Abstract
Background/aim
This research study was conducted to examine the clinical characteristics and post-splenectomy survival outcomes of patients diagnosed with hepatosplenic T-cell lymphoma (HSTCL).
Materials and methods
A total of 10 cases of HSTCL patients admitted to the Hematology Department of Fudan University Affiliated Huadong Hospital between January 2012 and December 2021 were included. In addition, we also included 30 other cases reported from domestic and international sources. All pathological specimens were stained with hematoxylin and eosin (H&E) and immunohistochemistry, with color development using DAB staining. Survival analysis was conducted using Kaplan-Meier curves and log-rank tests.
Results
In the 10 HSTCL patients, Epstein-Barr virus (EBV) infection was confirmed. Six patients had died, with 5 of them within 1 year of disease onset. Survival analysis showed poorer prognosis in patients with hemophagocytic syndrome and thrombocytopenia. Patients who underwent splenectomy followed by chemotherapy had a higher median and average survival time compared to those who only received chemotherapy. The study included a total of 40 HSTCL patients, with 29 males and 11 females, and an average age of onset at 42.3 years. All patients presented with fever, with some exhibiting emaciation and/or hemophagocytic syndrome. Splenomegaly, hepatomegaly, lymphadenopathy, and bone marrow involvement were found in the patients. Common laboratory findings included leukopenia, anemia, and thrombocytopenia. All patients exhibited elevated ferritin levels and decreased blood calcium levels.
Conclusion
Those patients suffering from hemophagocytic syndrome at the onset of this disease face greater treatment-related difficulties and a higher risk of mortality. Combined chemotherapy after splenectomy may improve HSTCL patient survival.
Keywords: Hepatosplenic T-cell lymphoma, Splenectomy, Chemotherapy, Survival outcomes, Prognostic factors
Introduction
Hepatosplenic T-cell lymphoma (HSTCL) is a rare disease, accounting for 1–2% of all peripheral T-cell lymphoma cases, with notably lower rates in Asia (0.2%) relative to Europe (2.3%) or the Americas (3%) [1]. Immunophenotyping can enable the classification of cases into αβ- and γδ-T-cell lymphoma cases, but no significant differences in clinical presentation or prognostic outcomes have been noted when comparing the two [2]. HSTCL cases do not exhibit distinctive clinical manifestations, which often include hepatic and splenic enlargement, decreased blood counts, damaged liver function, and potential bone marrow involvement. The most common genetic features of this disease are related to chromosome 7q or triploid chromosome 8 [3, 4]. Genomic sequencing has revealed that mutations in the STAT5B, STAT3, and PIK3CD genes are present in up to 6% of HSTCL cases [5]. The treatment of affected patients generally centers on some combination of splenectomy, CHOP- or L-asparaginase-based chemotherapy regimens, and hematopoietic stem cell transplantation. Even with these interventions, however, a majority of patients still die within one year of initial diagnosis [6].
In this study, we present the clinicopathologic, immunophenotypic, and clinical data of 10 patients who developed HSTCL. We also searched the literature and identified 30 additional cases of HSTCL. We summarize and analyze the clinical data from HSTCL patients admitted to our hospital in recent years to provide an overview of this disease.
Materials and methods
Study group
We searched the files of the department of Hematopathology at the Fudan University Affiliated Huadong Hospital between January 2012 and December 2021. The diagnosis of HSTCL fulfilled the criteria specified in the World Health Organization. Clinical data were collected from review of the medical records, which included laboratory indicators, pathological reports, and bone marrow biopsy results imaging studies for evidence of splenomegaly, hepatomegaly or lymphadenopathy, therapy and clinical follow-up.Telephone-based follow-up with patients or their families was performed to evaluate their treatment plans and survival outcomes.
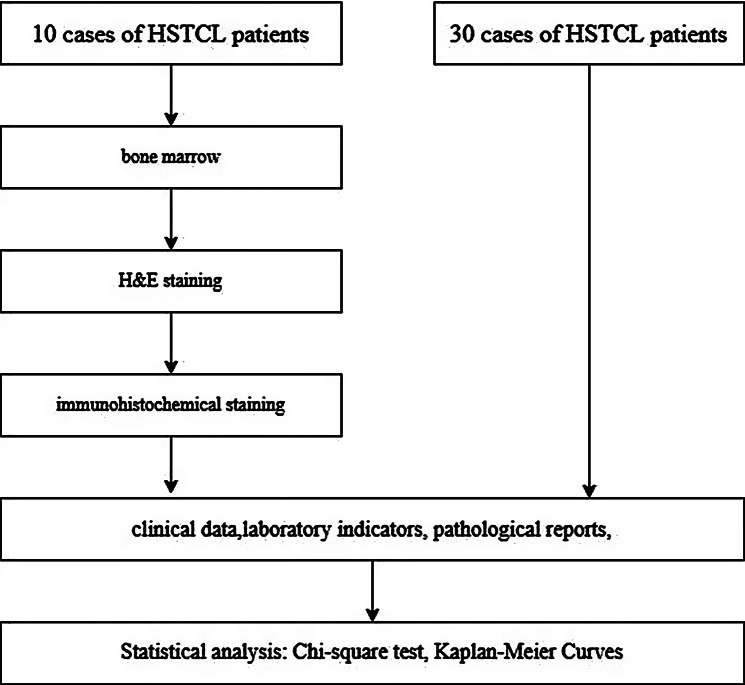
Although the study initially included only 10 patients. To mitigate this limitation, we have retrospectively reviewed the clinical and survival data from an additional 30 cases of HSTCL reported both domestically and internationally. This combined dataset of 40 cases allows for more robust statistical analysis and a broader representation of the disease’s clinical spectrum (Fig. 1).
