOI] [Google Scholar]
- Zakaria A. S.; Nasr-El-Din H. A.; Ziauddin M. Flow of emulsified acid in carbonate rocks. Ind. Eng. Chem. Res. 2015, 54, 4190–4202. 10.1021/ie504167y. [DOI] [Google Scholar]
- Osmond J. C. Dolomites in Silurian and Devonian of East-Central Nevada. Am. Assoc. Pet. Geol. Bull. 1954, 38, 1911–1956. 10.1306/5CEAE052-16BB-11D7-8645000102C1865D. [DOI] [Google Scholar]
- Dong K.; Zhu D.; Hill A. D. The role of temperature on optimal conditions in dolomite acidizing: An experimental study and its applications. J. Pet. Sci. Eng. 2018, 165, 736–742. 10.1016/j.petrol.2018.03.018. [DOI] [Google Scholar]
- Su W.; Liu Y.; Pi J.; Chai R.; Li C.; Wang Y. Effect of water salinity and rock components on wettability alteration during low-salinity water flooding in carbonate rocks. Arab. J. Geosci. 2018, 11, 1–5. 10.1007/s12517-018-3611-6. [DOI] [Google Scholar]
- Xie Y.; Khishvand M.; Piri M. Impact of Connate Brine Chemistry on in Situ Wettability and Oil Recovery: Pore-Scale Experimental Investigation. Energy Fuels 2020, 34, 4031–4045. 10.1021/acs.energyfuels.9b03787. [DOI] [Google Scholar]
- Sari A.; Chen Y.; Xie Q.; Saeedi A. Low salinity water flooding in high acidic oil reservoirs: Impact of pH on wettability of carbonate reservoirs. J. Mol. Liq. 2019, 281, 444–450. 10.1016/j.molliq.2019.02.081. [DOI] [Google Scholar]
- Xie Y.; Khishvand M.; Piri M. Wettability of Calcite Surfaces: Impacts of Brine Ionic Composition and Oil Phase Polarity at Elevated Temperature and Pressure Conditions. Langmuir 2020, 36, 6079–6088. 10.1021/acs.langmuir.0c00367. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Dong M.; Asghari K.; Tu Y. Wettability alteration by magnesium ion binding in heavy oil/brine/chemical/sand systems - Analysis of electrostatic forces. J. Pet. Sci. Eng. 2007, 59, 147–156. 10.1016/j.petrol.2007.03.010. [DOI] [Google Scholar]
- Haagh M. E. J.; et al. Aging brine-dependent deposition of crude oil components onto mica substrates, and its consequences for wettability. Fuel 2020, 274, 117856. 10.1016/j.fuel.2020.117856. [DOI] [Google Scholar]
- Morrow N. R.; Lim H. T.; Ward J. S. Effect of Crude-Oil-Induced Wettability Changes on Oil Recovery. SPE Form. Eval. 1986, 1, 89–103. 10.2118/13215-PA. [DOI] [Google Scholar]
- Liu M.; et al. Ion-induced oil-water wettability alteration of rock surfaces. Part II: Base interactions between oil and solid. Chem. Eng. Sci. 2021, 236, 116521. 10.1016/j.ces.2021.116521. [DOI] [Google Scholar]
- Xie Q.; et al. pH effect on wettability of oil/brine/carbonate system: Implications for low salinity water flooding. J. Pet. Sci. Eng. 2018, 168, 419–425. 10.1016/j.petrol.2018.05.015. [DOI] [Google Scholar]
- Buckley J. S.; Takamura K.; Morrow N. R. Influence of electrical surface charges on the wetting properties of crude oils. SPE Reserv. Eng. Society Pet. Eng. 1989, 4, 332. 10.2118/16964-PA. [DOI] [Google Scholar]
- Al Maskari N. S.; Sari A.; Hossain M. M.; Saeedi A.; Xie Q. Response of non-polar oil component on low salinity effect in carbonate reservoirs: Adhesion force measurement using atomic force microscopy. Energies 2020, 13, 77. 10.3390/en13010077. [DOI] [Google Scholar]
- Zhang Y.; Yuan L.; Sadrzadeh M.; Dehghanpour H. Effects of Electro-Oxidation Process on Tight-Rock Wettability and Imbibition Oil Recovery. Energy Fuels 2022, 36, 6771–6784. 10.1021/acs.energyfuels.2c00434. [DOI] [Google Scholar]
