Abstract
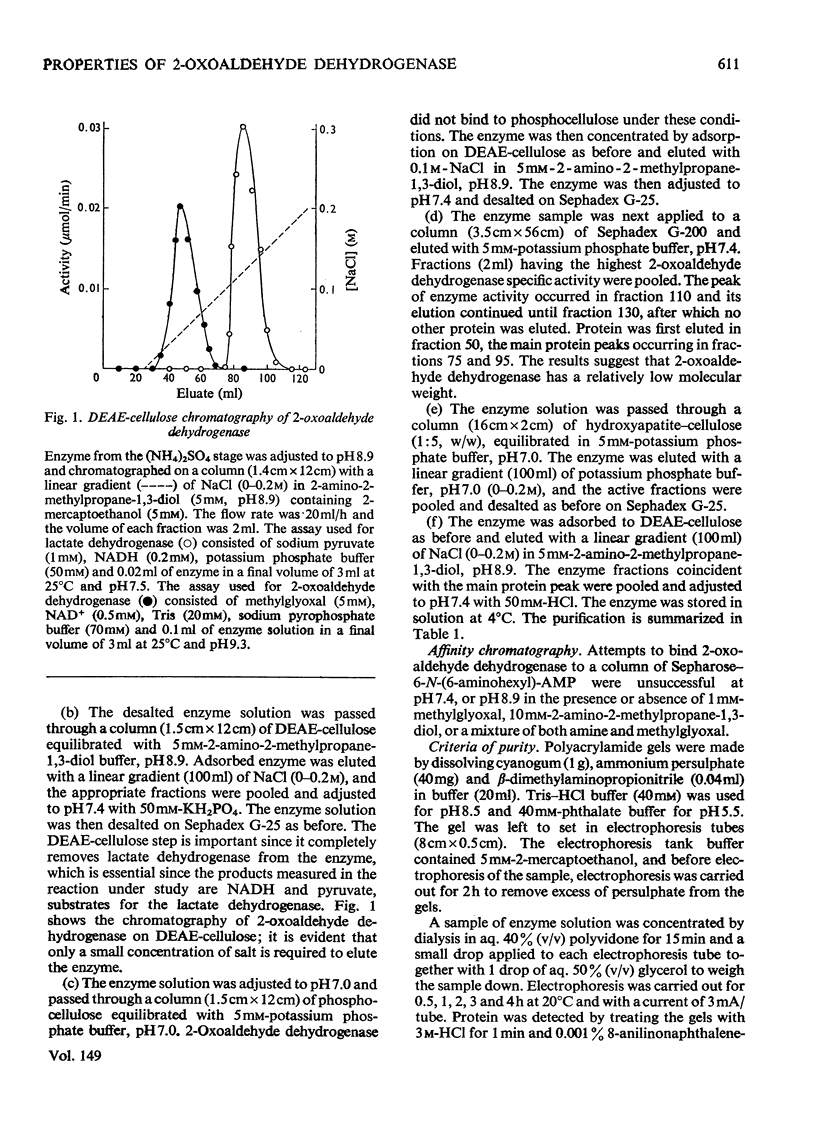
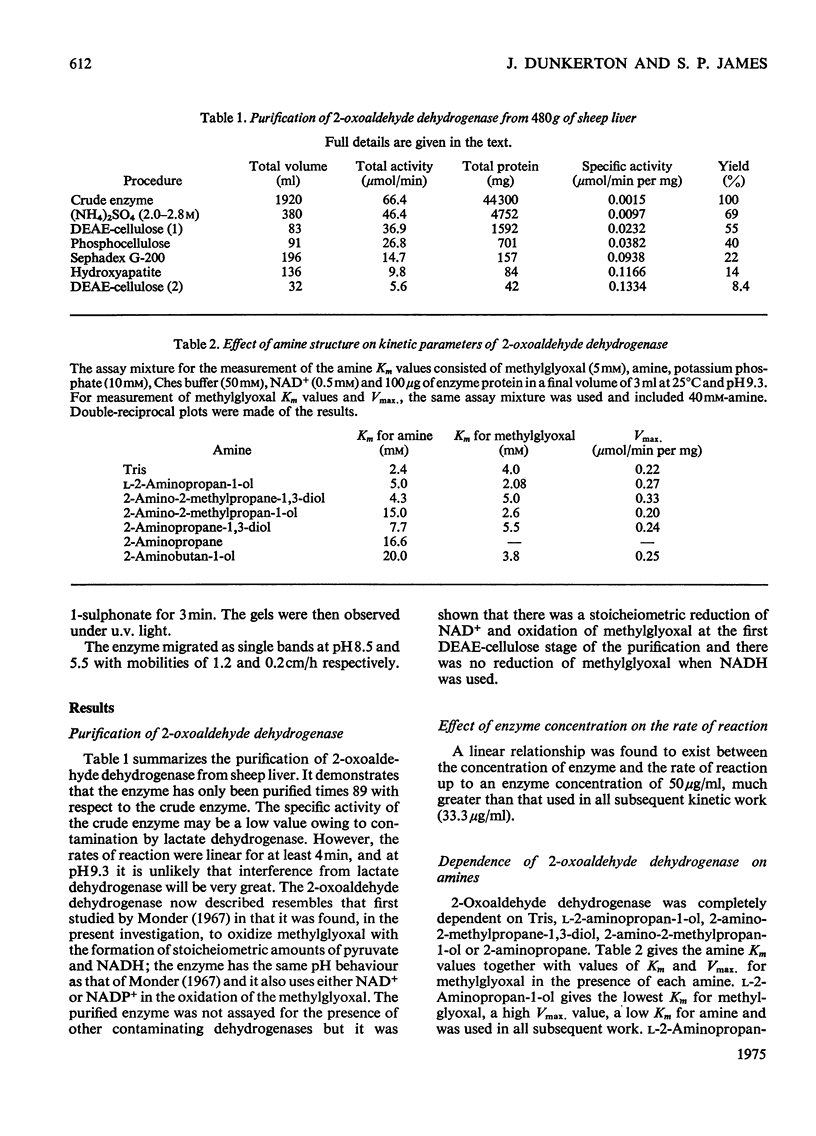
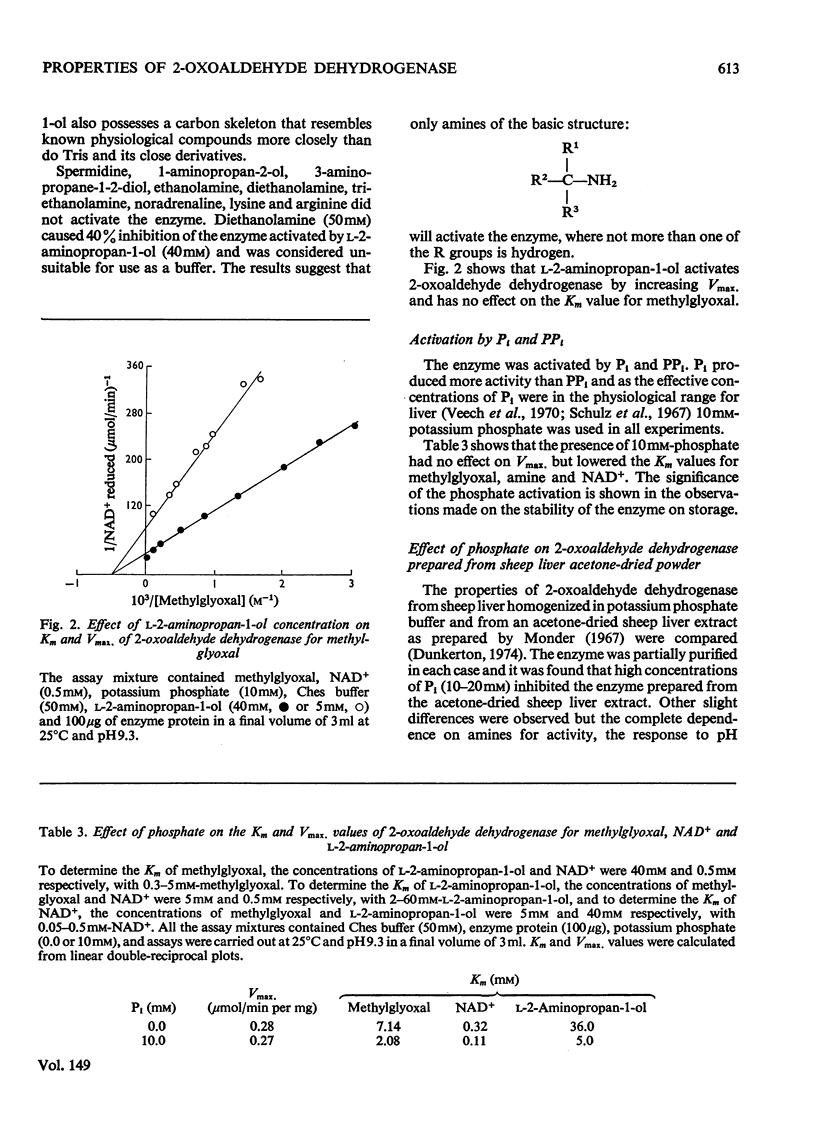
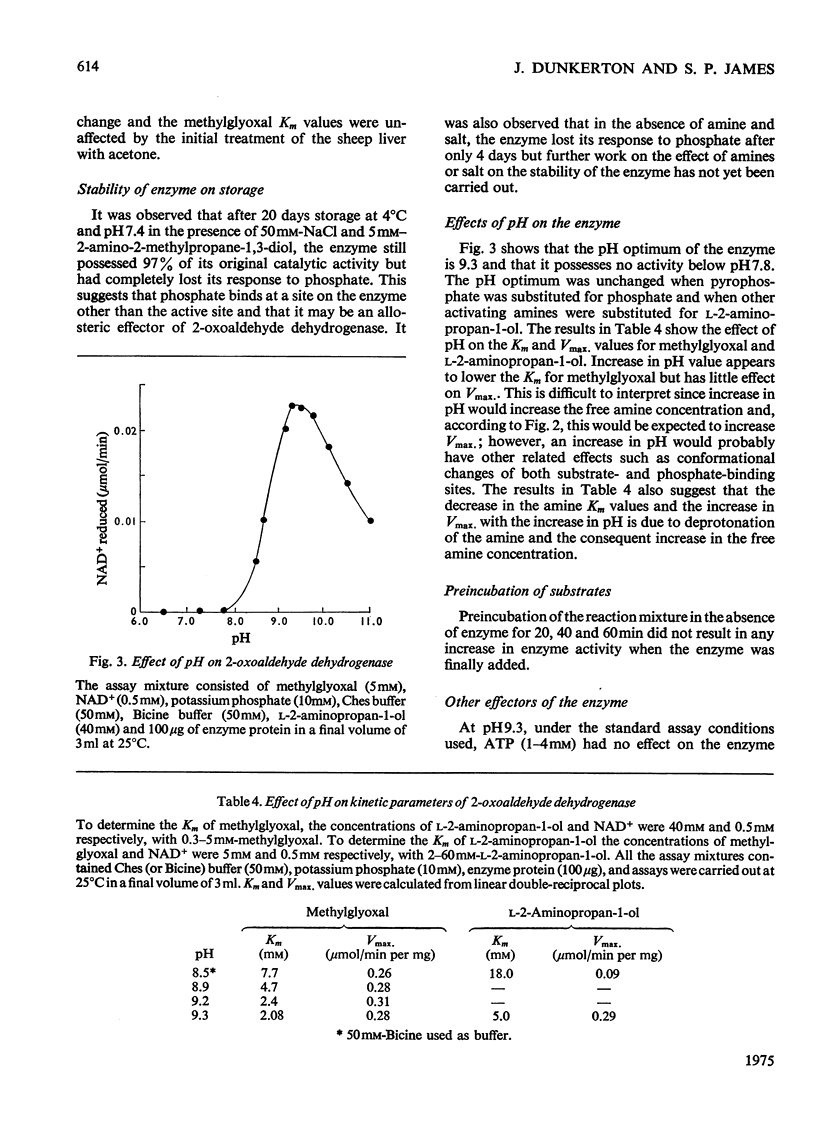
1. 2-Oxoaldehyde dehydrogenase was purified from sheep liver and gave one band on polyacrylamide-gel electrophoresis. 2. The enzyme was completely dependent for its activity on the presence of Tris or one of a number of related amines, all of general structure: (See article). When more than one R group was hydrogen no enzyme activity was observed. 3. Only one of these amines is known to exist in living tissues and large concentrations of all amines were required for maximum activity. L-2-Aminopropan-1-ol was the most effective amine on the basis of substrate Km and Vmax. values and the amine Km values. 4. The enzyme was activated by phosphate which lowered the Km values for methylglyoxal, amine and NAD+. 5. The pH optimum of the enzyme was 9.3 and there was no activity at pH values below 7.8. A search for activators that might produce activity at pH 7.4 proved unsuccessful. 6. The enzyme was inhibited by rather large concentrations of barbiturates (6-46 mM) and nitro-alcohol analogues of the activating amines (66-139 mM).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. M., Boyer J. L. A rapid assay for the glyoxalase enzyme system. Anal Biochem. 1971 May;41(1):29–38. doi: 10.1016/0003-2697(71)90188-6. [DOI] [PubMed] [Google Scholar]
- Bonsignore A., Leoncini G., Ricci D., Siri A. Aminic compounds tested as catalysts for glyceraldehyde conversion into methylglyoxal. Ital J Biochem. 1972 Jul-Aug;21(4):169–178. [PubMed] [Google Scholar]
