Abstract
Background
California’s relatively low smoking rate (10.1% in 2019–2020) (About CHIS, UCLA Center for Health Policy Research, 2024) masks deep disparities among low-income populations, where smoking rates are nearly double that of their middle- to upper-income peers. Low-income smokers report a similar desire to quit and similar rates of recent quit attempts as smokers from other groups; yet, they often face barriers in accessing effective resources to facilitate successful cessation.
Methods
Our team will conduct a pragmatic stepped-wedge cluster, randomized controlled trial of Enhanced Multicomponent Proactive Navigator-Assisted Cessation of Tobacco Use (EMPACT-Us), a suite of tobacco cessation services supported by patient navigators, designed in close partnership with patients, providers, and community stakeholders. The study will take place at Family Health Centers of San Diego (FHCSD), the largest federally qualified health center (FQHC) in San Diego. Eight primary care clinics are included, where 70% (n = 13,496) of smokers at FHCSD receive care.
Discussion
We hypothesize that multiple points of engagement and integration of navigation services into the workflow of existing staff will improve utilization and cessation success. This study will examine if the enhanced suite of services offers insights on how to best integrate evidence-based tobacco treatment services into usual care.
Trial registration
ClinicalTrials.gov, NCT05750537, Registered on March 1, 2023.
Supplementary information
The online version contains supplementary material available at 10.1186/s12889-024-20997-6.
Keywords: Tobacco use, Smoking cessation, Electronic Health Record (EHR), Kick It California (KIC), Low-income populations, Evidence-based tobacco treatment
Background
Despite persistent tobacco control efforts, the prevalence of smoking among low-income smokers has not declined in parallel with that among smokers in other groups [1]. Tobacco use thus remains consistently and disproportionately concentrated among low-income populations [2]. In 2022, the percentage of low-income Californians who currently smoke included 14% of men and 6% of women compared to 6% and 3% among higher income men and women respectively [3]. Currently, 1,558,000 Californians living below 200% of the federal poverty limit smoke [3].
This disparity persists despite similar cessation motivation and rates of recent quit attempts for low income as found among other smokers [4]. Rather, evidence suggests that low-income smokers lack access to tobacco treatment services shown to increase quit attempts, and when access does exist, there often are systematic barriers to engagement [5]. Over 28 million Americans – representing a third of the low-income population– receive their medical care at Federally Qualified Health Centers (FQHC) [6]. Funded by the Health Resources and Services Administration, FQHCs are community-based healthcare providers that offer primary care services in underserved areas, regardless of a patient's ability to pay. Tobacco use is higher among FQHC patients than the general population and among smokers served in these clinics, 82% express a desire to quit, making this clinical setting a vital entry point into a harmonized, comprehensive suite of evidence-based tobacco treatment services. The need to deliver more intensive treatments for low-income smokers is reflected in the 2020 Surgeon General’s report on smoking cessation, which highlights FQHCs as the community centric clinical environment where a focus on improving tobacco treatment services is essential [2].
Family Health Centers of San Diego (FHCSD), one of the ten largest FQHCs in the nation and the fourth largest in California, provides healthcare for over 160,000 predominately low-income patients annually, with more than 1 million visits each year. The overwhelming majority of patients at FHCSD belong to low-income and minority groups: 87% live under 200% of the federal poverty line, 16% are homeless, 41% lack insurance, and 83% are from racial or ethnic minority backgrounds. Black and Latino patients make up 9% and 59% of the patient base, respectively, figures which are disproportionately high compared to the demographic composition of San Diego County. Approximately 19,000 of FHCSD’s adult patients have tobacco use documented as an active medical problem in their electronic health records (EHR). This represents roughly 11.8% of the adult patient population, in contrast with California’s adult smoking rate of 9% [7]. In this setting, as in other underserved communities across the country that bear a disproportionate burden of smoking-related morbidity and mortality, there is a need to implement effective strategies tailored to meet the unique needs of low-income, underserved, and minority tobacco users within the primary care setting.
Efforts to understand the under-utilization of quitlines, telephone-based services that offer counseling and other resources on quitting the use of tobacco [8], and support for the acceptability of patient navigation [9] suggest potential pathways to improved tobacco treatment outcomes. Several studies have examined systems for enhancing the engagement of FQHC patients in telephone quit lines. The QUITSmart Utah is an ongoing pragmatic trial that is testing the impact of EHR-prompting of quitline referrals with or without short message service (SMS) on quitline engagement for patients referred by FQHCs [10]. Broadening Ask, Advise, and Connect workflow trainings to include nurses and medical assistants (MAs) making electronic referrals (eReferrals) to quit lines has shown promise in low-income primary care (PCP) setting with increases in referrals from < 1% to 30%, and up to 19% engagement in tobacco treatment [11]. In contrast, the comparison intervention with targeted clinical training of physicians to provide a teachable moment was used in only 8% of visits with smokers and failed to increase treatment delivery [12]. We are aware of other recent trials [13, 14] examining comprehensive referral systems that include eReferral to quit lines, but we are not aware of trials focused on treatment engagement through staff-level patient navigation for combined behavioral and pharmacological interventions within a FQHC setting.