- Gbadamosi A.; et al. Recent advances on the application of low salinity waterflooding and chemical enhanced oil recovery. Energy Reports 2022, 8, 9969–9996. 10.1016/j.egyr.2022.08.001. [DOI] [Google Scholar]
- Tian H.; Wang M. Electrokinetic mechanism of wettability alternation at oil-water-rock interface. Surf. Sci. Rep. 2017, 72, 369–391. 10.1016/j.surfrep.2018.01.001. [DOI] [Google Scholar]
- McMillan M. D.; Rahnema H.; Romiluy J.; Kitty F. J. Effect of exposure time and crude oil composition on low-salinity water flooding. Fuel 2016, 185, 263–272. 10.1016/j.fuel.2016.07.090. [DOI] [Google Scholar]
- Moosavi S. R.; Rayhani M.; Malayeri M. R.; Riazi M. Impact of monovalent and divalent cationic and anionic ions on wettability alteration of dolomite rocks. J. Mol. Liq. 2019, 281, 9–19. 10.1016/j.molliq.2019.02.078. [DOI] [Google Scholar]
- Strand S.; Høgnesen E. J.; Austad T. Wettability alteration of carbonates - Effects of potential determining ions (Ca2+ and SO42-) and temperature. Colloids Surf., A 2006, 275, 1–10. 10.1016/j.colsurfa.2005.10.061. [DOI] [Google Scholar]
- Chandrasekhar S.; Sharma H.; Mohanty K. K. Dependence of wettability on brine composition in high temperature carbonate rocks. Fuel 2018, 225, 573–587. 10.1016/j.fuel.2018.03.176. [DOI] [Google Scholar]
- Rashid S.; Mousapour M. S.; Ayatollahi S.; Vossoughi M.; Beigy A. H. Wettability alteration in carbonates during ‘Smart Waterflood’: Underling mechanisms and the effect of individual ions. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 487, 142–153. 10.1016/j.colsurfa.2015.09.067. [DOI] [Google Scholar]
- Lashkarbolooki M.; Ayatollahi S.; Riazi M. The impacts of aqueous ions on interfacial tension and wettability of an asphaltenic-acidic crude oil reservoir during smart water injection. J. Chem. Eng. Data 2014, 59, 3624–3634. 10.1021/je500730e. [DOI] [Google Scholar]
- Song L.; Ning Z.; Duan l. The surface wettability of brine in a tight oil reservoir. Pet. Sci. Technol. 2018, 36, 398–403. 10.1080/10916466.2018.1427109. [DOI] [Google Scholar]
- Mugele F.; et al. Charge control and wettability alteration at solid-liquid interfaces. In Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2014; Vol. 3, pp 1563–1576. [Google Scholar]
- Alshaikh M.; Mahadevan J.. Impact of brine composition on carbonate wettability: A sensitivity study. In Society of Petroleum Engineers - SPE Saudi Arabia Section Technical Symposium and Exhibition; OnePetro, 2014; 10.2118/172187-ms. [DOI] [Google Scholar]
- Lu Y.; Najafabadi N. F.; Firoozabadi A. Effect of Temperature on Wettability of Oil/Brine/Rock Systems. Energy Fuels 2017, 31, 4989–4995. 10.1021/acs.energyfuels.7b00370. [DOI] [Google Scholar]
- Han X.; et al. CO2-water-rock reaction transport via simulation study of nanoparticles-CO2 flooding and storage. Sustain. Energy Technol. Assessments 2022, 50, 101736. 10.1016/j.seta.2021.101736. [DOI] [Google Scholar]
- Liu Y.; Rui Z.; Yang T.; Dindoruk B. Using propanol as an additive to CO2 for improving CO2 utilization and storage in oil reservoirs. Appl. Energy 2022, 311, 118640. 10.1016/j.apenergy.2022.118640. [DOI] [Google Scholar]