Fig. 1.
Flow chart of study design and methodology
Histopathologic assessment
All pathological specimens were fixed with 10% neutral formalin, routinely dehydrated, paraffin-embedded, and cut into 4µ M-thick sections. Sections were subjected to hematoxylin and eosin (H&E).
Immunophenotypic analysis
Immunohistochemical analysis was performed using formalin-fixed, paraffin-embedded tissue sections at the referring institutions or in our own laboratory at the time of diagnosis. At our institution, we used 4-µm thick tissue sections, heat-induced epitope retrieval, and an avidin-biotin complex detection method. 3,3′-Diaminobenzidine was used as a chromogen, and staining was performed in an automated immunostainer as described previously. Antibodies specific for the following antigens were used. All antibodies used for this study were purchased from Fuzhou Maixin Company.
Follow-up time
The follow-up time for our patients was a minimum of 6 months, with the majority of patients followed for over a year. This duration is sufficient to assess short-term survival and initial response to treatment. For patients who survived beyond one year, we have continued to track their survival status and any recurrence of disease, with the longest follow-up extending up to 34 months post-diagnosis. This extended follow-up time allows us to evaluate long-term survival and recurrence rates, providing a more comprehensive understanding of treatment outcomes.
Statistical analysis
SPSS 26.0 software was utilized for all data analyses. The Student’s t-test and chi-square test were used for the analysis between two groups. Kaplan-Meier curves with log-rank tests was used for survival analysis. A p-value of < 0.05 was considered statistically significant for all comparisons made.
Results
Clinical data
The 10 patients analyzed in this study included 7 males and 3 females, with an average age of 42.8 years and respective minimum and maximum ages at disease onset of 18 and 74 years. In all 10 cases, the disease initially began with a fever, with accompanying emaciation in 3 cases. Patients did not experience obvious night sweating. There were also 5 cases of combined hemophagocytic syndrome and 1 case of a systemic rash. Splenomegaly was evident in 10 cases, while 5 patients exhibited liver enlargement, 7 exhibited lymph node enlargement, and 5 exhibited bone marrow involvement. There were 7 cases of leukopenia at disease onset, as well as 8 cases of anemia (Hb < 120 g/L), and 5 cases of thrombocytopenia (PLT < 100 × 109/L). In all 10 cases, elevated ferritin and decreased blood calcium levels were observed. Eight cases exhibited varying degrees of impaired liver function, while 3 exhibited elevated uric acid levels, 8 presented with significantly decreased lactate dehydrogenase levels (LDH > 300 U/L), and 5 cases had elevated β 2 microglobulin levels (β 2 microglobulin > 3.5 mg/L). Epstein-Barr virus (EBV) infections were confirmed in 2 patients (EBV copies > 103). Of these 10 patients, 6 had died as of the time of manuscript preparation, of whom 5 died within 1 year of disease onset. One patient experienced recurrent disease after autologous hematopoietic stem cell transplantation and died 34 months following disease onset as shown in Table 1.
Table 1.
Clinical characteristics of 10 patients with hepatosplenic lymphoma
| Serial Number | Gender | Age | Peripheral abnormal hemogram | Liver damage | LDH abnormality | Hemophagocytic syndrome | Bone marrow infiltration | Treatment | Follow up results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 29 | - | - | - | - | - | Splenectomy + GDP | 9 m alive |
| 2 | F | 34 |
Agranulocytosis Anemia |
+ | + | + | - | Splenectomy + VP16 + Cyclosporin | 10 m alive |
| 3 | F | 55 | Anemia | + | + | + | NA(Not available) | Splenectomy + CHOP | 10 m alive |
| 4 | F | 66 | Anemia | + | + | - | + | Splenectomy + CHOP | 24 m alive |
| 5 | M | 29 |
Agranulocytosis Anemia Thrombocytopenia |
+ | - | + | - | Splenectomy + GDLP | 6 m died |
| 6 | M | 64 |
Agranulocytosis Thrombocytopenia |
+ | + | + | + | Splenectomy + Lucitinib | 2.5 m died |
| 7 | M | 74 |
Agranulocytosis Anemia Thrombocytopenia |
+ | + | + | + | Splenectomy + VP16 | 1 m died |
| 8 | M | 26 |
Agranulocytosis Anemia |
- | + | - | + | Splenectomy + Hyper-CVAD | 7.5 m died |
| 9 | M | 33 |
Agranulocytosis Anemia Thrombocytopenia |
+ | + | + | + | Splenectomy + CHOP | 1 m died |
| 10 | M | 18 |
Agranulocytosis Anemia Thrombocytopenia |
+ | + | - | - | Splenectomy + Hyper-CVAD + Autologous stem cell transplantation | 34 m died |
Note NA indicates missing data
Immunophenotypic characteristics
Of these 10 patients, all were positive for CD3. In addition, three cases were CD2 positive, five cases were CD5 positive, three cases were CD7 positive, and nine cases were CD56 positive. Moreover, two cases were positive for EBER, while all were Ki67 positive. Seven were positive for TIA-1 (cytotoxic T cell immunophenotype 1), while six were positive for granzyme B, five were positive for perforin, six were negative for IGH rearrangement, and two were positive for TCR rearrangement as shown in Table 2; Figs. 2, 3, 4 and 5.
