Abstract
Increasing evidence highlights the role of disrupted circadian rhythms in the neural dysfunctions and sleep disturbances observed in autism spectrum disorder and attention-deficit/hyperactivity disorder. However, the causality and directionality of these associations remain unclear. In this study, we employed a bidirectional two-sample Mendelian randomization framework, leveraging genome-wide association study data from the UK Biobank (n = 85,670) and FinnGen (n = 377,277). Genetic variants served as instrumental variables to infer causation, and objective accelerometer-derived metrics identified circadian rhythm and sleep genetic instruments. The results showed that the timing of the most active 10 h was significantly linked to higher odds of autism spectrum disorder and attention-deficit/hyperactivity disorder. Independently, higher sleep efficiency predicted a lower risk of autism spectrum disorder, while attention-deficit/hyperactivity disorder was linked to an increase in nocturnal sleep episodes. Heterogeneity and sensitivity analyses confirmed these findings. Our study establishes causal links between circadian alterations and autism spectrum disorder and attention-deficit/hyperactivity disorder, distinguishing the independent and protective role of sleep efficiency in autism spectrum disorder from circadian rhythms. In attention-deficit/hyperactivity disorder, however, disrupted sleep appears as a consequence, not a cause. These insights highlight divergent interactions with sleep factors in autism spectrum disorder and attention-deficit/hyperactivity disorder, laying the groundwork for tailored therapeutic strategies that recognize the distinct influences of sleep quality and circadian rhythms in each disorder.
Lay abstract
Research shows that people with autism spectrum disorder and attention-deficit/hyperactivity disorder often have sleep issues and problems with the body’s natural daily rhythms, known as circadian rhythms. By exploring the genetic variants associated with these rhythms and the conditions, this study reveals that these rhythm changes and sleep patterns are directly linked to autism spectrum disorder and attention-deficit/hyperactivity disorder. It found that the timing of one’s most active hours can increase the likelihood of having both autism spectrum disorder and attention-deficit/hyperactivity disorder. Importantly, it also shows that good sleep quality might protect against autism spectrum disorder, while disturbed sleep in people with attention-deficit/hyperactivity disorder seems to be a result rather than the cause of the condition. This understanding can help doctors and researchers develop better treatment approaches that focus on the specific ways sleep and body rhythms affect those with autism spectrum disorder and attention-deficit/hyperactivity disorder, considering their unique associations with circadian rhythms and sleep patterns. Understanding these unique links can lead to more effective, personalized care for those affected by these conditions.
Keywords: attention-deficit/hyperactivity disorder, autism spectrum disorder, chronotype, circadian rhythm, Mendelian randomization, sleep disorder
Introduction
Autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD) are prevalent lifelong neurodevelopmental disorders with distinctive yet overlapping symptoms. ASD, affecting approximately one in 100 individuals, is characterized by challenges in social interaction, communication and repetitive behaviours (Zeidan et al., 2022). ADHD, impacting about 5%–7% of children, is defined by persistent symptoms of impulsivity, inattention and hyperactivity (Polanczyk et al., 2007). Typically identified in early childhood, the implications of these disorders extend into adulthood, influencing overall functioning.
Recent studies suggest a substantial link between circadian rhythm (CR) alternations and psychiatric disorders (Levandovski et al., 2011; Takahashi et al., 2008), with increasing evidence highlighting the role of disrupted CRs in the neural dysfunctions and sleep disturbances observed in ASD (Carmassi et al., 2019; Geoffray et al., 2016) and ADHD (Bijlenga et al., 2019; Coogan & McGowan, 2017). CRs which encompass the body’s natural 24-h cycles of physiological, behavioural and psychological processes are crucial for regulating hormones and sleep-wake patterns. Chronotype, an individual’s tendency towards earlier or later sleep times, reflects the alignment of internal circadian cycles with sleep requirements, and is considered a valuable tool in studying CRs (Jones et al., 2016; Jones, Lane, et al., 2019). This CR disorders hypothesis (Bijlenga et al., 2019; Geoffray et al., 2016; Wimpory et al., 2002) explains certain observations related to ASD and ADHD. For instance, consistent evidence links ADHD to a late chronotype and phase delays in circadian markers, such as the onset of melatonin production under dim light conditions and delayed sleep onset (Coogan & McGowan, 2017). In addition, sleep disturbances, abnormal melatonin profiles and circadian sleep dysfunctions (e.g. irregular sleep-wake patterns and delayed sleep phase) have been linked to the severity of symptoms in ASD (Carmassi et al., 2019; Tordjman et al., 2013). Moreover, both ASD and ADHD exhibit a similar sleep impairment profiles, marked by longer sleep onset latency, reduced sleep efficiency, more frequent awakenings during sleep, and poorer self-reported sleep quality (Lugo et al., 2020; Ming & Walters, 2009), suggesting a circadian component as the underlying mechanism in these sleep disturbances (Bijlenga et al., 2019). However, it is also important to note that certain sleep disturbances found in ASD and ADHD, such as sleep-related movement and breathing disorders, insomnia and hypersomnia, cannot solely be attributed to CR dysfunctions (Mehta et al., 2019; Ming & Walters, 2009).
Despite the evidence, the exact mechanisms by which CRs and sleep contribute to the pathophysiology of disorders such as ASD and ADHD remain unclear. Current research, which is largely observational, has not definitively established a causal link between these factors. However, interventions targeting CRs, including light therapy and melatonin supplementation, have proven effective in alleviating sleep disturbances in ASD and ADHD, yet their impact on cognitive and behavioural outcomes in these disorders presents inconsistent findings (der Heijden et al., 2007; Esposito et al., 2019; Parvataneni et al., 2020; Rzepka-Migut & Paprocka, 2020). In addition, this gap in research methodology also highlights the potential for reverse causality or the influence of confounding factors. Recent developments in this field suggest a potentially bidirectional relationship between circadian disturbances and the behavioural and cognitive symptoms typical of ASD and ADHD. For example, circadian dysfunction might aggravate core symptoms such as stereotypical behaviours and attention deficits (Cortese et al., 2009). In contrast, the intrinsic challenges of ASD and ADHD, like difficulties in social interaction and sensory processing, may in turn exacerbate disruptions in circadian and sleep patterns (Geoffray et al., 2016; Imeraj et al., 2012).