- Liao G.; et al. Discussion on the limit recovery factor of carbon dioxide flooding in a permanent sequestration scenario. Pet. Explor. Dev. 2022, 49, 1463–1470. 10.1016/S1876-3804(23)60364-7. [DOI] [Google Scholar]
- Jiang Y.; Luo Y.; Lu Y.; Qin C.; Liu H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. 10.1016/j.energy.2015.12.124. [DOI] [Google Scholar]
- Kang X.; et al. Stability influence factors and mechanism of produced emulsion from CO2 flooding. J. Mol. Liq. 2021, 333, 115974. 10.1016/j.molliq.2021.115974. [DOI] [Google Scholar]
- Pruess K.; Müller N. Formation dry-out from CO2 injection into saline aquifers: 1. Effects of solids precipitation and their mitigation. Water Resour. Res. 2009, 45, 3402. 10.1029/2008WR007101. [DOI] [Google Scholar]
- Tovar F. D., Barrufet M. A.; Schechter D. S.. Long term stability of acrylamide based polymers during chemically assisted CO2WAG EOR. In Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2014; Vol. 1, pp 310–318. [Google Scholar]
- Yekeen N.; Padmanabhan E.; Sevoo T. A.; Kanesen K. A.; Okunade O. A. Wettability of rock/CO2/brine systems: A critical review of influencing parameters and recent advances. J. Ind. Eng. Chem. 2020, 88, 1–28. 10.1016/j.jiec.2020.03.021. [DOI] [Google Scholar]
- Seyyedi M.; Sohrabi M.; Farzaneh A. Investigation of Rock Wettability Alteration by Carbonated Water through Contact Angle Measurements. Energy Fuels 2015, 29, 5544–5553. 10.1021/acs.energyfuels.5b01069. [DOI] [Google Scholar]
- Yang D.; Gu Y.; Tontiwachwuthikul P. Wettability determination of the crude oil-reservoir brine-reservoir rock system with dissolution of CO2 at high pressures and elevated temperatures. Energy Fuels 2008, 22, 2362–2371. 10.1021/ef800012w. [DOI] [Google Scholar]
- Yang D.; Gu Y.; Tontiwachwuthikul P. Wettability determination of the crude oil-reservoir brine-reservoir rock system with dissolution of CO2 at high pressures and elevated temperatures. Energy Fuels 2008, 22, 2362–2371. 10.1021/ef800012w. [DOI] [Google Scholar]
- Kavousi A.; Torabi F.; Chan C. W.; Shirif E. Experimental measurement and parametric study of CO2 solubility and molecular diffusivity in heavy crude oil systems. Fluid Phase Equilib. 2014, 371, 57–66. 10.1016/j.fluid.2014.03.007. [DOI] [Google Scholar]
- Emera M. K.; Sarma H. K. Prediction of CO2 Solubility in Oil and the Effects on the Oil Physical Properties. Energy Sources, Part A 2007, 29, 1233–1242. 10.1080/00908310500434481. [DOI] [Google Scholar]
- Hagedorn K. D.; Orr F. M. Component partitioning in CO2/crude oil systems: effects of oil composition on CO2 displacement performance. SPE Adv. Technol. Ser. 1994, 2, 177–184. 10.2118/25169-PA. [DOI] [Google Scholar]
- Li X.; Chi P.; Guo X.; Sun Q. CO2-induced asphaltene deposition and wettability alteration on a pore interior surface. Fuel 2019, 254, 115595. 10.1016/j.fuel.2019.06.003. [DOI] [Google Scholar]
- Cruz A. A.; et al. CO2 influence on asphaltene precipitation. J. Supercrit. Fluids 2019, 143, 24–31. 10.1016/j.supflu.2018.08.005. [DOI] [Google Scholar]
- Aftab A.; Hassanpouryouzband A.; Xie Q.; Machuca L. L.; Sarmadivaleh M. Toward a Fundamental Understanding of Geological Hydrogen Storage. Ind. Eng. Chem. Res. 2022, 61, 3233–3253. 10.1021/acs.iecr.1c04380. [DOI] [Google Scholar]
- Magnie R. L.Recovery of crude oil utilizing hydrogen. U.S. Patent US4241790A, 1979.
