Abstract
Background
Recently, International Diabetes Federation position statement has adopted one-hour post-load glucose (1hPG) ≥ 8.6 mmol/L for diagnosing intermediate hyperglycemia. We aimed to assess the association of 1hPG ≥ 8.6 mmol/L with metabolic syndrome (MetS) and its components, as well as interaction between sex and 1hPG ≥ 8.6 mmol/L on MetS and its components in Chinese people at high risk of diabetes.
Methods
The cross-sectional study was conducted in DaQing city of HeiLongJiang Province, China between August, 2023 and January, 2024. Eligible individuals with fasting glucose of 5.6–6.9 mmol/L and age of 25–55 years in health checkup data in the year of 2023 or with at least one risk factor of diabetes were invited to receive the oral glucose tolerance test and biochemical examinations. Individuals with self-reported presence of diabetes or usage of glucose-lowering medication were excluded. MetS was defined as presence of at least three of the five components according to the Chinese Diabetes Society criteria. Logistic regression was performed to evaluate the association of 1hPG ≥ 8.6 mmol/L with MetS and its components. Additive interaction was estimated using the relative excess risk due to interaction, attributable proportion due to interaction (AP), and synergy index.
Results
A total of 2419 subjects comprising 1465 men (60.6%) with a mean age of 45.77 ± 6.20 years were included, and the prevalence of MetS was 46.8%, with 59.7% in men and 27.1% in women. 1hPG ≥ 8.6 mmol/L was associated with MetS (aOR = 4.40, 95% CI 3.26–6.01), elevated blood pressure (aOR = 1.46, 95% CI 1.13–1.89), hyperglycemia (aOR = 15.46, 95% CI 11.56–20.98), and reduced HDL-C (aOR = 1.51, 95% CI 1.07–2.15) in the overall population, whereas no significant association between 1hPG ≥ 8.6 mmol/L and elevated blood pressure in men (aOR = 1.36, 95% CI 0.97–1.91) or dyslipidemia in women (elevated TG: aOR = 0.81, 95% CI 0.47–1.39; reduced HDL-C: aOR = 1.08, 95% CI 0.49–2.37). Additive interaction effect between sex and 1hPG ≥ 8.6 mmol/L on MetS was observed, with 31% attributed to the interaction effect between men and 1hPG ≥ 8.6 mmol/L (AP = 0.31, 95% CI 0.06–0.49).
Conclusions
There was an additive interaction effect between sex and 1hPG on MetS among Chinese people at high risk of diabetes. 1hPG test and sex-specific strategies should be taken into consideration in cardiometabolic disorder identification and management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-024-01544-0.
Keywords: One-hour post-load glucose, Metabolic syndrome, Interaction effect, Oral glucose tolerance test
Background
Metabolic syndrome (MetS) encompasses a cluster of cardiometabolic risk factors, including abdominal obesity, elevated blood pressure (BP), hyperglycemia, elevated triglyceride (TG), and reduced high-density lipoprotein-cholesterol (HDL-C) [1]. These cardiometabolic risk factors, also refer to as MetS components, are common risk factors for diabetes and cardiovascular diseases [2, 3]. As a result of population aging, industrialization, and unhealthy lifestyles, the prevalence of MetS has increased globally [3, 4]. In China, the prevalence of MetS increased from 13.7% in 2000–2001[5] to 31.1% in 2015–2017[6] according to The Adult Treatment Panel III of the National Cholesterol Education Program (NCEP-ATP III) criteria. Thus, it is worthwhile to identify valuable risk factors for MetS to achieve early identification, management of MetS and prevention of adverse outcomes related to MetS.
Traditionally, fasting plasma glucose (FPG), and two-hour post-load glucose (2hPG) are commonly used to identify cardiometabolic risk, of which post-load hyperglycemia has a stronger association with incident diabetes or cardiovascular outcomes and mortality [7, 8]. Notably, there is heterogeneity of cardiometabolic risk in subjects with normal FPG and 2hPG, while one-hour post-load glucose (1hPG) could be a valuable indicator for identifying populations with increased cardiometabolic risk among individuals with normoglycemia. Previous studies have demonstrated that individuals with elevated 1hPG had more severe insulin resistance [9], which also plays a significant role in the pathophysiology of MetS [10]. Recently, International Diabetes Federation position statement has adopted 1hPG ≥ 8.6 mmol/L for diagnosing intermediate hyperglycemia [11]. In addition, the higher waist circumference (WC), body mass index (BMI), BP, serum uric acid (SUA), liver enzymes, and decreased estimated glomerular filtration rate (eGFR), as well as worse lipid profile, were observed in individuals with normoglycemia but 1hPG ≥ 8.6 mmol/L [11–14]. Therefore, 1hPG could be potentially associated with MetS.
Previous studies explored the association between 1hPG and MetS [13, 15, 16], however, these studies defined MetS using Joint Interim Statement or NCEP-ATP III criteria, without consideration for post-load glucose when defining hyperglycemia. Post-load hyperglycemia is the main subtype of hyperglycemia, and more than half of all cases of hyperglycemia can be undiagnosed using FPG for diagnosis alone in China [17]. Besides, because of the differences in genetic architecture, sex chromosomes, sex hormones, and the gut microbiota between sexes, there are sex differences in BP, lipid and glucose metabolism, and MetS [18, 19]. However, how sex and 1hPG affect the risk of prevalent MetS and its components remains unknown. Hence, it is necessary to explore the associations between 1hPG and MetS or its components by using diagnostic criteria suitable for the Chinese population. In this study, we aimed to assess the associations of 1hPG with MetS and its components via the Chinese Diabetes Society criteria in people at high risk of diabetes, and further explore the interaction effects of sex and 1hPG on MetS and its components.
Methods
Study design and participants
The participants were from the “Daqing Diabetes Prevention Study II (Daqing DPS-II)”, which included the high-risk population screening stage and the lifestyle intervention stage, and this study analyzed the data from the former stage by the two-step screening process. First, individuals were recruited from the health checkup data available for the year of 2023, and those with FPG of 5.6–6.9 mmol/L and age of 25–55 years were invited to visit a digital risk assessment questionnaire for initial eligibility assessment. Additionally, we recruited 231 participants aged 25–55 who did not have health checkup data for 2023, but had anyone of the following: overweight/obese (BMI ≥ 24 kg/m2); family history of diabetes; fasting capillary blood glucose between 5.6 mmol/L and 6.9 mmol/L; random capillary blood glucose between 7.8 mmol/L and 11.0 mmol/L. Second, eligible individuals were invited to receive an oral glucose tolerance test, structural questionnaire, anthropometric measurements, and other biochemical examinations between August, 2023 and January, 2024.
