Abstract
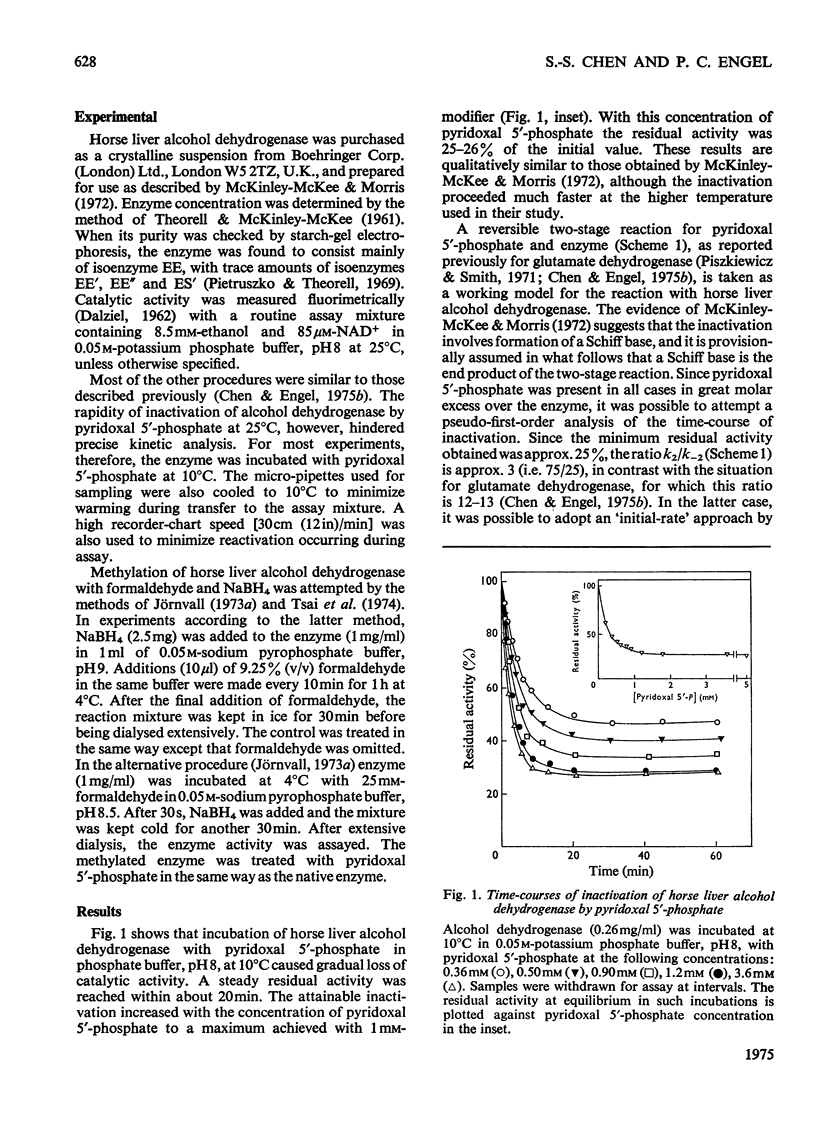
1. The inactivation of horse liver alcohol dehydrogenase by pyridoxal 5'-phosphate in phosphate buffer, pH8, at 10 degrees C was investigated. Activity declines to a minimum value determined by the pyridoxal 5'-phosphate concentration. The maximum inactivation in a single treatment is 75%. This limit appears to be set by the ratio of the first-order rate constants for interconversion of inactive covalently modified enzyme and a readily dissociable non-covalent enzyme-modifier complex. 2. Reactivation was virtually complete on 150-fold dilution: first-order analysis yielded an estimate of the rate constant (0.164min-1), which was then used in the kinetic analysis of the forward inactivation reaction. This provided estimates for the rate constant for conversion of non-covalent complex into inactive enzyme (0.465 min-1) and the dissociation constant of the non-covalent complex (2.8 mM). From the two first-order constants, the minimum attainable activity in a single cycle of treatment may be calculated as 24.5%, very close to the observed value. 3. Successive cycles of modification followed by reduction with NaBH4 each decreased activity by the same fraction, so that three cycles with 3.6 mM-pyridoxal 5'-phosphate decreased specific activity to about 1% of the original value. The absorption spectrum of the enzyme thus treated indicated incorporation of 2-3 mol of pyridoxal 5'-phosphate per mol of subunit, covalently bonded to lysine residues. 