This complex interplay necessitates a comprehensive understanding of CR’s role in these disorders, pivotal for both pathogenesis comprehension and chronotherapeutic strategy development. Genetic analyses identifying variants robustly associated with putative risk factors, such as circadian timing and sleep, can improve causal understanding by providing genetic instruments for use in Mendelian randomization (MR) analyses, which can minimize the effect of both reverse causality and confounding bias (Lawlor et al., 2008). MR calculates the proportion of the change explained by genetics in the exposure (e.g. chronotype and sleep) and the change explained by the same genetic factors on the outcome (e.g. ASD and ADHD). It is possible to calculate how changes in the exposure influence the outcome. This approach is useful to avoid reverse causality or confounding issues and can provide a more robust understanding of the associations of CRs, sleep disturbances and these disorders and may offer insights into novel therapeutic targets for managing their comorbidities (Burgess et al., 2023).
A recent genome-wide association study (GWAS) using objective accelerometer data from 85,670 UK Biobank participants has advanced our understanding of the genetic basis of sleep (Jones, van Hees, et al., 2019). This study uncovered 47 independent genetic associations (single-nucleotide polymorphisms, SNPs) with eight distinct sleep traits, falling into three different categories: circadian timing, sleep quality and duration. Accelerometers, aligning closely with polysomnography and sleep diaries (van Hees et al., 2015, 2018), are crucial for accurately measuring sleep patterns (Van de Water et al., 2011) and CR activity levels (Vitale et al., 2015) outside laboratory settings. This approach is particularly beneficial in large epidemiological studies, overcoming biases often present in self-reported chronotype and sleep assessments (Bianchi et al., 2013; Lauderdale et al., 2008). In addition, a subsequent comparison with self-reported chronotype measures and a GWAS meta-analysis, excluding UK Biobank participants with prior accelerometer data (Jones, Lane, et al., 2019; Jones, van Hees, et al., 2019), confirmed the impact of chronotype SNPs on sleep timing and circadian metrics. These analyses validate these genetic variants for MR analysis, facilitating the exploration into the intricate connections between CRs and sleep, and its implications for ASD and ADHD.
Therefore, in this study, using the SNPs identified from the GWAS study mentioned above, we conducted a bidirectional two-sample MR analysis to clarify the role of CRs in the aetiology of ASD and ADHD. By analysing genetic variants derived from objective, accelerometer-based measurements, we explored the associations between various circadian timing, along with sleep quality and quantity traits, and ASD and ADHD, focusing on establishing causality and directionality in these relationships.
Method
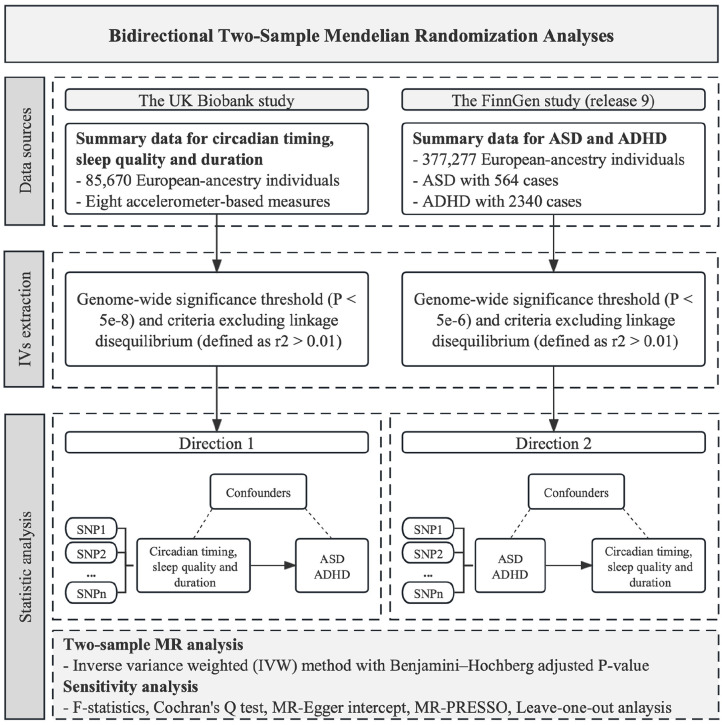
This two-sample bidirectional MR investigation was based on publicly available genome-wide association studies. Figure 1 shows the overview design of the study.
Figure 1.
The flowchart illustrates the study design methodology. Rectangles represent key steps or processes.
CRs and sleep data source
Genetic associations with eight measures of chronotype and sleep were derived from the large-scale GWAS of multiple sleep traits, which estimated objective, accelerometer data from 85,670 UK Biobank participants (Jones, van Hees, et al., 2019), who wore research-grade activity monitors (accelerometers) continuously for up to 7 days.
A total of eight accelerometer-based measures of circadian timing and sleep included measures representative of (1) circadian timing, including sleep midpoint (N = 84,810), timing of the least-active 5 h (L5) (N = 85,205) and timing of the most active 10 h (M10) (N = 85,670); (2) sleep quality, including sleep efficiency (sleep duration divided by the time between the start and end of the first and last nocturnal inactivity period, respectively) (N = 84,810) and the number of nocturnal sleep episodes (N = 84,810); and (3) sleep duration, including diurnal inactivity (N = 84,757), nocturnal sleep duration (N = 85,449) and variability (N = 84,441). Phenotypes were rank-normalized and adjusted for age, sex, location and season when activity-monitor worn and genotyping chip (Jones, van Hees, et al., 2019). It should also be noted that the indicators of sleep quality and sleep duration are uncorrelated with circadian timing indicators (Jones, van Hees, et al., 2019). Detailed information on circadian timing and sleep data is available in Supplementary Table 1.