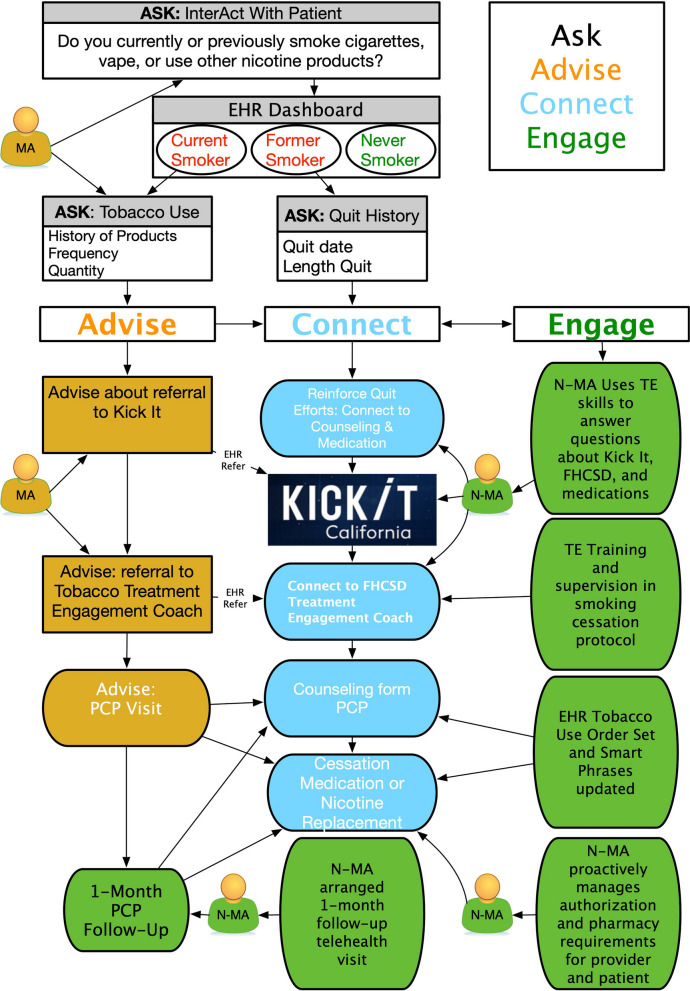
The present study is rooted in the Chronic Care Model (CCM) and the Ask, Advise, and Connect [15] approach to tobacco treatment. The study is designed to maintain a patient-focused and community-informed approach to tobacco treatment within a framework based in the CCM [16] that is oriented towards an informed patient paired with proactive and sustained clinical care that leads to self-management support. Input from multiple stakeholders including service users, care providers, and administrators is sought at each design step. A detailed breakdown of the CCM and the Ask, Advise and Connect approach in regard to the study can found in Fig. 1. Major domains of the CCM guide our design and organize input to prioritizing comprehensive systems of tobacco control strategies for low-income smokers. The CCM has been successfully applied in tobacco treatment activities in a variety of settings [16]. The Engage component is a critical extension of current best practices and the component addition to be tested saliva in this trial. The context of the FQHC suggests the need to incorporate how clinicians communicate, adapt to workflow demands, and streamline effective documentation of activities both within clinical encounters and through interactions such as behavioral counseling services.
Fig. 1.
The chronic care model and the ask, advice and connect approach in relation to the study
Objective
The primary objective is to compare the effectiveness of EMPACT-Us versus newly enhanced usual care (EUC). EMPACT-Us utilizes proactive treatment engagement and patient navigation to increase the efficacy of a suite of tobacco treatment and cessation services designed in close partnership with patients, providers, and community stakeholders. On the other hand, EUC entails changes in the electronic health record (E-HR) to highlight to providers patient nicotine use status and improve access to pharmacological or eReferral options. This objective will examine whether EMPACT-Us will improve engagement in evidence-based tobacco treatments (i.e. utilization of medications and behavioral counseling) and increase quit attempts and biochemically verified abstinence from tobacco when compared with EUC. Our secondary objective explores tailoring EMPACT-Us based on the effect it has among low-income patients, sexual and gender minorities, racial/ethnic minorities, and those with comorbid medical, substance use, and/or mental health concerns. The tertiary objective is to examine implementation processes and fidelity, including acceptability and perceived feasibility by patients, clinic staff, clinic providers, and community stakeholders.
Methods
The SPIRIT figure for this protocol can be found in Table 1 for reference by clinicians and patients. Our Community Advisory Board (CAB) will be the primary vehicle for individual community members not on the research team and community-based organizations not involved in the project to provide input during all stages of the study. If the investigators are unable to come to a consensus, the document describing the disagreement will be shared with our existing CAB. CAB members will discuss proposed resolutions to the disagreement and arrive at a final resolution by consensus. Any required and unplanned protocol amendments will be reported to the Institutional Review Board (IRB) and reflected in the trial registration.
Table 1.
The overview of the study period including training schedule of clinics, interventions and assessments
| STUDY PERIOD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Enrollment | Allocation | Post-allocation | |||||||
| TIMEPOINT | 0 | 0 | 0 M | 6 M | 12 M | 18 M | 24 M | 30 M | 36 M |
| Cluster ENROLLMENT: | |||||||||
| Cluster 1 | X | X | X | X | X | X | X | ||
| Cluster 2 | X | X | X | X | X | X | |||
| Cluster 3 | X | X | X | X | X | ||||
| Cluster 4 | X | X | X | X | |||||
| Patient ENROLLMENT: | |||||||||
| Eligibility | X | ||||||||
| Broad Consent | X | ||||||||
| Allocation | X | X | X | X | X | X | X | ||
| INTERVENTIONS: | |||||||||
| [Intervention A] | |||||||||
| EMPACT-Us | X | X | X | X | X | X | |||
| [Intervention B] | |||||||||
| SMS Campaign | X | X | X | X | X | X | |||
| ASSESSMENTS: | |||||||||
| Staff Interviews | X | X | X | X | X | X | |||
| Patient Surveys | X | X | X | X | X | X | |||
| Cotinine Testing | X | X | X | X | X | X | |||
Study design
We will implement our approach utilizing a single blind pragmatic stepped-wedge cluster randomized controlled trial of Enhanced Multicomponent Proactive Navigator-Assisted Cessation of Tobacco Use (EMPACT-Us). In accordance with the step wedge design, all clinics outside the active clusters serve as controls in this study and are engaged in the enhanced usual care (EUC) model. The EUC pathway includes the same existing staff as those in EMPACT-Us, however, MAs and PCPs will not have received training on the EHR-guided clinical processes until their clinic enters the trial as an active cluster-pair. Each clinic pair (cluster) starts out with enhanced usual care (EUC) and pairs of clinics are then randomized to one of four intervention sequences that determine the period in which they will begin EMPACT-Us. All the clinics will lack any E-HR changes, staff or clinician training or other adjustments occurring with the EUC and EMPACT-Us.
