Abstract
Brain radiation necrosis is a serious adverse effect of radiotherapy in patients with malignant brain metastases. There is currently no standard treatment for brain radiation necrosis; however, there are advantages to using bevacizumab. Nonetheless, due to the risk of severe bleeding when bevacizumab is used in patients with squamous cell lung carcinoma, relevant clinical studies are lacking; therefore, there is no clear conclusion on the use of bevacizumab to treat brain radiation necrosis in patients with squamous cell carcinoma of the lung with brain metastases. The present study described the case of a patient treated with bevacizumab after brain radiation injury with pathological manifestations diagnosed as squamous cell carcinoma of the lung. Through the evaluation of clinical symptoms and imaging data, the patient was diagnosed with cerebral radiation necrosis a few months after receiving local radiotherapy for intracranial metastatic lesions. After four cycles of treatment with bevacizumab (7.5 mg/kg once every 3 weeks, intravenous drip), the clinical and imaging manifestations of the patient were considerably improved with no significant adverse effects. The favorable efficacy and safety profiles of this patient suggest that bevacizumab holds potential as a future therapeutic option for managing radiation-induced brain necrosis in patients with squamous cell lung cancer.
Keywords: brain radiation necrosis, bevacizumab, squamous cell lung carcinoma, pathophysiological mechanism, radiotherapy, dose, case report
Introduction
Brain radiation necrosis is a severe consequence of intracranial radiotherapy, which can result in central nervous system injury and patient mortality (1). The global incidence rate of brain radiation necrosis has been reported to be 14–15% in patients following conventional radiotherapy modalities, with high levels detected after stereotactic radiosurgery treatment (24–68.8%) (2). Traditionally, glucocorticoids have been employed as the standard treatment for brain radiation necrosis (3); however, due to its complex pathophysiological processes, including vascular-inflammatory responses, recent studies have proposed the advantages of bevacizumab in the treatment of brain radiation necrosis (4,5). However, in patients with brain metastases from squamous cell carcinoma of the lung, there are few studies or case reports regarding this treatment, and a lack of relevant clinical data due to its safety limitations (6,7). The present study describes the case of a patient who received bevacizumab after a diagnosis of brain radiation necrosis following radiotherapy treatment for brain metastasis from squamous cell lung cancer. The patient was treated with four cycles of bevacizumab that resulted in the improvement of clinical and imaging manifestations. The present study discusses the safety of bevacizumab for the treatment of brain radiation necrosis in patients with brain metastasis from squamous cell lung cancer, and further reviews the mechanisms, treatment efficacy and clinical practice relating to brain radiation necrosis.
Case report
The present case report was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (approval no. 2024ER253-1; Nanchong, China). The participant provided written informed consent to participate in the study, and written informed consent was also obtained from the individual for the publication of any potentially identifiable images or data included in this article.
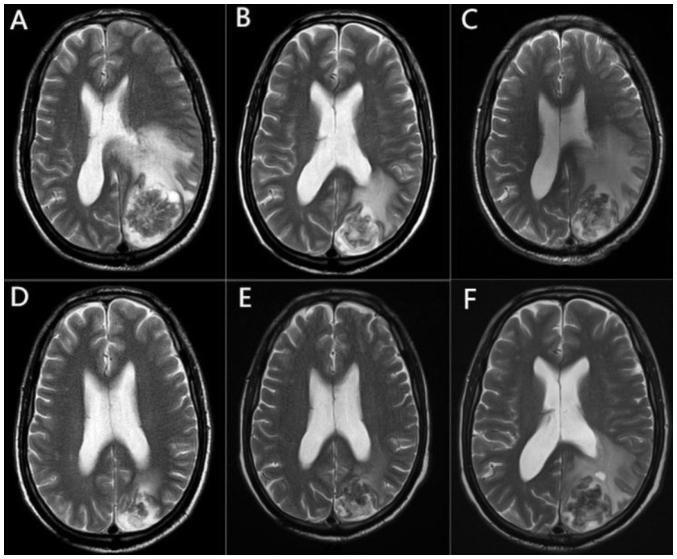
The patient was a 67-year-old man with no history of food or drug allergies, heart disease, hypertension or diabetes mellitus who presented to the Affiliated Hospital of North Sichuan Medical College in July 2019. The patient was diagnosed with squamous cell carcinoma of the upper lobe of the left lung with mediastinal lymph node metastasis cT4N2M0 stage IIIB in August 2019, using fiberoptic bronchoscopy, pathological biopsy, computed tomography, ultrasound and whole-body bone imaging, and other auxiliary examinations. The patient was treated with three cycles of paclitaxel + cisplatin (paclitaxel 150 mg/kg + cisplatin 75 mg/kg, once every 3 