- Mirchi V.; Dejam M.; Alvarado V. Interfacial tension and contact angle measurements for hydrogen-methane mixtures/brine/oil-wet rocks at reservoir conditions. Int. J. Hydrogen Energy 2022, 47, 34963–34975. 10.1016/j.ijhydene.2022.08.056. [DOI] [Google Scholar]
- Hjelmeland O. S.; Larrondo L. E. Experimental Investigation of the Effects of Temperature, Pressure, and Crude Oil Composition on Interfacial Properties. SPE Reserv. Eng. (Society Pet. Eng. 1986, 1, 321–328. 10.2118/12124-PA. [DOI] [Google Scholar]
- Hamouda A. A.; Gomari K. A. R.. Influence of temperature on wettability alteration of carbonate reservoirs. In Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2006; Vol. 2, pp 848–859. [Google Scholar]
- Liu Y.; Buckley J. S. Evolution of wetting alteration by adsorption from crude oil. SPE Form. Eval. 1997, 12, 5–11. 10.2118/28970-PA. [DOI] [Google Scholar]
- Al-Aulaqi T.; Grattoni C.; Fisher Q.; Musina Z.; Al-Hinai S.. Effect of temperature, oil asphaltene content, and water salinity on wettability alteration. In Society of Petroleum Engineers - SPE/DGS Saudi Arabia Section Technical Symposium and Exhibition 2011; OnePetro, 2018; pp 256–274, 10.2118/149071-ms. [DOI] [Google Scholar]
- Kim T. W.; Kovscek A. R. Wettability alteration of a heavy oil/brine/carbonate system with temperature. Energy Fuels 2013, 27, 2984–2998. 10.1021/ef400204k. [DOI] [Google Scholar]
- Escrochi M.; Nabipour M.; Ayatollahi S.; Mehranbod N.. Wettability alteration at elevated temperatures: The consequenses of asphaltene precipitation. In Proceedings - SPE International Symposium on Formation Damage Control; OnePetro, 2008; Vol. 2, pp 536–542. [Google Scholar]
- Luo P.; Li S.; Knorr K. D.; Nakutnyy P.. Underlying mechanisms of tight reservoir wettability and its alteration. In Proceedings - SPE Symposium on Improved Oil Recovery; OnePetro, 2018; Vol 2018, pp 14–18. [Google Scholar]
- Wang W.; Gupta A.. Investigation of the Effect of Temperature and Pressure on Wettability Using Modified Pendant Drop Method. In SPE Annual Technical Conference and Exhibition, Dallas, TX, Oct 1995, OnePetro, 1995; SPE-30544-MS; pp 22–25, 10.2118/30544-ms. [DOI]
- Sari A.; Al Maskari N. S.; Saeedi A.; Xie Q. Impact of surface roughness on wettability of oil-brine-calcite system at sub-pore scale. J. Mol. Liq. 2020, 299, 112107. 10.1016/j.molliq.2019.112107. [DOI] [Google Scholar]
- Isah A.; Mahmoud M.; Arif M.; Al Jawad M.; Amao A. O. Influence of surface cleaning on the wettability of calcite/oil/brine systems. Fuel 2023, 351, 128908. 10.1016/j.fuel.2023.128908. [DOI] [Google Scholar]
- Treiber L.E.; Owens W.W. A Laboratory Evaluation of the Wettability of Fifty Oil-Producing Reservoirs. Soc. Pet Eng. J. 1972, 12, 531–540. 10.2118/3526-PA. [DOI] [Google Scholar]
- Al-Ameer M. A.; et al. A Guide for Selection of Aging Time and Temperature for Wettability Alteration in Various Rock-Oil Systems. ACS Omega 2023, 8, 30790. 10.1021/acsomega.3c00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Morrow N. R.; Ma S. Interrelationship of wettability, initial water saturation, aging time, and oil recovery by spontaneous imbibition and waterflooding. SPE J. 2000, 5, 199–207. 10.2118/62507-PA. [DOI] [Google Scholar]