ASD and ADHD data source
Summary-level genetic data on ASD with 564 cases and ADHD with 2340 cases were sourced from the most recent release (Release 9) of FinnGen data (Kurki et al., 2023). We used independent sources for exposure and outcome to avoid biases caused by sample overlap (Burgess et al., 2016). The FinnGen research project represents a collaborative effort between the public and private sectors, amalgamating genotype data from Finnish biobanks and digital health record information from Finnish health registries to establish a cohort of 500,000 participants. Genome-wide association analyses with FinnGen were adjusted for sex, age, genetic components and genotyping batch. As of May 2023 (Release 9), 2272 endpoints and 377,277 samples have been analysed (Kurki et al., 2023). The endpoints of ASD and ADHD were defined by International Classification of Diseases, Ninth revision (ICD-9) and Tenth revision (ICD-10) codes. Detailed diagnostic codes in FinnGen are listed in Supplementary Table 2.
Instrumental variable selection
Genetic instrumental variables (IVs) for circadian timing and sleep were identified based on the genome-wide significance threshold (p < 5 × 10−8) and criteria excluding linkage disequilibrium (defined as r2 < 0.01). As for the reversed direction of MR, no SNPs associated with ASD and ADHD reached the conventional threshold. For instrument selection, we opted for variants with p < 5 × 10−6 and r2 < 0.01. It is crucial to emphasize that all these SNPs underwent verification to confirm non-proximity, and any palindrome SNPs were deliberately excluded from consideration.
Statistical analysis
The two-sample MR analysis was conducted by the inverse-variance weighted (IVW) method. Using a weighted average, the IVW method is the most powerful estimator under the assumption of all the IVs being valid (Burgess et al., 2023), and its significance can be interpreted as evidence of a causal association. In this analysis, a Benjamini–Hochberg (BH)-adjusted p < 0.05 was considered indicative of a significant causal exposure-outcome relationship (Benjamini & Hochberg, 1995), while a nominal p < 0.05 but BH-adjusted p > 0.05 suggested a potential relationship. To assess the robustness of the IVW results, four sensitivity analyses were conducted. These included F-statistics (Burgess & Thompson, 2011), MR-Egger intercept test (Bowden et al., 2017), MR-PRESSO (global and outlier test) (Verbanck et al., 2018), and leave-one-out (LOO) analysis (Hemani et al., 2018), with the aim of identifying insufficient instruments and potential horizontal pleiotropy that could compromise the MR assumptions. Heterogeneity tests for IVW method were performed to estimate the variability in the effect estimated by each variant using Cochran’s Q test (Greco et al., 2015). All analyses were performed using R version 4.3.1 with the R packages ‘TwoSampleMR’ and ‘MR-PRESSO’ (R Core Team, 2023).
Community involvement
This study was conceptualized by the authors who have extensive clinical experience in providing behavioural assessment and intervention services to children with autism. Recognizing the vital importance of community engagement, efforts were made to incorporate insights and perspectives from the autistic community and their advocates to ensure that the research aligns with the needs and values of those it aims to serve. We acknowledge the diversity within the autistic community and strive to contribute to a body of knowledge that supports inclusive and respectful practices.
Results
From circadian timing and sleep traits to ASD and ADHD
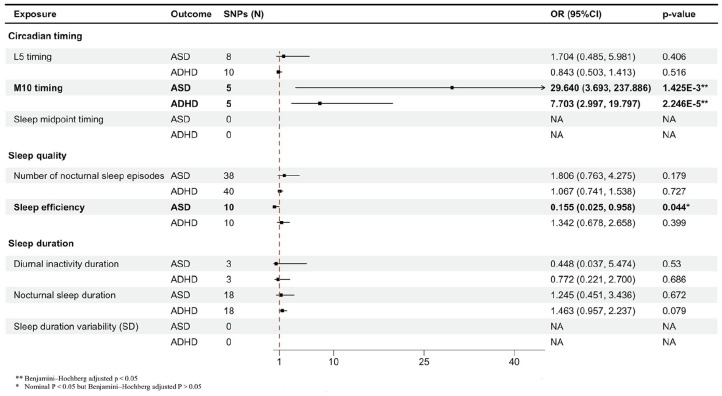
This set of analyses investigated the causal role of eight accelerometer-based measures of circadian timing and sleep (exposure) on ASD and ADHD (outcome). In the final analysis, 10 SNPs strongly associated with L5 timing, five with M10 timing, 40 with number of nocturnal sleep episodes, 10 with sleep efficiency, three with diurnal inactivity duration, and 18 with nocturnal sleep duration met the recruitment threshold. The F-statistics for each IV exceeded 10, indicating a robust association between CR and sleep exposure (Supple-mentary Table 3).
Genetically predicted M10 timing was linked to higher odds of ASD (odds ratio (OR) = 29.640, 95% confidence interval (CI) = [3.693, 237.886]; p = 1.425×10–3, BH adjusted p < 0.01) and ADHD (OR = 7.703, 95% CI = [2.997, 19.797]; p = 2.246 × 10–5, BH-adjusted p < 0.001). In the case of using sleep efficiency as the exposure, a potentially protective effect on ASD (OR = 0.155, 95% CI = [0.025, 0.958]; p < 0.05, BH-adjusted p > 0.05) was identified. The results of the sensitivity analysis for these significant associations were consistent, with no heterogeneity, horizontal pleiotropy, or outlier SNP detected (Supplementary Table 4). MR-PRESSO detected 2 outliers in the analysis from L5 timing and number of nocturnal sleep episodes to ASD; however, the association remained insignificant after the removal of these SNPs (Figure 2).
Figure 2.
The forest plot visually represents the associations between genetic liability to circadian timing and sleep traits measured by accelerometers and the risks of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD), expressed as odds ratios.