Table 2.
Immunophenotypic characteristics of 10 patients with hepatosplenic T-cell lymphoma
| Serial Number | CD2 | CD3 | CD5 | CD7 | CD56 | CD4 | CD8 | EBER | Ki67 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | - | - | 30%+ |
| 2 | + | A few+ | Scattered weak + | + | red pulp + | - | Dense area 30%+ | ||
| 3 | partly+ | partly+ | + | + | partly+ | - | + | ||
| 4 | partly+ | partly+ | A few+ | partly+ | - | +/- | + | ||
| 5 | + | - | + | -/+ | + | + | + | ||
| 6 | + | partly+ | + | scattered + | partly+ | A few+ | - | 60%+ | |
| 7 | + | + | - | + | - | littoral cell + | - | 30%+ | |
| 8 | - | + | + | + | - | - | - | 70%+ | |
| 9 | + | + | - | - | + | 25%+ | |||
| 10 | + | - | + | 30%+ |
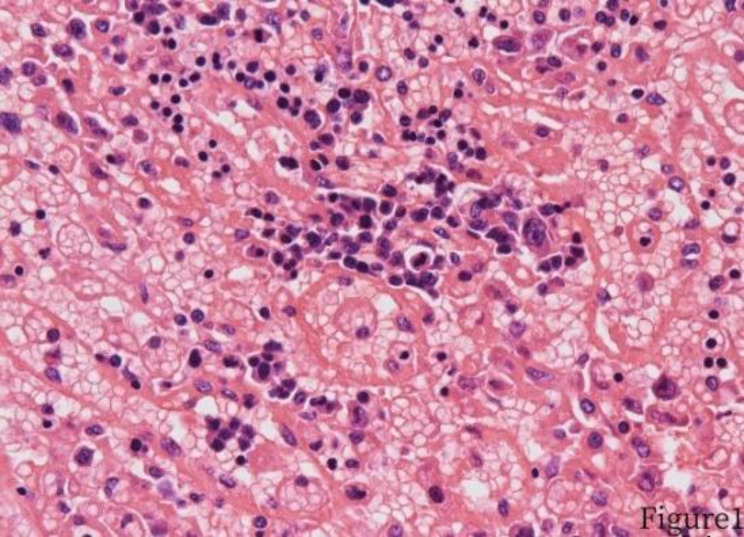
Fig. 2.
Tumor cells invasion of the splenic sinus cord
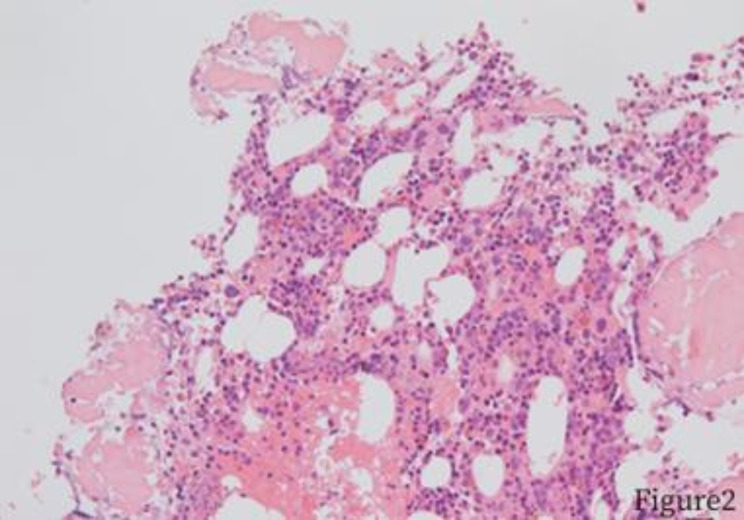
Fig. 3.