- Jia D., Buckley J. S.; Morrow N. R.. Control of core wettability with crude oil. In SPE International Symposium on Oilfield Chemistry; OnePetro, 1991; pp 401–409, 10.2118/21041-ms. [DOI] [Google Scholar]
- Yu T.; Li Q.; Hu H.; Tan Y.; Xu L. Molecular dynamics simulation of the interfacial wetting behavior of brine/sandstone with different salinities. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 632, 127807. 10.1016/j.colsurfa.2021.127807. [DOI] [Google Scholar]
- Baghel V.; Ranjan M. Numerical estimation of droplet motion on linear wettability gradient surface in microgravity environment. Mater. Today Commun. 2022, 32, 103916. 10.1016/j.mtcomm.2022.103916. [DOI] [Google Scholar]
- Nasr H.; McLaughlin J. B.; Ahmadi G.; Jia X.. Drop Motion Simulation on a Surface Due to a Wettability Gradient. Proceedings of the ASME 2006 Joint U.S.-European Fluids Engineering Summer Meeting Collocated with the 14th International Conference on Nuclear Engineering, Miami, FL, July 17–20, 2006, pp 547–550, 10.1115/FEDSM2006-98176. [DOI]
- Russo A.; et al. Numerical simulation of droplet impact on wettability-patterned surfaces. Phys. Rev. Fluids 2020, 5, 074002. 10.1103/PhysRevFluids.5.074002. [DOI] [Google Scholar]
- Qu J.; Yang X.; Wang Z. Numerical simulations on the self-motion of droplets in hydrophobic microchannels driven by wettability gradient surfaces. Int. Commun. Heat Mass Transfer 2020, 119, 104961. 10.1016/j.icheatmasstransfer.2020.104961. [DOI] [Google Scholar]
- Boampong L. O.; Rafati R.; Sharifi Haddad A. A calibrated surface complexation model for carbonate-oil-brine interactions coupled with reservoir simulation - Application to controlled salinity water flooding. J. Pet. Sci. Eng. 2022, 208, 109314. 10.1016/j.petrol.2021.109314. [DOI] [Google Scholar]
- Chowdhury I. U.; Mahapatra P. S.; Sen A. K. Shape evolution of drops on surfaces of different wettability gradients. Chem. Eng. Sci. 2021, 229, 116136. 10.1016/j.ces.2020.116136. [DOI] [Google Scholar]
- Ashoke Raman K. Dynamics of simultaneously impinging drops on a dry surface: Role of inhomogeneous wettability and impact shape. J. Colloid Interface Sci. 2018, 516, 232–247. 10.1016/j.jcis.2018.01.063. [DOI] [PubMed] [Google Scholar]
- Rostami P.; Sharifi M.; Aminshahidy B.; Fahimpour J. The effect of nanoparticles on wettability alteration for enhanced oil recovery: micromodel experimental studies and CFD simulation. Pet. Sci. 2019, 16, 859–873. 10.1007/s12182-019-0312-z. [DOI] [Google Scholar]
- Iyi D.; Balogun Y.; Oyeneyin B.; Faisal N. Numerical modelling of the effect of wettability, interfacial tension and temperature on oil recovery at pore-scale level. J. Pet. Sci. Eng. 2021, 201, 108453. 10.1016/j.petrol.2021.108453. [DOI] [Google Scholar]
- Saputra I. W. R.; Schechter D. S.. Comprehensive Workflow for Laboratory to Field-Scale Numerical Simulation to Improve Oil Recovery in the Eagle Ford Shale by Selective Testing and Modelling of Surfactants for Wettability Alteration. SPE/AAPG/SEG Unconventional Resources Technology Conference 2018; URTC, 2018; pp 23–25, 10.15530/URTEC-2018-2884598. [DOI] [Google Scholar]
- Delshad M.; Najafabadi N. F.; Anderson G. A.; Pope G. A.; Sepehrnoori K. Modeling wettability alteration by surfactants in naturally fractured reservoirs. SPE Reserv. Eval. Eng. 2009, 12, 361–370. 10.2118/100081-PA. [DOI] [Google Scholar]
