Abstract
We examined the reactivity of a panel of anti-GM1 immunoglobulin M monoclonal antibodies (MAbs) cloned from multifocal motor neuropathy patients with lipopolysaccharides (LPSs) of Campylobacter jejuni strains, including serotype O:41 strains associated with Guillain-Barré syndrome. The MAbs reacted with ganglioside GM1 to different degrees, and these differences in fine specificities for GM1 were reflected in the different degrees of reactivity with each of the C. jejuni LPSs tested. Antibodies could also be discriminated by the varying patterns of inhibition by cholera toxin (a GM1 ligand) in LPS binding studies. These results indicate that there is a substantial heterogeneity among C. jejuni O:41 strains in their expression of GM1-like epitopes and among the fine specificities of different neuropathy-associated anti-GM1 antibodies.
Guillain-Barré syndrome (GBS), an acute polyneuropathy, is now the most common cause of generalized paralysis and in two-thirds of cases is preceded by a respiratory or gastrointestinal infection (8, 12). Campylobacter jejuni, a leading cause of acute gastroenteritis, has been identified as the most frequent infectious agent associated with the development of GBS (up to 66% of patients) (8, 17). C. jejuni can be serotyped based on differences in the polysaccharide structure (O side chain and core oligosaccharide [OS]) of the lipopolysaccharide (LPS) (O antigen) of the bacterium (16). Reports have shown that the C. jejuni isolates obtained from diarrheic patients prior to the onset of GBS belonged to serotype O:19, an uncommon serotype in gastroenteritis patients (6, 9, 23). Other C. jejuni serotypes commonly identified in association with GBS include O:2, O:2/44, O:4/59, O:15, O:18, O:21, O:24, O:30, O:37, and O:53 (6, 10, 12, 17).
Autoreactive antibodies to gangliosides, especially GM1, are found in 20% of GBS patient sera, particularly after C. jejuni infection (8, 12, 19, 25), and are also found in the sera of 50% of patients with the chronic neuropathy termed multifocal motor neuropathy (14). Neuropathy-associated GM1 antisera have been shown to cross-react with C. jejuni LPSs (19, 22, 23). It is thus currently hypothesized that antiganglioside antibodies may be induced as a result of molecular mimicry between peripheral nerve gangliosides and structurally similar C. jejuni LPS (19, 23). Although there are indications that anti-GM1 antibodies may lead to the activation of inflammatory pathways and act by disrupting membrane ion channel function at nodes of Ranvier (20), experimental proof of involvement in disrupting nerve function has been difficult to conclusively demonstrate. However, since anti-GM1 antibodies in human sera are likely to be a contributory factor in the induction of GBS, an important step in elucidating the pathogenesis of the disease is determining the structure of the immunogenic epitopes in ganglioside-mimicking C. jejuni LPS.
Chemical studies of the LPS extracted from C. jejuni O:19 have shown that the terminal regions of the LPS core mimic human gangliosides GM1, GD1a, GT1a, and GD3 (2, 9, 24). GM2-like OS structures occur in LPS from O:1, O:23, and O:36 (4), whereas the core OSs of C. jejuni O:4 and O:41 mimic gangliosides GD1a and GM1, respectively (4, 15). Mimicry of C. jejuni O:2 LPS is limited to a disaccharide present in a range of gangliosides (3).
The authors of several studies have previously investigated the reactivities of human and animal anti-GM1 antisera with C. jejuni LPS and demonstrated the principle of cross-reactivity. However, no information is available on the extent to which antibodies with different fine specificities of epitope recognition for GM1 are capable of binding GM1-like LPSs. In this study, we aimed to use a set of human monoclonal antibodies (MAbs) that are reactive with GM1 and have been characterized as structurally distinct (13), in conjunction with a panel of well-defined LPSs, to determine the degree to which ganglioside GM1 and C. jejuni LPSs share immunoreactive epitopes.