An open cohort design will be used, identifying all tobacco users in the clinics and including any new cases. The research will occur at 8 of the largest clinic sites at FHCSD. These well-established clinic populations will allow the project to easily access the adult smokers per target clinic site necessary to conduct the proposed clinical trial. Randomization will occur at the cluster level. Individual randomization of subjects was deemed impractical due to the large numbers of patients and pace of clinic visits. All clusters will be exposed to the intervention condition before the end of the trial. Transition periods of no data collection for two weeks will enable consistent training as two new clinics begin the EMPACT-Us protocol in each of the four periods. A new cluster will crossover every 6 months into EMPACT-Us, starting from month 6 until month 33.
Allocation is concealed from all clinic staff until the study group is assigned. Only the physicians and medical assistants involved in the delivery of the intervention are subsequently made aware of the allocation to the intervention condition and the ‘activation’ of their clinic cluster. Patients will have no knowledge of their provider’s potential use of the EHR enhancements and exposure to the re-vamped training curricula.
Participating FHCSD clinics were entered into an Excel sheet. The RAND() function was applied to a new column to generate a random number between zero and one for each clinic, and these values were saved by copying and pasting values only. The sheet was then arranged based on the ascending values from the RAND() function and this determined the order clinics beginning the EMPACT-Us intervention.
Suite of tobacco services
The EMPACT-Us suite of services will succinctly integrate a tobacco treatment training module for each activated clinic in the study. This module will efficiently educate every adult medicine PCP and MA in the intervention clinics, empowering them to effectively utilize the enhanced E-HR and InterAct workflow. Each clusters’ PCPs and MAs will receive separately tailored trainings two weeks prior to each cluster-pair entering the EMPACT-Us phase; each of the trainings curriculum has been developed based on learnings from the Tobacco-Related Disease Research Program (TRDRP) community partnered participatory research (CPPR) pilot study Feasibility and Acceptability of a Suite of Tobacco Cessation Services for Low-Income Populations (2020–2022).
Clusters’ PCPs and MAs will not receive training on the EHR-guided clinical processes until their clinic enters the trial as a cluster-pair. The trained N-MA, which includes every adult medicine MA in the intervention clinics, will be guided by EHR prompts during the patient rooming process to screen for tobacco use. Patients will be screened for desire to quit, and those who express interest in tobacco treatment will be offered an eReferral to KIC (the California telephone quitline) as well as to a Treatment Engagement (TE) Coach. An option to set a quit date is offered by the N-MA to motivated patients, and the N-MA will cue patients to further discuss their desire to quit with their PCP.
PCPs will receive a comprehensive one-time in-person tobacco treatment tailored video-based workshop training at each individual active clinic within the intervention. This training will be comprised of a series of four concise videos covering newly enhanced E-HR updates, utilization of a tobacco-use order-set, effective medication ordering, and proactive tobacco treatment support referrals. Moreover, the training will delineate between the two e-referral options within the E-HR system.
Additionally, and at time of training, PCPs are equipped with quick reference guides that highlight the newly enhanced features of E-HR. These guides outline key aspects such as the depiction of smoking status on the E-HR encounter dashboard, procedures for updating a patient's smoking status, setting a quit date, accessing tobacco-use order sets, details of the tobacco treatment medication list within the order sets, information about Kick It California (KIC) and the FHCSD Tobacco Treatment Support Program, follow-up options, educational handouts, and smart phrase templates.
The enhanced dashboard prompts trained PCPs to initiate a conversation with the patient regarding tobacco treatments. From the order sets, PCPs can select one of two pre-developed smart phrases prompting a motivational interview (MI) approach to guide the conversation, develop an assessment plan, order tobacco treatment medications with recommended dosages for Nicotine Replacement Therapies (NRTs) and varenicline or bupropion medications, initiate an e-referral to KIC or a newly trained FHCSD Health Educator (HE) Coach, and/or schedule a follow-up based on recommended tobacco treatment intervals for either a focused telemedicine or in-person visit. The newly enhanced e-referrals for the tobacco treatment behavior counseling options are designed to facilitate automatic transmission of referrals. Within one to three weeks after receiving an e-referral, patients’ referral status is categorized based on their response: unreachable, declined services, or having undergone a portion or the full intervention. Patient status data is batched and relayed back to the FHCSD EHR from KIC. Encounter template notes are accessible within the E-HR for patients referred to a FHCSD HE Coach. Additionally, if a patient sets a quit date with either counseling program, this information is prominently displayed in the EHR Dashboard.
The FHCSD TE Coach plays a vital role in engaging with patients referred to the program. Utilizing an adapted curriculum enhanced with MI techniques, the HE Coach establishes a supportive and collaborative relationship with each patient. Through this approach, the coach helps patients explore their motivations, barriers, and goals related to tobacco. As the coaching sessions progress, the HE Coach, along with E-HR loadable curriculum templates, works closely with patients to develop personalized strategies for quitting tobacco use. The coach also provides ongoing support and encouragement to help patients navigate challenges and maintain their commitment to quitting. In addition to providing behavioral counseling, the HE Coaches are able to use E-HR messaging to collaborate closely with the patient's PCP; if there is a need for further medical intervention or if the patient encounters difficulties during their quit journey, particularly with regards to medication adherence and/or dosing issues, the coach may facilitate communication and coordination between the patient and their PCP.
