Abstract
Recently, interest has surged in the environmental and biomedical applications of two-dimensional transition metal borides, commonly referred to as MBenes. These materials have emerged as promising candidates for energy storage devices, such as batteries and supercapacitors. Additionally, MBenes have shown remarkable catalytic activity due to their high surface area and tunable electronic properties. They exhibit significant promise in various catalytic applications, particularly in nitrogen reduction reactions (NRRs), electrocatalytic conversion of nitrogen oxides, and several electrochemical reactions such as the oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and hydrogen evolution reaction (HER). Notably, MBenes have shown great potential in water treatment and pollutant removal applications, such as desalination and water purification. Their high water permeability, ion selectivity, and excellent stability make them suitable for efficient water treatment processes. On the other hand, MBenes are emerging as versatile materials with significant potential in various biomedical applications, particularly in biosensing, cancer therapy, and the treatment of neurodegenerative diseases. However, several challenges hinder their practical implementation in biomedical and environmental fields. One significant issue is the scalability of synthesis methods; producing MBenes in large quantities while maintaining high purity and uniformity is often complex and costly. Moreover, the stability of MBenes and their composites under different environmental and biological conditions raises concerns, as they may undergo degradation or lose their functional properties over time, which could limit their long-term effectiveness. Additionally, there is a need for comprehensive toxicity assessments to ensure the safety of MBenes in biomedical applications, particularly when interacting with human tissues or biological systems. This review aims to systematically investigate the environmental and biomedical applications of MBenes and their composites, emphasizing their unique characteristics and potential roles in addressing pressing global challenges. Furthermore, the review will identify and discuss the existing challenges and limitations in the operational performance of MBenes and their composites, providing a critical assessment of their current state in various applications.
This review aims to systematically investigate the environmental and biomedical applications of MBenes and their composites.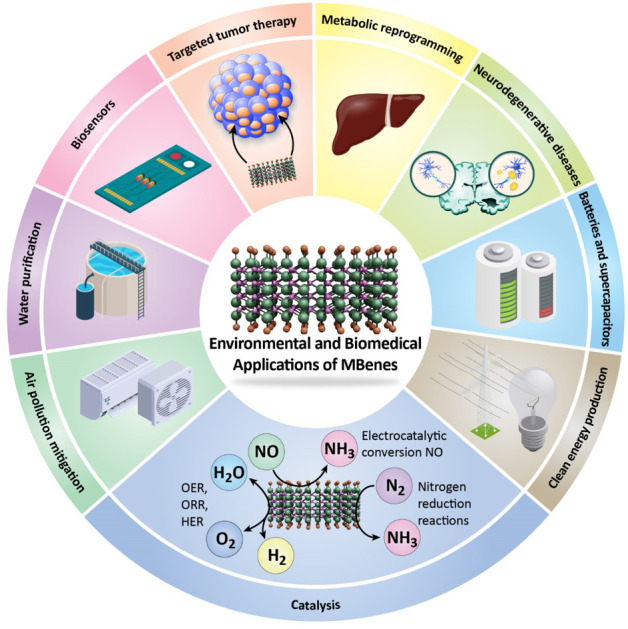
1. Introduction
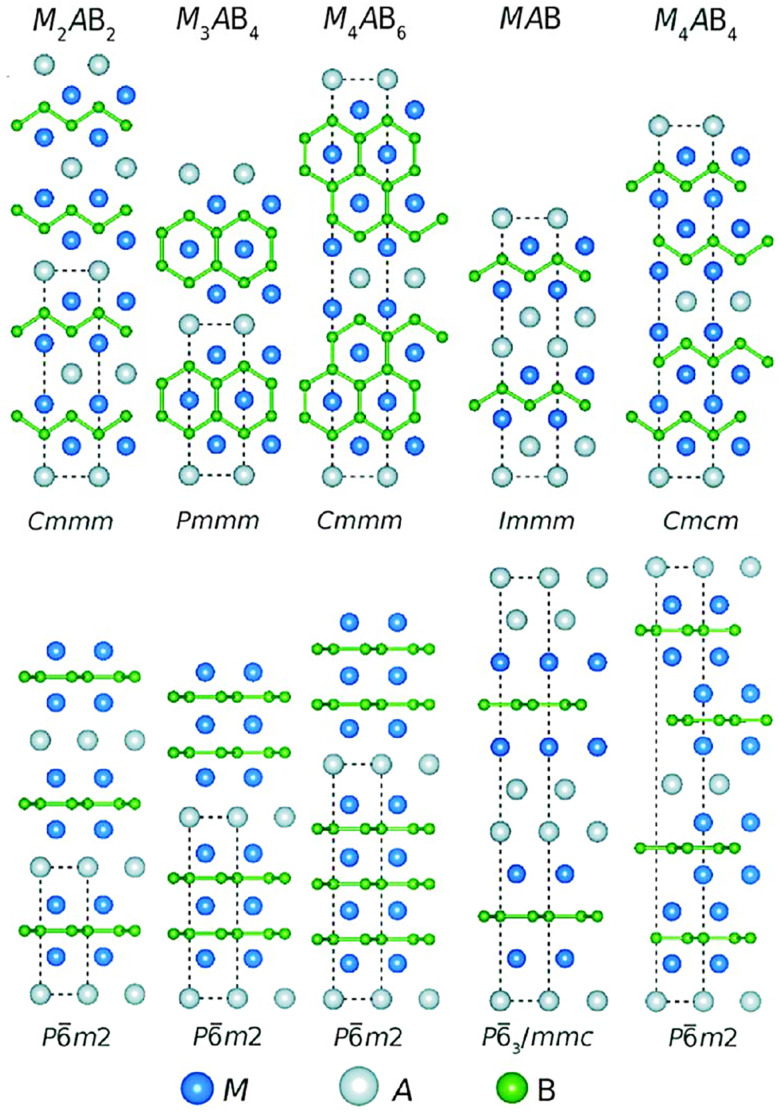
Two-dimensional (2D) transition metal borides, MBenes, have gained attention in environmental and biomedical applications due to their unique properties and versatile nature. These materials exhibit exceptional chemical stability, high thermal conductivity, and excellent mechanical strength, making them ideal candidates for various environmental challenges.1 MXenes and MBenes, both categorized as 2D materials, exhibit unique properties and structures that set them apart.2,3 Understanding these differences is crucial for researchers and engineers looking to harness their potential applications. Firstly, the composition of these materials varies significantly. MXenes are primarily derived from transition metal carbides, nitrides, or carbonitrides. The general formula for MXenes is expressed as Mn+1XnTx, (n = 1–4), where M represents transition metals (like titanium or zirconium), n indicates the number of transition metal layers, X refers to non-metallic elements (carbon and/or nitrogen), and T denotes surface terminations such as –O, –OH, and –F.4–7 In the realm of advanced materials, the emergence of MBenes marks a significant milestone. Inspired by the groundbreaking success of MXenes, researchers sought to expand the horizons of this innovative class. In 2017, an exciting discovery took place—the introduction of a new kind of 2D transition metal boride, aptly named MBenes. This catchy nomenclature highlights their unique 2D morphology, distinguishing them from their predecessors.8 Through meticulous first principles calculations, four distinct MBenes were identified: Cr2B2, Fe2B2, Mo2B2, and W2B2. These were derived from their corresponding precursors—Cr2AlB2, Fe2AlB2, MoAlB, and WAlB—using selective etching techniques.9 Remarkably, this theoretical foundation paved the way for practical applications. Subsequently, Zhang et al.10 and Alameda et al.11 successfully synthesized the 2D Cr2B2 and Mo2B2, respectively. These pioneering investigations opened the floodgates to an exciting new field of study. As a result, a plethora of orthorhombic and hexagonal MBenes were discovered, each contributing to the expanding landscape of 2D materials. Today, MBenes have burgeoned into a vast family, boasting over 50 members. Their general formula, MnB2n−2, succinctly encapsulates their structure, with M representing the transition metal, B denoting boron, and n ranging from 2 to 4. This diversity showcases the capabilities of MBenes in different applications, from catalysis and electronics to energy storage.9
MBene and MXene have structural similarities, as both feature 2D layered nanosheet structures with an accordion-like appearance. They also face the common limitation of self-stacking observed in most 2D materials. Furthermore, both materials have surfaces rich in functional groups, which promote efficient interaction with electrolyte ions. However, despite these similarities, MBene and MXene differ significantly in several respects. MXenes are composed of transition metal layers interleaved with carbon or nitrogen layers, forming a layered hexagonal structure. In contrast, MBenes consist of transition metal layers interleaved with boron layers and can adopt both orthorhombic and hexagonal structures (Scheme 1). MBenes exhibit superior properties, including a higher Young's modulus and greater anisotropy compared to MXenes and other 2D materials like MoS2. Additionally, MXenes are prone to oxidation and require storage in vacuum environments, while MBenes demonstrate exceptional anti-oxidation properties, making them more stable for catalytic and energy storage applications. These advantages position MBenes as promising materials with the potential to surpass MXenes in various fields.12
