Abstract
The rml genes are involved in dTDP-rhamnose synthesis in Streptococcus mutans. A gene fusion between gtfB and gtfC, which both encode extracellular water-insoluble glucan-synthesizing enzymes, accompanied by inactivation of the rml genes was observed for cells grown in the presence of sucrose. The survival rates of rml mutants isolated in the absence of sucrose were drastically reduced in the presence of sucrose. The rates were consistent with the frequency of spontaneous gene fusions between gtfB and gtfC, suggesting that the spontaneous recombinant organisms were selected in the presence of sucrose. The rml mutants with a gtfB-gtfC fusion gene had markedly reduced water-insoluble glucan synthetic activity and lost the ability to colonize glass surfaces in the presence of sucrose. These results suggest that the rml mutants of S. mutans, which are defective in dTDP-rhamnose synthesis, can survive only in the absence of water-insoluble glucan synthesis.
Water-insoluble glucan, which is primarily composed of α1,3-linked glucose residues, plays an especially important role in the cariogenicity of Streptococcus mutans (4, 9). Two genes coding for glucosyltransferase (GTF), which is responsible for water-insoluble glucan synthesis, gtfB (coding for GTF-I) and gtfC (coding for GTF-SI), have been isolated from S. mutans (1, 5). In experiments using specific-pathogen-free rats, it has been shown that the expression of both genes in addition to production of the water-soluble glucan-synthesizing enzyme (GTF-S) encoded by gtfD is required for maximal in vivo virulence of the organism (20, 21).
On the other hand, rhamnose-containing cell wall polysaccharides on the S. mutans cell surface are major cell surface antigens and determine the organism’s serological properties. In vitro stimulation of human monocytes with the serotype f-specific polysaccharide antigen induces the release of inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β (15). Furthermore, the antigen provokes nitric oxide production in the rat aorta (10). Recently, we isolated four genes (rmlA, rmlB, rmlC, and rmlD) involved in dTDP-l-rhamnose synthesis and subsequently determined their roles in cell wall polysaccharide synthesis, since dTDP-l-rhamnose is an immediate precursor of the poly-l-rhamnose backbone of the polysaccharides (17, 18). Inactivation of any of these four genes totally prevented cell wall polysaccharide synthesis.
In this study, we found that fusions between the gtfB and gtfC genes were observed in each of the rml mutants isolated previously. Further characterization of these mutants revealed that gtfB-gtfC gene fusions regularly accompanied rml gene inactivation only in the presence of sucrose. These results are discussed in relation to the simultaneous recombination of genes located in a locus distant from the inactivated gene.
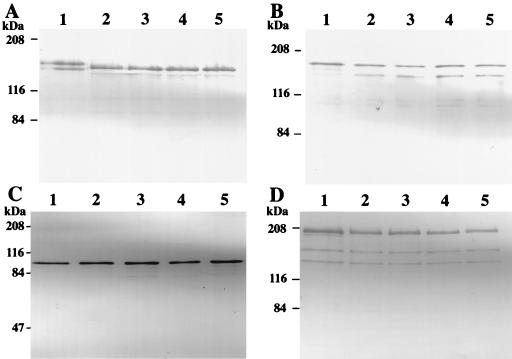
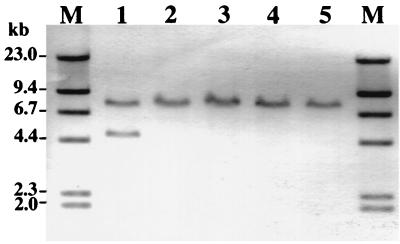
We previously constructed rml mutants of the serotype c S. mutans strain Xc (Xc23 [rmlA], Xc24 [rmlB], Xc21 [rmlC], and Xc26 [rmlD]) which were isolated on mitis salivarius agar plates (17, 18) (Table 1). All of these rml mutants showed similar colony morphology on mitis salivarius agar plates and were easily distinguished from the parental strain, Xc, by colony morphology. The mutant colonies were smaller than the colonies of the parental strain and were circular and convex with a dull surface which was smooth even in the presence of sucrose. When the mutants were propagated in liquid broth, their doubling times in the logarithmic growth phase were one-third of that of the wild-type strain, and this was reflected in the colony size. The morphological changes of the mutant colonies on sucrose-containing agar plates suggested changes in the production of extracellular polysaccharides or cell surface proteins. Therefore, we compared the expression of polysaccharide-synthesizing enzymes and the cell surface protein antigen with a molecular mass of 190 kDa (PAc) by the rml mutants with