When a patient follows up with the PCP, either via telemedicine or in-person, and has a predetermined Quit Date or indicates recent cessation of tobacco use in E-HR, they are flagged within InterAct section of the E-HR. InterAct prompts for the MA to screen for and update their smoking status. For patients who had previously set a quit date with an MA, TE coach, or KIC, the E-HR's InterAct prompts the MA to inquire about their quit success. Smoking treatment tools are intentionally integrated throughout the E-HR system and care teams. This deliberate integration aims to prevent reliance on single points of contact, reducing the risk of disruption to implementation and sustainability, particularly in the face of frequent staff turnover, which is common in FQHCs. The sections below provide more details on the intervention and an overview of the changes implemented throughout the study can be found in Table 2.
Table 2.
Comparison of EMPACT-Us vs. EUC
| EMPACT-Us | Enhanced Usual Care (EUC) | Description | |
|---|---|---|---|
| E-HR Enhancements | X | X | Creation of an order set, smart phrase, and dashboard modifications |
| Health Educator with new treatment engagement + MI curriculum | X | X | A revamped role for smoking cessation class (SCC) health educators who provided health education to patients referred to behavior counseling within FHCSD’s system. New curriculum adapted for delivery in an FQHC setting, using a motivational interview framework |
| Enhanced Navigator training for MAs | X | Completed version 1.0 of the training video which highlights the two behavioral treatment services, as well as ability to begin a referral for the patient. Version 2.0 was modified based on feedback from the MAs | |
| Provider training | X | Version 1.0 detailed the behavioral treatment services, NRTs and their possible combinations, and available smart phrase. Version 2.0 was developed based on feedback from providers | |
| Biochemically verified cessation | X | X | Performed a passive drool saliva sample collection in patients who reported nicotine abstinence of five days or more through either the Qualtrics survey or the SMS campaign |
SMS campaign
Patients who have had an index appointment within the eight clinics will receive a text message regarding their smoking status. Once the patient responds, there will be an automated message inquiring about whether they would like to enroll in motivational SMS messages created to support patients as they engage in the EMPACT-Us suite of services. If the patient enrolls in the motivational campaign regarding tobacco use, there will be a motivational message sent every day for fourteen days. If the patient chooses not to engage in the campaign, they will receive the initial automated message inquiring about their smoking status again. All patients will be offered the opportunity to enroll in or op-out for the motivational campaign.
Setting
We selected eight of the largest FHCSD clinics within greater San Diego, CA. The included clinics were selected based on size, special populations represented, and staff size to ensure diversity in the types of patients served. Additionally, 70% of the smokers were represented in these eight clinics.
Participants
Active patients from the 8 selected clinics will be recruited for the study. These clinics represent roughly 70% of the tobacco users at FHCSD. Participant recruitment for this study is still ongoing. We have been actively recruiting eligible patients since 07/1/2022 and will continue to recruit until 02/28/2025 to ensure a comprehensive and representative sample for our research on the effectiveness of tobacco cessation services in low-income communities. The target for the enhanced tobacco suite of services are patients who are currently tobacco smokers. To maximize its efficacy, adult patients in eight of the largest FHCSD clinics who are due for a tobacco screening will be prompted with the updated InterAct screening portion. If the patient reports being a current tobacco smoker, the trained MA can create a referral to tobacco cessation services. Furthermore, the provider can engage the patient in the topic of tobacco cessation by utilizing the suite of tobacco services EMPACT-Us has curated.
Inclusion criteria
Inclusion criteria for patients includes (1) current tobacco smoker, (2) ≥ 18 years or older, (3) speaks English or Spanish, (4) attends adult and/or general medicine encounter in one of the eight participating clinics and (5) have a current FHCSD signed broad consent. FHCSD broad consent includes authorizing their Protected Health Information to be used for research purposes.
Exclusion criteria
Exclusion criteria for patients involved in the study includes those that have not signed or do not have an active FHCSD broad consent. Pregnant or breastfeeding women and patients with a lung cancer diagnosis will be excluded. No exclusions due to nationality, mental health status, substance use, race, sexual orientation, or ethnicity will be made.
Randomization, allocation concealment and blinding
Our approach is centered around an open cohort design that includes identification of all adult tobacco users in the clinics and can include new cases. Each clinic pair (cluster) starts out with EUC and pairs of clinics are then randomized to one of four intervention sequences that determine the period in which they will begin EMPACT-Us. Our single-blind design blinds patients to which intervention group they are assigned. Allocation is concealed from all clinic staff until the study group is assigned. Only the physicians and medical assistants involved in the delivery of the intervention are subsequently made aware of the allocation to the intervention condition and the ‘activation’ of their clinic cluster. Patients will have no knowledge of their provider’s potential use of the EHR enhancements and exposure to the training curricula.
Retention and withdrawal
To promote clinic participation, we will contact the clinical directors to organize a training among the adult general medicine providers and medical assistants to maximize the number of present personnel. Training will be provided to all corresponding personnel, and all present staff will be logged. Educational materials designed to reinforce learnings and promote continued engagement will be sent out four weeks after the completion of each cluster training, (post two-week blackout data collection period).
Measures
We will assemble demographic information, smoking characteristics, physical and mental health related questions, substance abuse and most proximal Patient Health Questionnaire-9 through EHR. We will identify tobacco users at each period and extract tobacco workflow events from each encounter.