4. NAD+ and NADH protected the enzyme completely against inactivation by pyridoxal 5'-phosphate, but ethanol and acetaldehyde were without effect. 5. Pyridoxal 5'-phosphate used as an inhibitor in steady-state experiments, rather than as an inactivator, was non-competitive with respect to both NADH and acetaldehyde. 6. The partially modified enzyme (74% inactive) showed unaltered apparent Km values for NAD+ and ethanol, indicating that modified enzyme is completely inactive, and that the residual activity is due to enzyme that has not been covalently modified. 7. Activation by methylation with formaldehyde was confirmed, but this treatment does not prevent subsequent inactivation with pyridoxal 5'-phosphate. Presumably different lysine residues are involved. 8. It is likely that the essential lysine residue modified by pyridoxal 5'-phosphate is involved either in binding the coenzymes or in the catalytic step. 9. Less detailed studies of yeast alcohol dehydrogenase suggest that this enzyme also possesses an essential lysine residue.
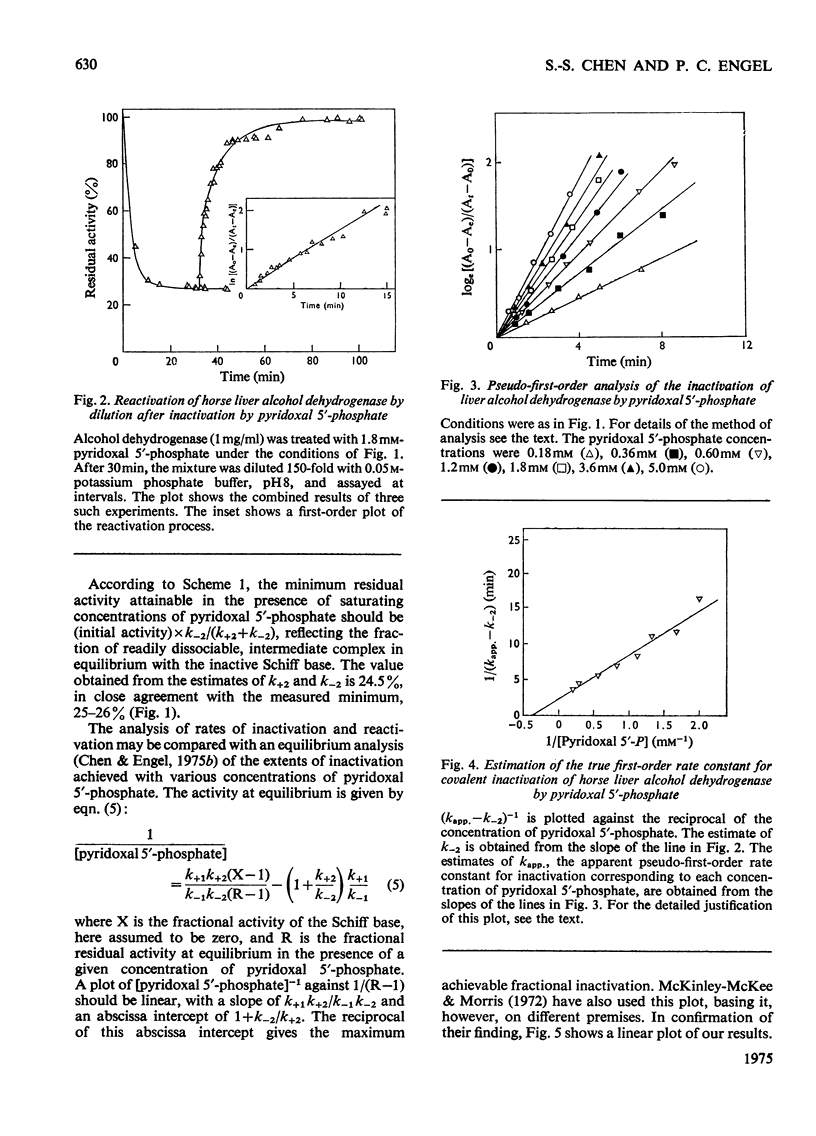
Full text
PDF


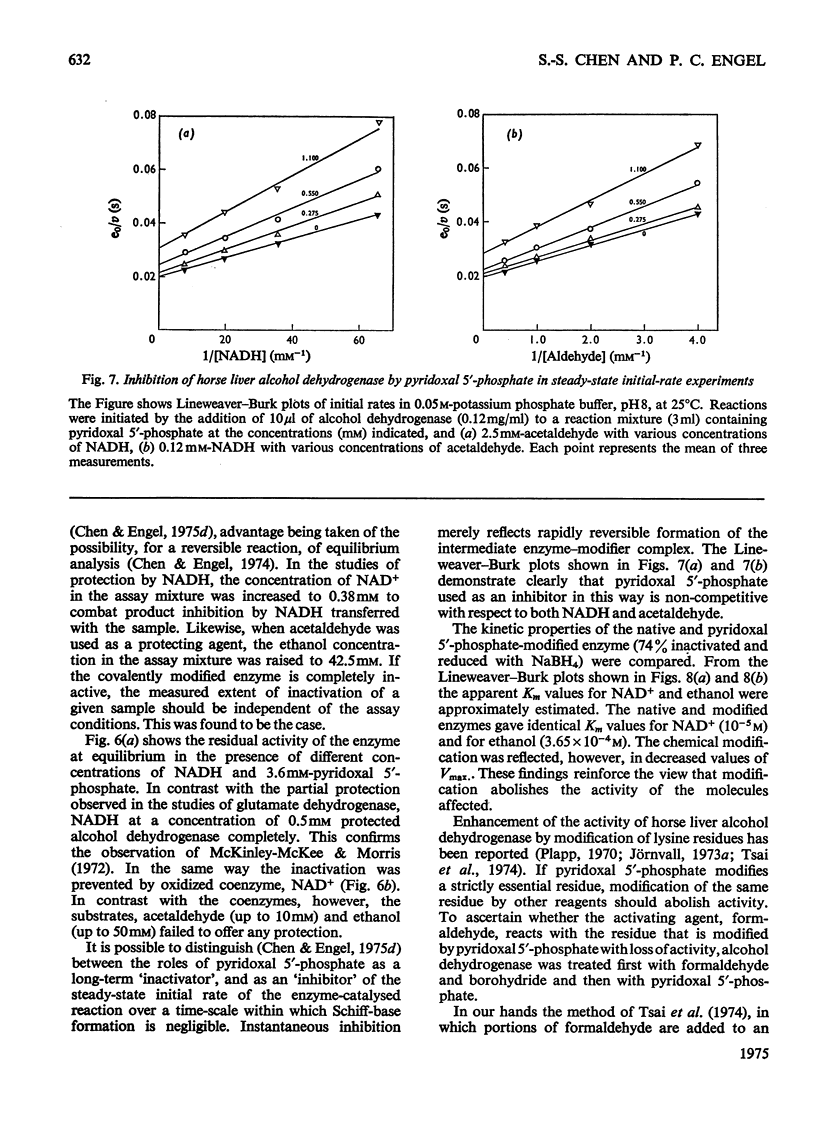
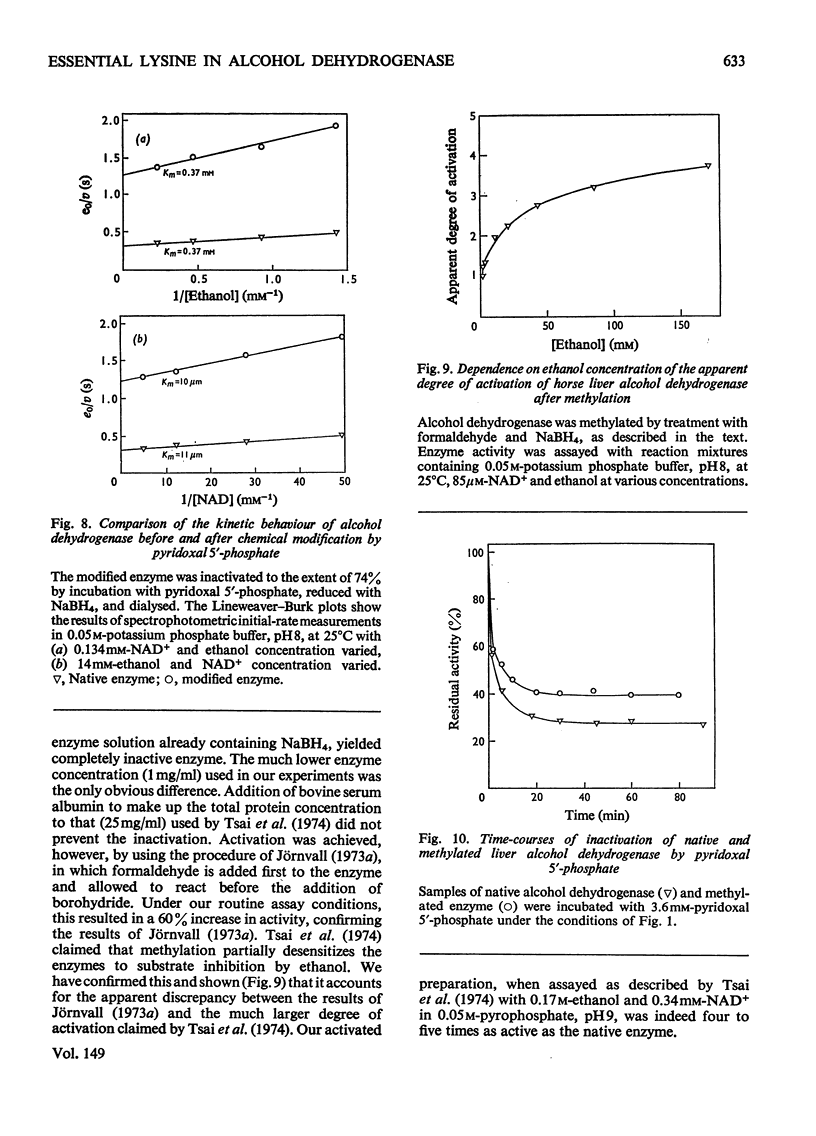


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S. S., Engel P. C. Inactivation of nicotinamide--adenine dinucleotide-linked dehydrogenases by pyridoxal 5'-phosphate. Biochem Soc Trans. 1975;3(1):80–82. doi: 10.1042/bst0030080. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. Modification of pig M4 lactate dehydrogenase by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1975 Jul;149(1):107–113. doi: 10.1042/bj1490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. The equilibrium position of the reaction of bovine liver glutamate dehydrogenase with pyridoxal5'-phosphate. A demonstration that covalent modification with this reagent completely abolishes catalytic activity. Biochem J. 1975 May;147(2):351–358. doi: 10.1042/bj1470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Engel P. C. Protection of glutamate dehydrogenase by nicotinamide-adenine dinucleotide against reversible inactivation by pyridoxal 5'-phosphate as a sensitive indicator of conformational change induced by substrates and substrate analogues. Biochem J. 1974 Dec;143(3):569–574. doi: 10.1042/bj1430569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. F. A comparison of the kinetics and stoichiometry of proton uptake with aldehyde reduction for liver alcohol dehydrogenase under single turnover conditions. Biochemistry. 1974 Mar 12;13(6):1146–1151. doi: 10.1021/bi00703a015. [DOI] [PubMed] [Google Scholar]
- Dworschack R., Tarr G., Plapp B. V. Identification of the lysine residue modified during the activation of acetimidylation of horse liver alcohol dehydrogenase. Biochemistry. 1975 Jan 28;14(2):200–203. doi: 10.1021/bi00673a002. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Brändén C. I. The structure of horse liver alcohol dehydrogenase. FEBS Lett. 1974 Aug 25;44(2):200–204. doi: 10.1016/0014-5793(74)80725-8. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase. The reductive amination of 2-oxoglutarate. Biochem J. 1970 Jul;118(3):409–419. doi: 10.1042/bj1180409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin B. R., Frieden C. The effect of pyridoxal phosphate modification on the catalytic and regulatory properties of bovine liver glutamate dehydrogenase. J Biol Chem. 1972 Apr 10;247(7):2139–2144. [PubMed] [Google Scholar]
- Johnson G. S., Deal W. C., Jr Inactivation of tetrameric rabbit muscle pyruvate kinase by specific binding of 2 to 4 moles of pyridoxal 5'-phosphate. J Biol Chem. 1970 Jan 25;245(2):238–245. [PubMed] [Google Scholar]
- Jörnvall H. Partial similarities between yeast and liver alcohol dehydrogenases. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2295–2298. doi: 10.1073/pnas.70.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Reactive lysine residues in horse liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1069–1076. doi: 10.1016/0006-291x(73)90036-3. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- McKinley-McKee J. S., Morris D. L. The lysines in liver alcohol dehydrogenase. Chemical modification with pyridoxal 5'-phosphate and methyl picolinimidate. Eur J Biochem. 1972 Jun 23;28(1):1–11. doi: 10.1111/j.1432-1033.1972.tb01877.x. [DOI] [PubMed] [Google Scholar]