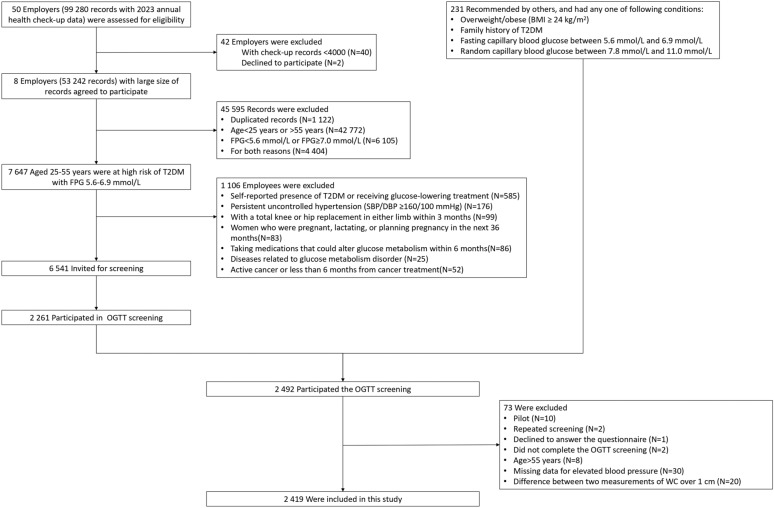
In terms of the lifestyle interventions in the future, we restricted the conditions of individuals invited to be screened. The exclusion criteria were as follows: self-reported presence of diabetes or regular use of glucose medication; persistent uncontrolled hypertension, i.e., systolic BP (SBP) ≥ 160 mmHg or diastolic BP (DBP) ≥ 100 mmHg; with a total knee or hip replacement in either limb within 3 months; women who were pregnant, lactating, or planning pregnancy in the next 36 months; taking medications that could alter glucose metabolism within 6 months; diseases related to glucose metabolism disorders (e.g., Cushion's syndrome); active cancer or less than 6 months from cancer treatment. Individuals with missing data on post-load glucose, WC, BP, or lipid profiles and other reasons were excluded in this analysis (Fig. 1).
Fig. 1.
Flow chart for participants selection
All participants signed written informed consent form, and ethics approval was obtained from the Institutional Ethics Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS&PUMC-IEC-2022-061).
Data collection and variable classification
Participants' sociodemographic characteristics (age, sex, marital status, education, and personal income), personal history and medication for hypertension, fatty liver, and hyperlipidemia, family history of diabetes, and lifestyle factors (smoking status, frequency of alcohol consumption, dietary quality, physical activity, and sleep duration) were obtained by professional interviewers through face-to-face questionnaires. Smoking status was divided into current smoking and non-current smoking (never/former smokers). If participants consumed alcohol at least once a week in the past 12 months, they were classified as habitual alcohol consumers. Information on dietary quality and physical activity was collected via Chinese Diet Quality Questionnaire [20] and the International Physical Activity Questionnaire Short Form [21], respectively. Height, weight, WC, and BP were measured by trained staff. BMI was calculated as weight (kg) divided by height squared (m2), and overweight/obesity was defined by BMI ≥ 24.0 kg/m2. WC was measured for twice with the difference between the two measurements ≤ 1 cm, and the final measurement was recorded as the average of the two measurements. BP was measured three times with a one-minute interval between measurements following a minimum duration of 5 min’ rest, and the average of the last two measurements was used for analyses [22]. The details were available in Additional file: Supplementary Methods and Table S1.
Laboratory measurements
All subjects fasted for at least 8 h, and their fasting blood and random urine were collected at 7–9 a.m. the next day. After fasting blood was collected, the participants were asked to drink 300 mL of water containing 75 g of anhydrous glucose within 5 min before 1- and 2-h venous blood was collected to test the corresponding glucose and insulin levels. Plasma glucose was measured by the hexokinase method, and insulin was measured by radio-immunoassay. Total cholesterol (TC), TG, HDL-C, low-density-lipoprotein cholesterol (LDL-C) were measured by enzymatic methods. Other biochemical indicators included SUA, liver enzymes (ALT, AST, GGT), and serum creatinine. Insulin resistance was evaluated using homeostasis model assessment of insulin resistance (HOMA-IR), where HOMA-IR = fasting insulin (μU/mL) × FPG (mmol/L)/22.5, and the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [23].
Definition of MetS
MetS was defined by the 2020 version of the Guidelines for the Prevention and Treatment of Type 2 Diabetes in China. Individuals who had three or more of the following five components were classified as having MetS: abdominal obesity: WC ≥ 90 cm for men or ≥ 85 cm for women; elevated BP: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, or diagnosed hypertension and on antihypertensive therapy; hyperglycemia: FPG ≥ 6.1 mmol/L or 2hPG ≥ 7.8 mmol/L; elevated TG: ≥ 1.7 mmol/L; reduced HDL-C: < 1.04 mmol/L [24].
Statistical analysis
We conducted several rounds of program training before collecting the data, and performed the rigorous procedure according to our Standard Operation Procedures (SOPs). All data were analyzed using the statistical software R version 4.2.2. Continuous data was presented as mean ± standard deviation for normally distributed variables or median and interquartile range for nonnormally distributed variables. Differences in continuous variables were tested using independent sample Student’s t test (parametric variables) or Wilcoxon test (nonparametric variables). Categorical variables were described as n (%) and were tested with the chi-square (χ2) test.
We made a bar chart to visualize the levels of 1hPG by the number of MetS components (0, 1, 2, 3, 4, and 5) and sex. Pearson correlation analysis was performed to evaluate the correlation of 1hPG with normally distributed variables (WC, SBP, DBP, FPG, 2hPG, HDL-C), and Spearman correlation analysis was performed to evaluate the correlation of 1hPG with TG, which remained skewed despite log10 transformation. Steiger’s Z test was used to compare correlation coefficients [25]. Logistic regression was used to investigate the associations of 1hPG with MetS and its components. In the fully adjusted model, sociodemographic characteristics (age, sex, education, personal income), fatty liver, family history of diabetes, lifestyle factors (smoking status, frequency of alcohol consumption, dietary quality, physical activity, sleep duration), and important indicators (BMI, TC, LDL-C, ALT, AST, GGT, SUA, and eGFR) were considered as covariates. Variance inflation factors (VIFs) and tolerances were calculated to assess the collinearity assumption, with VIFs less than 5 and tolerances greater than 0.2 considered to indicate no significant collinearity (upon testing, no significant multicollinearity was found), odds ratios (ORs) and 95% confidence intervals (CIs) were presented.