C. jejuni serostrains O:2 (ATCC 43430), O:3 (ATCC 43431), O:4 (ATCC 43432), O:19 (ATCC 43446), and O:41 (ATCC 43460) were obtained from the American Type Culture Collection (Manassas, Va.). The details concerning three GBS patients and one enteritis patient from whom C. jejuni O:41 strains (16971.94GSH, 28134.94GSH, 260.94RXH, and 176.83, respectively) were isolated have been described previously (7, 15). Isolates and serostrains were routinely cultured on blood agar under microaerobic conditions at 37°C for 48 h, bacterial biomass was harvested, and the bulk extraction of LPS was performed by the phenol-water extraction procedure (11). In addition, LPSs from two GBS isolates, C. jejuni OH4382 and OH4384, which exhibit mimicry of gangliosides GD3 and GT1a, respectively (2), were a generous gift from G. O. Aspinall (York University, Toronto, Ontario, Canada). The immunoglobulin M (IgM) anti-GM1 MAbs termed BO1-1, BO3-1, SM1-8, and WO1-4 were cloned from peripheral blood lymphocytes of three multifocal motor neuropathy patients, all of whom had abnormally elevated anti-GM1 antibody titers, and have been described previously (13, 21). The MAbs were purified by the ultrafiltration of culture supernatants and checked for monoclonality by isoelectric focusing (21).
Gangliosides (Sigma Chemical Co., St. Louis, Mo.) and LPSs were analyzed by thin-layer chromatography (TLC) on precoated silica gel 60 glass plates (Merck, Darmstadt, Germany) by using solvent systems of chloroform–methanol–0.22% CaCl2 · 2H2O (50:45:10 [vol/vol/vol]) (18) and n-propanol–water–25% NH4OH (60:30:10 [vol/vol/vol] (19) as developers for gangliosides and LPSs, respectively. Chemical staining was performed with a resorcinol-HCl reagent (19), and immunostaining was performed by using the procedure of Saito et al. (18), as modified by Schwerer et al. (19), with MAbs diluted to a concentration of 10 μg/ml as the primary antibody and peroxidase-conjugated anti-human IgM (Dako, Cambridge, United Kingdom) diluted 1:1,000 as the secondary antibody. Binding experiments with cholera toxin-peroxidase conjugate (CT-HRP; Sigma) and peanut agglutinin (PNA)-HRP conjugate (Kem-En-Tec, Copenhagen, Denmark) were carried out with only one TLC overlay step by using CT-HRP or PNA-HRP at dilutions of 1:1,000 and 1:50, respectively. Inhibition experiments were performed by using the B subunit of CT (Sigma) at 1 μg/ml to overlay separated gangliosides on TLC plates and incubating the plates at room temperature for 1 h before the addition of MAbs. Preliminary PNA blocking experiments, in which unlabeled PNA was used to block PNA-HRP, failed to reproducibly demonstrate a blocking effect, and hence, PNA blocking experiments were not conducted further.
Two experiments were performed to test the reaction of the MAbs with the core OS of C. jejuni O:41 LPS. First, lipid A was liberated from C. jejuni 176.83 LPS by acid hydrolysis (11), the resultant free lipid A (1 to 4 μg) was dotted onto TLC plates and overlaid with the anti-GM1 MAbs (BO1-1, BO3-1, and SM1-8), and immunoreactants were detected as described for immunostaining. Second, the saccharide mixture from the acid hydrolysis of LPS was fractionated by gel permeation chromatography on Bio-Gel P6 (Bio-Rad Laboratories, Hercules, Calif.) and TSK-40 columns (5, 15), yielding core OS in the second peak which was subsequently freeze-dried. The MAbs (10 μg/ml) were immunoadsorbed with 200 μg of core OS (37°C for 3 h), immunoprecipitates were removed by centrifugation (10,000 × g for 10 min), and the supernatants were reacted with intact C. jejuni 176.83 LPS.