From ASD and ADHD to circadian timing and sleep traits
We inverted the direction of MR in this set of analysis. Table 1 displays the β estimates for ASD and ADHD exposure with sleep and activity as the outcome. As outlined in the ‘Methods’ section, we used a reduced p < 5 × 10−6 threshold and identified 10 instruments with ASD and 18 with ADHD after clumping and harmonization with the summary data for circadian timing and sleep. All F-statistics suggested IVs were strongly associated with ASD and ADHD exposure (F > 100, see Supplementary Table 3). Genetic liability to ADHD was associated with the number of nocturnal sleep episodes (β = 0.017, 95% CI = [0.001, 0.033]; p < 0.05, BH-adjusted p > 0.05), suggesting a potential causal influence from ADHD to poorer sleep quality. No heterogeneity, horizontal pleiotropy, or outlier was detected (Supplementary Table 5).
Table 1.
Associations of genetic liability to ASD and ADHD with eight accelerometer-based measures of circadian timing and sleep quality and duration traits.
| Exposure | Outcome | No. of used SNPs | β | p value |
|---|---|---|---|---|
| ASD | L5 timing | 10 | –0.006 (–0.020, 0.009) | 0.440 |
| M10 timing | 10 | –0.001 (–0.013, 0.010) | 0.807 | |
| Sleep midpoint timing | 10 | –0.005 (–0.017, 0.007) | 0.416 | |
| Number of nocturnal sleep episodes | 10 | 0.004 (–0.008, 0.015) | 0.533 | |
| Sleep efficiency | 10 | –0.004 (–0.016, 0.008) | 0.486 | |
| Diurnal inactivity duration | 10 | 0.003 (–0.009, 0.016) | 0.624 | |
| Nocturnal sleep duration | 10 | –0.003 (–0.013, 0.008) | 0.597 | |
| Sleep duration variability (SD) | 10 | 0.002 (–0.008, 0.013) | 0.663 | |
| ADHD | L5 timing | 18 | –0.013 (–0.029, 0.003) | 0.114 |
| M10 timing | 18 | 0.001 (–0.014, 0.017) | 0.857 | |
| Sleep midpoint timing | 18 | 0.003 (–0.013, 0.019) | 0.735 | |
| Number of nocturnal sleep episodes | 18 | 0.017 (0.001, 0.033) | 0.036* | |
| Sleep efficiency | 18 | –0.013 (–0.029, 0.003) | 0.101 | |
| Diurnal inactivity duration | 18 | 0.009 (–0.007, 0.024) | 0.268 | |
| Nocturnal sleep duration | 18 | 0.007 (–0.009, 0.023) | 0.374 | |
| Sleep duration variability (SD) | 18 | 0.002 (–0.014, 0.018) | 0.816 |
Nominal p < 0.05 but Benjamini–Hochberg-adjusted p > 0.05.
Discussion
In this study, we conducted a comprehensive bidirectional MR analysis to investigate the causal relationship between various CRs along with sleep quality and quantity traits, and both ASD and ADHD. Our findings reveal significant evidence supporting a causal link between certain CR patterns and these disorders. Notably, using GWAS data derived from objective, accelerometer-based estimates of circadian timing and sleep measures, we established a causal relationship between genetically predicted M10 timing and both ASD and ADHD. M10 timing serves as a key indicator of chronotype, with higher values corresponding to a late chronotype preference. This finding suggests a potential causal impact of late chronotype preference in the development of ASD and ADHD. Moreover, our study offers new insights into the role of sleep efficiency, revealing a potential protective effect independently from circadian timing against ASD but not ADHD. In a novel observation, our bidirectional MR analyses indicated that ADHD led to an increased number of sleep episodes, highlighting a potential one-way causal relationship. However, the reverse scenario, where sleep disruptions cause ADHD, was not supported by our data. This distinction underlines the unique pathways through which the genetically predicted alterations in CRs and sleep interact with ASD and ADHD. To our knowledge, this investigation is the first to robustly demonstrate these specific causal relationships and their directions. Our findings imply that circadian alternation is causally linked with both ASD and ADHD. Interestingly, while sleep quality appears to play a causally independent role exclusively in ASD, it emerges as a consequence rather than a cause in ADHD (Figure 3).
Figure 3.
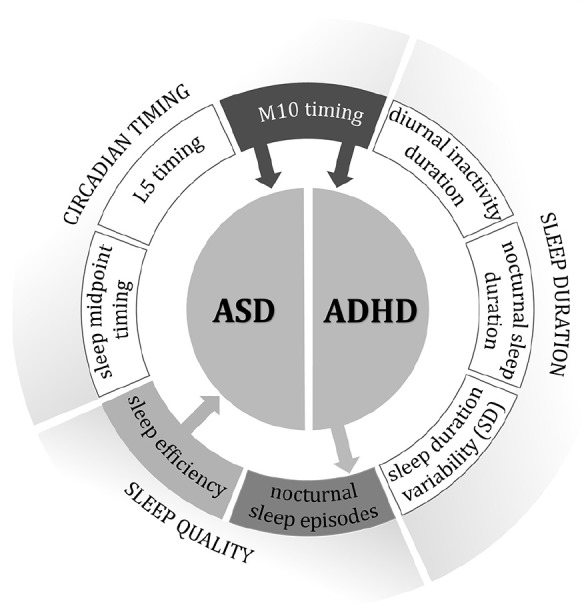
This figure summarizes the key causal associations identified in the study between genetic liability to circadian timing and sleep traits, and the risks of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD). Each arrow signifies a causal pathway.