For our primary outcome, engagement in evidence-based cessation treatment (yes/no) is define d as at least one dose/session of evidence-based pharmacotherapy or behavioral counseling (e.g. KIC reports, clinic contacts) for tobacco treatment monitored using EHR. Medical charts will be reviewed, to confirm attendance on tobacco treatment visits. FHCSD EHR-recorded intentions to attempt cessation will trigger an SMS-based protocol to assess tobacco use status and treatment use on a weekly basis for 1-month. The N-MA will be notified of all referrals or stated attempts to quit.
Our secondary outcome is quit attempts. Given the potential difficulty of defining a quit attempt uniformly, we selected a definition that included both a stated intention to achieve long-term cessation and an ability to refrain from smoking as evidenced by a self-reported 24-h period of no smoking (e.g. C-TUQ: How long has it been since you last smoked a cigarette (even one or two puffs)? [17]). Self-reported attempts to stop smoking and duration of abstinence will be recorded in the EHR (i.e., first and last day of a quit attempt) at each FHCSD visit (typically 2-times during each 6- month assessment period).
Our third outcome is prolonged abstinence, with a subsample providing biochemical verification with cotinine for this outcome of interest [18]. As patients participate in the Qualtrics surveys and SMS campaigns, we will contact those that self-report to being abstinent from tobacco use to schedule a saliva sample collection. We will conduct a cooperative and purposeful sample as opposed to a random sample to ensure a complete pool.
Data collection and generation methods
Cotinine test collection
We will evaluate the success of a small subset of patients engaged with the EMPACT-Us study protocol by biochemically verifying self-reported abstinence from tobacco use. Cotinine test kits, produced by Salimetrics, will be utilized to detect the absence of cotinine (< 10 ng/ml) in patients that have consumed some form of tobacco for up to four days prior to the testing [18]. Patients reporting abstinence of five days or more either through the Qualtrics survey or the SMS campaign (described below), will be invited to provide a saliva sample. These patients must already have a signed FHCSD broad consent prior to being contacted. Study personnel will contact the patient and explain the purpose of the cotinine test, as well as the benefits in participating in this part of the study. If the patient wishes to provide a sample, both parties will organize to meet. After obtaining written consent, patients will provide a one-time passive drool saliva sample into a Salimetrics cryovial and be compensated for their contribution and time.
The study personnel and patients will agree on a set meeting location and date for the saliva sample collection. Confirmation of the details will be sent via text message to the patient through a Health Insurance Portability Accountability Act (HIPPA) compliant workspace account phone number. When both parties meet, we will obtain a signed consent form. The patient will be assigned a study identification number upon enrollment to identify their sample. This process will continue until 50 patients have provided a saliva sample.
Patient surveys
Each patient that engages with tobacco treatment will be contacted to complete a survey via a unique Qualtrics link, designed to elicit feedback on the available tobacco treatment options and medical experience. Should a patient answer “no” to the initial inquiry of whether they have used tobacco products in the past six months, they will be disqualified from completing the survey. With every completed survey, we contact the patient to disperse an incentive. This will help address and identify any gaps within the tobacco treatment services provided to the patients.
Staff interviews
MAs and PCPs working with the EUC protocols will be asked if they would like to participate in a 1-on-1 interview with study staff designed to expand on the impacts to clinical workflow. If the participant agrees to take part in the interview, their experience during the EMPACT-Us phases of the study will be solicited. The interview will be scheduled and conducted via video conference. Research staff will obtain verbal or written consent prior to the interview. MA, HE, and PCP in-depth interviews will cover knowledge, attitudes, and experiences with the EMPACT-US training and suite of services (e.g., EHR changes, InterAct questions, templates, referrals to behavioral counseling). PCPs additionally will be queried about their decision process when addressing tobacco use with patients. All participants will be probed for their thoughts on the usefulness and fit of the intervention for their unique patient base and will also be asked about sustainability of the intervention.
Data privacy, management and quality assurance
Throughout our research collaboration, data generated will be owned by Family Health Centers of San Diego (FHCSD) but shared with UCSD Co-Principal Investigator under an existing data use agreement. This agreement permits FHCSD to share protected health information (PHI) and limited data sets (LDS) with designated UCSD colleagues, subject to IRB notification and additional data use agreements as needed.
EHR data will contain identifiable information such as medical record numbers, dates of birth, and contact details. Saliva samples will only be identified by a study ID number and collection date, with a key linking them to deidentified data from medical records and surveys. Participant confidentiality will be strictly maintained through secure storage and encrypted electronic systems, following HIPAA guidelines and FHCSD policies. All paper and electronic records will use subject study IDs to uphold confidentiality. Secure web-based tools will facilitate data management, with both FHCSD and UCSD having access. Trained personnel will conduct regular data integrity checks, and manual fidelity checks will ensure completeness and accuracy.
All information will be gathered and supervised using secure, password-protected, web-based tools equipped with programming features for data integrity checks. These checks include predefined ranges for acceptable values, notifications for unexpected values, and a summary of skipped measures. Both entities involved in the ongoing research (FHCSD, UCSD) have access to these tools. Trained research personnel will systematically scrutinize data provided by clinicians and patients to detect any issues that can be promptly addressed after the intended data collection. Data generated by trained research staff through chart review and administrative extraction will undergo manual fidelity checks conducted by an additional member of the research team, ensuring thorough evaluation for completeness and accuracy. The current study, which is neither exceptionally large nor long-term, has no scheduled interim analyses for efficacy or futility. Consequently, there is no plan to appoint a Data Safety and Monitoring Board.