weeks, intravenous drip) and radiotherapy targeting lung tumor lesions [planning-gross tumor volume (P-GTV): 63.8 Gy/29 Fx; volume-GTV node: 63.8 Gy/29 Fx]. After completion of radiotherapy, a follow-up examination in January 2020 showed that the lung lesion was stable and without distant metastasis. The patient declined further chemotherapy and treatment was suspended. In March 2020, the patient experienced limb weakness without any obvious triggers, with no dizziness, headache or other discomfort. This same month, contrast-enhanced magnetic resonance imaging (MRI) suggested that a left parieto-occipital lobe mass was accompanied by obvious peripheral cerebral edema (Fig. 1A), which was considered a brain metastasis and staged as cT4N2M1b stage IVA. The patient declined surgery, and local radiation therapy was initiated 10 days after MRI for brain metastasis with the following dosage and division pattern: P-GTV: 39 Gy/13 Fx, 3 Gy/Fx, with mannitol (0.25 g mg/kg, every 6 h, intravenous drip) dehydration to reduce intracranial pressure and other symptomatic treatments (such as cough suppressants and expectorants). Because the lung lesion appeared to be stable and no other distant metastases were found except for in the brain, three cycles of the original chemotherapy regimen (paclitaxel 150 mg/kg + cisplatin 75 mg/kg, once every 3 weeks, intravenous drip) were administered after the completion of intracranial radiotherapy, and the treatment ended in July 2020.
Figure 1.
Brain MRI changes in the patient throughout the course of the disease. (A) MRI presentation at the earliest detection of brain metastases. (B) MRI after radiotherapy treatment of the brain lesion, showing reduced metastasis and surrounding edema. (C) MRI at the time of brain radiation necrosis diagnosis, showing a significant increase in edema around the lesion. (D) MRI after two cycles of bevacizumab treatment, showing a decrease in edema around the lesion. (E) MRI after four cycles of bevacizumab treatment, with a slight decrease in the perifocal edema and a significant reduction in edema compared to before bevacizumab treatment. (F) MRI at diagnosis of recurrent brain radiation necrosis, showing an increase in edema around the intracranial lesion. MRI, magnetic resonance imaging.
In August 2020, chest computed tomography with contrast enhancement showed progression of the lung lesion, whereas cranial contrast-enhanced MRI showed that the intracranial lesion had been reduced and the peripheral cerebral edema was reduced (Fig. 1B). After comprehensive evaluation, and consultation of the relevant guidelines (8) and the wishes of the patient's family, second-line docetaxel chemotherapy (75 mg/kg, intravenous drip) was administered for one cycle. The patient was injected with polyethylene glycolated recombinant human granulocyte colony-stimulating factor at the end of chemotherapy and developed febrile neutropenia after the injection. The treatment was adjusted to afatinib (40 mg, once a day, oral administration, cycle every 28 days, 14 cycles in total) and the lung mass was markedly reduced.
In November 2020, the patient reported dizziness and headache with no obvious cause, and a review of the cranial contrast-enhanced MRI showed that the area of cerebral edema around the lesion had considerably increased (Fig. 1C). Taking into account the treatment history, brain radiation necrosis was considered, and auxiliary examinations did not reveal other metastases throughout the body. After mannitol (0.25 g mg/kg, every 6 h, intravenous drip) dehydration to reduce intracranial pressure and 1 month of treatment with glucocorticoids (1.0 g, once daily, intravenously for 3 days, tapering until discontinued), the symptoms were not significantly relieved, and the efficacy and safety of using bevacizumab in this patient were discussed. The current lung lesions were small, did not invade large blood vessels and had no obvious coagulation abnormalities; therefore, the use of bevacizumab was considered safe. After consultation with the patient and their family, bevacizumab was administered at 7.5 mg/kg once every 3 weeks (intravenous drip) starting in December 2020.