Our research offers important supporting evidence for the CR disorder hypothesis, demonstrating that certain CR patterns, particularly a late chronotype preference as indicated by M10 timing, are causally linked to both ASD and ADHD. This finding is in line with previous studies that reported an atypical CR pattern in these populations (Arns et al., 2021; Carmassi et al., 2019; Coogan & McGowan, 2017). Our study, using objective measures, expands upon these findings by establishing a causal relationship. There is growing evidence from epidemiological studies suggesting that circadian traits are linked with a range of psychiatric disorders, both general and specific (Arns et al., 2021; Schuch et al., 2018; von Schantz et al., 2021). This circadian alteration may explain certain sleep disturbances commonly observed in ASD and ADHD, such as prolonged sleep onset, nocturnal awakenings and early morning arousal (Cortese et al., 2009; Cortesi et al., 2010; Glickman, 2010; Lugo et al., 2020). These sleep challenges might be indicative of broader neurobehavioral and endocrine dysfunctions, as evidenced by abnormal cortisol and melatonin profiles in children with these disorders (Tordjman et al., 2005). Despite these insights, interventions like bright light therapy and melatonin supplementation have shown inconsistent results in improving behaviours and functions in children with ASD and ADHD (Coogan & McGowan, 2017; Rzepka-Migut & Paprocka, 2020), which may be attributable to variations in intervention duration or melatonin dosage (Rzepka-Migut & Paprocka, 2020). Under the assumption of an MR approach, we found that genetically predicted M10 timing is linked to an increased risk of ASD and ADHD. This contrasts with a prior MR study (Sun et al., 2022), which reported no association between chronotype and psychiatric disorders. Notably, the SNPs for morning chronotype in the previous study were based on self-reported data, whereas our study used SNPs calculated from objective accelerometer data, potentially providing a more accurate chronotype assessment.
In contrast to earlier research suggesting a bidirectional relationship between CR patterns and ASD (Geoffray et al., 2016) and ADHD (Imeraj et al., 2012), our findings indicate a unidirectional causal relationship. This primarily emphasizes the role of a consistently late chronotype in the onset and aggravation of these disorders, rather than the reverse. More importantly, our research provides a strong mechanistic basis for treatments aimed at modifying CRs, highlighting their potential not only in managing circadian sleep issues but also in directly influencing the disorders themselves. Therefore, promoting such interventions could be crucial in addressing complex sleep-related challenges and the broader symptoms of ASD and ADHD. The underlying mechanistic basis through which the circadian pathways influence the risk and progression of ASD and ADHD can be multifaceted. For instance, alterations in CRs can disrupt neurodevelopment and affect immune-inflammatory, metabolic, nitro-oxidative stress and neurotransmitter pathways, potentially through epistatic interactions or epigenetic modifications (Abdul et al., 2022), thereby contributing to the neurobiological changes associated with ASD. CR disruptions might affect the expression of key molecules like neurexins and neuroligins, known to play a role in the aetiology of these disorders. These disruptions are likely to occur during critical periods of brain development and plasticity (Zafeiriou et al., 2013). Evidence suggests that ASD and ADHD may be linked to CR disorders during critical periods of brain development (Geoffray et al., 2016), which could also include foetal and postnatal periods. Given the significant interplay between maternal and child CRs during these periods (Serón-Ferré et al., 2001), further research is essential to control for maternal and postnatal CRs and sleep patterns of the mothers, and to clarify the precise role of disrupted CRs in the onset and progression of these conditions. Future research should include longitudinal studies tracking circadian patterns from early foetal development into later life stages, and investigations into genetic and epigenetic markers associated with CR dysregulation in individuals with ASD and ADHD to further clarify the pathway. By enhancing our understanding of how CR disruptions impact neurodevelopment and behaviour, we can better design interventions to mitigate the effects of these disorders.
Our study suggests that sleep quality measures appear to independently influence ASD, differing from its role in ADHD, where it primarily acts as a consequence rather than a cause. This distinction contributes a new perspective to our understanding of these disorders, challenging previous notions of bidirectional interactions between sleep disturbances and ASD/ADHD (Owens et al., 2013; Whelan et al., 2022). While CRs affect sleep phenotypes and sleep-wake disorders, and the genetic determinants of these tend to emerge (Dijk & Archer, 2010), it is important to note that our measures of sleep quality and duration are proven to be independent of CR indicators as shown in the previous GWAS study (Jones, van Hees, et al., 2019). This underscores that sleep quality’s protective role in ASD does not stem from shared mechanisms with CRs, highlighting sleep quality’s unique contribution to ASD. Conversely, in ADHD, impaired sleep quality appears to be a result rather than a contributing factor. This, however, does not diminish the relevance of sleep disturbances in ADHD; rather, it suggests that non-circadian aspects of sleep disruption such as sleep quality and duration, as measured in our study, do not directly lead to ADHD. The circadian sleep disturbances can still act as the mediators from CRs to these disorders. A meta-analysis of 42 studies (Wagner et al., 2004) investigating sleep disturbances in adults with ASD and ADHD reveals similar patterns of sleep impairment in both groups, including longer sleep onset latency, reduced sleep efficiency, more frequent awakenings and lower self-perceived sleep quality compared to healthy controls. In ASD, poor sleep quality and insomnia, not linked to CR dysfunction, may exacerbate specific symptoms. In ADHD, hyperactivity might intensify sleep fragmentation. These insights underscore the necessity for disorder-specific interventions: enhancing sleep quality could benefit individuals with ASD, whereas in ADHD, addressing the core symptoms may mitigate sleep issues.
The relationship between psychiatric disorders and both short and long sleep duration is well-documented (Lugo et al., 2020; Mayes et al., 2009; Parvataneni et al., 2020; Singh & Zimmerman, 2015). However, our study suggested that in ASD, it is sleep quality, as measured by sleep efficiency, rather than sleep duration, which plays a crucial contributing role. In ADHD, the increased number of sleep episodes, indicative of fragmented sleep, also reflects sleep quality issues. Therefore, sleep quality emerges as a more comprehensive aspect than sleep duration alone. While total sleep hours (duration) are important, the restfulness of sleep is more critical in disorders like ASD and ADHD. Focusing solely on sleep duration may overlook the complexities of sleep disturbances in these disorders.