Intramedullary sinus invasion of the bone marrow
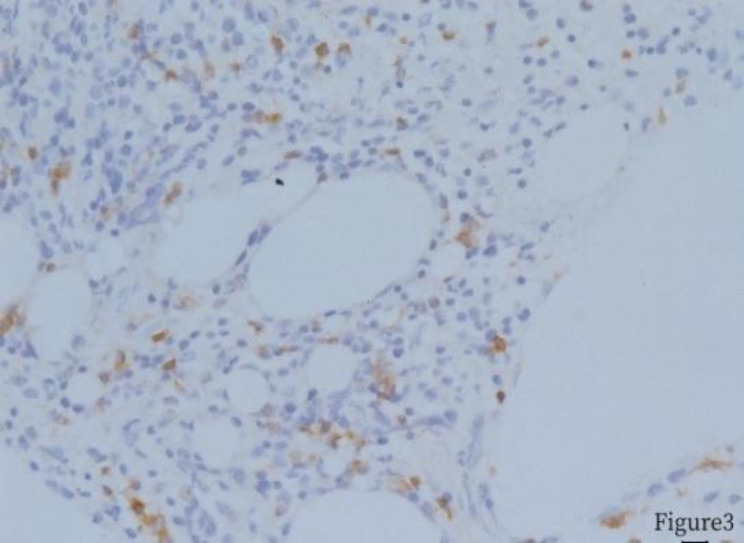
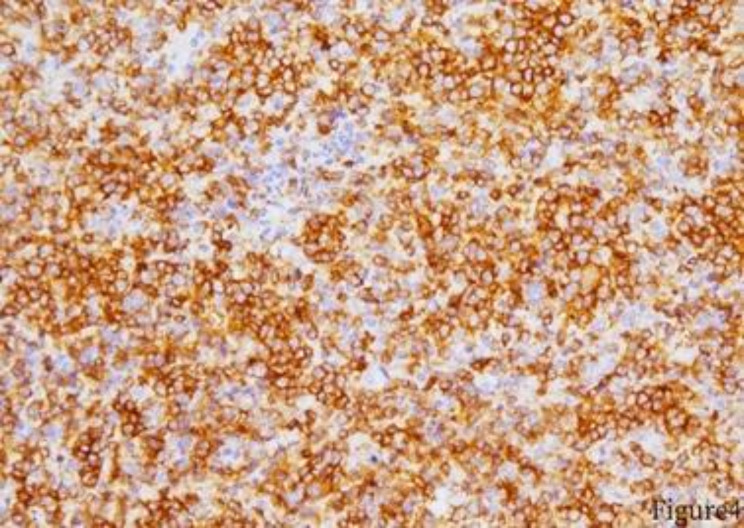
Fig. 4.
Bone marrow biopsy, CD3 positive
Fig. 5.
Spleen biopsy, CD56 positive
Survival analysis
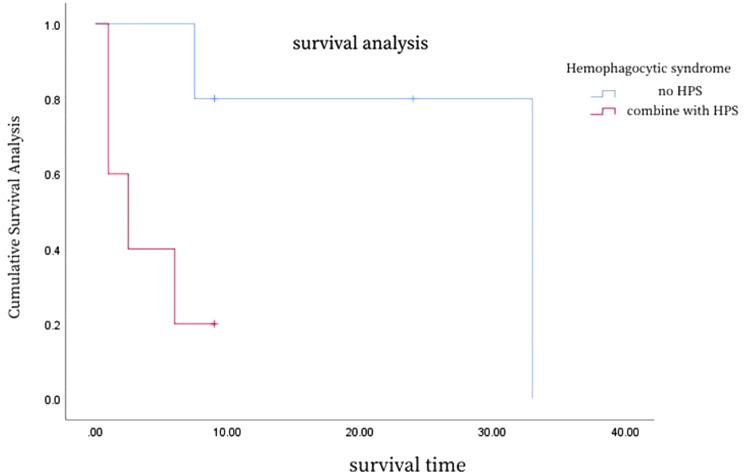
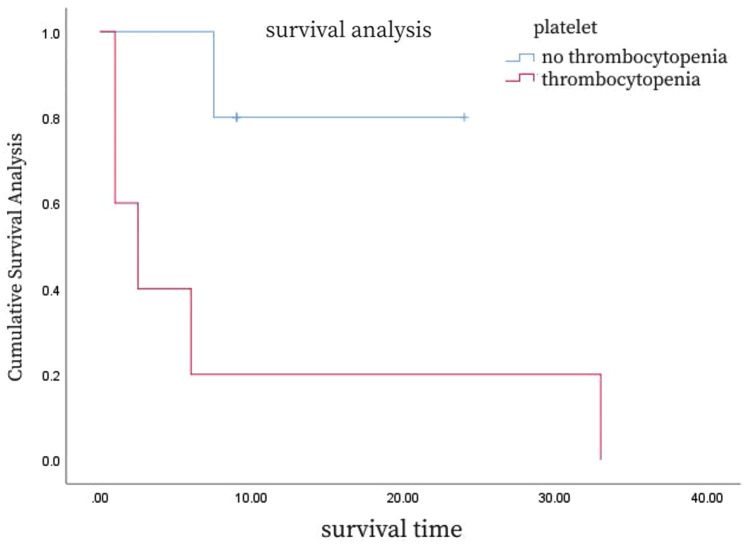
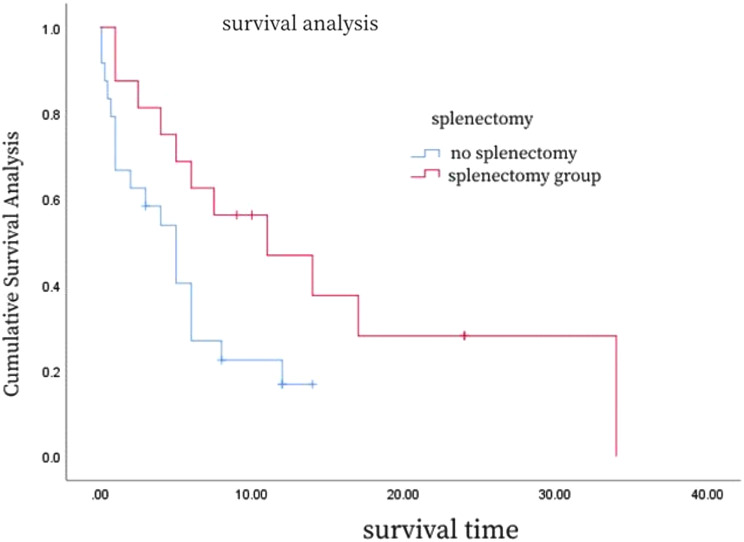
A detailed analysis of the survival curves (Figs. 6, 7 and 8; Table 3) reveals the impact of different clinical characteristics and treatment modalities on patient survival. For example, Fig. 6 shows that HSTCL patients with hemophagocytic syndrome at disease onset may have a shorter median survival time, while Fig. 7 indicates that patients with thrombocytopenia at disease onset have a poorer prognosis. Figure 8 demonstrates that patients who underwent splenectomy followed by chemotherapy have a higher survival rate compared to those who received chemotherapy alone. To provide more precise survival data, we calculated the median and mean survival times for these patient groups and found that patients who underwent splenectomy combined with chemotherapy had increased median and mean survival times.