After two cycles of treatment, the headache and dizziness symptoms had improved, and cranial contrast-enhanced MRI showed markedly reduced edema around the lesion (Fig. 1D). After four treatment cycles, the symptoms improved considerably. Cranial contrast-enhanced MRI showed no significant changes in the intracranial lesion, and the edema around the lesion was slightly reduced (Fig. 1E), which confirmed the clinical efficacy of bevacizumab in this patient with brain radiation necrosis. No significant adverse reactions were observed during bevacizumab treatment and patient adherence was good. Subsequently, bevacizumab treatment was discontinued, while afatinib treatment was continued with regular follow-up. In August 2021, follow-up cranial MRI showed increased edema around the intracranial lesion (Fig. 1F) and a diagnosis of recurrence of radiological cerebral necrosis was considered. However, due to the lack of clinical symptoms and the care being unaffordable for the patient, they declined further bevacizumab treatment. Another follow-up cranial MRI showed that the intracranial lesion had stabilized without significant changes. The last follow-up in November 2021 was assessed as stable disease, and the patient was subsequently lost to follow-up.
Discussion
The classification of radiation-induced brain necrosis is based on the temporal onset of symptoms, resulting in three categories: Acute, semi-delayed and late (9). The most prevalent type of radiation-induced brain necrosis is late necrosis, which is characterized by the presence of central nervous system injury and imaging changes 6 months after the completion of radiotherapy. The incidence of radiation necrosis is considered to be associated with various factors, including the total radiotherapy dose, the fractionated dose, a prior history of whole-brain radiotherapy and the tumor lesion volume. Specifically, a higher total or fractionated dose, and a larger lesion volume are associated with an increased incidence of radiation necrosis, which tends to manifest earlier (10,11). Symptoms of radiation-induced brain necrosis include vertigo, cephalalgia, mental health conditions, motor or sensory impairments, amnesia, changes in personality, cognitive impairment and seizures (12,13), which greatly reduce the quality of life of patients. Glucocorticoids are the conventional treatment for brain radiation necrosis; however, their effectiveness is insufficient to meet therapeutic requirements, and there is a high occurrence of negative long-term effects, such as medical hyperadrenocorticism, infections, diabetes and peptic ulcers (10,14). A growing body of evidence has suggested that bevacizumab could be considered a viable therapeutic option for the management of brain radiation necrosis (15–17).
Previous studies have shown that the development of brain radiation necrosis is a complex pathophysiological process caused by the interaction of multiple factors (18–22). Ionizing radiation induces the production of reactive oxygen species in glial cells and destroys cellular structures, such as single-stranded and double-stranded DNA and cell membranes, leading to cell necrosis or apoptosis, which in turn leads to a series of inflammatory reactions, disruption of the blood-brain barrier and the formation of cerebral edema (18,19). In particular, radiotherapy-induced endothelial cell damage, resulting in increased cerebrovascular permeability and perivascular edema, is one of the main pathogenic mechanisms during the acute response phase (20). In this pathophysiological process, vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) may serve important roles. Vascular endothelial cell injury causes impaired tissue oxygen exchange, and tissue hypoxia further leads to the upregulation of HIF-1α expression, which promotes the secretion of VEGF by astrocytes and other cells, which in turn promotes focal angiogenesis. However, the newly formed blood vessels are highly permeable and easily damaged, leading to increased local inflammation and edema, forming a vicious cycle of hypoxia-edema-hypoxia, ultimately leading to focal brain necrosis (21,22).