Strengths and limitations
A major strength of our study lies in the MR design, using GWAS data leveraging objective accelerometer-based estimates of circadian timing and sleep measures. The use of MR design minimizes the residual confounding and reverse causality inherent in observational studies, and has allowed us to explore potential pathways of CRs, sleep disturbances and these disorders with greater accuracy. Moreover, unlike most other MR studies that rely on subjective self-reported sleep measures, our approach utilizes accelerometer data from the UK Biobank to provide a more precise and comprehensive picture of sleep patterns. Previous large-scale genetic studies of sleep traits have depended heavily on self-reported measures, often constrained to a limited set of questions that can only approximate a few sleep traits and are prone to biases like misperception and recall errors. These biases, including social desirability bias and the influence of current mood on recall, can significantly skew the accuracy of self-reported sleep data. Our method, by contrast, offers a more objective and nuanced understanding of sleep and CRs, free from such subjective biases. Our comprehensive GWAS analysis, the largest scale to date using accelerometer data, identified numerous genetic associations across circadian timing, sleep duration and quality, offering novel insights into sleep biology that were not detectable in larger studies relying on self-reported sleep traits.
Despite its strengths, our study has limitations. First, the genetic analyses were confined to individuals of European ancestry, restricting the generalizability of the findings and leaving unexplored associations on individuals of non-European ancestry who might be at risk. In addition, due to the absence of sex-stratified GWAS, we could not examine potential causal differences by sex, a significant factor commonly associated with psychiatric disorders. Second, the associations identified reflect cumulative lifetime exposure effects. However, it is plausible that personal CR, sleep quality and duration may vary with age. Unfortunately, the current analyses lack the capability to stratify MR effects by age, raising the possibility that causal effects may differ for younger and older adults. Notably, our study does not consider potential nonlinear effects of sleep-associated traits on psychiatric disorders due to the summary-data-level nature of MR analyses. Future research could investigate sleep-associated traits at different life stages to understand the impact of sleep changes on psychiatric disorder risk. Furthermore, chronotype measured as the most active 10 h period in the study, influenced by an interplay of CRs, innate sleep homeostatic mechanisms and societal pressures (Jones, Lane, et al., 2019), may not fully capture the complexities of circadian misalignment. Future research should focus on discerning whether it is circadian misalignment, rather than chronotype per se, that exhibits a stronger association with ASD and ADHD.
Conclusion
Using bidirectional MR analysis and GWAS data derived from objective accelerometer-based measures, our study provides solid evidence to reveal a causal link between late chronotype and both ASD and ADHD. Notably, we find that sleep quality independently influences ASD but emerges as a consequence rather than cause in ADHD, highlighting distinct interactions between CRs and sleep disturbances with each disorder. These findings emphasize the need for tailored approaches in studying and managing ASD and ADHD, considering their unique associations with sleep patterns and CRs. Our research, by identifying specific causal relationships, lays the groundwork for more focused therapeutic strategies.
Supplemental Material
Supplemental material, sj-docx-1-aut-10.1177_13623613241258546 for The role of circadian rhythms and sleep in the aetiology of autism spectrum disorder and attention-deficit/hyperactivity disorder: New evidence from bidirectional two-sample Mendelian randomization analysis by Xiaotian Dai, Gareth J. Williams, John A. Groeger, Gary Jones, Keeley Brookes, Wei Zhou, Jing Hua and Wenchong Du in Autism
Footnotes
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Gareth J. Williams  https://orcid.org/0000-0001-7689-1231
https://orcid.org/0000-0001-7689-1231
Wenchong Du  https://orcid.org/0000-0002-5115-7214
https://orcid.org/0000-0002-5115-7214
Supplemental material: Supplemental material for this article is available online.
References
- Abdul F., Sreenivas N., Kommu J. V. S., Banerjee M., Berk M., Maes M., Leboyer M., Debnath M. (2022). Disruption of circadian rhythm and risk of autism spectrum disorder: Role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways. Reviews in the Neurosciences, 33(1), 93–109. 10.1515/revneuro-2021-0022 [DOI] [PubMed] [Google Scholar]
- Arns M., Kooij J. J. S., Coogan A. N. (2021). Review: Identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. Journal of the American Academy of Child & Adolescent Psychiatry, 60(9), 1085–1095. 10.1016/j.jaac.2020.12.035 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bianchi M. T., Williams K. L., Mckinney S., Ellenbogen J. M. (2013). The subjective–objective mismatch in sleep perception among those with insomnia and sleep apnea. Journal of Sleep Research, 22(5), 557–568. [DOI] [PubMed] [Google Scholar]
- Bijlenga D., Vollebregt M. A., Kooij J. J. S., Arns M. (2019). The role of the circadian system in the etiology and pathophysiology of ADHD: Time to redefine ADHD? ADHD Attention Deficit and Hyperactivity Disorders, 11(1), 5–19. 10.1007/s12402-018-0271-z [DOI] [PubMed] [Google Scholar]