that by the parental strain, Xc, using Western blotting of the whole culture broth, which was precipitated with acetone and included cells and extracellular components as described previously (16, 23). For all of the rml mutants, the GTF-I and GTF-SI bands were not detected and a single band at a position intermediate between GTF-I and GTF-SI reacted with anti-GTF-I antiserum (Fig. 1A). On the other hand, production of GTF-S (Fig. 1B), fructosyltransferase (FTF) (Fig. 1C), and PAc (Fig. 1D) did not differ greatly from that for Xc, except that an additional band that reacted weakly with anti-GTF-S serum was seen at a position 20 kDa smaller than GTF-S for the rml mutants. Rabbit anti-GTF-I serum and anti-GTF-S serum were kindly provided by K. Fukushima, Nihon University, Matsudo Dental School, Matsudo, Japan. Rabbit anti-PAc serum was prepared as described previously (13). FTF was purified from 5 liters of Xc100L (Table 1) culture supernatant. The FTF protein in the culture supernatant was purified by using a preparative electrophoresis apparatus (Nippon Eido Co., Tokyo, Japan) according to the procedure used for purification of the gtfB-gtfC fusion gene product (21). The fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for protein (8) and water-soluble polysaccharide synthetic activity with raffinose used as a substrate. Rabbit anti-FTF serum was raised by subcutaneous injection of the purified FTF protein. The production of GTF-I and GTF-SI in the rml mutants is similar to that in UA101 and SP2 (19, 21), which both contain a spontaneous gtfB-gtfC fused gene as the result of an event of homologous recombination between the gtfB and gtfC genes. To evaluate the status of gtfB and gtfC in the rml mutants, EcoRI-digested chromosomal DNA was analyzed by Southern blotting with a digoxigenin (DIG)-labeled PCR probe (probe I) corresponding to the 1.6-kb BamHI fragment of gtfB as described previously (21, 23). While EcoRI digests of strain Xc chromosomal DNA exhibited two positive bands of 4.7 and 7.6 kb, a single 7.6-kb band was observed in EcoRI digests of chromosomal DNA from all the rml mutants (Fig. 2). These results indicate that the gtfB and gtfC genes were combined into a single fusion gene in the rml mutants, as seen in UA101 and SP2 previously (19, 21).
TABLE 1.
S. mutans strains used in this study
| Strain | Genotype or relevant characteristicsa | Reference |
|---|---|---|
| Xc | c wild-type strain | 7 |
| Xc21 | Emr; Xc carrying P15A replicon and the gene encoding Emr inserted into rmlC | 18 |
| Xc23 | Emr; Xc carrying P15A replicon and the gene encoding Emr inserted into rmlA | 18 |
| Xc24 | Emr; Xc carrying P15A replicon and the gene encoding Emr inserted into rmlB | 18 |
| Xc26 | Emr; Xc carrying P15A replicon and the gene encoding Emr inserted into rmlD | 17 |
| Xc21R | Emr; rmlC mutant strain transformed with chromosome of Xc21, which was isolated on tryptic soy agar plate | This study |
| Xc23R | Emr; rmlA mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate | This study |
| Xc24R | Emr; rmlB mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate | This study |
| Xc26R | Emr; rmlD mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate | This study |
| Xc21RS | Emr; rmlC mutant strain transformed with chromosome of Xc21, which was isolated on tryptic soy agar plate containing 5% sucrose | This study |
| Xc23RS | Emr; rmlA mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate containing 5% sucrose | This study |
| Xc24RS | Emr; rmlB mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate containing 5% sucrose | This study |
| Xc26RS | Emr; rmlD mutant strain transformed with chromosome of Xc23, which was isolated on tryptic soy agar plate containing 5% sucrose | This study |
| Xc100L | Subculture variant of Xc showing decreased levels of PAc, GTF-I, and GTF-SI production and increased levels of GTF-S and FTF production | 23 |
Emr, erythromycin resistance. To obtain and maintain erythromycin-resistant mutants, 10 μg of erythromycin/ml was used.