Bevacizumab is a recombinant human monoclonal IgG1 antibody that can competitively inhibit the binding of VEGF to endothelial cell surface receptors by binding to VEGF, reducing endothelial cell proliferation and neovascularization, and decreasing vascular permeability. Bevacizumab has been widely used in non-small cell lung cancer and other malignant tumors for targeted antiangiogenic therapy (23). Due to the aforementioned pathophysiological mechanisms of brain radiation necrosis, the inhibitory effects of bevacizumab on angiogenesis and its ability to lower vascular permeability have been suggested as the mechanisms underlying its palliative effects on brain radiation necrosis and localized cerebral edema; this was first suggested in a phase I clinical study in 2007 (24). Since 2007, several case reports and early clinical studies have reported the use of bevacizumab in brain radiation necrosis (25–29). In 2011, Levin et al (30) published a prospective, placebo-controlled, randomized clinical study in which 14 patients with symptomatic MRI-supported brain radiation necrosis were randomized into placebo and bevacizumab (7.5 mg/kg every 3 weeks) groups. MRI was performed after two doses, and patients showing effective treatment and tolerance were re-assessed after two more doses. All patients in the bevacizumab group experienced varying degrees of relief of subjective symptoms and imaging signs, whereas there was no significant change in the placebo group. Furthermore, the safety profile of the bevacizumab group was favorable (30). In 2018, Xu et al (15) included 121 cases of brain radiation necrosis in a multicenter randomized controlled study, where patients were randomly assigned to a bevacizumab (5 mg/kg every 2 weeks, 4 cycles) and a glucocorticoid group. Treatment responses were markedly higher in the bevacizumab group than in the glucocorticoid group (65.5 vs. 31.5%), and clinical improvement was detected in more patients in the bevacizumab group than in the glucocorticoid group (62.1 vs. 42.6%) (15). In addition, several retrospective studies have provided supportive evidence for the use of bevacizumab in brain radiation necrosis. In 2015, Sadraei et al (16) published the results of a retrospective study in 24 patients with radiation brain necrosis, showing that all patients treated with bevacizumab achieved imaging improvements and reduced glucocorticoid use. Another retrospective study in 2016 supported the same conclusion; in 14 patients with brain radiation necrosis treated with bevacizumab, 13 patients showed reduced necrotic brain volumes (92.86%), and 10 out of 12 symptomatic patients achieved marked symptomatic improvements with no clinically significant adverse effects (17). All of the aforementioned studies provide effective and supportive clinical evidence for the use of bevacizumab in the treatment of brain radiation necrosis, but the number of cases reported in clinical studies to date is generally small, and randomized clinical studies with larger sample sizes are still needed for further validation.
Despite the clinical and preclinical evidence, there is lack of relevant reports on squamous cell carcinoma of the lung. Bevacizumab treatment has been reported to be associated with a high risk of severe pulmonary hemorrhage in patients with squamous cell carcinoma of the lung in a phase II randomized clinical (30); based on this study, almost all subsequent clinical studies on bevacizumab for non-small cell lung cancer have excluded patients with squamous cell carcinoma. Thus, there is a lack of clinical data on the use of bevacizumab for brain radiation necrosis in patients with squamous carcinoma of the lung. In 2017, Remon et al (31) reported the first case of bevacizumab treatment for brain radiation necrosis in squamous carcinoma of the lung. In this previous report, a patient with cerebellar metastasis developed radioencephalic necrosis after stereotactic radiotherapy and received six cycles of bevacizumab (5 mg/kg every 3 weeks), achieving a marked improvement in clinical symptoms and imaging manifestations. This tentatively suggested the efficacy and safety of bevacizumab for the treatment of brain radiation necrosis in patients with squamous cell lung cancer. In the present case report, a patient with brain metastasis from squamous cell lung cancer exhibited central nervous system symptoms and imaging changes 8 months after receiving brain radiotherapy, which was consistent with brain radiation necrosis. After receiving four cycles of bevacizumab (7.5 mg/kg every 3 weeks), the patient was in double remission, both regarding clinical symptoms and imaging manifestations, and there were no adverse reactions detected, such as pulmonary hemorrhage, further demonstrating the efficacy and safety of the treatment.
To the best of our knowledge, there are still no clinical studies on bevacizumab for the treatment of brain radiation necrosis in patients with squamous cell lung cancer, and this treatment regimen remains experimental and needs to be validated by additional clinical studies. Although the present case report suggested that it may be effective and safe, there is still a lack of relevant clinical data in this population. Severe pulmonary hemorrhage remains a serious adverse effect, requiring a high degree of vigilance when using this drug in patients with squamous cell lung cancer. Before using bevacizumab in patients with brain radiation necrosis and squamous cell cancer of the lung, the risk of pulmonary hemorrhage should be comprehensively assessed, and factors such as lung tumor invasion of large blood vessels, coagulation function and the baseline tumor cavity should be considered (32). Over the course of treatment, the patient should be continuously and closely observed for the development of hemoptysis; if this occurs, bevacizumab should be discontinued and symptomatic treatments should be immediately administered.