Scheme 1. Schematic image of different types of MAB phases. Reprinted from ref. 13 under the terms of the Creative Commons CC BY license. Copyright 2023, Wiley.
The metal–B bonds in MBene have lower bond energies than the metal–C and metal–N bonds in MXene, simplifying the etching of Al atom layers to create its layered structure. Additionally, MBene's high surface activation energy enhances the adaptability and modifiability of its functional groups, further facilitating electrolyte interaction, promoting electrochemical reactions, and boosting performance. MBene exhibits superior electrochemical energy storage performance compared to MXene that is related to the several key differences between the two materials. First, the bond energy of the metal–B bond in MBene is lower than that of the metal–C and metal–N bonds in MXene. This makes it easier to remove the aluminum (Al) atom layer during the etching process, forming a 2D layered, accordion-like MBene structure. Additionally, MBene has higher surface activation energy, making its surface functional groups more adaptable and easier to modify. These abundant surface functional groups facilitate optimal contact with electrolyte ions, enhancing electrochemical reactions and improving overall performance. Moreover, MBene possesses lower resistance, leading to better conductivity and enabling the rapid transport of electrons.13–15
MBenes, specifically orthorhombic and hexagonal transition metal borides, derive from MAB phases 9 and can be synthesized using a variety of innovative methods, each contributing to their unique properties and structures.16 Key techniques used for the production of these compounds include selective topochemical deintercalation, which allows for controlled ion removal; chemical vapor deposition (CVD), known for producing high-quality films; and magnetron sputtering, which provides precise control over film composition and thickness. Additionally, chemical etching is employed to create MBenes with specific structural characteristics, while microwave-assisted and ultrasound-assisted chemical etching enhance efficiency and uniformity in the synthesis process. These diverse methods collectively offer distinct advantages, paving the way for optimizing different properties of MBenes and expanding their applications across various fields.16
Structurally, MBenes display alternate stacking of transition metal and boron layers, creating unique configurations that differentiate them from other materials. To categorize MBenes based on their crystal symmetries, researchers have established two primary groups: orthorhombic MBenes (orth-MBenes) and hexagonal MBenes (hex-MBenes). The origins of these classifications trace back to their precursors, with orth-MBenes stemming from orthorhombic MAB (orth-MAB) and hex-MBenes emerging from hexagonal MAB (hex-MAB). Some researchers have previously referred to them as “boride MXenes” 17,18 or “boridene”.19 However, these terms fail to capture the fundamental distinctions between MBenes and MXenes. The phrase “boride MXenes” overlooks the structural and chemical formula differences, while “boridene” encompasses all 2D borides, indiscriminately. Thus, the term “MBenes” emerges as the most appropriate designation. This name not only elucidates the compositions of these materials but also emphasizes their relationship to MXenes—highlighting both their similarities and distinct characteristics.9,17,18,20
Beyond the widely recognized MnB2n−2-type MBenes, a fascinating array of predicted compounds exists. Notably, there are fourteen predicted 2D transition metal borides of the form 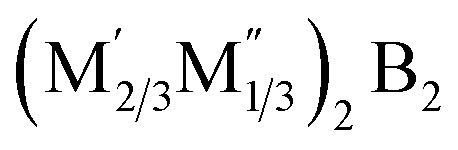 . In this formulation, M′ encompasses elements such as Ti, Cr, Mn, Fe, Mo, and W, while M′′ includes Sc, Y, Zr, Nb, and Hf. Additionally, there is one synthesized variant, M4/3B2, where M equates to Mo. These fifteen compounds derive from their corresponding quaternary hex-MAB phases, specifically
. In this formulation, M′ encompasses elements such as Ti, Cr, Mn, Fe, Mo, and W, while M′′ includes Sc, Y, Zr, Nb, and Hf. Additionally, there is one synthesized variant, M4/3B2, where M equates to Mo. These fifteen compounds derive from their corresponding quaternary hex-MAB phases, specifically 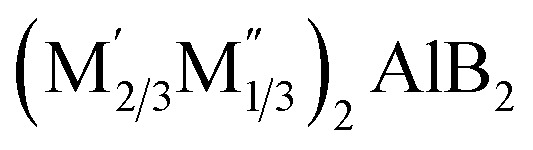 . Their structures exhibit similarities to hex-MBenes, featuring in-plane ordered double metal elements or vacancies. Among the fourteen predicted
. Their structures exhibit similarities to hex-MBenes, featuring in-plane ordered double metal elements or vacancies. Among the fourteen predicted 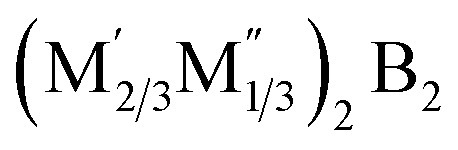 compounds, one stands out, the (Fe2/3Sc1/3)2B2, which belongs to the P6mmm space group. Given these structural characteristics, they should also be classified within the MBenes category, specifically as in-plane ordered hex-MBenes.21,22 However, it's essential to clarify that MBenes do not encompass all 2D transition metal borides. This situation mirrors the distinction between MXenes and all 2D transition metal carbides. For instance, compounds like 2D TiC and MoC2, although 2D transition metal carbides, do not fall under the MXene classification.23,24 To delineate MBenes from other 2D transition metal borides, several key factors must be considered. Firstly, compounds like 2D Ti2B, Mo2B, and Hf2B must be classified as novel boron-containing MXenes. They exhibit identical structures and chemical formulas to conventional M2X MXenes. Secondly, 2D LiB and NiB lack the layered structures typically characterized by alternating stacks of transition metal and boron layers. Furthermore, compounds like 2D MgB2, FeB2, TiB2, and tetragonal Mn2B2 do not have corresponding MAB precursors, further disqualifying them from the MBenes family. Moreover, other 2D transition metal borides, including M5B2, M10B4, and MB6, possess distinctly different structures and compositions, reinforcing their exclusion from the MBenes classification.9