FIG. 1.
Western blot analyses of GTF-I, GTF-SI, GTF-S, FTF, and PAc in acetone-precipitated whole culture broth of rml mutants. (A to D) Results obtained with rabbit anti-GTF-I, anti-GTF-S, anti-FTF, and anti-PAc sera, respectively. Lanes 1 to 5 are for S. mutans Xc, Xc23, Xc24, Xc21, and Xc26, respectively. The molecular mass standards (expressed in kilodaltons) are shown on the left.
FIG. 2.
Southern blot analysis of chromosomal DNA from the rml mutants. EcoRI-digested chromosomal DNA was hybridized with DIG-labeled probe I. Lanes 1 to 5 contain chromosomal DNA of Xc, Xc23, Xc24, Xc21, and Xc26, respectively. M: HindIII-digested and DIG-labeled lambda DNA. The target DNA was hybridized with the probe overnight at 42°C in the presence of 50% formamide. The numbers on the left are size markers.
The original rml mutants were isolated on mitis salivarius agar containing 5% sucrose. It is possible that the sucrose content of the agar used for transformation might be related to the occurrence of fusions between the gtfB and gtfC genes in the transformants, because the gtfB and gtfC gene products are enzymes involved in synthesizing water-insoluble glucan from sucrose. The rml mutants were reconstructed by using tryptic soy agar plates in the absence of sucrose with chromosomal DNA from the original rml mutants (Xc23, Xc24, Xc21, and Xc26) isolated on mitis salivarius agar plates (17, 18). Chromosomal DNA was prepared from the original rml mutant strains and the wild-type strain (Xc) was transformed with the chromosomal DNA according to the methods described previously (14, 22). Ten transformants were isolated in each case, and Western blot analyses using antisera against PAc, GTF-I, GTF-S, and FTF were performed. All of the transformants showed similar results in the Western blot analyses, and 1 transformant in each set of 10 transformants was randomly selected. The resulting four transformants were designated Xc23R, Xc24R, Xc21R, and Xc26R, according to the origin of the chromosomal DNA used for transformation as described in Table 1. Similarly, the reconstructed mutant strains that were obtained on tryptic soy agar plates containing 5% sucrose were designated Xc23RS, Xc24RS, Xc21RS, and Xc26RS (Table 1). The reconstructed rml mutants were confirmed to have lost serotype c-specific antigenicity accompanied by a drastic decrease of the amounts of rhamnose and glucose in the cell wall. These were detected by immunodiffusion analysis with serotype c-specific antiserum and high-pressure liquid chromatography as described previously (18).
The colony morphology of each of the mutants isolated on agar plates in the presence or absence of 5% sucrose was identical to that of the original rml mutants grown on tryptic soy agar and mitis salivarius agar plates. Western blot analyses of the polysaccharide-synthesizing enzymes of the rml mutants reconstructed on sucrose-containing agar plates produced results completely identical to those (shown in Fig. 1) obtained for the original rml mutants (data not shown). The rml mutants selected on sucrose-free agar plates also showed the same results as those observed with the original rml mutants (data not shown), except for the result obtained with anti-GTF-I serum. Western blot analysis showed that Xc23R, Xc24R, Xc21R, and Xc26R produced the same levels of both GTF-I and GTF-SI proteins as the wild-type strain (data not shown). Southern blot analysis confirmed that these mutants had intact gtfB and gtfC genes (data not shown).