- Drexler S.; Hoerlle F.; Godoy W.; Boyd A.; Couto P. Wettability alteration by carbonated brine injection and its impact on pore-scale multiphase flow for carbon capture and storage and enhanced oil recovery in a carbonate reservoir. Appl. Sci. 2020, 10, 6496. 10.3390/app10186496. [DOI] [Google Scholar]
- Zhang L.; Lu X.; Liu X.; Yang K.; Zhou H. Surface Wettability of Basal Surfaces of Clay Minerals: Insights from Molecular Dynamics Simulation. Energy Fuels 2016, 30, 149–160. 10.1021/acs.energyfuels.5b02142. [DOI] [Google Scholar]
- Shi K.; et al. Effect of wettability of shale on CO2 sequestration with enhanced gas recovery in shale reservoir: Implications from molecular dynamics simulation. J. Nat. Gas Sci. Eng. 2022, 107, 104798. 10.1016/j.jngse.2022.104798. [DOI] [Google Scholar]
- Zhou J.; Zhang J.; Yang J.; Jin Z.; Luo K. H. Mechanisms for kerogen wettability transition from water-wet to CO2-wet: Implications for CO2 sequestration. Chem. Eng. J. 2022, 428, 132020. 10.1016/j.cej.2021.132020. [DOI] [Google Scholar]
- Yu X.; et al. Molecular dynamics computations of brine-CO2/CH4-shale contact angles: Implications for CO2 sequestration and enhanced gas recovery. Fuel 2020, 280, 118590. 10.1016/j.fuel.2020.118590. [DOI] [Google Scholar]
- Myshakin E. M.; Saidi W. A.; Romanov V. N.; Cygan R. T.; Jordan K. D. Molecular dynamics simulations of carbon dioxide intercalation in hydrated Na-montmorillonite. J. Phys. Chem. C 2013, 117, 11028–11039. 10.1021/jp312589s. [DOI] [Google Scholar]
- Tenney C. M.; Cygan R. T. Molecular simulation of carbon dioxide, brine, and clay mineral interactions and determination of contact angles. Environ. Sci. Technol. 2014, 48, 2035–2042. 10.1021/es404075k. [DOI] [PubMed] [Google Scholar]
- Ahmadi M.; Chen Z. Molecular Dynamics Investigation of Wettability Alteration of Quartz Surface under Thermal Recovery Processes. Molecules 2023, 28, 1162. 10.3390/molecules28031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; et al. Molecular dynamics simulation and experimental characterization of anionic surfactant: Influence on wettability of low-rank coal. Fuel 2020, 279, 118323. 10.1016/j.fuel.2020.118323. [DOI] [Google Scholar]
- Chen J.; Min F.-f.; Liu L.-y. The interactions between fine particles of coal and kaolinite in aqueous, insights from experiments and molecular simulations. Appl. Surf. Sci. 2019, 467–468, 12–21. 10.1016/j.apsusc.2018.10.130. [DOI] [Google Scholar]
- Ma Y.; Lu G.; Shao C.; Li X. Molecular dynamics simulation of hydrocarbon molecule adsorption on kaolinite (0 0 1) surface. Fuel 2019, 237, 989–1002. 10.1016/j.fuel.2018.10.063. [DOI] [Google Scholar]
- Zhong J.; et al. Adsorption mechanism of oil components on water-wet mineral surface: A molecular dynamics simulation study. Energy 2013, 59, 295–300. 10.1016/j.energy.2013.07.016. [DOI] [Google Scholar]
- Underwood T.; Erastova V.; Greenwell H. C. Wetting Effects and Molecular Adsorption at Hydrated Kaolinite Clay Mineral Surfaces. J. Phys. Chem. C 2016, 120, 11433–11449. 10.1021/acs.jpcc.6b00187. [DOI] [Google Scholar]
- Wang J.; Kalinichev A. G.; Kirkpatrick R. J.; Cygan R. T. Structure, energetics, and dynamics of water adsorbed on the muscovite (001) surface: A molecular dynamics simulation. J. Phys. Chem. B 2005, 109, 15893–15905. 10.1021/jp045299c. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Yi H.; Zhao Y.; Min F.; Song S. Study on the differences of Na- and Ca-montmorillonites in crystalline swelling regime through molecular dynamics simulation. Adv. Powder Technol. 2016, 27, 779–785. 10.1016/j.apt.2016.03.005. [DOI] [Google Scholar]