compounds, one stands out, the (Fe2/3Sc1/3)2B2, which belongs to the P6mmm space group. Given these structural characteristics, they should also be classified within the MBenes category, specifically as in-plane ordered hex-MBenes.21,22 However, it's essential to clarify that MBenes do not encompass all 2D transition metal borides. This situation mirrors the distinction between MXenes and all 2D transition metal carbides. For instance, compounds like 2D TiC and MoC2, although 2D transition metal carbides, do not fall under the MXene classification.23,24 To delineate MBenes from other 2D transition metal borides, several key factors must be considered. Firstly, compounds like 2D Ti2B, Mo2B, and Hf2B must be classified as novel boron-containing MXenes. They exhibit identical structures and chemical formulas to conventional M2X MXenes. Secondly, 2D LiB and NiB lack the layered structures typically characterized by alternating stacks of transition metal and boron layers. Furthermore, compounds like 2D MgB2, FeB2, TiB2, and tetragonal Mn2B2 do not have corresponding MAB precursors, further disqualifying them from the MBenes family. Moreover, other 2D transition metal borides, including M5B2, M10B4, and MB6, possess distinctly different structures and compositions, reinforcing their exclusion from the MBenes classification.9
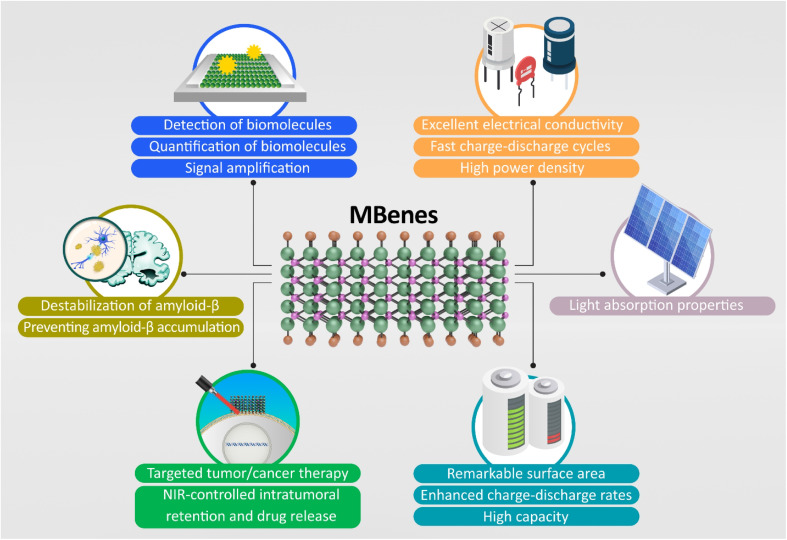
The advent of MBenes has opened new avenues in both environmental and biomedical fields (Fig. 1). These 2D transition metal borides exhibit remarkable properties that make them suitable for various applications. Their unique structure, high surface area, and tunable chemical composition enhance their functionality.25 MBene membranes represent a promising solution for addressing the challenges of contaminated wastewater treatment. Their robustness, high rejection efficiency, and flexible applications position them as a vital tool in the quest for cleaner water and sustainable environmental practices.26 MBene family materials emerge as high-efficient and low-cost co-catalysts for photocatalytic hydrogen production. Their distinct properties, especially their unique optical, electronic, and thermal properties, and exceptional performance, open new possibilities for enhancing solar energy technologies. Indeed, MBene materials could absorb light, strongly, across a broad spectrum, including visible and near-infrared light that allows them to harness solar energy more efficiently compared to conventional materials, making them highly suitable for applications such as solar steam generation and photocatalysis. The excellent photothermal properties of MBenes enable efficient conversion of solar energy into heat that makes them ideal for solar-driven interfacial steam generation systems and improving energy efficiency. MBenes possess favorable electronic band structures that enable efficient charge separation, a critical process for photocatalytic hydrogen production. Moreover, these innovations pave the way for cleaner and more sustainable energy solutions, significantly contributing to the global shift toward renewable energy sources.27,28 Moreover, MBenes are capturing heightened interest because of their remarkable electrical conductivity, solid structural and mechanical attributes, and impressive chemical stability. These qualities position them as highly advantageous candidates for use as electrode materials in electrochemical storage technologies. Their layered structure provides abundant active sites and facilitates rapid ion transport, which is essential for high-rate performance. MBene-based electrodes can achieve improved energy density and cycling stability by forming hybrid structures or composites with other materials. These properties make MBenes highly effective in applications like lithium-ion batteries, sodium-ion batteries, and supercapacitors for efficient energy storage.29 In-depth theoretical and experimental investigations highlight the promise of MBenes in lithium (Li)-ion and sodium-ion batteries, as well as in supercapacitors. Supercapacitors, which bridge the gap between conventional capacitors and batteries, have become key players in modern energy storage systems. Their ability to charge and discharge rapidly, due to the electrostatic separation of charges, is significantly influenced by the selection of electrode materials. Transition metal borides, commonly referred to as MBenes, have gained significant attention in this context. These materials offer tunable properties based on their composition, enabling performance enhancements that help overcome the energy density limitations typically associated with supercapacitors. With the growing need for high-performance energy storage, MBenes are positioned as transformative materials in supercapacitor technology. For example, the discovery of 2D HfBO has underscored its potential as a superior electrode material for supercapacitors, highlighting its suitability for energy storage applications. Additionally, 2D MBenes have demonstrated efficiency as co-catalysts in photocatalytic hydrogen production under visible light, emphasizing their versatility in light-responsive supercapacitor systems. Their high surface area, chemical reactivity, and rapid charge carrier mobility further solidify their promise in supercapacitor applications. Moreover, the MBene family has been explored for use as anode materials in lithium-ion batteries, reinforcing their relevance and versatility in advanced energy storage technologies.15,30–32 Yet, despite the increasing focus and multiple studies, a considerable gap persists in existing literature. This gap pertains to a thorough and systematic evaluation of MBenes' roles in electrochemical energy storage systems. Addressing this gap is crucial for fully understanding their capabilities, optimizing performance, and facilitating the practical implementation of MBenes in sustainable energy solutions.29
Fig. 1. Environmental and biomedical applications of MBenes, with their unique properties.