It is worth noting that, because of the difficulty in regenerating central nervous tissue, the pathological damage following brain radiation necrosis cannot be reversed, and recurrence has been observed in a number of studies after bevacizumab discontinuation (28,33,34). In the present case, the patient was also observed to have an imaging manifestation suggestive of recurrence 5 months after discontinuing bevacizumab. It is generally accepted that, because bevacizumab acts on neovascularization around the lesion, as long as the necrotic tissue exists, its peripheral vasculature can continue to reactively proliferate after bevacizumab discontinuation, continuing the pathophysiological process of local hypoxia and edema (35). Furthermore, when recurrence occurs in patients after stopping bevacizumab, repeated treatment with bevacizumab seems to be effective (36). However, the current case report was unable to observe the efficacy of bevacizumab for recurrent brain radiation necrosis. Notably, there is still a lack of clinical data on patients with recurrent radiation brain necrosis and research remains in the preliminary stages.
There is no standardized dose of bevacizumab for treatment of radiation brain necrosis, and most doses used in clinical studies and case reports are 5–7.5 mg/kg once every 2–3 weeks for ≥2 cycles. The present case used a treatment regimen of 7.5 mg/kg every 3 weeks for four cycles, because the patient wanted to reduce the frequency of hospital admissions. However, considering the possible serious adverse effects, such as hypertension, thromboembolism and hemorrhage (37), lower doses of bevacizumab may be a superior treatment option. In recent years, several clinical studies have shown support for low-dose bevacizumab in the treatment of brain radiation necrosis. A clinical study in 2023 demonstrated that, in 13 patients treated with a low-dose bevacizumab regimen (400 mg loading dose, then 100 mg every 4 weeks) for brain radiation necrosis, 12 achieved clinical improvement, all patients achieved imaging improvement and no clinical adverse effects were observed (38). In addition, a recently published retrospective study comparing the efficacy and safety of high-dose (≥5 mg/kg) and low-dose (<5 mg/kg) bevacizumab for the treatment of brain radiation necrosis showed that clinical and imaging improvement did not significantly differ between the groups, but the use of high-dose bevacizumab was associated with a higher incidence of grade 3 and higher adverse reactions (39). By contrast, two phase II prospective clinical studies used ultra-low-dose (1 mg/kg every 3 weeks for ≥3 doses) and single low-dose (2.5 mg/kg) treatment regimens of targeted infusions, and both were clinically efficacious with favorable safety profiles (40,41). In summary, a low-dose regimen may be a better choice than the standard bevacizumab dose, but to the best of our knowledge, there are no prospective studies comparing different regimens, and more evidence is needed on optimal dosages and cycle durations.
In conclusion, the clinical efficacy of bevacizumab as a potentially recommended drug for the treatment of brain radiation necrosis has been recognized, but most current clinical evidence has limitations. For example, the safety of its use in patients with squamous cell lung cancer, the tendency to relapse after drug discontinuation, and the optimal dosage and duration of its use still need to be further discussed with a higher level of clinical evidence. In clinical practice, the treatment of radiation brain necrosis remains challenging, particularly in patients with squamous cell lung cancer. Although the present case report suggested that bevacizumab may offer therapeutic potential for this condition, further evidence is needed to confirm its safety. When bevacizumab use is warranted, an experimental approach is recommended, carefully assessing the bleeding risk of the patient, and tailoring the dosage and duration of therapy to the needs of the individual. Close monitoring for adverse effects throughout treatment is also essential.
Acknowledgements
Not applicable.