- Fang T.; Zhang Y.; Ding B.; Yan Y.; Zhang J. Static and dynamic behavior of CO2 enhanced oil recovery in nanoslits: Effects of mineral type and oil components. Int. J. Heat Mass Transfer 2020, 153, 119583. 10.1016/j.ijheatmasstransfer.2020.119583. [DOI] [Google Scholar]
- Kim S. Y.; Li J. Machine learning of metal-ceramic wettability. J. Mater. 2022, 8, 195–203. 10.1016/j.jmat.2021.03.014. [DOI] [Google Scholar]
- Al Dhaif R.; Ibrahim A. F.; Elkatatny S. Prediction of Surface Oil Rates for Volatile Oil and Gas Condensate Reservoirs Using Artificial Intelligence Techniques. J. Energy Resour. Technol. Trans. ASME 2022, 144, 033001. 10.1115/1.4051298. [DOI] [Google Scholar]
- Castillo E.; Hadi A. S.; Gutiérrez J. M.; Lacruz B. Some applications of functional networks in statistics and engineering. Technometrics 2001, 43, 10–24. 10.1198/00401700152404282. [DOI] [Google Scholar]
- Hagan M.; Menhaj M. Training Feedforward Networks with the Marquardt Algorithm. IEEE Trans. Neural Netw. 2004, 5 (6), 989–993. 10.1109/72.329697. [DOI] [PubMed] [Google Scholar]
- Cho Y.; Kim S.; Park C. H. Surface Wettability Prediction Using Image Analysis and an Artificial Neural Network. Langmuir 2022, 38, 7208–7217. 10.1021/acs.langmuir.2c00539. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. F. Prediction of coal wettability using machine learning for the application of CO2 sequestration. Int. J. Greenh. Gas Control 2022, 118, 103670. 10.1016/j.ijggc.2022.103670. [DOI] [Google Scholar]
- Tariq Z.Data-Driven Machine Learning Modeling of Mineral/CO2/Brine Wettability Prediction: Implications for CO2 Geo-Storage. SPE Middle East Oil Gas Show Conf. MEOS, Proc.; SPE, 2023, 10.2118/213346-MS. [DOI]
- Tariq Z.; et al. Predicting wettability of mineral/CO2/brine systems via data-driven machine learning modeling: Implications for carbon geo-sequestration. Chemosphere 2023, 345, 140469. 10.1016/j.chemosphere.2023.140469. [DOI] [PubMed] [Google Scholar]
- Zhang H.; et al. Improving predictions of shale wettability using advanced machine learning techniques and nature-inspired methods: Implications for carbon capture utilization and storage. Sci. Total Environ. 2023, 877, 162944. 10.1016/j.scitotenv.2023.162944. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. F. Application of various machine learning techniques in predicting coal wettability for CO2 sequestration purpose. Int. J. Coal Geol. 2022, 252, 103951. 10.1016/j.coal.2022.103951. [DOI] [Google Scholar]
- Kordijazi A.; Behera S.; Patel D.; Rohatgi P.; Nosonovsky M. Predictive Analysis of Wettability of Al-Si Based Multiphase Alloys and Aluminum Matrix Composites by Machine Learning and Physical Modeling. Langmuir 2021, 37, 3766–3777. 10.1021/acs.langmuir.1c00358. [DOI] [PubMed] [Google Scholar]
- Khosravi V.; Mahmood S. M.; Zivar D.; Sharifigaliuk H. Investigating the applicability of molecular dynamics simulation for estimating the wettability of sandstone hydrocarbon formations. ACS Omega 2020, 5, 22852–22860. 10.1021/acsomega.0c02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama G.; Cheng P. Effects of interface wettability on microscale flow by molecular dynamics simulation. Int. J. Heat Mass Transfer 2004, 47, 501–513. 10.1016/j.ijheatmasstransfer.2003.07.013. [DOI] [Google Scholar]