Their ability to form stable photothermal systems with excellent solar-to-thermal conversion efficiency makes them highly effective in solar-driven applications like interfacial steam generation and photothermal therapy. Compared to graphene, MBenes show greater versatility due to their customizable band structure and superior thermal stability under operational conditions. Their tunable bandgap makes them more flexible for tailored absorption across wavelengths. Besides, it exhibits higher robustness in harsh environmental conditions, enhancing long-term performance.15,33 In biomedicine, near-infrared (NIR)-photothermal MBenes showed the capability of converting irradiated light into heat through non-radiative relaxation due to their highly conductive structure and surface plasmon resonance effect, which are ideal for photothermal applications. Indeed, MBene's tunable structure allows modulation of the light absorption properties, optimizing it for the NIR region (typically between 700 and 1300 nm) for deep tissue penetration. They could also represent photo-controlled drug released pattern that introduced it as a groundbreaking nanoplatform for high-efficacy tumor-targeted drug delivery and controlled drug release in cancer therapy.34 Their unique structural properties allow for the conjugation of specific ligands, enhancing selective drug delivery directly to tumor sites, while minimizing systemic exposure to healthy tissues. Upon exposure to NIR light, MBenes generate localized heat that can trigger the controlled release of encapsulated drugs and directly ablate cancer cells through hyperthermia. Additionally, their favorable biocompatibility makes them suitable for in vivo applications. As research continues to optimize their synthesis and functionalization, NIR-photothermal MBenes hold immense potential for transforming cancer treatment strategies by integrating targeted drug delivery with effective thermal therapy.34
The aim of this review is to thoroughly investigate and analyze the multifaceted environmental and biomedical applications of MBenes and their composites, shedding light on their unique properties and potential to address critical challenges in these fields. By examining recent advancements, we aim to highlight the innovative use of MBenes in air pollution mitigation, energy storage systems, biosensing technologies, catalysis for nitrogen fixation, biomedical potentials, among others. Additionally, this review seeks to identify the current challenges faced in the synthesis, stability, and integration of MBenes into practical applications, providing insights that could guide future research directions.
2. MBene: synthesis and properties
MBenes share significant similarities with MXenes, differing mainly in that boron replaces the carbon and/or nitrogen atoms. However, MAB-MBenes cannot be exclusively correlated with MAX-MXenes pairs due to variations in stoichiometry, 2D layer intercalation patterns, and structural transformations. Layered MBenes are derived from their parent MAB phases through chemical etching. These MAB phases exhibit diverse chemical formulas, such as MAB, M2AB2, M3AB4, and M4AB6. The M–A bond in these structures is metallic, while the M–B bond exhibits a hybrid of covalent, metallic, and ionic characteristics, closely resembling the M–X bond in MAX phases. By leveraging the difference in bond strengths between M–A and M–B, 2D MBenes can be produced by selectively etching the Al layers in MAB phases, the same as the process used for isolating MXenes.35 Besides, there are other methods, as well, for the fabrication of MBenes, which are categorized into two main classes, top-down methods and bottom-up methods,29 that some of them are similar to methods used for the synthesize of MXene. For instance, etching methods are among the most common types of top-down methods used for the fabrication of MXenes, which are used for the fabrication of MBenes, as well. Hydrofluoric acid (HF)-etching (used for the synthesis of Mo4/3B2), acid/salt hybrid etching (produced MoB), HCl-etching (used for the production of 2D layered CrB MBene), and NaOH-etching (applied for producing 2D MoB nanosheets) are among the etching methods used for producing MBenes. This method could be used for the production of MBenes in large scale with few-layer or single-layer nanosheets.36
Mechanical exfoliation is also a type of top-down method in which physical methods, like mechanical forces, are used to peel off thin layers of borides from bulk materials. The material's layers are mechanically peeled or cleaved to produce a single- or few-layered structure. By optimizing factors such as pressure, temperature, and the type of adhesive tape used, the exfoliation process can be enhanced, enabling the production of MBenes. This method preserves the inherent properties of bulk precursors, such as high mechanical strength and intrinsic conductivity. It is a safe, simple, and low-cost method that has limitation in producing high-yield of material and controlling layer thickness and uniformity.15
Solid-state synthesis is another method used for the fabrication of MBenes that is based on the direct reaction of transition metals with boron at high temperatures to produce layered borides, which are then exfoliated into MBenes. Its simplicity and scalability make it an attractive choice for large-scale production. Additionally, the solid-state method enables precise control over the stoichiometry and phase purity of the final product, which is crucial for achieving desired material properties. While this method may lack the atomic-level precision of chemical vapor deposition (CVD), it is particularly advantageous for producing MBenes for applications in energy storage, catalysis, and electronic devices, where bulk material properties are prioritized. It is a cost-effective method used for the production of bulk layered borides that has some challenges in providing high temperature, precise control over the conditions of reaction, and achievement to a uniform exfoliation.16,29
CVD, is a bottom-up synthesis method works based on the introduction of a transition metal precursor (e.g., metal chloride or organometallic compounds) and a boron source (e.g., diborane (B2H6) or boron trichloride (BCl3)) into a high-temperature reaction chamber under a controlled atmosphere (inert gases like argon) and high temperatures, facilitating the formation of 2D borides directly on the substrate. This method leads to the production of high-quality defect-free thin films with excellent control over thickness and composition. On the other hand, MBenes synthesized through CVD typically exhibit enhanced tunability and scalability due to the precise regulation of growth conditions and defect engineering enabled by the chemical deposition process; however, it has limitations in scalability and cost of equipment and precursors. This method allows for the tailoring of electronic properties, including enhanced conductivity and uniformity in thin films. Additionally, CVD facilitates the growth of large-area MBenes, which are essential for applications like flexible electronics and quantum devices. Understanding the complex interplay between deposition techniques and the resulting electrical properties is crucial for optimizing the performance of MBene-based electronic devices in diverse applications, ranging from flexible electronics to quantum computing.37,38
Magnetron sputtering is a type physical vapor deposition (PVD) technique employed for the synthesis of MBenes, in which material from a target is ejected and deposited onto a substrate under a high-energy plasma environment. This method allows precise control over film thickness, composition, and uniformity, making it suitable for producing high-quality thin films of MBenes. One of the key advantages of magnetron sputtering is its scalability and ability to create defect-free films with tailored stoichiometry, essential for applications in electronics and optoelectronics. However, the process is equipment-intensive and requires high vacuum conditions, which can increase operational costs. Additionally, sputtering may not be suitable for producing large quantities of bulk materials due to its focus on thin films. The properties of MBenes synthesized via magnetron sputtering, such as electrical conductivity, mechanical stability, and surface morphology, can be finely tuned by adjusting deposition parameters, such as power, pressure, and gas flow rates. These customizable properties are particularly beneficial for energy storage, catalysis, and sensing applications, where surface and structural characteristics play a critical role.39
The choice of synthesis method directly affects properties like conductivity, defect density, layer uniformity, and scalability. For instance, CVD and magnetron sputtering are ideal for producing high-quality thin films, while chemical etching and mechanical exfoliation are better suited for bulk synthesis and rapid prototyping. By understanding the interplay between synthesis methods and material properties, researchers can optimize MBenes for specific applications, ranging from energy storage to biomedical devices. These insights emphasize the importance of tailoring synthesis techniques to achieve desired properties, paving the way for advanced applications of MBenes in various fields.