- Bonsignore A., Leoncini G., Siri A., Ricci D. The lysine catalysed reaction transforming glyceraldehyde into methylglyoxal. Ital J Biochem. 1970 Jul-Aug;19(4):284–301. [PubMed] [Google Scholar]
- CLIFFE E. E., WALEY S. G. The mechanism of the glyoxalase I reaction, and the effect of ophthalmic acid as an inhibitor. Biochem J. 1961 Jun;79:475–482. doi: 10.1042/bj0790475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970 Dec 11;11(4):273–276. doi: 10.1016/0014-5793(70)80546-4. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. H. Amino-acetone; its isolation and role in metabolism. Nature. 1959 Apr 11;183(4667):1051–1052. doi: 10.1038/1831051a0. [DOI] [PubMed] [Google Scholar]
- Együd L. G., Szent-Györgyi A. Cell division, SH, ketoaldehydes, and cancer. Proc Natl Acad Sci U S A. 1966 Feb;55(2):388–393. doi: 10.1073/pnas.55.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. T. Inhibition of mammalian cell division by glyoxals. Exp Cell Res. 1968 Apr;50(1):65–72. doi: 10.1016/0014-4827(68)90394-7. [DOI] [PubMed] [Google Scholar]
- Jellum E., Elgjo K. S-Alkylglutathione, methylglyoxal metabolism and cell division. Biochem Biophys Res Commun. 1970 Feb 20;38(4):575–582. doi: 10.1016/0006-291x(70)90620-0. [DOI] [PubMed] [Google Scholar]
- Jellum E. Metabolism of the ketoaldehyde 2-keto-3-deoxyglucose. Biochim Biophys Acta. 1968 Oct 15;165(3):357–363. doi: 10.1016/0304-4165(68)90213-4. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. The molecular biology of Euglena gracilis. V. Enzyme localization. Exp Cell Res. 1968 Jul;51(1):150–156. doi: 10.1016/0014-4827(68)90165-1. [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Ikeda Y., Tsuiki S. Inhibition of L-glutamine:D-fructose-6-phosphate aminotransferase by methylglyoxal. Biochim Biophys Acta. 1972 Dec 7;289(2):303–310. doi: 10.1016/0005-2744(72)90081-2. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Lindström L., Bártfai T. Partial purification and characterization of glyoxalase I from porcine erythrocytes. Eur J Biochem. 1972 Sep 18;29(2):276–281. doi: 10.1111/j.1432-1033.1972.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Monder C. Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate. J Biol Chem. 1967 Oct 25;242(20):4603–4609. [PubMed] [Google Scholar]
- Patterson R. L., Mottram D. S. The occurrence of volatile amines in uncured and cured pork meat and their possible role in nitrosamine formation in bacon. J Sci Food Agric. 1974 Nov;25(11):1419–1425. doi: 10.1002/jsfa.2740251110. [DOI] [PubMed] [Google Scholar]
- SZENT-GYOERGYI A. CELL DIVISION AND CANCER. Science. 1965 Jul 2;149(3679):34–37. doi: 10.1126/science.149.3679.34. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A., HEGYELI A., McLAUGHLIN J. A. Cancer therapy: a possible new approach. Science. 1963 Jun 28;140(3574):1391–1392. doi: 10.1126/science.140.3574.1391. [DOI] [PubMed] [Google Scholar]
- Schulz D. W., Passonneau J. V., Lowry O. H. An enzymic method for the measurement of inorganic phosphate. Anal Biochem. 1967 May;19(2):300–314. doi: 10.1016/0003-2697(67)90166-2. [DOI] [PubMed] [Google Scholar]