- Bowden J., Del Greco M. F., Minelli C., Davey Smith G., Sheehan N., Thompson J. (2017). A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Statistics in Medicine, 36(11), 1783–1802. 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Davey Smith G., Davies N. M., Dudbridge F., Gill D., Glymour M. M., Hartwig F. P., Kutalik Z., Holmes M. V., Minelli C., Morrison J. V., Pan W., Relton C. L., Theodoratou E. (2023). Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Research, 4, 186. 10.12688/wellcomeopenres.15555.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Davies N. M., Thompson S. G. (2016). Bias due to participant overlap in two-sample Mendelian randomization. Genetic Epidemiology, 40(7), 597–608. 10.1002/gepi.21998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Thompson S. G. (2011). Avoiding bias from weak instruments in Mendelian randomization studies. International Journal of Epidemiology, 40(3), 755–764. 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- Carmassi C., Palagini L., Caruso D., Masci I., Nobili L., Vita A., Dell’Osso L. (2019). Systematic review of sleep disturbances and circadian sleep desynchronization in autism spectrum disorder: Toward an integrative model of a self-reinforcing loop. Frontiers in Psychiatry, 10, Article 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan A. N., McGowan N. M. (2017). A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. ADHD Attention Deficit and Hyperactivity Disorders, 9(3), 129–147. 10.1007/s12402-016-0214-5 [DOI] [PubMed] [Google Scholar]
- Cortese S., Faraone S. V., Konofal E., Lecendreux M. (2009). Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 894–908. 10.1097/CHI.0b013e3181ac09c9 [DOI] [PubMed] [Google Scholar]
- Cortesi F., Giannotti F., Ivanenko A., Johnson K. (2010). Sleep in children with autistic spectrum disorder. Sleep Medicine, 11(7), 659–664. 10.1016/j.sleep.2010.01.010 [DOI] [PubMed] [Google Scholar]
- der Heijden K. B. V. A. N., Smits M. G., Van Someren E. J. W., Ridderinkhof K. R., Gunning W. B. (2007). Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. Journal of the American Academy of Child & Adolescent Psychiatry, 46(2), 233–241. [DOI] [PubMed] [Google Scholar]
- Dijk D.-J., Archer S. N. (2010). PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Medicine Reviews, 14(3), 151–160. 10.1016/j.smrv.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Esposito S., Laino D., D’Alonzo R., Mencarelli A., Di Genova L., Fattorusso A., Argentiero A., Mencaroni E. (2019). Pediatric sleep disturbances and treatment with melatonin. Journal of Translational Medicine, 17(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffray M. M., Nicolas A., Speranza M., Georgieff N. (2016). Are circadian rhythms new pathways to understand Autism Spectrum Disorder? Journal of Physiology Paris, 110(4), 434–438. 10.1016/j.jphysparis.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Glickman G. (2010). Circadian rhythms and sleep in children with autism. Neuroscience and Biobehavioral Reviews, 34(5), 755–768. 10.1016/j.neubiorev.2009.11.017 [DOI] [PubMed] [Google Scholar]
- Greco M. F., Del Minelli C., Sheehan N. A., Thompson J. R. (2015). Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Statistics in Medicine, 34(21), 2926–2940. 10.1002/sim.6522 [DOI] [PubMed] [Google Scholar]
- Hemani G., Zheng J., Elsworth B., Wade K. H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R., Tan V. Y., Yarmolinsky J., Shihab H. A., Timpson N. J., Evans D. M., Relton C., Martin R. M., Davey Smith G., Gaunt T. R., Haycock P. C. (2018). The MR-Base platform supports systematic causal inference across the human phenome. eLife, 7, Article e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeraj L., Sonuga-Barke E., Antrop I., Roeyers H., Wiersema R., Bal S., Deboutte D. (2012). Altered circadian profiles in attention-deficit/hyperactivity disorder: An integrative review and theoretical framework for future studies. Neuroscience & Biobehavioral Reviews, 36(8), 1897–1919. 10.1016/j.neubiorev.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Jones S. E., Lane J. M., Wood A. R., van Hees V. T., Tyrrell J., Beaumont R. N., Jeffries A. R., Dashti H. S., Hillsdon M., Ruth K. S. (2019). Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nature Communications, 10(1), 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. E., Tyrrell J., Wood A. R., Beaumont R. N., Ruth K. S., Tuke M. A., Yaghootkar H., Hu Y., Teder-Laving M., Hayward C. (2016). Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLOS Genetics, 12(8), Article e1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. E., van Hees V. T., Mazzotti D. R., Marques-Vidal P., Sabia S., van der Spek A., Dashti H. S., Engmann J., Kocevska D., Tyrrell J., Beaumont R. N., Hillsdon M., Ruth K. S., Tuke M. A., Yaghootkar H., Sharp S. A., Ji Y., Harrison J. W., Freathy R. M., . . .Wood A. R. (2019). Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nature Communications, 10(1), 1–12. 10.1038/s41467-019-09576-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki M. I., Karjalainen J., Palta P., Sipilä T. P., Kristiansson K., Donner K. M., Reeve M. P., Laivuori H., Aavikko M., Kaunisto M. A., Loukola A., Lahtela E., Mattsson H., Laiho P., Della Briotta Parolo P., Lehisto A. A., Kanai M., Mars N., Rämö J., . . .Palotie A. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature, 613(7944), 508–518. 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale D. S., Knutson K. L., Yan L. L., Liu K., Rathouz P. J. (2008). Self-reported and measured sleep duration: How similar are they? Epidemiology, 19, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. A., Harbord R. M., Sterne J. A. C., Timpson N., Davey Smith G. (2008). Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Statistics in Medicine, 27(8), 1133–1163. [DOI] [PubMed] [Google Scholar]
- Levandovski R., Dantas G., Fernandes L. C., Caumo W., Torres I., Roenneberg T., Hidalgo M. P. L., Allebrandt K. V. (2011). Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology International, 28(9), 771–778. [DOI] [PubMed] [Google Scholar]
- Lugo J., Fadeuilhe C., Gisbert L., Setien I., Delgado M., Corrales M., Richarte V., Ramos-Quiroga J. A. (2020). Sleep in adults with autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic review and meta-analysis. European Neuropsychopharmacology, 38, 1–24. 10.1016/j.euroneuro.2020.07.004 [DOI] [PubMed] [Google Scholar]
- Mayes S. D., Calhoun S., Bixler E. O., Vgontzas A. N. (2009). Sleep problems in children with autism, ADHD, anxiety, depression, acquired brain injury, and typical development. Sleep Medicine Clinics, 4(1), 19–25. 10.1016/j.jsmc.2008.12.004 [DOI] [Google Scholar]
- Mehta T. R., Gurung P., Nene Y., Fayyaz M., Bollu P. C. (2019). Sleep and ADHD: A review article. Current Developmental Disorders Reports, 6, 228–234. [Google Scholar]
- Ming X., Walters A. S. (2009). Autism spectrum disorders, attention deficit/hyperactivity disorder, and sleep disorders. Current Opinion in Pulmonary Medicine, 15(6), 578–584. [DOI] [PubMed] [Google Scholar]