To explore the additive interaction effect, we used three parameters: the relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and synergy index [26]. According to the suggestion [27], women with 1hPG < 8.6 mmol/L were set as reference category. The RERI can be interpreted as an additional risk due to interaction and is calculated as the difference between the expected and observed risks: RERI = OR11–OR10–OR01 + 1, where OR11 refers to the OR of the MetS and its components for the exposure of both men and 1hPG ≥ 8.6 mmol/L, OR10 is the OR of the MetS and its components for men with 1hPG < 8.6 mmol/L, OR01 is the OR of the MetS and its components for women with 1hPG ≥ 8.6 mmol/L. The AP is the proportion attributed to the interaction among men with 1hPG ≥ 8.6 mmol/L: AP = RERI/OR11. The synergy index can be interpreted as the excess risk from exposure to both factors when there is interaction relative to the risk from exposure without interaction: synergy index = (OR11–1)/[(OR10–1) + (OR01–1)]. RERI > 0, AP > 0, and synergy index > 1 indicate an additive interaction between sex and 1hPG, and significance was considered when 95%CIs did not contain 0, 0, and 1 for RERI, AP, and synergy index, respectively. All analyses were two-sided tests, with P value < 0.05 indicating statistical significance.
Results
Characteristics of the study participants
Among the individuals recruited from the health checkup data, 6541 subjects were invited to participant in screening and 2261 subjects received the screening, no significant difference in sex and FPG between the two populations (Additional file: Table S2–S3). In total, 2419 subjects were included in this analysis (Fig. 1). The characteristics of the study participants stratified by sex and MetS are presented in Table 1 and Additional file: Table S4. Overall, the mean age was 45.77 ± 6.20 years, men comprised 60.6% (n = 1 465), and 46.8% (n = 1 133) had MetS, with 874 (59.7%) in men and 259 (27.1%) in women, respectively. Subjects with MetS were older, and had lower education levels than the counterparts without MetS (P < 0.001). With respect to lifestyle factors, subjects with MetS were more likely to have unhealthy lifestyles such as current smoking, habitual alcohol, insufficient physical activity, and were less likely to consume all five recommended food groups than those without MetS (P < 0.05). Stratified by sex, significant differences in current smoking, habitual alcohol consumption, and physical activity between individuals with MetS and those without MetS were only found in men. In terms of cardiometabolic profiles, subjects with MetS had significantly higher BMI, FPG, 1hPG, 2hPG, HOMA-IR, SBP, DBP, TC, TG, SUA, and liver enzyme, as well as lower HDL-C level than the counterparts without MetS (all P < 0.001), with similar results in both sexes.
Table 1.
Characteristics of the study participants in men and women by MetS
| Variables | Men (N = 1 465) | Women (N = 954) | ||||
|---|---|---|---|---|---|---|
| MetS (n = 874) | Non-MetS (n = 591) | P | MetS (n = 259) | Non-MetS (n = 695) | P | |
| Age (years) | 46.83 ± 5.92 | 46.36 ± 6.89 | 0.168 | 44.96 ± 5.09 | 44.24 ± 5.96 | 0.066 |
| Education, n (%) | 0.050 | < 0.001 | ||||
| High school or below | 434 (49.7) | 260 (44.0) | 131 (50.6) | 253 (36.4) | ||
| Associate degree | 185 (21.2) | 125 (21.2) | 55 (21.2) | 161 (23.2) | ||
| Bachelor’s degree or above | 255 (29.2) | 206 (34.9) | 73 (28.2) | 281 (40.4) | ||
| Married, n (%) | 774 (88.6) | 515 (87.1) | 0.461 | 229 (88.4) | 598 (86.0) | 0.394 |
| Personal income, n (%) | 0.823 | 0.234 | ||||
| < 5000 CNY/month | 212 (24.3) | 145 (24.5) | 139 (53.7) | 330 (47.5) | ||
| 5000–9999 CNY/month | 594 (68.0) | 395 (66.8) | 110 (42.5) | 333 (47.9) | ||
| ≥ 10,000 CNY/month | 68 (7.8) | 51 (8.6) | 10 (3.9) | 32 (4.6) | ||
| Fatty liver, n (%)a | 658 (75.5) | 336 (57.1) | < 0.001 | 200 (77.5) | 272 (39.2) | < 0.001 |
| Family history of diabetes, n (%)b | 366 (43.2) | 197 (35.0) | 0.002 | 134 (53.4) | 329 (48.7) | 0.229 |
| Current smoking, n (%) | 464 (53.1) | 244 (41.3) | < 0.001 | 11 (4.2) | 17 (2.4) | 0.211 |
| Habitual alcohol consumer, n (%) | 410 (46.9) | 245 (41.5) | 0.045 | 11 (4.2) | 39 (5.6) | 0.498 |
| All-5 score = 1, n (%) | 263 (30.1) | 207 (35.0) | 0.054 | 98 (37.8) | 263 (37.8) | 1.000 |