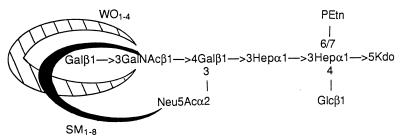
The binding patterns of the four MAbs to ganglioside GM1 and C. jejuni LPSs are summarized in Table 1. The specificities of the MAbs were confirmed by their binding to GM1 and are consistent with previous results (13, 21). The MAbs reacted to different degrees with each of the serotype O:41 LPSs tested. BO1-1 and BO3-1 both recognized the LPS of the enteritis strain (176.83), whereas BO1-1 and BO3-1 separately bound LPSs of two different GBS-associated strains (28134.94GSH and 260.94RXH, respectively). In addition, both MAbs bound serostrain O:2 LPS but did not react with serostrain O:41 LPS or with any of the other C. jejuni LPSs. SM1-8, which reacted with ganglioside GM1 only, recognized all C. jejuni O:41 LPSs with different intensities, including those from the three GBS-associated strains (Fig. 1), but did not bind to any of the other C. jejuni LPSs. WO1-4 recognized three of the four serotype O:41 LPSs (excluding that from the enteritis strain) and the serostrain O:41 LPS but did not react with the other C. jejuni LPSs. Previously, we undertook investigations of the structures of LPSs from the C. jejuni O:41 strains and, in particular, established chemically that the core OS structure of C. jejuni 16971.94GSH shared a tetrasaccharide with ganglioside GM1 (15). The putative regions of binding of MAbs SM1-8 and WO1-4 in C. jejuni 16971.94GSH LPS are shown in Fig. 2. MAbs BO1-1 and BO3-1 are not indicated in Fig. 2, since no reaction was observed with this particular C. jejuni O:41 LPS. However, the binding patterns of BO1-1 and BO3-1 are more comparable to that of MAb WO1-4 than that of MAb SM1-8. Therefore, ganglioside GM1 and C. jejuni O:41 LPSs share an immunoreactive epitope, but because the patterns of MAb reactivity varied with individual C. jejuni O:41 LPSs (including those MAbs which were specific for GM1 only), there must be a degree of structural variability present within this epitope(s). Alternatively, there may be differences in the surface topography or density of the GM1-like molecule(s) in the C. jejuni O:41 LPSs. Furthermore, the influence of an O side chain in C. jejuni O:41 LPS on MAb binding can be excluded, since electrophoretic and immunoblotting analyses have shown that C. jejuni O:41 strains produce low-Mr LPS without an O chain (15) and chemical analyses indicate the presence of only high-Mr extracellular polysaccharides independent of LPS (5, 15).
TABLE 1.
Binding and inhibition studies with anti-GM1 antibodies and C. jejuni LPS or gangliosidesa
| Antigen | CT binding | PNA binding | Level of staining for:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BO1-1
|
BO3-1
|
SM1-8
|
WO1-4
|
|||||||
| Control | CT block | Control | CT block | Control | CT block | Control | CT block | |||
| Ganglioside GM1 | ++++ | ++ | ++ | − | +++ | − | +++ | − | ++++ | − |
| C. jejuni LPS | ||||||||||
| 16971.94GSH | ++++ | ++ | − | ND | − | ND | +++ | − | + | − |
| 28134.94GSH | +++ | +++ | +++ | − | − | ND | +++ | − | +++ | +++ |
| 260.94RXH | ++++ | ++ | − | ND | ++ | − | +++ | − | +++ | − |
| 176.83 | ++++ | ++ | ++ | − | ++ | − | ++ | − | − | ND |
| Serostrain O:41 | +++ | − | − | ND | − | ND | ++ | ND | ++ | ND |
| Serostrain O:2 | ++ | +++ | + | ND | ++ | ++ | − | ND | − | ND |
| Serostrain O:3 | − | − | − | ND | − | ND | − | ND | − | ND |
| Serostrain O:4 | +++ | − | − | ND | − | ND | − | ND | − | ND |
| Serostrain O:19 | ++++ | + | − | ND | − | ND | − | ND | − | ND |
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; −, no reaction. ND, not determined.
FIG. 1.
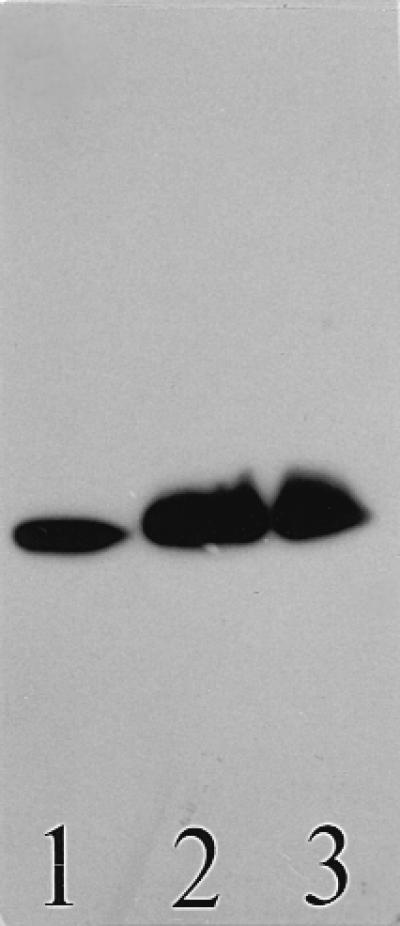
Binding of human IgM MAb SM1-8 to purified C. jejuni O:41 LPSs. Lane 1, C. jejuni 16971.94GSH LPS; lane 2, C. jejuni 260.94RXH LPS; and lane 3, C. jejuni 28134.94GSH LPS. The immunostained chromatogram was overlaid first with MAb and subsequently with anti-human IgM. A sample of 1 μg was applied per lane.