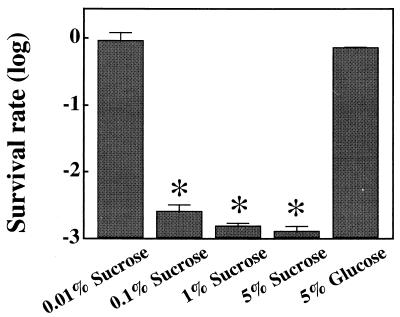
To elucidate the mechanism of the gtfB-gtfC gene fusion, the survival rates of the rml mutants were examined in the presence of 0 to 5% sucrose or 5% glucose. The survival rate of Xc24 was not greatly affected, even in the presence of 5% glucrose. Compared with the survival rate of cells grown in the presence of 5% glucose, the survival rate (1.2 × 10−3 ± 0.7 × 10−3) of Xc24R cells was decreased significantly when they were grown in the presence of 0.1% or more sucrose but not in the presence of 0.01% sucrose (Fig. 3). The survival rates of the other rml mutants (Xc21R, Xc23R, and Xc26R) did not decrease greatly even in the presence of 0.1% sucrose. However, the majority of colonies were very tiny and rough with irregular margins, and a few colonies exhibited the typical rml mutant colony morphology. In addition, these tiny background colonies were not observed when cells were grown in the presence of 1% or more sucrose, and the survival rates of these mutants decreased to the same level (1.1 × 10−3 to 2.0 × 10−3) as observed for Xc24R. We previously observed that Xc24 is sensitive to osmolarity stress (24). Sucrose osmolarity stress may cause the low survival rate of Xc24R in the presence of sucrose observed in this study. Therefore, we examined the effects of 5% glucose on the survival rates of strain Xc and the rml mutants. Glucose, however, had barely any effect on the survival rate of any of the rml mutant strains, including Xc24R (Fig. 3), suggesting that the reduced survival of the rml mutants reconstructed in the absence of sucrose on sucrose-containing plates is not due to osmolarity stress.
FIG. 3.
Survival rates of S. mutans Xc24R cells grown in the presence of sucrose or glucose. S. mutans cells were grown in brain heart infusion broth until an optical density at 550 nm of about 0.4 was attained. The broth was sonicated three times for 10 s at 20% pulse power with a sonicator (Cell Disrupter model W-225R; Heat Systems, Inc., Farmingdale, N.Y.) and spread on tryptic soy agar supplemented with 0 to 5% (wt/vol) sucrose or 5% (wt/vol) glucose. The survival rates for cells grown at each concentration of added sucrose or glucose were calculated by dividing the number of CFU by the number of CFU for cells grown on medium without additional glucose or sucrose. Each column and bar represents the mean percent survival ± standard deviation for four different experiments. The statistical differences in survival rates were analyzed by using the Mann-Whitney U test. ∗, P < 0.05 (compared to survival rate of cells grown in the presence of 5% glucose).
The water-insoluble glucan production activity of the rml mutants was visually analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels as described previously (21). Xc23R, Xc24R, Xc21R, and Xc26R produced the same level of water-insoluble glucan as the wild-type strain (Xc) (data not shown). On the other hand, Xc23RS, Xc24RS, Xc21RS, and Xc26RS produced barely detectable levels of water-insoluble glucan (data not shown). Furthermore, the in vitro sucrose-dependent adherence ability of the rml mutants was examined. In glass tubes, S. mutans cells were grown to stationary phase in brain heart infusion broth in the absence of sucrose. At the stationary phase, the cells were ultrasonicated with a sonicator and sucrose was added to the broth at a final concentration of 1% (wt/vol). After incubation at 37°C for 12 h, cells adhering to the glass surfaces were stained with Coomassie brilliant blue R-250. Xc24R and Xc26R could colonize glass surfaces in the presence of 1% sucrose like the wild-type strain, while Xc24RS and Xc26RS could not (data not shown).