| Physical activity (MET·min/week) | 960.0 (396.0–1980.0) | 1371.0 (495.0–2502.0) | 0.001 | 840.0 (297.0–1519.5) | 924.0 (396.0–1767.0) | 0.318 |
| Sleep duration (h/day) | 6.53 ± 1.04 | 6.57 ± 1.03 | 0.539 | 6.57 ± 1.15 | 6.64 ± 1.13 | 0.414 |
| BMI (kg/m2) | 28.4 ± 3.4 | 25.2 ± 3.0 | < 0.001 | 29.1 ± 4.4 | 24.2 ± 3.5 | < 0.001 |
| FPG (mmol/L) | 6.5 ± 1.2 | 5.9 ± 0.6 | < 0.001 | 6.4 ± 0.9 | 5.7 ± 0.6 | < 0.001 |
| 1hPG (mmol/L) | 12.5 ± 3.0 | 9.9 ± 2.9 | < 0.001 | 12.3 ± 2.6 | 9.3 ± 2.6 | < 0.001 |
| 2hPG (mmol/L) | 9.8 ± 3.5 | 7.3 ± 2.6 | < 0.001 | 10.5 ± 3.1 | 7.4 ± 2.4 | < 0.001 |
| HOMA-IR | 7.00 (4.83–9.29) | 3.46 (2.82–5.82) | < 0.001 | 7.76 (5.80–9.88) | 3.23 (2.53–5.44) | < 0.001 |
| SBP (mmHg) | 135.5 ± 14.0 | 126.0 ± 13.1 | < 0.001 | 132.5 ± 14.9 | 117.5 ± 13.8 | < 0.001 |
| DBP (mmHg) | 90.4 ± 9.8 | 83.2 ± 9.2 | < 0.001 | 88.3 ± 9.3 | 78.2 ± 9.2 | < 0.001 |
| TC (mmol/L) | 5.14 ± 1.00 | 4.82 ± 0.91 | < 0.001 | 5.19 ± 0.99 | 5.01 ± 0.89 | 0.009 |
| TG (mmol/L) | 2.37 (1.77–3.44) | 1.30 (0.95–1.62) | < 0.001 | 2.05 (1.69–2.89) | 1.11 (0.84–1.48) | < 0.001 |
| HDL-C (mmol/L) | 1.08 ± 0.23 | 1.29 ± 0.29 | < 0.001 | 1.18 ± 0.26 | 1.51 ± 0.34 | < 0.001 |
| LDL-C (mmol/L) | 3.29 ± 0.93 | 3.22 ± 0.80 | 0.165 | 3.39 ± 0.91 | 3.28 ± 0.81 | 0.113 |
| SUA (μmol/L) | 412.0 ± 87.0 | 374.4 ± 78.9 | < 0.001 | 344.7 ± 76.3 | 287.1 ± 64.8 | < 0.001 |
| ALT (U/L) | 23.6 (19.2–29.5) | 20.5 (17.4–24.7) | < 0.001 | 20.7 (16.6–30.2) | 17.9 (15.4–21.2) | < 0.001 |
| AST (U/L) | 31.1 (23.6–46.1) | 22.2 (16.4–31.0) | < 0.001 | 24.8 (16.6–41.0) | 15.2 (11.7–21.4) | < 0.001 |
| GGT (U/L) | 46.0 (31.0–68.8) | 27.0 (20.0–40.6) | < 0.001 | 28.0 (19.0–41.6) | 16.0 (12.0–24.0) | < 0.001 |
| eGFR (mL/min/1.73m2) | 97.1 ± 12.7 | 97.6 ± 11.7 | 0.437 | 109.9 ± 10.7 | 109.2 ± 10.0 | 0.337 |
| Overweight/obesity, n (%) | 820 (93.8) | 396 (67.0) | < 0.001 | 234 (90.3) | 334 (48.1) | < 0.001 |
| Abdominal obesity, n (%) | 694 (79.4) | 140 (23.7) | < 0.001 | 184 (71.0) | 107 (15.4) | < 0.001 |
| Elevated BP, n (%)c | 740 (84.7) | 270 (45.7) | < 0.001 | 201 (77.6) | 167 (24.0) | < 0.001 |
| Hyperglycemia, n (%) | 717 (82.0) | 193 (32.7) | < 0.001 | 234 (90.3) | 249 (35.8) | < 0.001 |
| Elevated TG, n (%) | 698 (79.9) | 122 (20.6) | < 0.001 | 194 (74.9) | 96 (13.8) | < 0.001 |
| Reduced HDL-C, n (%) | 426 (48.7) | 75 (12.7) | < 0.001 | 82 (31.7) | 20 (2.9) | < 0.001 |
Data are presented as mean ± standard deviation or median (interquartile range). CNY, Chinese Yuan; BMI, Body mass index; FPG, Fasting plasma glucose; 1hPG, One-hour post-load glucose; 2hPG, Two-hour post-load glucose; HOMA-IR, Homeostasis model assessment of insulin resistance; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; TC, Total cholesterol; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; SUA, Serum uric acid; ALT, Alanine aminotransferase; AST, Aspartate transaminase; GGT, Gamma glutamyl transferase; eGFR, Estimated glomerular filtration rate; BP, Blood pressure; MetS, Metabolic syndrome
a 3, 3, 1, and 2 persons were excluded analysis because the answer was unknown in men with MetS, men without MetS, women with MetS, and women without MetS, respectively
b 27, 28, 8 and 19 persons were excluded analysis because the answer was unknown in men with MetS, men without MetS, women with MetS, and women without MetS, respectively
c Systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg, or diagnosed and on antihypertensive therapy
Distribution of 1hPG by the number of MetS components and sex
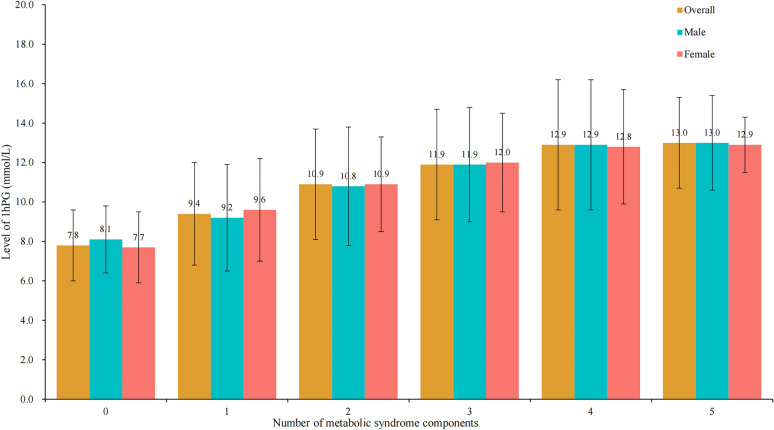
Figure 2 showed the mean and standard deviation of 1hPG by number of MetS components (0, 1, 2, 3, 4, and 5) and sex. Overall, there was higher level of 1hPG in individuals with more MetS components (Ptrend < 0.001), and similar results were observed in men and women separately.
Fig. 2.
Distribution of 1hPG by the number of metabolic syndrome components and sex
Associations of 1hPG with MetS and its components by sex
Correlation coefficients between 1hPG and MetS indices were presented in Table 2. 1hPG correlated with all MetS indices (WC, SBP, DBP, FPG, 2hPG, TG, and HDL-C) in overall population. Stratified by sex, the correlation coefficients of 1hPG with FPG and 2hPG were similar between the sex-specific populations, while the other MetS indices were correlated stronger with 1hPG in women than those in men (PSteiger < 0.01).