Funding Statement
Financial support was received for the research, authorship and/or publication of this article. This work was supported by the Sichuan Science and Technology Program (grant no. 2022NSFSC1554), the Research Project of North Sichuan Medical College (grant no. CBY20-QA-Z11) and the Doctoral Research Startup Fund Project of the Affiliated Hospital of North Sichuan Medical College (grant no. 2019-248).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
KG and BZ conceptualized the study and wrote the original manuscript. BZ conducted a relevant survey of the background to this study and provided grant support. BZ and HC searched the literature and obtained case-related data. KG, SM and DM analyzed data and relevant literature. BZ and DM reviewed and edited the final draft. DM was responsible for managing this research project. KG and BZ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was conducted according to the guidelines of The Declaration of Helsinki and was approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College (approval no. 2024ER253-1; 2024-04-03).
Patient consent for publication
Written informed consent was provided by the patient to obtain clinical data and information, as well as for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899–904. doi: 10.3171/jns.2001.94.6.0899. [DOI] [PubMed] [Google Scholar]
- 2.Ali FS, Arevalo O, Zorofchian S, Patrizz A, Riascos R, Tandon N, Blanco A, Ballester LY, Esquenazi Y. Cerebral radiation necrosis: Incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21:66. doi: 10.1007/s11912-019-0818-y. [DOI] [PubMed] [Google Scholar]
- 3.Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: Implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67:1799–815. doi: 10.1227/NEU.0b013e3181fa775b. discussion 1815. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Ren H, Fu J. Treatment of radiation-induced brain necrosis. Oxid Med Cell Longev. 2021;2021:4793517. doi: 10.1155/2021/4793517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao G, Khan M, Zhao Z, Arooj S, Yan M, Li X. Bevacizumab treatment of radiation-induced brain necrosis: A systematic review. Front Oncol. 2021;11:593449. doi: 10.3389/fonc.2021.593449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS. Toxicities of antiangiogenic therapy in non-small-cell lung cancer. Clin Lung Cancer. 2006;8((Suppl 1)):S23–S30. doi: 10.3816/CLC.2006.s.010. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, III, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Hanna N, Johnson D, Temin S, Baker S, Jr, Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl NB, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484–3515. doi: 10.1200/JCO.2017.74.6065. [DOI] [PubMed] [Google Scholar]
- 9.Martino A, Krainik A, Pasteris C, Hoffmann D, Chabardes S, Berger F, Le Bas JF, Cantin S, Attye A, Grand S. Neurological imaging of brain damages after radiotherapy and/or chimiotherapy. J Neuroradiol. 2014;41:52–70. doi: 10.1016/j.neurad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16:903–914. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 11.Matuschek C, Bölke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, Gerber PA, Rusnak E, Lammering G, Budach W. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187:135–139. doi: 10.1007/s00066-010-2184-4. [DOI] [PubMed] [Google Scholar]
- 12.Cheung MC, Chan AS, Law SC, Chan JH, Tse VK. Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer. 2003;97:2019–2026. doi: 10.1002/cncr.11295. [DOI] [PubMed] [Google Scholar]
- 13.Wang XS, Ying HM, He XY, Zhou ZR, Wu YR, Hu CS. Treatment of cerebral radiation necrosis with nerve growth factor: A prospective, randomized, controlled phase II study. Radiother Oncol. 2016;120:69–75. doi: 10.1016/j.radonc.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Jiang CL, Liu L, Li Z, Buttgereit F. The novel strategy of glucocorticoid drug development via targeting nongenomic mechanisms. Steroids. 2015;102:27–31. doi: 10.1016/j.steroids.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Rong X, Hu W, Huang X, Li Y, Zheng D, Cai Z, Zuo Z, Tang Y. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: A randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101:1087–1095. doi: 10.1016/j.ijrobp.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 16.Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, Suh JH, Peereboom DM, Stevens GH, Ahluwalia MS. Treatment of cerebral radiation necrosis with bevacizumab: The cleveland clinic experience. Am J Clin Oncol. 2015;38:304–310. doi: 10.1097/COC.0b013e31829c3139. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, Wang X, Yuan Z, Wang P. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. 2016;6:24364. doi: 10.1038/srep24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20:485–502. doi: 10.1016/j.jocn.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–1969. doi: 10.1016/0360-3016(86)90133-1. [DOI] [PubMed] [Google Scholar]