All of the rml mutants reconstructed in the presence of 5% sucrose exhibited recombination between gtfB and gtfC, whereas no rml mutant with the gtfB-gtfC fusion gene was isolated in the absence of sucrose. This suggests that sucrose in the selection medium has an obvious effect on the status of gtfB and gtfC in rml mutants. Moreover, the cells of the Xc24RS strain and of the other rml mutant strains surviving in the presence of ≥0.1% or ≥1% sucrose, respectively, possessed the gtfB-gtfC fusion gene (data not shown), indicating that gene fusion between gtfB and gtfC is necessary for the rml mutants to survive in the presence of sucrose. In spite of the fact that strains Xc21R, Xc23R, and Xc26R seemed to be slightly more resistant to the presence of sucrose than Xc24R, their survival rates in the presence of 1% or more sucrose were similar to that of Xc24R. It was previously reported that in vitro spontaneous recombination between gtfB and gtfC occurs at a frequency ranging from 1 × 10−3 to 3 × 10−3 (14, 19), which is in agreement with the survival rates of the rml mutants grown in the presence of sucrose. It is reasonable to speculate that fusion between the gtfB and gtfC genes in the rml mutants in the presence of sucrose results from the selection of the spontaneous recombinant organisms and not from an increased frequency of recombination.
In addition to the gtfB and gtfC gene products, S. mutans produces GTF-S and FTF extracellularly. These enzymes catalyze production of extracellular water-soluble glucan and fructan, respectively, from sucrose. No remarkable change in GTF-S or FTF production was observed in any rml mutants (Fig. 1B and C), suggesting that water-soluble polysaccharides may not be related to the viability of the rml mutants. Since S. mutans strains with the gtfB-gtfC fusion gene are less able to produce water-insoluble glucan than strains without the fusion gene, while water-soluble glucan production is not affected (19, 21), water-insoluble glucan synthesis may be the determining factor interrupting the growth of the rml mutants that have intact gtfB and gtfC genes. For Xc24R, the difference between 0.01 and 0.1% sucrose had a dramatic effect on the survival rate, and 0.01% sucrose did not affect the survival rate of the rml mutant greatly. These results are consistent with the Km values (around 3 to 10 mM) reported for GTF-I and GTF-SI (2, 3, 11, 12), because 0.01% sucrose is much lower than the Km values of these enzymes for sucrose. With such a low concentration of the substrate, neither GTF-I nor GTF-SI is likely to produce enough water-insoluble glucan to suppress the growth of Xc24R.
The reason for the difference in viability between Xc24R and the other rml mutants (Xc21R, Xc23R, and Xc26R) in the presence of 0.1% sucrose remains to be elucidated. Although the other rml mutants do survive in the presence of 0.1% sucrose, even if they have intact gtfB and gtfC genes, their growth is severely inhibited. It seems that 0.1% is close to the critical sucrose concentration for the survival of these rml mutants. The suggested survival mechanism for Xc24 seems to be applicable to the survival of strains Xc21R, Xc23R, and Xc26R.
We concluded that dTDP-rhamnose synthesis-deficient mutants of S. mutans cannot grow in the presence of sucrose unless the ability to produce water-insoluble glucan is reduced by a spontaneous recombination between gtfB and gtfC. However, we could not determine whether dTDP-rhamnose itself or a glucose-rhamnose polysaccharide is required for the survival of the rml mutants in the presence of sucrose. Recently, Hazlett et al. (6) reported that inactivation of the gbpA gene encoding an S. mutans glucan-binding protein promotes the in vivo recombination between gtfB and gtfC. Although they did not determine the mechanism for the accumulation of the gbpA mutants with a gtfB-gtfC fusion gene, the cell surface structure seems to be important for maintaining a steady state of intact gtfB and gtfC genes.
S. mutans strains with the gtfB-gtfC fusion gene have a reduced sucrose-dependent ability to adhere to glass surfaces, and the rml mutants with the gtfB-gtfC fusion gene failed to adhere to glass surfaces even in the presence of 1% sucrose. It is interesting that the dTDP-rhamnose synthesis pathway is necessary for S. mutans to survive in the presence of water-insoluble glucan synthesis, which is implicated in the cariogenicity of the organism. Disruption of the dTDP-rhamnose synthesis pathway triggers a virulence-attenuating gene recombination in S. mutans. In the future, this pathway could become the target of a novel class of antimicrobial agents for caries preventive therapy.