Table 2.
Correlation coefficients (95%CI) between 1hPG and MetS indices by sex
| MetS indices | Overall population | Men | Women | Z | PSteiger |
|---|---|---|---|---|---|
| WC | 0.346 (0.311–0.381) | 0.250 (0.202–0.298) | 0.355 (0.298–0.409) | − 2.778 | 0.005 |
| SBP | 0.305 (0.268–0.341) | 0.204 (0.154–0.252) | 0.348 (0.291–0.403) | − 3.748 | < 0.001 |
| DBP | 0.269 (0.232–0.306) | 0.172 (0.122–0.222) | 0.309 (0.251–0.366) | − 3.495 | < 0.001 |
| FPG | 0.724 (0.705–0.743) | 0.721 (0.696–0.745) | 0.705 (0.671–0.735) | 0.781 | 0.435 |
| 2hPG | 0.815 (0.801–0.828) | 0.814 (0.796–0.830) | 0.827 (0.806–0.846) | − 0.955 | 0.340 |
| TG+ | 0.336 (0.299–0.372) | 0.251 (0.202–0.299) | 0.354 (0.296–0.410) | − 2.725 | 0.006 |
| HDL-C | − 0.254 (− 0.291–− 0.217) | − 0.154 (− 0.204–− 0.104) | − 0.258 (− 0.317–− 0.198) | 2.610 | 0.009 |
MetS, Metabolic syndrome; 1hPG, One-hour post-load glucose; 2hPG, Two-hour post-load glucose; WC, Waist circumference; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FPG, Fasting plasma glucose; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol
* Pearson correlation coefficient, or otherwise specified
+ Spearman correlation coefficient
The associations of 1hPG with MetS and its components are detailed in Table 3 and Additional file: Table S5. In the total population, individuals with 1hPG ≥ 8.6 mmol/L had a higher risk of prevalent MetS than those with 1hPG < 8.6 mmol/L (aOR = 4.40, 95% CI 3.26–6.01) after adjusting for the covariates. For the MetS components, the aORs (95% CIs) of hyperglycemia, elevated BP, and reduced HDL-C in individuals with 1hPG ≥ 8.6 mmol/L compared to those with 1hPG < 8.6 mmol/L were 15.46 (11.56–20.98), 1.46 (1.13–1.89), and 1.51 (1.07–2.15), respectively. However, no significant associations were observed between 1hPG ≥ 8.6 mmol/L and abdominal obesity (aOR = 1.30, 95% CI 0.93–1.82) or elevated TG (aOR = 1.30, 95% CI 0.94–1.80). Stratified by sex, a stronger association of 1hPG ≥ 8.6 mmol/L with hyperglycemia was found in women (aOR = 26.73, 95% CI 16.35–45.64) than that in men (aOR = 11.19, 95% CI 7.74–16.50). In addition, significant association of 1hPG ≥ 8.6 mmol/L with an increased risk of elevated BP was observed only in women (aOR = 1.65, 95% CI 1.08–2.54), whereas the association of 1hPG ≥ 8.6 mmol/L with an increased risk of dyslipidemia was significant only in men (elevated TG: aOR = 1.62, 95% CI 1.05–2.51; reduced HDL-C: aOR = 1.55, 95% CI 1.05–2.32).
Table 3.
Association between 1hPG ≥ 8.6 mmol/L and MetS or its components by sex [OR (95%CI)]
| Dependent variables | Overall population | Men | Women |
|---|---|---|---|
| MetS | 4.40 (3.26–6.01) | 3.88 (2.70–5.63) | 5.97 (3.32–11.43) |
| Abdominal obesity | 1.30 (0.93–1.82) | 1.26 (0.84–1.90) | 1.36 (0.72–2.59) |
| Elevated BP | 1.46 (1.13–1.89) | 1.36 (0.97–1.91) | 1.65 (1.08–2.54) |
| Hyperglycemia | 15.46 (11.56–20.98) | 11.19 (7.74–16.50) | 26.73 (16.35–45.64) |
| Elevated TG | 1.30 (0.94–1.80) | 1.62 (1.05–2.51) | 0.81 (0.47–1.39) |
| Reduced HDL-C | 1.51 (1.07–2.15) | 1.55 (1.05–2.32) | 1.08 (0.49–2.37) |
MetS, Metabolic syndrome; BP, Blood pressure; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol
Adjusted for age, education, marital status, personal income, fatty liver, family history of diabetes, lifestyle factors (including current smoking, habitual alcohol, dietary quality, physical activity, sleep duration), BMI, TC, LDL-C, SUA, ALT, AST, GGT, eGFR for MetS, and further adjusted other MetS components for specific MetS component
Additive interaction effect between sex and 1hPG on MetS and its components
Individuals were categorized into four groups based on sex and 1hPG level: Group A (control group; women with 1hPG < 8.6 mmol/L), Group B (women with 1hPG ≥ 8.6 mmol/L), Group C (men with 1hPG < 8.6 mmol/L), and Group D (men with 1hPG ≥ 8.6 mmol/L). The adjusted ORs for MetS and its components are presented in Table 4. Compared to Group A, the aOR (95% CI) of MetS was 10.59 (5.59–20.06) for Group D, and the corresponding aORs (95% CIs) of hyperglycemia, reduced HDL-C, elevated BP, and abdominal obesity were 18.08 (10.61–30.81), 5.06 (2.71–9.44), 2.72 (1.81–4.09), and 2.50 (1.43–4.39), respectively.
Table 4.