Fig. 6.
Survival curves for patients with hemophagocytic syndrome
Fig. 7.
Survival curves for patients with and without thrombocytopenia at HSTCL onset
Fig. 8.
Survival curves for HSTCL patients that did and did not undergo splenectomy
Table 3.
Univariate analyses of survival outcomes in 10 cases of hepatosplenic lymphoma
| Item | χ2 | P value |
|---|---|---|
| Gender | 3.268 | 0.070 |
| Stage | 0.759 | 0.384 |
| IPI | 2.902 | 0.088 |
| leukocyte disorder | 1.793 | 0.181 |
| Anemia | 0.002 | 0.962 |
| Thrombocytopenia | 4.620 | 0.032 |
| Hepatic insufficiency | 2.068 | 0.150 |
| Elevated LDH | 0.013 | 0.910 |
| Elevated uric acid | 0.286 | 0.593 |
| Elevated β 2-MG | 1.330 | 0.249 |
| Bone marrow infiltration | 2.618 | 0.106 |
| Combined hemophagocytic syndrome | 4.620 | 0.032 |
| Treatment with CHOP regimen | 0.469 | 0.493 |
Prognostic factors
Next, data were compiled from 45 case reports of HSTCL cases reported domestically and internationally from 2012 to 2021. After excluding reports with incomplete data or missing follow-up information, 30 remaining cases were identified for analysis [7–31]. These cases were merged with our 10 patients discussed above, yielding a cohort consisting of 29 males and 11 females, with an average age of 42.3 years and respective minimum and maximum ages at disease onset of 15 and 75 years. To identify independent prognostic factors, we conducted a multivariate Cox regression analysis. The results of the analysis showed that splenectomy (P = 0.038, HR = 1.784) and chemotherapy (P = 0.038, HR = 1.784) are independent factors affecting the prognosis of HSTCL patients. Additionally, hemophagocytic syndrome (P = 0.032, HR = 2.013) and thrombocytopenia (P = 0.032, HR = 2.013) are also independent predictors of poor prognosis. These results emphasize the importance of combining splenectomy and chemotherapy in the treatment of HSTCL (Table 4).
Table 4.
Univariate survival analyses in 40 cases of hepatosplenic lymphoma
| Item | χ2 | P value |
|---|---|---|
| Gender | 0.515 | 0.473 |
| leukocyte disorder | 0.005 | 0.944 |
| Anemia | 0.231 | 0.631 |
| Thrombocytopenia | 2.041 | 0.153 |
| Hepatic insufficiency | 3.248 | 0.072 |
| Elevated LDH | 0.077 | 0.782 |
| Bone marrow infiltration | 1.757 | 0.185 |
| splenectomy | 4.291 | 0.038 |
| transplantation | 2.259 | 0.133 |
| Treatment with CHOP regimen | 0.587 | 0.443 |
Discussion
Hepatosplenic T-cell lymphoma (HSTCL) is an aggressive and rare form of peripheral T-cell lymphoma that predominantly affects males, with a median age of onset around 35 years [32]. In our study cohort, which included 10 patients, the gender distribution was 7 males and 3 females, with a median age at onset of 42.8 years, aligning with previous research. The etiology of HSTCL remains elusive, but it is postulated that chronic T cell antigen stimulation or the use of immunosuppressive drugs may play a role in its development. Individuals with inflammatory bowel disease, psoriasis, ankylosing spondylitis, or a history of organ transplantation are at a higher risk of developing this condition [33]. A study by Kotlyar et al.^34^ highlighted that long-term treatment with infliximab and azathioprine was present in some HSTCL patients, and chronic infections with EBV, malaria, or HBV are also associated with the disease [35]. In our study, only 2 out of 10 patients showed signs of EBV infection, both presenting with hemophagocytic syndrome, which complicates the determination of its relationship with the onset of malignancy. None of the patients were HBV carriers.