- 20.Levin VA, Edwards MS, Byrd A. Quantitative observations of the acute effects of X-irradiation on brain capillary permeability: Part I. Int J Radiat Oncol Biol Phys. 1979;5:1627–1631. doi: 10.1016/0360-3016(79)90786-7. [DOI] [PubMed] [Google Scholar]
- 21.Nonoguchi N, Miyatake SI, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Fukumoto M, Ono K. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105:423–431. doi: 10.1007/s11060-011-0610-9. [DOI] [PubMed] [Google Scholar]
- 22.Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, Hayasaki H, Kuroiwa T, Ono K, Shibayama Y, Miyatake S. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res. 2014;55:803–811. doi: 10.1093/jrr/rru017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94:63–68. doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 26.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng X, Zhao R, Wu S, Shen G, Ding L, Sun B, Wang J. Efficacy of repeated low-dose bevacizumab treatment with long-dosing interval for radiation-induced brain necrosis: A case report. Cancer Biol Ther. 2017;18:63–66. doi: 10.1080/15384047.2016.1276127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessandretti M, Buzaid AC, Brandão R, Brandão EP. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep Oncol. 2013;6:598–601. doi: 10.1159/000357401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang E, Zhang N, Dai J. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17:25. doi: 10.1186/2047-783X-17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remon J, Le Péchoux C, Caramella C, Dhermain F, Louvel G, Soria JC, Besse B. Brain Radionecrosis Treated with bevacizumab in a Patient with Resected squamous cell carcinoma of the Lung. J Thorac Oncol. 2017;12:e1–e3. doi: 10.1016/j.jtho.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 32.Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, Wang LI, Johnson DH. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Huang X, Jiang J, Hu W, Hu J, Cai J, Rong X, Cheng J, Xu Y, Wu R, et al. Clinical variables for prediction of the therapeutic effects of bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2018;100:621–629. doi: 10.1016/j.ijrobp.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Furuse M, Nonoguchi N, Kawabata S, Yoritsune E, Takahashi M, Inomata T, Kuroiwa T, Miyatake S. Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn J Clin Oncol. 2013;43:337–341. doi: 10.1093/jjco/hys231. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol Cancer. 2019;18:21. doi: 10.1186/s12943-019-0950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuse M, Kawabata S, Kuroiwa T, Miyatake SI. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: A report of 2 cases. J Neurooncol. 2011;102:471–475. doi: 10.1007/s11060-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 37.Taugourdeau-Raymond S, Rouby F, Default A, Jean-Pastor MJ, French network of pharmacovigilance centers Bevacizumab-induced serious side-effects: A review of the French pharmacovigilance database. Eur J Clin Pharmacol. 2012;68:1103–1107. doi: 10.1007/s00228-012-1232-7. [DOI] [PubMed] [Google Scholar]
- 38.Tijtgat J, Calliauw E, Dirven I, Vounckx M, Kamel R, Vanbinst AM, Everaert H, Seynaeve L, Van Den Berge D, Duerinck J, Neyns B. Low-dose bevacizumab for the treatment of focal radiation necrosis of the brain (fRNB): A single-center case series. Cancers (Basel) 2023;15:2560. doi: 10.3390/cancers15092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao M, Wang X, Wang X, Niu G, Liu X, Zhao S, Wang Y, Yu H, Huo S, Su H, et al. Can low-dose intravenous bevacizumab be as effective as high-dose bevacizumab for cerebral radiation necrosis? Cancer Sci. 2024;115:589–599. doi: 10.1111/cas.16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang H, Zhuang H, Shi S, Wang Y. Ultra-low-dose bevacizumab for cerebral radiation necrosis: A prospective Phase II clinical study. Onco Targets Ther. 2019;12:8447–8453. doi: 10.2147/OTT.S223258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dashti SR, Kadner RJ, Folley BS, Sheehan JP, Han DY, Kryscio RJ, Carter MB, Shields LBE, Plato BM, La Rocca RV, et al. Single low-dose targeted bevacizumab infusion in adult patients with steroid-refractory radiation necrosis of the brain: A phase II open-label prospective clinical trial. J Neurosurg. 2022;137:1676–1686. doi: 10.3171/2022.2.JNS212006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study are included in the figures and/or tables of this article.