Individual and combined association of sex and 1hPG with MetS and its components
| Group | MetS | Abdominal obesity | Elevated BP | Hyperglycemia | Elevated TG | Reduced HDL-C |
|---|---|---|---|---|---|---|
| Group A | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Group B | 5.65 (3.09–10.35) | 1.32 (0.78–2.25) | 1.71 (1.19–2.46) | 23.78 (14.68–38.54) | 1.24 (0.79–1.95) | 1.57 (0.85–2.90) |
| Group C | 2.64 (1.31–5.31) | 1.93 (1.07–3.50) | 2.09 (1.36–3.21) | 1.60 (0.87–2.96) | 1.06 (0.61–1.85) | 3.38 (1.75–6.54) |
| Group D | 10.59 (5.59–20.06) | 2.50 (1.43–4.39) | 2.72 (1.81–4.09) | 18.08 (10.61–30.81) | 1.43 (0.86–2.39) | 5.06 (2.71–9.44) |
MetS, Metabolic syndrome; BP, Blood pressure; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol. Adjusted for age, education, marital status, personal income, fatty liver, family history of diabetes, lifestyle factors (including current smoking, habitual alcohol, dietary quality, physical activity, sleep duration), BMI, TC, LDL-C, SUA, ALT, AST, GGT, eGFR for MetS, and further adjusted other MetS components for specific MetS component. Group A: women with 1hPG < 8.6 mmol/L group. Group B: women with 1hPG ≥ 8.6 mmol/L. Group C: men with 1hPG < 8.6 mmol/L. Group D: men with 1hPG ≥ 8.6 mmol/L
We further analyzed the additive scales to investigate whether the combined effect of sex and 1hPG ≥ 8.6 mmol/L on the risk of MetS and its components exceeded the sum of their individual effects. The results are presented in Table 5. Additive interaction effect was statistically significant between sex and 1hPG ≥ 8.6 mmol/L for the risk of MetS (RERI = 3.30, 95% CI 0.04–9.66). Specifically, the combined risk of MetS from exposure to men and 1hPG ≥ 8.6 mmol/L was 1.53 times than the sum of their individual risk (synergy index = 1.53, 95% CI 1.08–2.15), and 31% of the risk of MetS was attributed to the interaction effect of men and 1hPG ≥ 8.6 mmol/L (AP = 0.31, 95% CI 0.06–0.49). There was no significant additive interaction effect between sex and 1hPG ≥ 8.6 mmol/L for the risk of all MetS components.
Table 5.
Interaction effect between 1hPG and sex on the MetS and its components
| Parameters | MetS | Abdominal obesity | Elevated BP | Hyperglycemia | Elevated TG | Reduced HDL-C |
|---|---|---|---|---|---|---|
| RERI (95%CI) | 3.30 (0.04–9.66) | 0.25 (− 1.23–1.31) | − 0.08 (− 1.15–0.75) | − 6.30 (− 17.01–1.39) | 0.14 (− 0.77–0.68) | 1.11 (− 1.31–3.79) |
| AP (95%CI) | 0.31 (0.06–0.49) | 0.10 (− 0.34–0.44) | − 0.03 (− 0.39–0.23) | − 0.35 (− 0.88–0.02) | 0.10 (− 0.40–0.52) | 0.22 (− 0.13–0.47) |
| synergy index (95%CI) | 1.53 (1.08–2.15) | 1.20 (0.57–2.52) | 0.96 (0.61–1.50) | 0.73 (0.52–1.03) | 1.46 (0.15–14.02) | 1.38 (0.84–2.27) |
RERI, Relative excess risk due to interaction; CI, Confidence interval; AP, Attributable proportion due to interaction; MetS, Metabolic syndrome; BP, Blood pressure; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol
Adjusted for age, education, marital status, personal income, fatty liver, family history of diabetes, lifestyle factors (including current smoking, habitual alcohol, dietary quality, physical activity, sleep duration), BMI, TC, LDL-C, SUA, ALT, AST, GGT, eGFR for MetS, and further adjusted other MetS components for specific MetS component
Discussion
MetS is an aggregation of cardiometabolic risk factors characterized by insulin resistance, which significantly contributes to incident diabetes, cardiovascular diseases, and related mortality. Thus, early recognition and management of the risk factors for MetS are vital. In this study, we found that 1hPG was associated with higher risk of prevalent MetS and its components in Chinese population at high risk of diabetes, and additive interaction effect was observed between sex and 1hPG on prevalent MetS.
In our study, the prevalence of MetS was 46.8% according to the Chinese Diabetes Society criteria in individuals at high risk of diabetes, which was higher than the prevalence in general population [28] and lower than that in individuals with newly diagnosed diabetes [29, 30]. In terms of MetS components, elevated BP and hyperglycemia had the highest prevalence, followed by abdominal obesity, elevated TG, and reduced HDL-C. These results differed from previous study in which hyperglycemia had the lowest prevalence [6], possibly because the subjects included in this study were people at high risk of diabetes. Subjects with MetS were older and more likely to be men in our study. Previous studies presented mixed results in terms of sex differences, with some studies showing similar results with our study [28, 31]. Other studies reported a higher prevalence of MetS in women [5, 6], and the difference may be due to the effect of estrogen or menopausal status. Data from the China National Health and Nutrition Surveillance (2010–2012) Project revealed a higher prevalence of MetS in men than in women aged less than 50 years, and the opposite pattern was observed among those aged above 50 years [31]. Some studies demonstrated that a higher risk of MetS was observed in postmenopausal women with the decrease of estrogen level [32]. Although we did not collect the information on menopausal status, the average age of the women in this study was younger than 45 years old, which may help explain why men had a higher risk of MetS than women did in our study.
Individuals with elevated 1hPG have insulin resistance and impaired β-cell function, and 1hPG is an independent risk factor for diabetes, cardiovascular diseases, and mortality [33]. According to our analysis, the higher level of 1hPG was observed in individuals with more MetS components regardless of sex, which indicates that 1hPG may reflect the severity of MetS. 1hPG ≥ 8.6 mmol/L was associated with MetS and all components after adjusting for demographic characteristics and lifestyle factors, but was no longer independently associated with abdominal obesity and elevated TG when further adjusting for the important biochemical indicators. In contrast, 1hPG increased the risk of MetS, elevated BP, hyperglycemia, and reduced HDL-C after controlling for potential covariates. However, a previous study reported that 1hPG ≥ 8.0 mmol/L was independently associated with MetS, elevated BP and elevated TG, but not with reduced HDL-C after adjusting for age, sex, alcohol consumption, smoking, family history of diabetes and BMI in participants with normoglycemia[16]. A cross-sectional study conducted in 2 439 Caucasian overweight or obese subjects (28% men, 72% women) also found that BP, FPG, and TG were independently associated with 1hPG ≥ 8.6 mmol/L, whereas HDL-C showed no significance [13]. One possible interpretation for the difference in associations between 1hPG and dyslipidemia might be the difference in the study participants. For example, the participants included in our study were predominantly Asian men, while the study involving Caucasian subjects mainly included women [13]; more research is needed to explore the sex-specific associations of 1hPG with MetS and its components in other ethnic populations.