FIG. 2.
Regions of gangliosides asialo-GM1 (crosshatched) and GM1 (solid) containing the putative binding sites of MAbs WO1-4 and SM1-8 (13, 21) shown on the proposed structure of C. jejuni 16971.94GSH LPS (15). The MAb WO1-4 binds the terminal dissacharide Galβ1-3GalNAc, whereas MAb SM1-8 binds the same disaccharide in the presence of laterally attached N-acetylneuraminic acid (Neu5Ac). MAbs BO1-1 and BO3-1 are not indicated, since no reaction was observed with this LPS. PEtn, phosphoethanolamine; Kdo, 3-deoxy-d-manno-octulosonic acid; Hep, l-glycero-d-manno-heptose; Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine.
Interestingly, none of the anti-GM1 MAbs reacted with serostrain O:4 or O:19 LPSs. The core OS of serostrain O:4 LPS contains a pentasaccharide in common with ganglioside GD1a (4). None of the MAbs react with ganglioside GD1a (13, 21), and so the lack of a reaction with serostrain O:4 LPS is expected. Although GM1 mimicry, along with mimicry of ganglioside GD1a, is present in the core OS of serostrain O:19 LPS (2), the lack of MAb reactivity may be explained by the presence of an O side chain in this LPS (2, 10), which affects epitope accessibility. As expected, no reaction occurred when the MAbs were reacted with LPSs of the C. jejuni O:19 GBS-associated strains (OH4382 and OH4384) which have been shown to mimic GD3 and GT1a, respectively (2). Furthermore, none of the MAbs reacted with free lipid A, and MAb preparations immunoadsorbed with the isolated core OS of C. jejuni 176.83 did not bind with the intact LPS of this strain. This provides conclusive evidence that it is the core OS region to which the antibodies bind and not lipid A.
CT, a specific ligand for ganglioside GM1, and PNA, a ligand for the disaccharide moiety Galβ1-3GalNAc, were tested for their abilities to react with ganglioside GM1 and C. jejuni LPSs (Table 1). In addition to binding with gangliosides GM1 (Table 1) and asialo-GM1 (data not shown), which is consistent with previous results (13, 21), CT-HRP showed a strong reactivity with serostrain O:4 and O:19 LPSs and all C. jejuni O:41 LPSs, including that of the serostrain. This is consistent with the presence of a GM1-like structure in these LPSs. A weaker reaction was observed with serostrain O:2 LPS, whose core OS lacks GalNAc and contains a sialylated Galβ1-3Gal disaccharide (3). No reaction was detected with the LPS of serostrain O:3, a strain whose LPS is not sialylated, does not mimic gangliosides, and is not associated with the development of GBS (1, 11). PNA-HRP bound to GM1 and asialo-GM1 gangliosides and reacted avidly with O:2 LPS, suggesting that PNA can bind to Galβ1-3Gal in addition to Galβ1-3GalNAc. PNA-HRP did not react with serostrain O:41 LPS but reacted with the other C. jejuni serotype O:41 LPSs (Table 1). The latter suggests the presence of a Galβ1-3GalNAc or Galβ1-3Gal disaccharide in these serotype O:41 LPSs. PNA-HRP bound weakly to serostrain O:19 LPS but did not react with the LPSs of the two GBS-associated C. jejuni O:19 strains or with serostrain O:4 LPS (Table 1), reflecting the occurrence of terminal sialylation in these latter LPSs. No reaction was observed with the control C. jejuni O:3 LPS.
