Abstract
The isolation of two forms of hepatic zinc-thioneins after either zinc injection into rats or partial restriction of their food intake is described. The proteins differed slightly in their amino acid composition and electrophoretic mobilities. Increases in liver zinc content after both treatments were synchronous with, and associated almost completely with, increased zinc-binding to these proteins. The time-course for the appearance and disappearance of the zinc proteins is shown. It is suggested that metallothionein is involved in the normal metabolism of zinc, perhaps in some temporary storage or detoxication capacity.
Full text
PDF
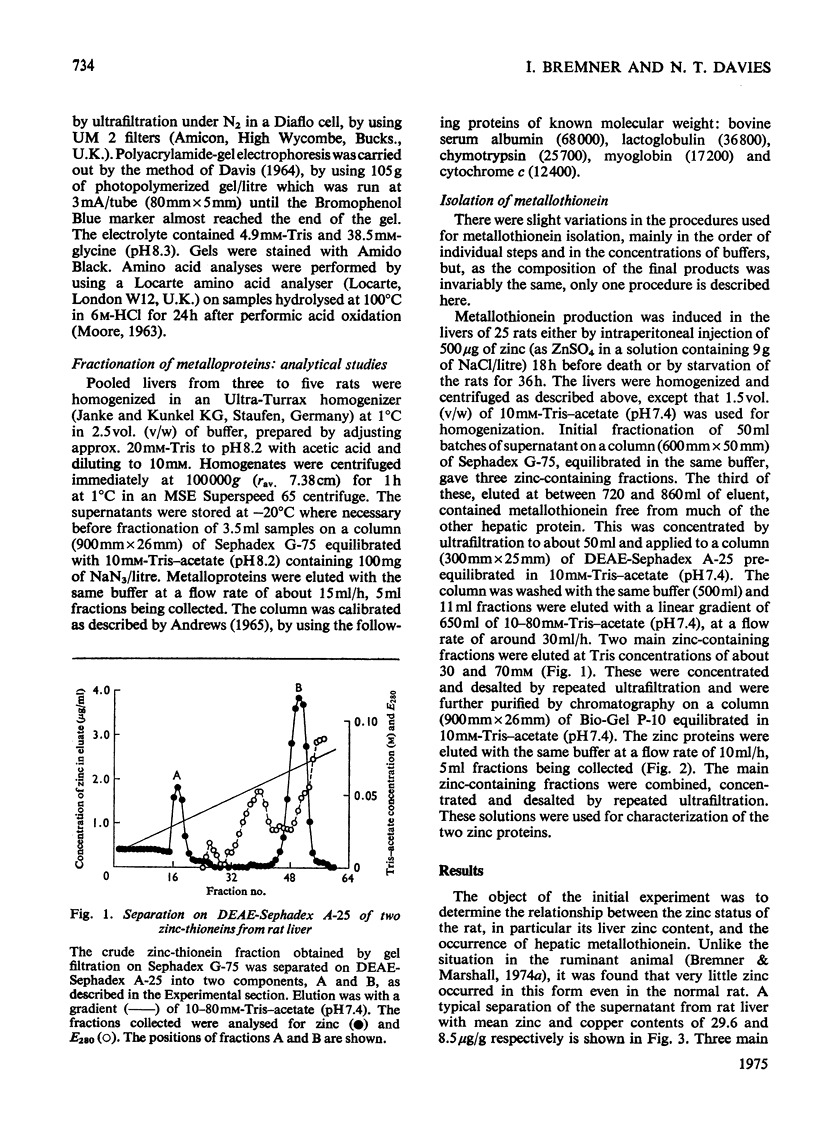
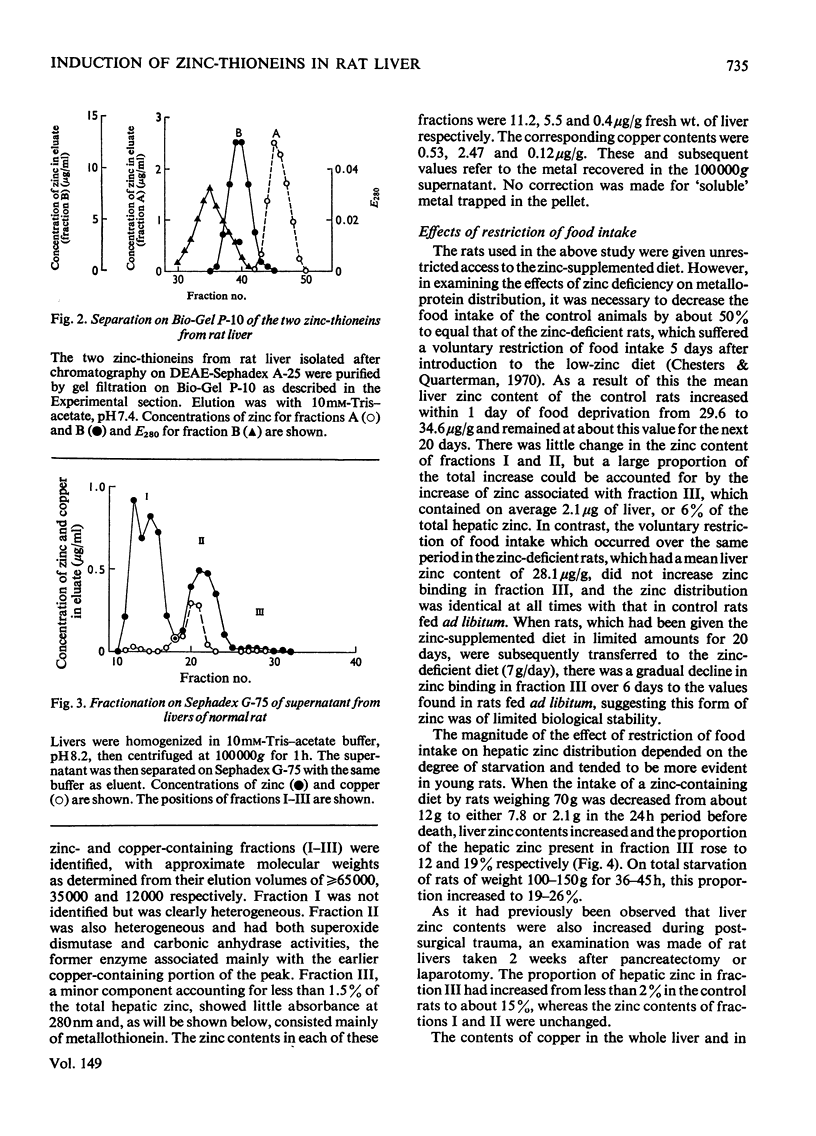
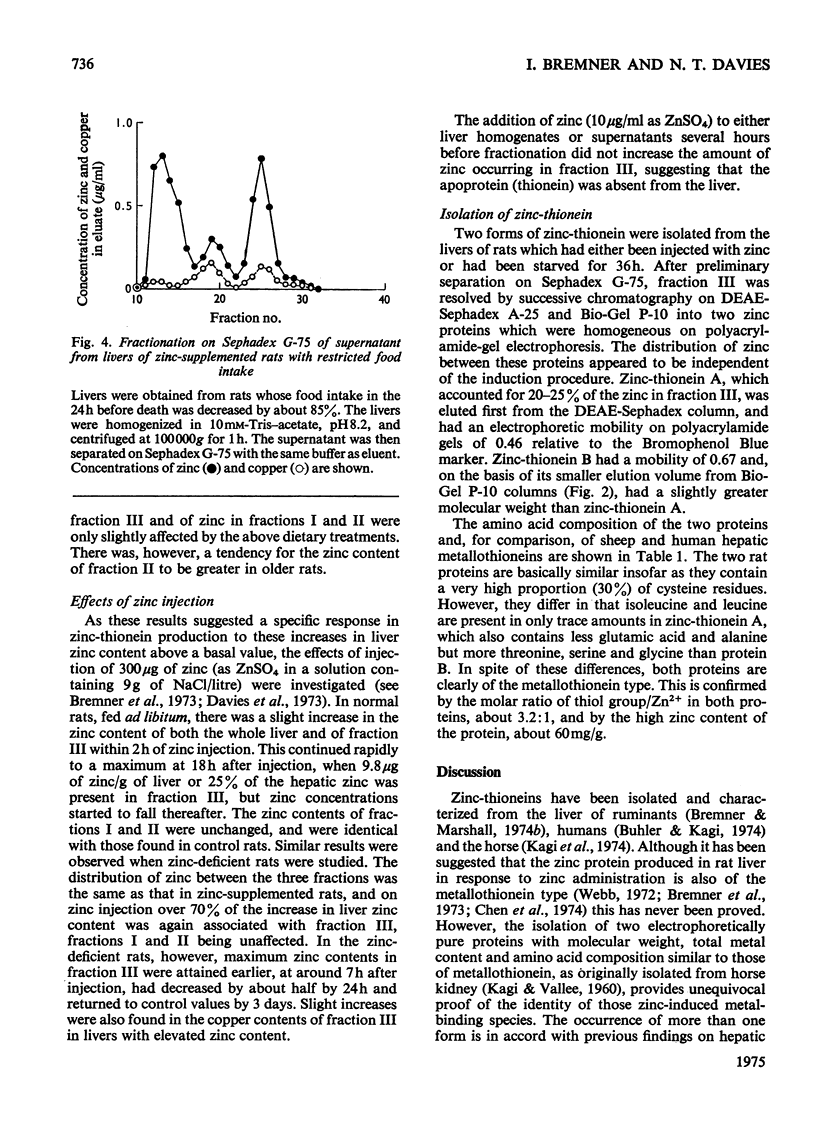
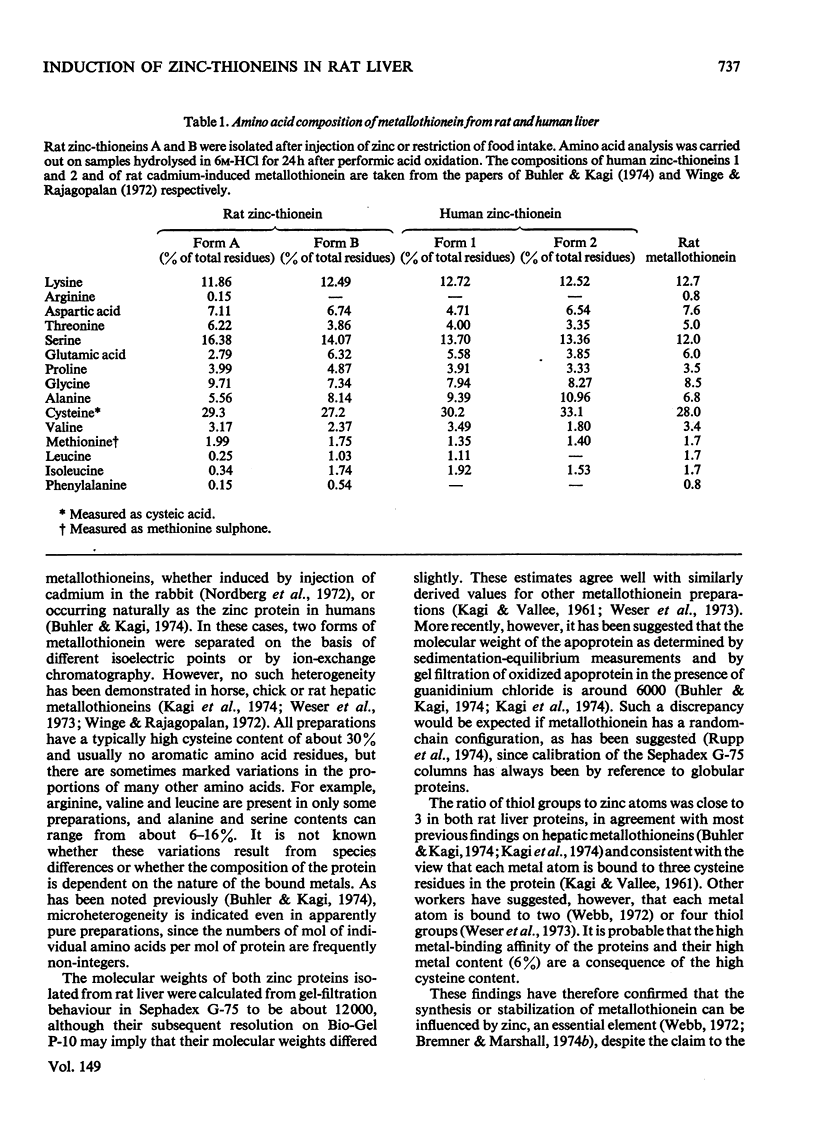

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Marshall R. B. Hepatic copper- and zinc-binding proteins in ruminants. 2. Relationship between Cu and Zn concentrations and the occurrence of a metallothionein-like fraction. Br J Nutr. 1974 Sep;32(2):293–300. doi: 10.1079/bjn19740082. [DOI] [PubMed] [Google Scholar]