Clinically, HSTCL patients almost always present with splenomegaly, and may also experience fever and hepatomegaly. Lymph node involvement is less common, and central nervous system involvement is rare, though it may manifest as ataxia or numbness [36]. Laboratory findings often include liver dysfunction, elevated bilirubin, increased LDH, and elevated β2-microglobulin levels, along with blood count abnormalities. Thrombocytopenia is a particularly significant indicator in these patients, with Belhadj et al. [37] noting a correlation between the severity of thrombocytopenia and tumor progression, suggesting its use as a marker for recurrence detection. In our study, patients presenting with thrombocytopenia at the onset of the disease had a poorer prognosis, although this correlation did not remain significant when combined with other reports, possibly due to patient ethnicity, comorbid conditions, or other factors [37].
Hemophagocytic syndrome is a frequent complication at disease onset, possibly due to antigenic stimulation, macrophage activation, and cytokine storm [38]. Six out of the 10 patients in our cohort exhibited this syndrome, which was associated with a significantly poorer prognosis [38]. The presence of hemophagocytic syndrome in HSTCL patients increases the risk of mortality and shortens the disease course to two months or less [38].
In terms of treatment, splenectomy is thought to reduce the risk of splenic rupture and improve blood counts, but not survival. Standard treatments include CHOP-based chemotherapy, gemcitabine, L-asparaginase, and hematopoietic stem cell transplantation. However, these treatments often result in only short-term responses, with many patients experiencing rapid relapse and death [39, 40]. Yajun et al. [41] analyzed data from 123 patients with hepatosplenic lymphoma, showing a median survival of 38.2% after a median follow-up of 9 months. In our study, the 1-year overall survival rate was 75% for patients who underwent surgery combined with radiotherapy and chemotherapy, compared to 63.6% for those who underwent radiotherapy combined with chemotherapy, and 49.2% for those who underwent chemotherapy alone. Cox regression analyses indicated that treatment methods significantly impact prognosis, with patients who underwent splenectomy combined with radiotherapy and chemotherapy having a better survival rate [41].
Rashidi et al. [42] reported a median survival of 68 months after allogeneic hematopoietic stem cell transplantation in 54 HSTCL cases, with an average recurrence-free interval of 18 months. Durani U et al. [43] compared outcomes of 136 patients who received traditional chemotherapy and 12 who underwent stem cell transplantation, including 4 cases of autologous transplantation, showing median OS intervals of 34.4 months and 6.7 months, respectively. In our hospital, only one patient underwent autologous hematopoietic stem cell transplantation and survived for 34 months, reflecting the highly malignant nature of HSTCL and the challenges in pre-transplant preparation. Transplantation is recommended when feasible, as it can significantly prolong patient survival [42, 43].
Conclusion
In conclusion, our study provides a comprehensive analysis of the clinical characteristics and treatment outcomes of patients with hepatosplenic T-cell lymphoma (HSTCL), emphasizing the significance of splenectomy in combination with chemotherapy on improving survival rates. The findings suggest that patients presenting with hemophagocytic syndrome or thrombocytopenia at the onset of HSTCL face greater treatment-related challenges and have a higher risk of mortality. The combination of splenectomy and chemotherapy may offer a more effective treatment strategy for these patients.
In this study, there are limitations that should be acknowledged. The retrospective nature of the study and the relatively small sample size, particularly in our primary cases, limit the generalizability and statistical power of our conclusions. Additionally, the use of a heterogeneous dataset from the literature introduces potential variability in the data, which may impact the consistency of our results.
Despite these limitations, our study benefits from a thorough analysis of a cohort of HSTCL patients, including an extensive review of the literature, which provides a more complete picture of the disease’s clinical course and treatment outcomes. The long-term follow-up of patients in our study also contributes valuable insights into the long-term effects of the treatment modalities used.
We hope that the insights provided by our study will guide future research and clinical practice in the management of HSTCL. Further studies with larger, prospectively collected datasets are needed to validate our findings and explore the potential for improving treatment strategies for this rare but aggressive form of lymphoma.
Author contributions
Mingyue-Chen:Conception and design, manuscript writing. Min Wu:Provision of study material. Yanhui-Xie:Collection and assembly of data, interpretation. Lin Shen:Data analysis and interpretation.
Funding
The work was not funded by any funding.
Data availability
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Human ethics and consent to participate
All experimental protocols were approved by the Ethics Committee of Huadong Hospital of Fudan University. Informed consent was obtained from all the participants. All methods were carried out in accordance with Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanhui-Xie, Email: Sylvia45sci@163.com.
Lin Shen, Email: shenlin1979@hotmail.com.
References
- 1.Yabe M, Miranda RN, Medeiros LJ. Hepatosplenic T-cell lymphoma: a review of clinicopathologic features, pathogenesis, and prognostic factors. Hum Pathol. 2018;74:5–16. [DOI] [PubMed] [Google Scholar]
- 2.Gaulard P, Jaffe ES, Krenacs L, Macon WR et al. Hepatosplenic T-cell lymphoma. In: Swerdlow SH, Campo E, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC, 2017:381-2.