The recognition of ganglioside GM1 by all of the MAbs was completely inhibited by the CT B subunit (Table 1). Thus, CT binds to ganglioside GM1 and thereby excludes the binding of the MAbs, inferring that CT and the MAbs must recognize closely related structures in ganglioside GM1. Furthermore, the binding of all the MAbs to the majority of C. jejuni O:41 LPSs was blocked by CT, again confirming that the MAbs and CT recognize a similar epitope in the C. jejuni O:41 LPSs. However, CT did not inhibit MAb WO1-4 from binding to C. jejuni 28134.94GSH LPS. This demonstrates that CT and MAb WO1-4 do not recognize the same epitope in this LPS, a further indication that differences occur in the C. jejuni O:41 LPSs. Likewise, CT did not inhibit the binding of BO3-1 to serostrain O:2 LPS.
This study demonstrates the cross-reactivity of human monoclonal IgM anti-GM1 antibodies with C. jejuni LPSs associated with GBS. The MAbs are known to have different specificities for ganglioside GM1 (13, 21); only some of them cross-react to varying degrees with the structurally related glycolipids asialo-GM1 and GD1b (21). Thus, each MAb recognizes a slightly different epitope within GM1, some of which may also occur in asialo-GM1 and GD1b. Our results are in accordance with those of others who demonstrated that IgM anti-GM1 MAbs from patients with chronic motor neuropathy reacted with LPSs of C. jejuni O:4, O:19, and O:50 serostrains (22). The MAbs also differed in their relative reactivities with the C. jejuni LPSs tested, in particular with regard to serotype O:41 LPSs. The results not only show the existence of a GM1-like epitope(s) in C. jejuni O:41 LPSs but also reveal the existence of differences within the serotype O:41 LPSs. The pattern of binding of the IgM anti-GM1 MAbs to C. jejuni O:41 LPSs indicates that either slight differences in sugar substitution occur in the core OS of the various C. jejuni O:41 LPSs or variation occurs in how the LPSs present these structures to the antibodies, possibly as a function of antigen density, and hence affect antibody recognition.
Furthermore, as in other studies, CT recognized serostrain O:2, O:4, and O:19 LPSs (19, 22, 23) and CT bound avidly to all C. jejuni O:41 LPSs, including that of the serostrain and the enteritis isolate (C. jejuni 176.83). Yuki et al. observed the binding of CT to serostrain O:19 LPS and deduced that it had a GM1-like structure (23), which has been confirmed by structural analyses (2). However, LPSs of two C. jejuni O:19 strains from GBS patients (OH4382 and OH4384) which mimic GD3 and GT1a, respectively (2), did not react with CT or any other ligands or antibodies used in this study. The binding of IgM MAbs to ganglioside GM1 and C. jejuni LPSs was inhibited by the CT B subunit; thus, CT and the MAbs recognize the same or a structurally overlapping epitope in ganglioside GM1 and GBS-associated C. jejuni LPSs. This concurs with the results in a previous report where the binding of GBS sera to O:19 LPS was blocked with CT (22). However, in this study, CT did not inhibit the binding of one of the MAbs to one serotype O:41 LPS, a further indication that slight differences in structure occur within the core OS of C. jejuni O:41 LPSs. Since our inhibition experiments using PNA were considered technically unreliable, we cannot confirm the evidence of others (22) that PNA does not inhibit the binding of anti-GM1 antibodies to either ganglioside GM1 or C. jejuni LPSs.
In conclusion, the evident mimicry between C. jejuni LPSs and gangliosides may act as a trigger to stimulate the production of antiganglioside antibodies which may play a role in the pathogenesis of GBS. The mimicry of gangliosides is not limited to those strains associated with GBS, as LPS from the C. jejuni O:41 enteritis isolate reacted in a way similar to that seen with LPSs from the GBS-associated strains. This phenomenon has previously been observed by us with C. jejuni O:19 LPS, whereby an enteritis isolate mimics both gangliosides GM1 and GD1a (9), suggesting that, in addition to mimicry, other host or bacterial factors are involved in disease pathogenesis and require further investigation.
Acknowledgments
This study was supported by grants from the Irish Health Research Board (to A.P.M.), the Guillain-Barré Syndrome Support Group of Great Britain (to H.J.W.), and the European Union Biomed Programme (to H.J.W.). H.J.W. is a Wellcome Trust Senior Research Leave Fellow.
We gratefully acknowledge Albert J. Lastovica at the University of Cape Town, Cape Town, South Africa, for providing the C. jejuni O:41 isolates used in this study.