For hyperglycemia, accumulating evidence showed that 1hPG was an independent factor for detecting and predicting hyperglycemia, and the International Diabetes Federation has adopted 1hPG ≥ 8.6 mmol/L for diagnosing intermediate hyperglycemia [11]. Consistently, we also found a significant association between 1hPG ≥ 8.6 mmol/L and hyperglycemia, with a stronger association in women than that in men. Similarly, a prospective study with 12-year follow-up found a better ability for 1hPG to predict incident diabetes in women (AUC = 0.82, 95% CI 0.74–0.89) than in men (AUC = 0.78, 95% CI 0.72–0.85)[34]. It could be interpreted that hyperglycemia was mainly attributed to elevated 2hPG [17], which is more commonly observed in women because of prolonged gut glucose absorption [35, 36]. Notably, the association of 1hPG with elevated BP was observed only in women after adjusting for covariates, whereas the association of 1hPG with dyslipidemia (including elevated TG and reduced HDL-C) was observed only in men. There was limited evidence on the sex differences in associations of 1hPG with MetS components; however, several studies supported the prominent associations of hypertension or pulse pressure with incident prediabetes in women [37, 38] and elevated TG with prediabetes in men [18]. The causes of these differences were unclear yet, but could be partially attributed to the differences in endogenous gonadal hormones [39]. Further investigation is essential to explore the etiology and intrinsic mechanisms that drive the sex-specific variances observed in the correlations between 1hPG and metabolic components. Such studies would be instrumental in the development of sex-specific approaches and strategies for the identification and management of metabolic disorders.
Our study further found a significant interaction effect between sex and 1hPG ≥ 8.6 mmol/L on MetS, while no interaction effects were observed for MetS components. Because of the differences in insulin resistance and metabolic disorders between sexes [19], how sex and 1hPG affect the risk of MetS is worth of exploration. We found the risk of prevalent MetS was significantly higher in men with 1hPG ≥ 8.6 mmol/L than the sum of individual risk in women with 1hPG ≥ 8.6 mmol/L and men with 1hPG < 8.6 mmol/L, and 31% of the risk of MetS could be attributed to the interaction effect of men and 1hPG ≥ 8.6 mmol/L. Previous studies rarely explored the interaction effect yet, which limited the comparison of our results with others. However, our findings could help with the precise identification and management of MetS, and further prevent diabetes, cardiovascular diseases and even mortality.
This study had several strengths. Initially, we used the Chinese Diabetes Society criteria to diagnose MetS, which incorporate 2hPG as a diagnostic marker for hyperglycemia, and the Chinese Diabetes Society criteria was more suitable for Chinese people, because the exclusion of 2hPG might underestimate the prevalence of hyperglycemia or MetS, especially in women [36]. Furthermore, we assessed the associations of 1hPG with MetS and its components, as well as the interaction effects between sex and 1hPG on MetS and its components. To the best of our knowledge, few previous studies explored the interaction effect between sex and 1hPG on MetS. However, several limitations should be taken into consideration. First, this study was a cross-sectional study, which could not explore the causal relationship between 1hPG and MetS or its components, further follow-up is needed to explore the role of 1hPG in the risk of incident MetS and its components. Second, we only included people at high risk of diabetes, limiting the generalization of the findings. However, the oral glucose tolerance test is not routinely recommended for the general population due to its complexity and time-intensive nature. Our study employed a two-step screening process to conduct the oral glucose tolerance test in individuals at high risk of diabetes, which is considered practical in the real world. Third, the study participants were only from northeast China, so further research in other regions is necessary to explore the associations of 1hPG with MetS and its components. Fourth, there could be some other potential confounders not included in this analysis, such as detailed information on smoking and alcohol consumption.
Conclusion
In summary, there was sex difference in associations of 1hPG with MetS and its components, and additive interaction effect was found between men and 1hPG ≥ 8.6 mmol/L on MetS among Chinese people at high risk of diabetes. 1hPG test and sex-specific strategies should be taken into consideration in cardiometabolic disorders identification and management.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- AP
Attributable proportion due to interaction
- BP
Blood pressure
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- eGFR
Estimated glomerular filtration rate
- FPG
Fasting plasma glucose
- GGT
Gamma glutamyl transferase
- HDL-C
High-density lipoprotein-cholesterol
- HOMA-IR
Homeostasis model assessment of insulin resistance
- LDL-C
Low-density-lipoprotein cholesterol
- MetS
Metabolic syndrome
- OR
Odd ratio
- 1hPG
One-hour post-load glucose
- RERI
Relative excess risk due to interaction
- SUA
Serum uric acid
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglyceride
- 2hPG
Two-hour post-load glucose
- WC
Waist circumference
Author contributions
XC conceptualized the study hypothesis, performed the data analysis, and wrote the main manuscript text. XC, YW, DL, DZ, KL, and CY contributed to data collection. JZ reviewed and edited the manuscript. JZ, QG, JW, and ZY contributed to project administration and project supervision. RS acquired the funding, and contributed to project administration. All authors read and approved the final manuscript.