- Sparkes B. G., Kenny C. P. Identification of a bacterial growth inhibitors from HeLa cells: a ketoaldehyde. Proc Natl Acad Sci U S A. 1969 Nov;64(3):920–922. doi: 10.1073/pnas.64.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi A. Bioelectronics. Intermolecular electron transfer may play a major role in biological regulation, defense, and cancer. Science. 1968 Sep 6;161(3845):988–990. doi: 10.1126/science.161.3845.988. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A., Együd L. G., McLaughlin J. A. Keto-aldehydes and cell division. Science. 1967 Feb 3;155(3762):539–541. doi: 10.1126/science.155.3762.539. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A., Hegyeli A., McLaughlin J. A. CONSTITUENTS OF THE THYMUS GLAND AND THEIR RELATION TO GROWTH, FERTILITY, MUSCLE, AND CANCER. Proc Natl Acad Sci U S A. 1962 Aug;48(8):1439–1442. doi: 10.1073/pnas.48.8.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Vander Jagt D. L., Han L. P., Lehman C. H. Kinetic evaluation of substrate specificity in the glyoxalase-I-catalyzed disproportionation of -ketoaldehydes. Biochemistry. 1972 Sep 26;11(20):3735–3740. doi: 10.1021/bi00770a011. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. J., McLean S. A. Double-isotope derivative assay of methylglyoxal. Anal Biochem. 1971 Oct;43(2):472–480. doi: 10.1016/0003-2697(71)90278-8. [DOI] [PubMed] [Google Scholar]
- Willetts A. J., Turner J. M. Threonine metabolism in a strain of Bacillus subtilis: enzymes acting on methylglyoxal. Biochim Biophys Acta. 1970 Dec 29;222(3):668–670. doi: 10.1016/0304-4165(70)90195-9. [DOI] [PubMed] [Google Scholar]