- Bremner I., Marshall R. B. Hepatic copper-and zinc-binding proteins in ruminants. 1. Distribution of Cu and Zn among soluble proteins of livers of varying Cu and Zn content. Br J Nutr. 1974 Sep;32(2):283–291. doi: 10.1079/bjn19740081. [DOI] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Chesters J. K., Quarterman J. Effects of zinc deficiency on food intake and feeding patterns of rats. Br J Nutr. 1970 Dec;24(4):1061–1069. doi: 10.1079/bjn19700109. [DOI] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- JOCELYN P. C. The effect of glutathione on protein sulphydryl groups in rat-liver homogenates. Biochem J. 1962 Dec;85:480–485. doi: 10.1042/bj0850480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski M., Piotrowski J., Trojanowska B. Binding of mercury in the rat: studies using 203HgCl2 and gel filtration. Toxicol Appl Pharmacol. 1970 May;16(3):743–753. doi: 10.1016/0041-008x(70)90080-3. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Kägi J. H., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. Purification, molecular weight, amino acid composition, and metal content. J Biol Chem. 1974 Jun 10;249(11):3537–3542. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nordberg G. F., Nordberg M., Piscator M., Vesterberg O. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972 Feb;126(3):491–498. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg G. F., Piscator M., Lind B. Distribution of cadmium among protein fractions of mouse liver. Acta Pharmacol Toxicol (Copenh) 1971;29(5):456–470. doi: 10.1111/j.1600-0773.1971.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Piotrowski J. K., Trojanowska B., Wiśniewska-Knypl J. M., Bolanowska W. Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: induction of metallothionein by mercury and cadmium. Toxicol Appl Pharmacol. 1974 Jan;27(1):11–19. doi: 10.1016/0041-008x(74)90169-0. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Calabrese L., Bossa F., Barra D., Agrò A. F., Mondovì B. Properties of the apoprotein and role of copper and zinc in protein conformation and enzyme activity of bovine superoxide dismutase. Biochemistry. 1972 May 23;11(11):2182–2187. doi: 10.1021/bi00761a027. [DOI] [PubMed] [Google Scholar]
- Roughton F. J., Booth V. H. The effect of substrate concentration, pH and other factors upon the activity of carbonic anhydrase. Biochem J. 1946;40(2):319–330. doi: 10.1042/bj0400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp H., Voelter W., Weser U. 270 MHz proton magnetic resonance spectra of metallothionein. FEBS Lett. 1974 Mar 15;40(1):176–179. doi: 10.1016/0014-5793(74)80921-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L. Zinc and cobalt alkaline phosphatases. Ann N Y Acad Sci. 1969 Oct 14;166(2):670–695. doi: 10.1111/j.1749-6632.1969.tb46426.x. [DOI] [PubMed] [Google Scholar]
- Webb M. Binding of cadmium ions by rat liver and kidney. Biochem Pharmacol. 1972 Oct 15;21(20):2751–2765. doi: 10.1016/0006-2952(72)90023-8. [DOI] [PubMed] [Google Scholar]
- Weser U., Rupp H., Donay F., Linnemann F., Voelter W., Voetsch W., Jung G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur J Biochem. 1973 Nov 1;39(1):127–140. doi: 10.1111/j.1432-1033.1973.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Williams R. B., Mills C. F. The experimental production of zinc deficiency in the rat. Br J Nutr. 1970 Dec;24(4):989–1003. doi: 10.1079/bjn19700102. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Rajagopalan K. V. Purification and some properties of Cd-binding protein from rat liver. Arch Biochem Biophys. 1972 Dec;153(2):755–762. doi: 10.1016/0003-9861(72)90395-5. [DOI] [PubMed] [Google Scholar]