This research, deemed minimal risk, ensures that potential harms are no greater than those typically encountered in medical care. Any reported harms will be addressed according to FHCSD's policies. Clinicians and patients will use digital tools with data fidelity checks, and trained team members will review and address issues promptly. Confidentiality is paramount, with only trained team members having access to patient data, treated with strict confidentiality under applicable laws and HIPAA regulations. All research staff will undergo CITI and FHCSD training for data security compliance. The study aligns with FHCSD's Data Security Plans.
Implementing comprehensive training sessions for healthcare providers and medical assistants is a crucial step in enhancing adherence to interventions within our tobacco cessation study. By equipping these professionals with updated knowledge on the latest tobacco cessation suite of services, we empower them to deliver more effective and personalized interventions to study participants. These training sessions not only focus on the technical aspects of the interventions but also emphasize the importance of empathetic communication and patient engagement.
Furthermore, ensuring that research staff strictly adhere to the study Standard Operating Procedures (SOP) is equally essential. This commitment to fidelity ensures consistency in the implementation of interventions, minimizing variations that could affect the study outcomes. By fostering a well-informed and standardized approach through training and SOP adherence, we create a foundation for success in our study, ultimately improving the overall efficacy and impact of the tobacco cessation interventions.
Quality assurance measures include comprehensive training for healthcare providers and adherence to Standard Operating Procedures (SOPs) to maintain consistency in intervention implementation. This approach aims to enhance the effectiveness and impact of tobacco treatments by empowering staff with updated knowledge and ensuring standardized procedures are followed.
In the context of our study, the consideration of provisions for ancillary and post-trial care, as well as compensation for potential harm resulting from trial participation, is not applicable due to the minimal risk associated with the research. Our comprehensive risk assessment has determined that the study poses minimal potential harm to participants, and as such, the need for specific provisions related to ancillary care and compensation is deemed unnecessary. Nonetheless, the welfare and safety of participants remain paramount, and appropriate measures are in place to ensure their well-being throughout the study duration.
Analysis
Statistical analysis
Primary analyses will describe tobacco users in EMPACT-Us and EUC on cessation milestones including a) intention to quit, b) acceptance of referral; c) engagement in treatment within 1-month; d) quit attempt; and e) a 24-h quit period. The generalized linear mixed effects model (GLMM) for limited categorical outcomes will include time-varying dummy codes for treatment arm, time (secular trend) and smoker characteristics as planned covariates when estimating differences in primary cessation milestones (24-h quit) and engagement outcomes. We will explore differential acceptance of referrals to specific cessation treatment and medications received. All models will include planned covariates for gender, ethnicity, education, and level of smoking. Our GLMM will include an examination of changes in the magnitude of between-group effects over time as longer-term outcomes accrue (6- and 12-month abstinence).
Qualitative analysis
Analysis of semi-structured interviews will take into account our conceptual framework (CCM), a priori concepts ( e.g. questions in the interview guide, including questions related to intervention outcomes [19]), and emergent themes (i.e.. issues raised by the respondents). A trained team will conduct guided analysis using a methodology of “Coding, Consensus, Co-occurrence, and Comparison” [20] that is rooted in grounded theory [21].
Clinics with a high concentration of patients who are sexual and gender minorities (Hillcrest FHC), middle eastern population (El Cajon-FHC), and with higher level of substance use and mental health concerns (Downtown-FHC) are part of the total clinics selected for randomization of cluster-pairs. Within primary outcome models for engagement, quit attempts and cessation, we will examine if treatment effects of EMPACT-Us compared to EUC or treatment effects over time differ according to baseline characteristics using interaction terms. Significant interactions will be followed by stratified models to estimate adjusted Odds Ratios relative to EUC to describe differences in effects within sub-groups.
Sample size
We conducted power/ sample size for this stepped-wedge design trial to address primary aims, using developed formulas [22] that account for unequal cluster size and package swCRT design [23]. The design includes eight large clinics (paired in clusters of two), at least one 6-month period in the EUC and four additional periods where assigned clinics can transition to EMPACT-Us at 6-month intervals. A 2-week transition time of no data collection will be planned while training new clusters. With 8 clinics that range in size, E-HR identified between 900 – 2495 current smokers who on average visit 10–15 times during a 32-month recruitment period. We estimated the sample needed to test a 10% difference in proportions of 24-h quit and engagement outcomes and a 5% increase in reported 6- and 12-month abstinence. Estimates from our previous pilot studies of the TE intervention demonstrated improvements (47% vs 45%) among veterans [24] and (37% vs 15%) psychiatric inpatients [25] and a large 20% increase in engagement in combined tobacco treatment. TE led to increased 7-day point prevalence abstinence (3-month assessment 30% vs 18%) among veterans [24]. Within FHCSD we expect EMPACT-Us will result in a 10% improvement over an observed base rate of 38% of engagement [26] and a 5% increase in rates of recorded cessation at 6- and 12-months after intervention. From the previous clustered logistic regression analysis of 20,119 FHCSD tobacco users we estimated standard deviation of random intercepts of 0.21 and intra-class correlation of 0.01 for engagement outcomes. With alpha = 0.05, four clusters (2 clinics per cluster = 8 clinics with n = 13,496) and five periods of transitions, we will maintain power > 0.84 to detect differences (EMPACT-Us vs EUC) of > 5% in rates of abstinence given a between clinic standard deviation of 0.05 in random treatment effects.
Patient loss to follow-up is not uncommon in the primary care setting. Mitigation efforts include appointment reminders and follow up by Navigator Trainers. Telemedicine visits will be offered to patients as an alternative to in-person visits. A 30% loss to follow-up has been factored into our sample size estimation. For covariates or confounders which are missing at 10% prevalence or less we will use multiple imputation or related EM likelihood-based procedures [27]. For subjects who are missing follow-up assessments of primary outcomes, we will use inverse probability weighting approaches [26].