3. Environmental applications
3.1. Water purification and pollutant removal
One of the key applications of MBenes lies in the development of advanced water purification systems. The high surface area of these 2D materials allows for efficient adsorption of contaminants, such as heavy metals and organic pollutants, from water sources.40 This leads to the creation of sustainable and cost-effective water treatment solutions. Solar-driven interfacial steam generation has emerged as a revolutionary technology aimed at addressing critical issues such as water scarcity and environmental degradation.41 At the heart of this technology lies the solar-to-heat conversion process, which significantly depends on the efficiency of photothermal materials.26 In this context, Chang et al.26 introduced a novel class of 2D layered photothermal materials based on MBenes. By combining these materials with thermally insulated nylon membranes, they created an effective solar-driven interfacial steam generation evaporator tailored for solar steam generation. The MBene membrane exhibited remarkable characteristics that enhanced its performance in solar steam generation. Its ability to absorb light across a broad spectrum enabled efficient photothermal conversion. When exposed to sunlight, the MBene membrane effectively converted solar energy into heat, facilitating localized heating. This process was crucial for generating steam from water, as it minimized heat loss and maximized the heating efficiency of the system. Rapid water transport was another significant advantage, enabling quick replenishment of water at the evaporation interface, which further increasing the evaporation rate. The performance of the MBene membrane in solar steam generation was impressive, demonstrating a high evaporation rate of 1.59 kg m−2 h−1. Additionally, the system achieved an efficiency of up to 96.66% under one-sun irradiation conditions. These metrics highlighted the potential of MBene materials in advancing solar-driven water purification technologies. In addition to its impressive solar steam generation capabilities, the MBene membrane exhibited exceptional robustness and versatility. It could efficiently treat various contaminated wastewater sources, including organic dyes, antibiotics, oil–water emulsions, and heavy metals. The membrane's high rejection efficiency ensured effective removal of harmful contaminants, making it a viable solution for wastewater treatment. Additionally, the MBene membrane's durability across a range of pH levels—whether acidic or alkaline—enhanced its applicability in diverse environments. This adaptability was essential for practical implementations, as wastewater characteristics could vary significantly. The solar-driven interfacial steam generation system utilizing MBene membranes also showed significant promise for sustainable freshwater production. By harnessing solar energy for the desalination of seawater and brine solutions, this technology can contribute to addressing global water scarcity.26
The effective removal of hazardous pollutants signifies a promising advancement in environmental remediation strategies. Liu et al.42 introduced an innovative approach, merging nanoscale zero-valent iron (nZVI) with MBenes. As a result, by integrating nZVI into the inter-layer structure of MBenes, the removal efficiency of U(vi) and Cr(vi) was significantly improved, compared to utilization of each compounds alone. MBenes not only avoided the oxidation of nZVI but also enhanced its reactivity. This dual functionality was crucial for achieving higher removal rates. In other word, the incorporation of nZVI into MBenes increased the interlayer distance, exposing more active sites for U(vi)/Cr(vi) adsorption. Additionally, MBenes served as an effective carrier, enhancing the dispersibility of nZVI and contributing to improved adsorption performance (via electrostatic interactions). In comparison to nZVI, MBenes showed lower removal efficiency resulting from the electrostatic repulsion between the negatively charged MBenes and the anionic Cr(vi) species. While in the case of U(vi), MBenes showed better removing performance. Additionally, kinetics and isotherm studies demonstrated that the adsorption data aligned well with the Langmuir and Pseudo-second-order kinetic models. The results indicated that the composite had a saturated capacity of 107.8 mg g−1 for U(vi) and 68.6 mg g−1 for Cr(vi). It was revealed that the targeted pollutants rapidly adhered to the composite through electrostatic interactions. Subsequently, the surface-bound nZVI acted as an electron donor, facilitating the reduction of Cr(vi) to Cr(iii) and U(vi) to U(iv).42
3.2. Air pollution mitigation strategies and reduction of environmentally toxic gases
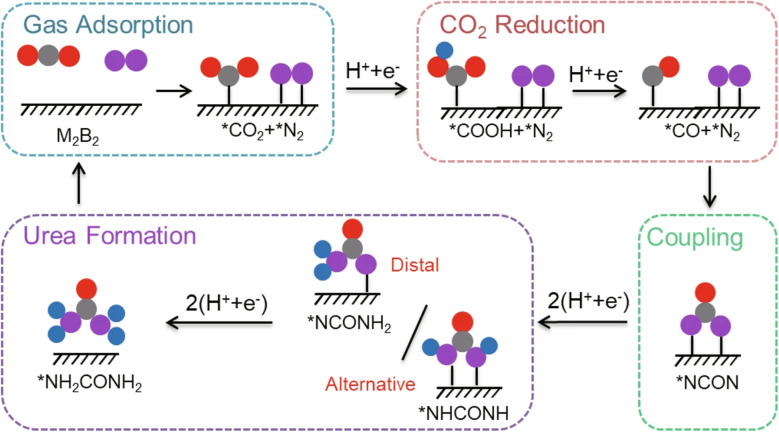