- Gan J.; et al. Experimental and molecular dynamics investigations of the effects of ionic surfactants on the wettability of low-rank coal. Energy 2023, 271, 127012. 10.1016/j.energy.2023.127012. [DOI] [Google Scholar]
- Chen L.; Wang S. Y.; Xiang X.; Tao W. Q. Mechanism of surface nanostructure changing wettability: A molecular dynamics simulation. Comput. Mater. Sci. 2020, 171, 109223. 10.1016/j.commatsci.2019.109223. [DOI] [Google Scholar]
- Jagadisan A.; Heidari Z. Molecular dynamic simulation of the impact of thermal maturity and reservoir temperature on the contact angle and wettability of kerogen. Fuel 2022, 309, 122039. 10.1016/j.fuel.2021.122039. [DOI] [Google Scholar]
- Xu C.; et al. Numerical simulation of the dynamic wetting of coal dust by spray droplets. Energy 2023, 270, 126667. 10.1016/j.energy.2023.126667. [DOI] [Google Scholar]
- Jung H. Y.; Onishi T.; Datta-Gupta A.. Numerical Simulation of EOR from Wettability Alteration in Tight Oil Reservoir with Multiple Hydraulic Fractures. In Proc. SPE Annu. Technol. Conf. Exhib., Sept 2018; Vol. 2018, pp 24–26.
- Yuan Z.; Wu X.; Chu F.; Wu R. Numerical simulations of guided self-propelled jumping of droplets on a wettability gradient surface. Appl. Therm. Eng. 2019, 156, 524–530. 10.1016/j.applthermaleng.2019.04.095. [DOI] [Google Scholar]
- Ghassemi A.; Pak A. Numerical study of factors influencing relative permeabilities of two immiscible fluids flowing through porous media using lattice Boltzmann method. J. Pet. Sci. Eng. 2011, 77, 135–145. 10.1016/j.petrol.2011.02.007. [DOI] [Google Scholar]
- Jiao Y.; Liu X.; Liu K. Wettability of laser textured surface: a parametric study based on numerical simulation and experimentation. Ind. Lubr. Tribol. 2018, 70, 977–983. 10.1108/ILT-01-2017-0008. [DOI] [Google Scholar]
- Han S.; Yang R.; Li C.; Yang L. The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Appl. Sci. 2019, Vol. 9, Page 566 2019, 9, 566. 10.3390/app9030566. [DOI] [Google Scholar]
- Lunkad S. F.; Buwa V. V.; Nigam K. D. P. Numerical simulations of drop impact and spreading on horizontal and inclined surfaces. Chem. Eng. Sci. 2007, 62, 7214–7224. 10.1016/j.ces.2007.07.036. [DOI] [Google Scholar]
- Ibrahim A. F.; Elkatatny S.. Application of Machine Learning to Predict Shale Wettability. In Proceedings of the Annual Offshore Technology Conference; OnePetro, 2023; Vol. 2023. [Google Scholar]
- Ibrahim A. F. Prediction of shale wettability using different machine learning techniques for the application of CO2 sequestration. Int. J. Coal Geol. 2023, 276, 104318. 10.1016/j.coal.2023.104318. [DOI] [Google Scholar]
- Zhang Y.; et al. Experimental study and artificial neural network simulation of the wettability of tight gas sandstone formation. J. Nat. Gas Sci. Eng. 2016, 34, 387–400. 10.1016/j.jngse.2016.07.002. [DOI] [Google Scholar]
- Ibrahim A. F.; Elkatatny S. Data-driven models to predict shale wettability for CO2 sequestration applications. Sci. Reports 2023 131 2023, 13, 1–14. 10.1038/s41598-023-37327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]