Dissemination plans
To communicate trial results effectively, our team plans to engage multiple channels, including presentations at conferences and health fairs, where we can share findings directly with healthcare professionals and the community. Additionally, we will utilize "Adelante," the FHCSD newsletter, to keep patients informed about our study results and relevant health information. Participants will have the option to opt in to receive this newsletter, ensuring they remain engaged with our research. This multifaceted approach will facilitate transparency and foster collaboration without any publication restrictions that could limit the dissemination of our findings.
Discussion
This research aims to offer crucial, high-quality evidence regarding the efficacy of a technology-enhanced, team-based care model for effectively managing tobacco treatment, while also offering insights into the viability, acceptance, and long-term durability of the approach. We expect an increase in knowledge on the most efficient method for supporting engagement in successful tobacco treatment, as well as facilitating available resources for those who wish to quit. Given that the enhanced suite of tobacco services will bring more awareness to what available resources and combination of treatments are available, it has a more comprehensive approach as opposed to the usual standard care. We expect that the knowledge and confidence of participating clinicians will increase over time with continuous training. This approach, which is clinician focused, is nonetheless likely to result in more rapid provision of high-quality care for patients. This research will demonstrate that integrating well-designed tobacco treatment tools within an electronic health record (EHR) system, combined with trainings that show clinicians the effectiveness of combining medication with behavioral counseling for tobacco cessation, leads to significantly improved patient outcomes and increased rates of successful tobacco cessation.
Trial status
This study was approved by the Office of IRB Administration (OIA) at the University of California, San Diego (Protocol Number 804666). This is protocol version 1.0 dated April 14, 2024. Recruitment began on February 26, 2023. This study has also been registered with ClinicalTrials.gov, with the ClinicalTrials.gov Identifier: NCT05750537, and was first posted on March 1, 2023. The estimated study completion date is July 1, 2025.
Supplementary Information
Acknowledgements
Above all, the authors would like to convey their sincere appreciation to the participants for generously sharing their time, trust, and valuable insights. The authors would like to express their sincere gratitude to the staff members at Family Health Centers of San Diego, Kick It California, and the University of California, San Diego Moore's Cancer Center for their support and collaboration in establishing this study. A special thank you is reserved to Carrie Kirby and Dr. Shu-Hong Zhu at Kick It California. We would also like to thank the Community Advisory Board for their time and contributions to this study.
Abbreviations
- EMPACT-Us
Enhanced Multicomponent Proactive Navigator-Assisted Cessation of Tobacco Use in Low-Income Patients
- FHCSD
Family Health Centers of San Diego
- FQHC
Federally Qualified Health Center
- E-HR
Electronic Health Record
- KIC
Kick It California
- CCM
Chronic Care Model
- SMS
Short Message Service
- MAs
Medical Assistants
- eReferrals
Electronic Referrals
- PCP
Primary Care Provider
- EUC
Enhanced Usual Care
- TAU
Treatment as Usual
- CAB
Community Advisory Board
- TRDRP
Tobacco Related Disease Research Program
- CPPR
Community-Partnered Participatory Research
- N-MA
Navigator Medical Assistant
- HE
Health Educator
- MI
Motivational Interview
- NRT
Nicotine Replacement Therapy
- SCC
Smoking Cessation Class
- HIPPA
Health Insurance Portability and Accountability Act
- PHI
Patient Health Information
- LDS
Limited Data Set
- IRB
Institutional Review Board
- SOP
Standard Operating Procedure
- GLMM
Generalized Linear Mixed Model
Appendix
Biological specimens
To collect saliva samples for cotinine testing, subjects will be provided with sterile saliva collection kits, ensuring that samples are obtained at consistent times to account for diurnal variation in cotinine levels. Participants will be instructed to avoid eating or drinking for at least 20 min prior to collection. The collected saliva will be immediately stored in pre-labeled, airtight cryovials. These containers will then be transported on ice to the laboratory for storage. To ensure sample integrity, all specimens will be stored at -20 °C or colder until analysis. Saliva samples will be collected following standardized protocols to ensure sample integrity and reliability. The collected samples will be stored at the UCSD Moore’s Cancer Center's biorepository until a total of 50 specimens have been accumulated. Once this threshold is reached, all samples will be shipped collectively to Salimetrics for testing. This approach of batch processing is intended to minimize variability in the results and ensure consistency in the analysis.
Authors’ contributions
DS and KGS are the principal investigators. DS and JL led the proposal and protocol development. GFR, NB, PM, AN, BS, ND, KB, MM,CS, JG, JL, KGS, and DS contributed to the protocol development and study implementation. GFR, NB, PM, KB, MM, JG, KGS, and DS wrote the manuscript. All authors have read and approved the final protocol.
Funding
This study was funded by the Tobacco-Related Disease Research Program (Community Partnered Participatory Research Award T32CF4745).
Data availability
The datasets analyzed during the current study, including the statistical code and the full protocol, will be made available by the corresponding author upon reasonable request. However, due to the inclusion of Protected Health Information (PHI), access to these datasets will require a formal data-sharing agreement in compliance with applicable privacy regulations, including the Health Insurance Portability and Accountability Act (HIPAA). All data will be de-identified to the extent possible, and any access provided will be subject to institutional review and approval to ensure patient confidentiality is maintained.
Declarations
Ethics approval and consent to participate
All patients will have completed FHCSD’s Broad Consent and authorized their Protected Health Information to be used for research purposes. This study has been approved by the Office of IRB Administration at University of California, San Diego (Protocol Number: 804666).