MBenes can be utilized in air pollution mitigation strategies as catalysts to convert harmful gases, such as nitrogen oxides and volatile organic compounds, into less toxic substances. Their high catalytic activity and stability make them valuable compounds in combating air pollution and improving air quality. The alarming increase in CO2 emissions has triggered a series of environmental crises, including global warming, the greenhouse effect, and ocean acidification. Thus, the conversion of CO2 into valuable chemical products, along with effective capture and storage methods, becomes paramount.43 In this context, MXenes and MBenes share remarkable characteristics that lend themselves to effective CO2 adsorption.44 Both MXenes and MBenes exhibit high surface areas and exceptional porosity that led to enhance their interaction with CO2, making them prime candidates for tackling climate change. Furthermore, their chemical functionality can be tailored, offering researchers the flexibility to optimize performance. Through advanced computational simulations, the comparative evaluation sheds light on the adsorption capacities of these materials. Experimental data supports the findings, demonstrating that both MXenes and MBenes outperform traditional carbon capture and storage materials. In addition to their impressive adsorption capabilities, the kinetic performance of MXenes and MBenes also stands out. The rapid uptake and release of CO2 significantly enhance their practicality in real-world applications. Moreover, stability is a key factor in determining the longevity of any carbon capture and storage material. Both MXenes and MBenes showcase remarkable stability, making them viable options for long-term CO2 capture. The discussion extends to the layer-thickness of these materials and their selective affinity toward CO2. Compared to conventional sorbents, MXenes and MBenes present distinct advantages, including easier regeneration and enhanced durability. As the findings illustrate the structure–property relationships, they pave the way for future advancements in carbon capture and storage technology.44 For instance, one study explored the potential of MBenes for CO2 capture and reduction.45 Using rigorous first-principles calculations, this study revealed the exceptional CO2 capture and activation capabilities of various M3B4-type MBenes. The underlying reason for this impressive performance was related to the lone pair of electrons that existed on the surface of MBenes. This unique electronic structure enhanced their interaction with CO2, laying the groundwork for effective reduction processes. An exciting aspect of the findings was the selective reduction of activated CO2 to methane (CH4) by the investigated MBenes. This capability was not just a theoretical possibility but a practical advantage. The ability to convert CO2 into valuable hydrocarbons exhibited a significant advancement in carbon utilization strategies. Moreover, the authors investigated a new linear scaling relationship concerning the adsorption energies of potential-determining intermediates, specifically *OCH2O and *HOCH2O, in relation to ΔG(*OCHO). This relationship provided insight into the CO2 reduction reaction limiting potential on MBenes. The different fitting slopes of ΔG(*OCH2O) and ΔG(*HOCHO) enabled significantly lower limiting potentials than those typically observed with traditional transition metals. Notably, two MBenes, Mo3B4 and Cr3B4, demonstrated exceptionally low limiting potentials of −0.48 V and −0.66 V, respectively. These values signified their potential as effective CO2 reduction reaction catalysts. Additionally, they exhibited competitive selectivity concerning HER, further solidifying their standing in this domain.45
The search for materials with high intrinsic catalytic activity for the carbon monoxide oxidation reaction (COOR) is of paramount importance. Such catalysts play a vital role in mitigating CO contamination, a significant environmental concern. Traditionally, COOR catalysts have depended heavily on noble metals and cerium dioxide (CeO2). However, the need for non-noble alternatives that can outperform CeO2 has driven extensive research in this area. In one study, Mir et al.46 investigated on MBenes (M2B2, where M = Sc, Ti, V, Cr, Mn, and Fe) for CO2 capture and activation. The findings revealed their static and dynamic stability, essential attributes for practical applications. These MBenes exhibited a metallic character along with impressive electrical conductivity, which enhanced their usability in various applications. Notably, the CO2 adsorption energy on these materials varied significantly, ranging from −1.04 to −3.95 eV. The order of effectiveness in CO2 adsorption followed the trend: Sc2B2 > Ti2B2 > V2B2 > Cr2B2 > Mn2B2 > Fe2B2. Interestingly, the spin-polarization calculations indicated a reduction in the adsorption energy for magnetic systems. This suggested that magnetic properties could influence CO2 interaction with the MBenes. Furthermore, Bader charge transfer analysis revealed the generation of a CO2δ− moiety on the surface of the MBenes, referred to as activated CO2. This activated form was crucial for facilitating reactions with other surface chemicals. Moreover, differential charge density plots illustrated remarkable charge accumulation around the CO2 molecule, reinforcing the interaction between the adsorbate and the substrate.46 The theoretical results of this study support the use of new MBenes as cost-effective catalysts for CO2 capture and activation. These findings not only contribute to the ongoing fight against climate change but also open new avenues for research and development in sustainable chemistry.