REFERENCES
- 1.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 2.Aspinall G O, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19. Structures of the core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structure of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanniffy, O. M., A. S. Shashkov, A. P. Moran, M. M. Prendergast, S. N. Senchenkova, Y. A. Knirel, and A. V. Savage. Chemical structure of a polysaccharide from Campylobacter jejuni 176.83 (serotype O:41) containing only furanose sugars. Submitted for publication. [DOI] [PubMed]
- 6.Kuroki S, Saida T, Nukina M, Haruta T, Yoshiota M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain β-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 7.Lastovica A J, Goddard E A, Argent A C. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O:41 strains. J Infect Dis. 1997;176(Suppl. 2):S139–S143. doi: 10.1086/513796. [DOI] [PubMed] [Google Scholar]
- 8.Mishu B, Blaser M J. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clin Infect Dis. 1993;17:104–108. doi: 10.1093/clinids/17.1.104. [DOI] [PubMed] [Google Scholar]
- 9.Moran A P, O’Malley D T. Potential role of lipopolysaccharides of Campylobacter jejuni in the development of Guillain-Barré syndrome. J Endotoxin Res. 1995;2:233–235. [Google Scholar]
- 10.Moran A P, Prendergast M M, Appelmelk B J. Molecular mimicry of host structures by bacterial lipopolysaccharide and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 11.Moran A P, Rietschel E T, Kosunen T U, Zahringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-d-glucose. J Bacteriol. 1991;173:618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nachamkin I, Allos B M, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson G, Wilson G, Kennedy P G E, Willison H J. Analysis of anti-GM1 antibodies cloned from motor neuropathy patients demonstrates diverse V region gene usage with extensive somatic mutation. J Immunol. 1995;155:3049–3059. [PubMed] [Google Scholar]
- 14.Pestronk A, Cornblath D R, Ilyas A A, Baba H, Quarles R H, Griffin J W, Alderson K, Adams R N. A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Ann Neurol. 1988;24:73–78. doi: 10.1002/ana.410240113. [DOI] [PubMed] [Google Scholar]
- 15.Prendergast M M, Lastovica A J, Moran A P. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect Immun. 1998;66:3649–3655. doi: 10.1128/iai.66.8.3649-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston M A, Penner J L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987;55:1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees J H, Soudain S E, Gregson N A, Hughes R A C. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Kasai N, Yu R K. In situ immunological determination of basic carbohydrate structures of gangliosides on thin-layer plates. Anal Biochem. 1985;148:54–58. doi: 10.1016/0003-2697(85)90627-x. [DOI] [PubMed] [Google Scholar]
- 19.Schwerer B, Neisser A, Polt R J, Bernheimer H, Moran A P. Antibody crossreactivities between gangliosides and lipopolysaccharides of Campylobacter jejuni serotypes associated with Guillain-Barré syndrome. J Endotoxin Res. 1995;2:395–403. [Google Scholar]
- 20.Takigawa T, Yasuda H, Kikkawa R, Shigeta Y, Saida T, Kitasata H. Antibodies against GM1 affect K+ and Na+ currents in isolated rat myelinated nerve fibres. Ann Neurol. 1995;37:436–442. doi: 10.1002/ana.410370405. [DOI] [PubMed] [Google Scholar]
- 21.Willison H J, Patterson G, Kennedy P G E, Veitch J. Cloning of human anti-GM1 antibodies from motor neuropathy patients. Ann Neurol. 1994;35:471–478. doi: 10.1002/ana.410350416. [DOI] [PubMed] [Google Scholar]
- 22.Wirguin I, Suturkova-Milosevic L, Della-Latta P, Fisher T, Brown R H, Jr, Latov N. Monoclonal IgM antibodies to GM1 and asialo-GM1 in chronic neuropathies crossreact with Campylobacter jejuni lipopolysaccharides. Ann Neurol. 1994;35:698–703. doi: 10.1002/ana.410350610. [DOI] [PubMed] [Google Scholar]
- 23.Yuki N, Handa S, Taki T, Kasama T, Takihashi M, Saito K, Miyatake T. Cross-reactive antigen between nervous tissue and a bacterium elicits Guillain-Barré syndrome: molecular mimicry between ganglioside GM1 and lipopolysaccharide from Penner’s serotype 19 of Campylobacter jejuni. Biomed Res. 1992;13:451–453. [Google Scholar]
- 24.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi T M, Saito M K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuki N, Yoshino H, Sato S, Miyatake T. Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology. 1990;40:1900–1902. doi: 10.1212/wnl.40.12.1900. [DOI] [PubMed] [Google Scholar]