- 3.Travert M, Huang Y, de Leval L, et al. Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood. 2012;119(24):5795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-hodgkin lymphoma. Nature. 2011;476(7360):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney M, Moffitt AB, Gaulard P, et al. The genetic basis of hepatosplenic T-cell lymphoma. Cancer Discov. 2017;7(4):369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Takata K, Kato S, Sato Y, Asano N, Ogino T, et al. Clinicopathological analysis of 17 primary cutaneous T-cell lymphoma of the γδ phenotype from Japan. Cancer Sci. 2014;105(7):912–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida Ramalho S, Abecasis F, Santos L, Leite V, Gonçalves D, Pintassilgo I, Broa AL. Hypercalcaemia: an extremely rare presentation of hepatosplenic T-cell lymphoma. EJCRIM. 2021;8. 10.12890/2020_002360. [DOI] [PMC free article] [PubMed]
- 8.Pateria P, Martin A, Khor TS, et al. Hepatosplenic T cell lymphoma presenting as multiorgan failure. BMJ Case Rep. 2019;12:e228186. 10.1136/bcr-2018-228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irappa M, Gaurang M, Harsha P et al. Hepatosplenic Gamma Delta T-Cell Lymphoma (HSGDTCL): Two Rare Case Reports from Western India. IJHOSCR 2017;11(4):296–300. [PMC free article] [PubMed]
- 10.Michelle FJ, Bailey A, Valerie P, Opipari, et al. Hepatosplenic Alpha/Beta T-cell Lymphoma as second malignancy in Young Adult patient with previously undiagnosed Ataxia-telangiectasia. J Pediatr Hematol Oncol. 2020;42(6):e463–5. 10.1097/MPH.0000000000001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abhijit S, Javier AS,Philip K. Progression of a hepatosplenic gamma delta T-cell leukemia/lymphoma on hyperCVAD/MTX and ara-C:literature review and our institutional treatment approach. Clin Case Rep. 2016;4(1):67–71. 10.1002/ccr3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.François K, Maya B, Laurence de L, et al. Purpura of the Face and Neck: an atypical clinical presentation revealing a hepatosplenic T cell lymphoma. Case Rep Dermatol. 2014;6:37–42. 10.1159/000360126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paes VR, Lima PP, Siqueira SAC. Hemophagocytic lymphohistiocytosis associated with hepatosplenic T-cell lymphoma: case report. Autopsy Case Rep [Internet]. 2014;4(4):19–24. 10.4322/acr.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui PJHJ-N, Li, et al. Successful second allogeneic stem-cell transplantation from the same sibling donor for a patient with recurrent hepatosplenic gamma-delta (g/d) T-cell lymphoma. Volume 97. Medicine; 2018. p. 44. e1294110.1097/MD0000000000012941. [DOI] [PMC free article] [PubMed]
- 15.Seiji T, Mitsuteru N,Hiromi F, et al. Hepatosplenic Gamma-delta T-cell Lymphoma Associated with Epstein-Barr Virus. Intern Med. 2014;53:2079–82. 10.2169/internalmedicine.53.2236. [DOI] [PubMed] [Google Scholar]
- 16.Maartje M, van de Meeberg,Lauranne AAP, Derikx,Harm AM, Sinnige, et al. Hepatosplenic T-cell lymphoma in a 47-year-old Crohn’s disease patient on thiopurine monotherapy. World J Gastroenterol. 2016;22(47):10465–70. 10.3748/wjg.v22.i47.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joana C, Vítor MP, Fernando J, et al. Hepatosplenic T-Cell lymphoma: a rare complication of Monotherapy with thiopurines in Crohn’s Disease. GE Port J Gastroenterol. 2019;26:279–84. 10.1159/000493350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrova M, Gomes MM, Carda JPN et al. Hepatosplenic T-cell lymphoma in a young immunocompetent man. BMJ Case Rep.2016;Published online: [please include Day Month Year].10.1136/bcr-2016-214414 [DOI] [PMC free article] [PubMed]
- 19.Jonah C, Eduardo H, Darshan K, et al. Hepatosplenic alpha/beta T-cell lymphoma masquerading as cirrhosis. J Gastrointest Oncol. 2013;4(2):131–6. 10.3978/j.issn.2078-6891.2013.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin L, Yusuke K, Kei F, et al. An unusual case of hepatosplenic T-cell lymphoma-like unclassifiable T/NK-cell lymphoma accompanied by acute myeloid leukemia. eJHaem. 2022;3:1335–8. 10.1002/jha2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippa A, Moosa P, Jenifer V et al. Hepatosplenic T-cell lymphoma: a case series.Hematology/Oncology and stem cell therapy.2015;8(2);78–84.10.1016/j.hemonc.2014.09.006 [DOI] [PubMed]
- 22.Yana P, Amy SC,Amanda LR et al. Targeting EZH2 for the treatment of hepatosplenic T-cell lymphoma.Blood advanceds.2020;4(7);1265–DOI1269:. 10.1182/bloodadvances.2019001256 [DOI] [PMC free article] [PubMed]
- 23.Lei CAO, Lei FAN, Li WANG, et al. Clinical analysis of one case of hepatosplenic γ/δ lymphoma [J]. Chin J Practical Intern Med. 2015;2(35):171–3. 10.7504/nk2015010601. [Google Scholar]
- 24.Rong,Bao LI, Xiupeng SHENYE, et al. A case of hepatosplenic γδT cell lymphoma and literature review[J]. J ClinHematol(China). 2015;28(11):990–2. 10.13201/j.issn.1004-2806.2015.11.022. [Google Scholar]
- 25.Yanfen LI, Yu ZHAO, Quanshun WANG, et al. HepatosplenicγδT-cell lymphoma: a report of 4 cases and literature review[J]. Acad J Chin PLA Med Sch. 2019;40(3):276–9. 10.3969/j.issn.2095-5227.2019.03.018. [Google Scholar]
- 26.Caixia WANG, CHANG Hong. HepatosplenicT cell lymphoma: report on a series of 2 patients and correlative literature Review[J]. West China Med J. 2009;24(3):590–2. [Google Scholar]
- 27.Saran FENG, Qian LIU. One case of hepotasplenic γδ lymphoma with systemic lupus erythematosus and literature review [J]. J Shandong University(Health Sciences). 2018;56(6):87–90. 10.6040/j.issn.1671-7554.0.2018.099.