- Owens J., Gruber R., Brown T., Corkum P., Cortese S., O’Brien L., Stein M., Weiss M. (2013). Future Research Directions in Sleep and ADHD. Journal of Attention Disorders, 17(7), 550–564. 10.1177/1087054712457992 [DOI] [PubMed] [Google Scholar]
- Parvataneni T., Srinivas S., Shah K., Patel R. S. (2020). Perspective on melatonin use for sleep problems in autism and attention-deficit hyperactivity disorder: A systematic review of randomized clinical trials. Cureus, 18, Article e8335. 10.7759/cureus.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., De Lima M. S., Horta B. L., Biederman J., Rohde L. A. (2007). The worldwide prevalence of ADHD: A systematic review and metaregression analysis. American Journal of Psychiatry, 164(6), 942–948. [DOI] [PubMed] [Google Scholar]
- R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [Google Scholar]
- Rzepka-Migut B., Paprocka J. (2020). Efficacy and Safety of melatonin treatment in children with autism spectrum disorder and attention-deficit/hyperactivity disorder – A review of the literature. Brain Sciences, 10(4), 219. 10.3390/brainsci10040219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch J. B., Genro J. P., Bastos C. R., Ghisleni G., Tovo-Rodrigues L. (2018). The role of CLOCK gene in psychiatric disorders: Evidence from human and animal research. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(2), 181–198. 10.1002/ajmg.b.32599 [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M., Torres-Farfán C., Forcelledo M. L., Valenzuela G. J. (2001). The development of circadian rhythms in the fetus and neonate. Seminars in Perinatology, 25(6), 363–370. 10.1053/sper.2001.29037 [DOI] [PubMed] [Google Scholar]
- Singh K., Zimmerman A. W. (2015). Sleep in autism spectrum disorder and attention deficit hyperactivity disorder. Seminars in Pediatric Neurology, 22(2), 113–125. 10.1016/j.spen.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Sun X., Liu B., Liu S., Wu D. J. H., Wang J., Qian Y., Ye D., Mao Y. (2022). Sleep disturbance and psychiatric disorders: A bidirectional Mendelian randomisation study. Epidemiology and Psychiatric Sciences, 31, Article e26. 10.1017/S2045796021000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J. S., Hong H.-K., Ko C. H., McDearmon E. L. (2008). The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nature Reviews Genetics, 9(10), 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S., Anderson G. M., Pichard N., Charbuy H., Touitou Y. (2005). Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biological Psychiatry, 57(2), 134–138. 10.1016/j.biopsych.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Tordjman S., Najjar I., Bellissant E., Anderson G., Barburoth M., Cohen D., Jaafari N., Schischmanoff O., Fagard R., Lagdas E., Kermarrec S., Ribardiere S., Botbol M., Fougerou C., Bronsard G., Vernay-Leconte J. (2013). Advances in the research of melatonin in autism spectrum disorders: Literature review and new perspectives. International Journal of Molecular Sciences, 14(10), 20508–20542. 10.3390/ijms141020508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water A. T. M., Holmes A., Hurley D. A. (2011). Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography: A systematic review. Journal of Sleep Research, 20(1, pt. 2), 183–200. [DOI] [PubMed] [Google Scholar]
- van Hees V. T., Sabia S., Anderson K. N., Denton S. J., Oliver J., Catt M., Abell J. G., Kivimäki M., Trenell M. I., Singh-Manoux A. (2015). A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLOS ONE, 10(11), Article e0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees V. T., Sabia S., Jones S. E., Wood A. R., Anderson K. N., Kivimäki M., Frayling T. M., Pack A. I., Bucan M., Trenell M. I. (2018). Estimating sleep parameters using an accelerometer without sleep diary. Scientific Reports, 8(1), 12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck M., Chen C.-Y., Neale B., Do R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics, 50(5), 693–698. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale J. A., Roveda E., Montaruli A., Galasso L., Weydahl A., Caumo A., Carandente F. (2015). Chronotype influences activity circadian rhythm and sleep: Differences in sleep quality between weekdays and weekend. Chronobiology International, 32(3), 405–415. [DOI] [PubMed] [Google Scholar]
- von Schantz M., Leocadio-Miguel M. A., McCarthy M. J., Papiol S., Landgraf D. (2021). Genomic perspectives on the circadian clock hypothesis of psychiatric disorders. In Advances in genetics (pp. 153–191). Elsevier. 10.1016/bs.adgen.2020.11.005 [DOI] [PubMed] [Google Scholar]
- Wagner M. L., Walters A. S., Fisher B. C. (2004). Symptoms of attention-deficit/hyperactivity disorder in adults with restless legs syndrome. Sleep, 27(8), 1499–1504. 10.1093/sleep/27.8.1499 [DOI] [PubMed] [Google Scholar]
- Whelan S., Mannion A., Madden A., Berger F., Costello R., Ghadiri Foroshani S., Leader G. (2022). Examining the relationship between sleep quality, social functioning, and behavior problems in children with autism spectrum disorder: A systematic review. Nature and Science of Sleep, 14, 675–695. 10.2147/NSS.S239622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpory D., Nicholas B., Nash S. (2002). Social timing, clock genes and autism: A new hypothesis. Journal of Intellectual Disability Research, 46(4), 352–358. 10.1046/j.1365-2788.2002.00423.x [DOI] [PubMed] [Google Scholar]
- Zafeiriou D. I., Ververi A., Dafoulis V., Kalyva E., Vargiami E. (2013). Autism spectrum disorders: The quest for genetic syndromes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(4), 327–366. [DOI] [PubMed] [Google Scholar]
- Zeidan J., Fombonne E., Scorah J., Ibrahim A., Durkin M. S., Saxena S., Yusuf A., Shih A., Elsabbagh M. (2022). Global prevalence of autism: A systematic review update. Autism Research, 15(5), 778–790. 10.1002/aur.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-aut-10.1177_13623613241258546 for The role of circadian rhythms and sleep in the aetiology of autism spectrum disorder and attention-deficit/hyperactivity disorder: New evidence from bidirectional two-sample Mendelian randomization analysis by Xiaotian Dai, Gareth J. Williams, John A. Groeger, Gary Jones, Keeley Brookes, Wei Zhou, Jing Hua and Wenchong Du in Autism