- Pietruszko R., Theorell H. Subunit composition of horse liver alcohol dehydrogenase. Arch Biochem Biophys. 1969 Apr;131(1):288–298. doi: 10.1016/0003-9861(69)90133-7. [DOI] [PubMed] [Google Scholar]
- Piszkiewicz D., Smith E. L. Bovine liver glutamate dehydrogenase. Equilibria and kinetics of inactivation by pyridoxal. Biochemistry. 1971 Nov 23;10(24):4538–4544. doi: 10.1021/bi00800a030. [DOI] [PubMed] [Google Scholar]
- Plapp B. V. Enhancement of the activity of horse liver alcohol dehydrogenase by modification of amino groups at the active sites. J Biol Chem. 1970 Apr 10;245(7):1727–1735. [PubMed] [Google Scholar]
- Ronchi S., Zapponi M. C., Ferri G. Inhibition by pyridoxal-phosphate of glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1969 Apr;8(3):325–331. doi: 10.1111/j.1432-1033.1969.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Equilibrium kinetic study of the catalytic mechanism of bovine liver glutamate dehydrogenase. Biochemistry. 1973 May 22;12(11):2164–2172. doi: 10.1021/bi00735a024. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Plapp B. V. Activation and inactivation of horse liver alcohol dehydrogenase with pyridoxal compounds. J Biol Chem. 1975 Jan 10;250(1):205–210. [PubMed] [Google Scholar]
- Tsai C. S., Tsai Y. H., Lauzon G., Cheng S. T. Structure and activity of methylated horse liver alcohol dehydrogenase. Biochemistry. 1974 Jan 29;13(3):440–443. doi: 10.1021/bi00700a007. [DOI] [PubMed] [Google Scholar]