Acknowledgments
This work was supported in part by Grant-in-Aid for Developmental Scientific Research (B) 09470474 from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu H K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukushima K, Ikeda T, Kuramitsu H K. Expression of Streptococcus mutans gtf genes in Streptococcus milleri. Infect Immun. 1992;60:2815–2822. doi: 10.1128/iai.60.7.2815-2822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada S, Horikoshi T, Minami T, Okahashi N, Koga T. Purification and characterization of cell-associated glucosyltransferase synthesizing water-insoluble glucan from serotype c Streptococcus mutans. J Gen Microbiol. 1989;135:335–344. doi: 10.1099/00221287-135-2-335. [DOI] [PubMed] [Google Scholar]
- 4.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazlett K R O, Michalek S M, Banas J A. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect Immun. 1998;66:2180–2185. doi: 10.1128/iai.66.5.2180-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga T, Asakawa H, Okahashi N, Takahashi I. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J Gen Microbiol. 1989;135:3199–3207. doi: 10.1099/00221287-135-12-3199. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin V, Kleschyov A L, Klein J-P, Beretz A. Induction of nitric oxide production by polyosides from the cell walls of Streptococcus mutans OMZ 175, a gram-positive bacterium, in the rat aorta. Infect Immun. 1997;65:2074–2079. doi: 10.1128/iai.65.6.2074-2079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukasa H, Shimamura A, Tsumori H. Purification and characterization of cell-associated glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. J Gen Microbiol. 1989;135:2055–2063. doi: 10.1099/00221287-135-7-2055. [DOI] [PubMed] [Google Scholar]
- 12.Mukasa H, Tsumori H, Shimamura A. Isolation and characterization of an extracellular glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. Infect Immun. 1985;49:790–796. doi: 10.1128/iai.49.3.790-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta H, Kato H, Okahashi N, Takahashi I, Hamada S, Koga T. Characterization of a cell-surface protein antigen of hydrophilic Streptococcus mutans strain GS-5. J Gen Microbiol. 1989;135:981–988. doi: 10.1099/00221287-135-4-981. [DOI] [PubMed] [Google Scholar]
- 14.Perry D, Wondrack L M, Kuramitsu H K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983;41:722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soell M, Lett E, Holveck F, Schöller M, Wachsmann D, Klein J-P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukioka Y, Yamashita Y, Nakano Y, Oho T, Koga T. Identification of a fourth gene concerned with dTDP-rhamnose synthesis in Streptococcus mutans. J Bacteriol. 1997;179:4411–4414. doi: 10.1128/jb.179.13.4411-4414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T. Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans. J Bacteriol. 1997;179:1126–1134. doi: 10.1128/jb.179.4.1126-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda S, Kuramitsu H K. Molecular basis for the spontaneous generation of colonization-defective mutants of Streptococcus mutans. Mol Microbiol. 1988;2:135–140. doi: 10.1111/j.1365-2958.1988.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita Y, Bowen W H, Kuramitsu H K. Molecular analysis of a Streptococcus mutans strain exhibiting polymorphism in the tandem gtfB and gtfC genes. Infect Immun. 1992;60:1618–1624. doi: 10.1128/iai.60.4.1618-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita Y, Takehara T, Kuramitsu H K. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J Bacteriol. 1993;175:6220–6228. doi: 10.1128/jb.175.19.6220-6228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita Y, Tsukioka Y, Nakano Y, Shibata Y, Koga T. Molecular and genetic analysis of multiple changes in the levels of production of virulence factors in a subcultured variant of Streptococcus mutans. FEMS Microbiol Lett. 1996;144:81–87. doi: 10.1111/j.1574-6968.1996.tb08512.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita Y, Tsukioka Y, Nakano Y, Tomihisa K, Oho T, Koga T. Biological function of UDP-glucose synthesis in Streptococcus mutans. Microbiology. 1998;144:1235–1245. doi: 10.1099/00221287-144-5-1235. [DOI] [PubMed] [Google Scholar]