Funding
This work was supported by the Disciplines Construction Project: Population Medicine (WH10022022010).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS&PUMC-IEC-2022–061). Written informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nwankwo M, Okamkpa CJ, Danborno B. Comparison of diagnostic criteria and prevalence of metabolic syndrome using WHO, NCEP-ATP III, IDF and harmonized criteria: a case study from urban southeast Nigeria. Diabetes Metab Syndr. 2022;16: 102665. 10.1016/j.dsx.2022.102665. [DOI] [PubMed] [Google Scholar]
- 2.Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine (Baltimore). 2017;96: e8491. 10.1097/md.0000000000008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neeland IJ, Lim S, Tchernof A, Gastaldelli A, Rangaswami J, Ndumele CE, et al. Metabolic syndrome. Nat Rev Dis Primers. 2024;10:77. 10.1038/s41572-024-00563-5. [DOI] [PubMed] [Google Scholar]
- 4.Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. 2017;17:101. 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–405. 10.1016/s0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 6.Yao F, Bo Y, Zhao L, Li Y, Ju L, Fang H, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients. 2021;13:4475. 10.3390/nu13124475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin A(1c) on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;42:1539–48. 10.2337/dc18-1390. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275–85. 10.1007/s00125-021-05592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcovecchio ML, Bagordo M, Marisi E, de Giorgis T, Chiavaroli V, Chiarelli F, et al. One-hour post-load plasma glucose levels associated with decreased insulin sensitivity and secretion and early makers of cardiometabolic risk. J Endocrinol Invest. 2017;40:771–8. 10.1007/s40618-017-0638-6. [DOI] [PubMed] [Google Scholar]
- 10.Giangregorio F, Mosconi E, Debellis MG, Provini S, Esposito C, Garolfi M, et al. A systematic review of metabolic syndrome: key correlated pathologies and non-invasive diagnostic approaches. J Clin Med. 2024. 10.3390/jcm13195880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman M, Manco M, Satman I, Chan J, Schmidt MI, Sesti G, et al. International diabetes federation position statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res Clin Pract. 2024;209: 111589. 10.1016/j.diabres.2024.111589. [DOI] [PubMed] [Google Scholar]
- 12.Briker SM, Hormenu T, DuBose CW, Mabundo LS, Chung ST, Ha J, et al. Metabolic characteristics of Africans with normal glucose tolerance and elevated 1-hour glucose: insight from the Africans in America study. BMJ Open Diabetes Res Care. 2020;8: e000837. 10.1136/bmjdrc-2019-000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haverals L, Van Dessel K, Verrijken A, Dirinck E, Peiffer F, Verhaegen A, et al. Cardiometabolic importance of 1-h plasma glucose in obese subjects. Nutr Diabetes. 2019;9:16. 10.1038/s41387-019-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagannathan R, Fiorentino TV, Marini MA, Sesti G, Bergman M. One-hour post-load glucose is associated with severity of hepatic fibrosis risk. Diabetes Res Clin Pract. 2022;189: 109977. 10.1016/j.diabres.2022.109977. [DOI] [PubMed] [Google Scholar]
- 15.Park SK, Ryoo JH, Oh CM, Choi JM, Jung JY. 1-Hour and 2-hour postload glucose level on oral glucose tolerance test and the risk of incident metabolic syndrome. J Clin Endocrinol Metab. 2019;104:539–49. 10.1210/jc.2018-01102. [DOI] [PubMed] [Google Scholar]
- 16.Pramodkumar TA, Priya M, Jebarani S, Anjana RM, Mohan V, Pradeepa R. Metabolic profile of normal glucose-tolerant subjects with elevated 1-h plasma glucose values. Indian J Endocrinol Metab. 2016;20:612–8. 10.4103/2230-8210.190532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui S, Zainal H, Harun SN, Sheikh Ghadzi SM, Ghafoor S. Gender differences in the modifiable risk factors associated with the presence of prediabetes: a systematic review. Diabetes Metab Syndr. 2020;14:1243–52. 10.1016/j.dsx.2020.06.069. [DOI] [PubMed] [Google Scholar]
- 19.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25:1657–66. 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Herforth AW, Vogliano C, Zou Z. Most commonly-consumed food items by food group, and by Province, in China: implications for diet quality monitoring. Nutrients. 2022;14:1754. 10.3390/nu14091754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. 10.1249/01.Mss.0000078924.61453.Fb. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004–18: findings from six rounds of a national survey. BMJ. 2023;380: e071952. 10.1136/bmj-2022-071952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49. 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35: e3158. 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 25.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–51. 10.1037/0033-2909.87.2.245. [Google Scholar]
- 26.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–9. 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 27.Knol MJ, VanderWeele TJ, Groenwold RH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26:433–8. 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Liu Q, Li Z, Du J, Wang C, Gao Y, et al. Prevalence of metabolic syndrome and risk factors among Chinese adults: results from a population-based study—Beijing China, 2017–2018. China CDC Wkly. 2022;4:640–5. 10.46234/ccdcw2022.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Cao C, Tang X, Yan X, Zhou H, Liu J, et al. Prevalence of metabolic syndrome and its determinants in newly-diagnosed adult-onset diabetes in china: a multi-center, cross-sectional survey. Front Endocrinol (Lausanne). 2019;10:661. 10.3389/fendo.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Chen ZY, Guo XL, Tu M. Monocyte to high-density lipoprotein and apolipoprotein A1 ratios: novel indicators for metabolic syndrome in chinese newly diagnosed type 2 diabetes. Front Endocrinol (Lausanne). 2022;13: 935776. 10.3389/fendo.2022.935776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He YN, Zhao WH, Zhao LY, Yu DM, Zhang J, Yang XG, et al. Prevalence of metabolic syndrome in Chinese adults in 2010–2012. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:212–5. 10.3760/cma.j.issn.0254-6450.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause. 2018;25:1155–64. 10.1097/gme.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 33.Peng M, He S, Wang J, An Y, Qian X, Zhang B, et al. Efficacy of 1-hour postload plasma glucose as a suitable measurement in predicting type 2 diabetes and diabetes-related complications: a post hoc analysis of the 30-year follow-up of the Da Qing IGT and diabetes study. Diabetes Obes Metab. 2024;26:2329–38. 10.1111/dom.15547. [DOI] [PubMed] [Google Scholar]
- 34.Saunajoki AE, Auvinen JP, Bloigu AH, Timonen MJ, Keinänen-Kiukaanniemi SM. Evaluating the 1-h post-load glucose level to predict future type 2 diabetes. Diabetes Res Clin Pract. 2020;160: 108009. 10.1016/j.diabres.2020.108009. [DOI] [PubMed] [Google Scholar]
- 35.Anderwald C, Gastaldelli A, Tura A, Krebs M, Promintzer-Schifferl M, Kautzky-Willer A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. 2011;96:515–24. 10.1210/jc.2010-1398. [DOI] [PubMed] [Google Scholar]
- 36.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–3. 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Wang F, Xiao X, Liu D, Deng Z. Non-linear relationship between pulse pressure and the risk of prediabetes: a 5-year cohort study in Chinese adults. Sci Rep. 2024;14:3824. 10.1038/s41598-024-52136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Cong X, Liu S, Zhang R, Li J. Relationship between blood pressure and prediabetes in Chinese adults: a prospective study. Chin J Diabetes Mellitus. 2022;46:38. [Google Scholar]
- 39.Chella Krishnan K, Mehrabian M, Lusis AJ. Sex differences in metabolism and cardiometabolic disorders. Curr Opin Lipidol. 2018;29:404–10. 10.1097/mol.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.