- 28.Ziyan HE, Junxia MENG, Chunlai LUAN, et al. One case of hepotasplenic eldly αβ lymphoma and literature review[J]. Clin Focus. 2018;33(3):255–61. 10.3969/j.issn.1004-583x.2018.03.016. [Google Scholar]
- 29.Ying MIAO, Xiumin QI, Chunjin HUANG, et al. Two cases of Hepatosplenic T-cell lymphoma. [J] J Clin Exp Pathol. 2010;26(5):640–1. [Google Scholar]
- 30.Li-ping ZHANG,XIA, Yun-jin,LONG, Zhi-guo et al. Hepatosplenic T-cell Lymphoma Complicated Viral Hepatitis Type B Secondary to Cirrhosis: A Case Report and Literature Review[J]. J HBUM.2011;30(4);495–499.
- 31.Yinli ZHANG, Pengfei SHI et al. YANG fan. Clinical Analysis of 4 Cases of Hepatosplenic T-cell Lymphoma [J]. Zhejiang Medicine.2022;44(17):1880–1882. 10.12056/j.issn.1006-2785.2022.44.17.2022-636
- 32.Calvaruso M, Gulino A, Buffa S, et al. Challenges and new prospects in hepatosplenic γδ T-cell lymphoma[J]. Leuk Lymphoma. 2015;55(11):2457–65. [DOI] [PubMed] [Google Scholar]
- 33.Deepak P, Sifuentes H, Sherid M. Et. T-cell non-hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha(TNF-alpha) inhibitors: results of the REFURBISH study[J]. Am J Gastroenterol. 2013;108(1):99–105. [DOI] [PubMed] [Google Scholar]
- 34.Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis[J]. Clin Gastroenterol Hepatol. 2015;13(5):847–58. [DOI] [PubMed] [Google Scholar]
- 35.Lu C, Tang Y, Yang Q, et al. Hepatosplenic T-cell lymphoma: clinicopathologic, immunophenotypic, and molecular characterization of17 Chinese cases[J]. Hum Pathol. 2011;42(12):1965–78. [DOI] [PubMed] [Google Scholar]
- 36.Yabe M, Medeiros LJ, Daneshbod Y, et al. Hepatosplenic T-cell lymphoma arising in patients with immunodysregulatory disorders: a study of 7 patients who did not receive tumor necrosis factor-alpha inhibitor therapy and literature review [J]. Ann Diagn Pathol. 2017;26:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients[J]. Blood. 2003;102(13):4261–9. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran S, Zaidi F, Aggarwal A et al. Recent advances in diagnostic and therapeutic guidelines for primary and secondary hemophagocytic lymphohistiocytosis[J]. Blood Cells Mol Dis 2017,64:53–7. [DOI] [PubMed]
- 39.Gowda L, Foss F. Hepatosplenic T-cell lymphomas. Cancer Treat Res. 2019;176:185–93. [DOI] [PubMed] [Google Scholar]
- 40.Krishnan M, Lunning M. Hepatosplenic gamma-delta T-Cell lymphoma: who is on your speed dial? J Oncol Pract. 2019;15(6):307–12. [DOI] [PubMed] [Google Scholar]
- 41.Yajun LK, Ch,Chaohui Z, et al. Survival Analysis of Hepatosplenic T Cell Lymphoma: a Population-based study using SEER. Int J Gen Med. 2021;14:8399–411. 10.2147/IJGM.S335464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rashidi A, Cashen AF. Outcomes of allogeneic stem cell transplantation in hepatosplenic T-cell lymphoma[J]. Blood Cancer J. 2015;5(6):e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durani U, Go RS. Incidence, clinical findings, and survival of hepatosplenic T-cell lymphoma in the United States.[J]. Am J Hematol. 2017;92(6):E99–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.