Consent for publication
Not applicable—no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. Informed consent materials are attached as supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang TW. Tobacco Product Use Among Adults — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67. [cited 2024 Jun 18]. Available from: https://www.cdc.gov/mmwr/volumes/67/wr/mm6744a2.htm. [DOI] [PMC free article] [PubMed]
- 2.U.S. Department of Health and Human Services., National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Office on Smoking and Health, 2020. Smoking Cessation: A Report of the Surgeon General. 2020:700. [PubMed]
- 3.California Health Interview Survey (CHIS). [cited 2024 Jun 18]. Available from: https://healthpolicy.ucla.edu/our-work/california-health-interview-survey-chis.
- 4.About CHIS | UCLA Center for Health Policy Research [Internet]. [cited 2024 Jun 18]. Available from: https://healthpolicy.ucla.edu/our-work/california-health-interview-survey-chis/about-chis.
- 5.Flocke SA, Vanderpool R, Birkby G, Gullett H, Seaman EL, Land S, et al. Addressing tobacco cessation at federally qualified health centers: current practices & resources. J Health Care Poor Underserved. 2019;30(3):1024–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC - 2017 BRFSS Survey Data and Documentation [Internet]. 2022 [cited 2024 Jun 18]. Available from: https://www.cdc.gov/brfss/annual_data/annual_2017.html.
- 7.California Tobacco Facts and Figures 2022.
- 8.Gonzales K, Berger AM, Fiandt K. Federally qualified health center use of the Nebraska Tobacco Quitline. Tob Prev Cessat. 2019;19(5):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levinson AH, Valverde P, Garrett K, Kimminau M, Burns EK, Albright K, et al. Community-based navigators for tobacco cessation treatment: a proof-of-concept pilot study among low-income smokers. BMC Public Health. 2015;15(1):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez ME, Schlechter CR, Del Fiol G, Gibson B, Kawamoto K, Siaperas T, et al. QuitSMART Utah: an implementation study protocol for a cluster-randomized, multi-level sequential multiple assignment randomized trial to increase reach and impact of tobacco cessation treatment in community health centers. Implement Sci IS. 2020;15(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flocke SA, Seeholzer E, Lewis SA, Gill IJ, Rose JC, Albert E, et al. 12-Month Evaluation of an EHR-Supported staff role change for provision of tobacco cessation care in 8 primary care safety-net clinics. J Gen Intern Med. 2020;35(11):3234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flocke SA, Albert EL, Lewis SA, Love TE, Rose JC, Kaelber DC, et al. A cluster randomized trial evaluating a teachable moment communication process for tobacco cessation support. BMC Fam Pract. 2021;22(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore M, Adsit R, Zehner M, McCarthy D, Lundsten S, Hartlaub P, et al. An electronic health record–based interoperable eReferral system to enhance smoking quitline treatment in primary care. J Am Med Inform Assoc JAMIA. 2019;26(8–9):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood-Medland EA, Stewart SL, Nguyen H, Avdalovic M, MacDonald S, Zhu SH, et al. Health system implementation of a tobacco quitline eReferral. Appl Clin Inform. 2019;10(4):735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlini BH, Schauer G, Zbikowski S, Thompson J. Using the chronic care model to address tobacco in health care delivery organizations: a pilot experience in Washington state. Health Promot Pract. 2010;11(5):685–93. [DOI] [PubMed] [Google Scholar]
- 17.Land SR, Warren GW, Crafts J, Hatsukami D, Ostroff JS, Willis G, et al. Cognitive testing of tobacco use items for administration to cancer patients and survivors in clinical research. Cancer. 2016;122(11):1728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2020;22(7):1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A systematic approach for using qualitative methods in primary prevention research - Willms - 1990 - Medical Anthropology Quarterly - Wiley Online Library [Internet]. [cited 2024 Jun 19]. Available from: https://anthrosource.onlinelibrary.wiley.com/doi/10.1525/maq.1990.4.4.02a00020.
- 21.Glaser B, Strauss A. Discovery of grounded theory: strategies for qualitative research. New York: Routledge; 2017. p. 282. [Google Scholar]
- 22.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182–91. [DOI] [PubMed] [Google Scholar]
- 23.Hughes J, Hakhu NR, Voldal E, Xia and F. swCRTdesign: Stepped Wedge Cluster Randomized Trial (SW CRT) Design. 2023 [cited 2024 Jun 19]. Available from: https://cran.r-project.org/web/packages/swCRTdesign/index.html.
- 24.Myers MG, Strong DR, Chen TC, Linke SE. Enhancing engagement in evidence-based tobacco cessation treatment for smokers with mental illness: a pilot randomized trial. J Subst Abuse Treat. 2020;111:29–36. [DOI] [PubMed] [Google Scholar]
- 25.Strong D, Schonbrun Y, Uebelacker L. Pilot randomized trial of a brief intervention to facilitate engagement of smoking cessation treatment among hospitalized smokers with depression. 2012; Society for the Research of Nicotine and Tobacco.
- 26.Liu J, Brighton E, Tam A, Godino J, Brouwer KC, Smoot CB, et al. Understanding health disparities affecting utilization of tobacco treatment in low-income patients in an urban health center in Southern California. Prev Med Rep. 2021;1(24):101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Who do Americans trust most for information about COVID-19? [Internet]. Yale Program on Climate Change Communication. [cited 2024 Jun 19]. Available from: https://climatecommunication.yale.edu/publications/who-do-americans-trust-most-for-information-about-covid-19/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study, including the statistical code and the full protocol, will be made available by the corresponding author upon reasonable request. However, due to the inclusion of Protected Health Information (PHI), access to these datasets will require a formal data-sharing agreement in compliance with applicable privacy regulations, including the Health Insurance Portability and Accountability Act (HIPAA). All data will be de-identified to the extent possible, and any access provided will be subject to institutional review and approval to ensure patient confidentiality is maintained.