Novel MBenes were introduced as high-efficient catalysts for CO2 electro-reduction, utilizing the DFT approach.47 The recently synthesized MBenes demonstrate high electrical conductivity and stability, making them promising candidates for the development of catalysts for CO2 electrocatalytic reduction (RR). Despite their potential, achieving a reaction mechanism that facilitates the formation of hydrocarbon species at low overpotentials presents a significant challenge. Their findings revealed that only C1 hydrocarbon products—such as methane (CH4), methanol (CH3OH), formaldehyde (HCHO), carbon monoxide (CO), and formic acid (HCOOH)-were generated, highlighting the high stability, catalytic performance, and selectivity of these MBenes in CO2 reduction while effectively suppressing the HER competing. These MBenes exhibited metallic characteristics that could be adjusted, making them suitable catalysts for CO2RR. This tunability allowed for better control of selectivity and catalytic activity, providing valuable insights into optimizing their performance for CO2 electro-reduction applications.47 Additionally, a systematic investigation was performed on 18 oxygen-functionalized MBenes featuring both orthorhombic and hexagonal crystal structures.48 These materials were denoted as orth-M2B2O2 and hex-M2B2O2, where M represents various transition metals, including Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, and W. Using high-throughput first-principles calculations, their COOR catalytic activities were assessed. It was revealed that hex-Mo2B2O2, orth-Mo2B2O2, hex-V2B2O2, and hex-Cr2B2O2 exhibited higher catalytic performance than CeO2 while maintaining structural stability at temperatures below 1000 K. This characteristic positioned them as promising candidates to replace CeO2 as substrates for COOR catalysts. Additionally, hex-Cr2B2O2, orth-Mo2B2O2, and hex-V2B2O2 exhibited greater COOR catalytic performance compared to conventional catalysts like Pt–CeO2 and Au–CeO2. This elevated their potential for direct application as COOR catalysts in various industrial applications. An interesting aspect of this research was identifying the generation energy of oxygen vacancies as a key descriptor for COOR catalytic performance. This insight significantly simplified the catalyst screening process, minimizing the number of calculations required during the evaluation of potential catalysts.48
Shukla et al.49 focused on the 2D 1T-2H phase of Mo2B and its functionalized derivatives, Mo2BX2 (where X can be H, OH, or O). Utilizing first-principles calculations, they investigated the structural, electronic, and adsorption behaviors of these materials toward toxic gases. Notably, both pristine and functionalized MBenes demonstrated significant dynamic and thermal stability. Furthermore, they maintained a metallic nature across both phases, which was pivotal for their applications in gas sensing. When examining the adsorption behavior, they discovered that pristine MBenes acted as desirable adsorbents for gases such as NO2, SO2, and CO2. In contrast, the functionalized MBenes–NH3 systems revealed moderate adsorption energies, indicating a good sensitivity for detecting NH3 gas. Particularly, the 2H–Mo2BH2 variant exhibited a notable charge transfer of −0.11 e and an appropriate adsorption energy of −0.30 eV, causing a shorter recovery time. This characteristic was critical for effective sensing applications. Density of states calculations further illustrated the electrical conducting behavior of MBenes, underpinning their suitability for NH3 detection with rapid recovery times.49 The findings delivered the first insights into how surface functionalization affects the structural and electronic properties of MBenes. In another study, a thorough investigation was conducted into the adsorption characteristics of monolayer MoB concerning various gas molecules, specifically CO, NO, SO, and SO2.50 The findings revealed intriguing patterns in the adsorption behavior. CO and NO exhibited a tendency to be adsorbed at the top of the molybdenum (Mo) atoms, showcasing a strong interaction with this transition metal. Conversely, SO and SO2 preferentially were adsorbed at the top of boron (B) sites. The adsorption energies for all examined gas molecules were negative, indicating favorable interactions, with SO displaying the smallest adsorption energy among them. All gas molecules acted as charge acceptors upon adsorption. Notably, the SO2/MoB system exhibited the highest charge transfer value, measuring 0.977 eV. This significant charge transfer further highlighted the effectiveness of the monolayer MoB in gas adsorption. According to transition state theory, the recovery time for monolayer MoB after CO adsorption was the shortest, indicating a reversible process. This reversibility suggested that monolayer MoB had promising potential as a CO gas detection sensor. However, at room temperature, monolayer MoB struggles to regain its initial state after adsorbing NO, SO, and SO2. This inability to recover highlighted a crucial aspect: while monolayer MoB demonstrated high sensitivity for NO, SO, and SO2 due to its low adsorption energies, it also indicated the potential for these gases to be detected irreversibly.50
3.3. Energy storage and conversion
MBenes are emerging as versatile materials in energy storage and conversion, demonstrating significant potential in various applications. In Li-ion batteries, they show promise as anode materials due to their remarkable surface area, enhancing charge–discharge rates, and overall capacity.51 Additionally, MBenes are being explored for sodium-ion batteries, where their structure accommodates larger sodium ions, improving performance. Their excellent electrical conductivity makes them ideal candidates for supercapacitors, offering fast charge–discharge cycles and high-power density. Furthermore, MBenes can enhance the efficiency of fuel cells by acting as effective catalysts in the oxygen reduction reaction (ORR). Lastly, their light absorption properties can be harnessed in photovoltaic technologies to improve solar energy conversion. As research progresses, MBenes show great promise in advancing sustainable energy solutions across these diverse fields.16,52
3.3.1. Clean energy production
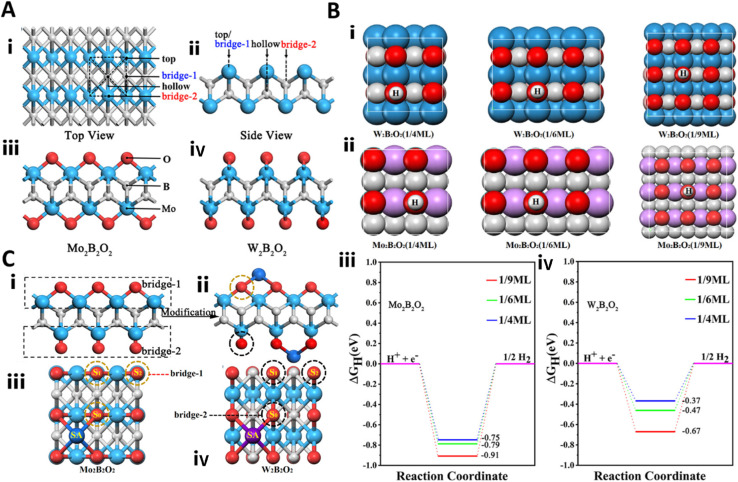
MBenes can be used as catalysts in fuel cells for clean energy production. In this context, 2D photo- and electrocatalysts are pivotal in facilitating hydrogen formation via water splitting. Extensive investigations have focused on identifying a cost-effective and efficient alternative to the noble-metal Pt. A novel approach was introduced, involving the incorporation of various transition-metal atoms to modulate the catalytic features of MBenes and their composites.53 Utilizing density functional theory (DFT) calculations, it was uncovered that the strength of H–O bonding could be attenuated through charge transfer between the oxygen atom and the single-metal atom introduced. This bond weakening significantly enhanced the catalytic activity of the MBene in driving the HER. Notably, the Gibbs free energy (|ΔGH|) of W2B2O2 experiences a reduction from |−0.67| to 0.013 eV upon the inclusion of a V adatom (Fig. 2).53 This innovative strategy holds promise for broadening the applications of MBene in the realms of green catalysis and the energy sector. The findings presented in this study are poised to pave the way for advancements in sustainable catalysis methodologies and energy generation technologies.
Fig. 2. (A): (i) A top-down perspective of the MBene displays four distinct adsorption locations: top, bridge-1, hollow, and bridge-2 sites. (ii) A side-view schematic representation of the MBene is presented. (iii and iv) Diagrams illustrating the structures of Mo2B2O2 and W2B2O2 are shown, respectively. (B): Hydrogen coverage levels of 1/4 ML, 1/6 ML, and 1/9 ML are examined: (i) for W2B2O2 and (ii) for Mo2B2O2. (iii) The computed free energy (ΔGH) for Mo2B2O2 and (iv) for W2B2O2 is presented, where the red, yellow, and blue lines correspond to hydrogen coverages of 1/9, 1/6, and 1/4, respectively. (C): (i and ii) A schematic illustration depicts the O-terminated MBene structure along with the alteration of the MBene surface through a single-metal adatom. (iii and iv) Three hydrogen adsorption locations (S0, S1, and S2) are identified, which correspond to the O atom adsorbed on the bridge-1 and bridge-2 sites, respectively. Reproduced with permission from ref. 53. Copyright 2020 American Chemical Society.
3.3.2. Batteries and supercapacitors
The advent of MBenes has developed the landscape of energy storage solutions, especially in the realm of batteries.54 MBenes are created by selectively etching away the A layer from layered transition metal borides, commonly referred to as MAB phases. This innovative approach yields materials that are not only stable but also exhibit isotropic and ultrahigh Young's modulus.8 In one study, among the standout MBenes, Mo2B2 and Fe2B2 captured attention due to their metallic nature and remarkable electronic conductivity.8 Such properties are highly sought after, especially for applications in Li-ion batteries and electrocatalysis. Furthermore, these materials showcased an omnidirectional small diffusion energy barrier alongside a high storage capacity for Li atoms. This combination underscored the potential of MBenes as exceptional electrode materials for Li-ion batteries, paving the way for advancements in energy storage technology. In addition to their energy storage capabilities, Fe2B2 MBene shined in its catalytic activity for the HER. With hydrogen adsorption Gibbs free energy hovering near the optimal value of 0 eV, this material emerged as a promising candidate for electrocatalysts in hydrogen formation.8 Additionally, Y2B2 MBene have emerged as significant candidates for use as electrode materials in Li-ion batteries and sodium (Na)-ion batteries.55 Extensive studies on the performance of Y2B2 revealed its dynamic and thermal stability through phonon and molecular dynamics simulations. First-principles calculations indicated that the material had excellent electron conductivity during charging, which was crucial for efficient battery operation. Notably, the low diffusion barriers of 0.013 eV for Li and 0.008 eV for Na highlight the rapid ion mobility within the material, with diffusivity values of 0.010 cm2 s−1 for Li and 0.013 cm2 s−1 for Na suggesting extremely fast charge–discharge rates. Furthermore, the impressive capacities of 806.31 mA h g−1 for Li and 403.16 mA h g−1 for Na surpassed those of other MBenes, indicating a strong potential for energy storage applications. The low initial open-circuit voltages of 0.43 V for Li and 0.45 V for Na also allowed for a larger voltage window in battery operations, enhancing overall performance.55
The recent arrival of MBenes has ignited significant curiosity surrounding 2D metal-borides. These materials are expected to demonstrate impressive electrochemical properties, paving the way for innovative applications in energy storage and conversion technologies. The structural stabilities of Ti2B functionalized with B, C, and N were explored, focusing particularly on the stable structure Ti2BN2.56 Consequently, the Ti2BN2's potential as an anode material was evaluated for a variety of ion-batteries, including those using Li, Na, K, Mg, Ca, and Zn, employing first-principles calculations. The dynamic and thermal stability of Ti2BN2 was confirmed through phonon dispersion curves and ab initio molecular dynamics simulations. Moreover, the examination of the band structure and density of states demonstrated the intrinsic metallic characteristics and non-magnetic nature of Ti2BN2. Remarkably lightweight, Ti2BN2 showcased an energetically favorable bilayer for Na adsorption, resulting in an impressive theoretical capacity of 797 mA h g−1 for Na-ion batteries. This capacity provided a substantial edge over many current 2D anode materials. Additionally, with a low diffusion barrier of 0.34 eV and a favorable open circuit voltage of 0.27 V, Ti2BN2 could be considered as a strong contender for SIB anodes. Thus, this study not only uncovered an electrode material with significant capacity for Na-ion batteries but also advanced the research surrounding N-functionalized MBenes, paving the way for future innovations in battery technology.56 In another study, by employing evolutionary search techniques alongside ab initio calculations, the MoBx (where x = 1, 3, and 4) monolayers were identified as dynamically, mechanically, and thermally stable 2D metal-borides.57 Given their metallic characteristics, their potential applications as anode materials were explored for Li-ion batteries. As a result, the MoB and MoB3 monolayers exhibited impressive capabilities, offering high Li-specific capacities of 670 and 418 mA h g−1, respectively, while also maintaining low Li diffusion barriers of 0.10 and 0.13 eV.57 Such properties highlight the promise of MoBx monolayers as efficient anode materials, paving the way for advancements in Li-ion battery technology. Xie et al.58 conducted an investigation on the fascinating world of hexagonal boron ring structures, specifically focusing on metal-covered M4B6 configurations, where M represents metals such as Mg, Zr, Nb, Mo, Hf, Ta, and W. Through meticulous first-principles calculations, they have uncovered intriguing insights regarding these materials. Notably, the stable Mg4B6 monolayer with its unique planar boron rings exhibited exceptional electrical conductivity. This characteristic was crucial for applications in modern technology, particularly in energy storage systems. Consequently, Mg4B6 could be deployed as a high-capacity electrode material with remarkable performance in Li-ion batteries. The Mg4B6 monolayer sustained a steady average open-circuit voltage while exhibiting an extraordinary theoretical capacity of up to 4299 mA h g−1. Moreover, the Mg4B6 monolayer could serve as a highly promising candidate for anode materials, particularly due to its excellent rate capacity.58
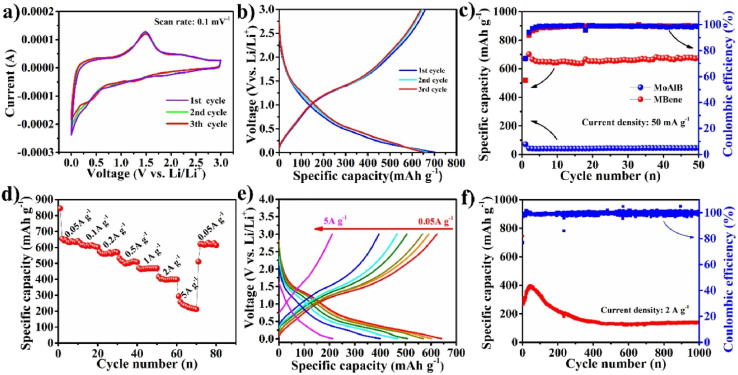
A fluorine-free hydrothermal-assisted alkane solution etching strategy was deployed for synthesizing the MoB MBene from MoAlB. This innovative approach successfully produced MoB MBene, showing remarkable electrochemical performance as an anode material in Li-ion batteries. Specifically, the 2D MoB MBene achieved a reversible specific capacity of 144.2 mA h g−1 after 1000 cycles at a current density of 2 A g−1, surpassing many previously reported MXene anodes (Fig. 3).54 This fluorine-free synthesis technique displayed suitable environmentally friendly advantage for exploring a range of MBene-based materials. In another study, a series of orthogonal MBenes were introduced for Li/Na ion batteries with high performance.59 Derived from first-principles DFT, these materials were obtained by mechanically stripping an MBene from a larger MAB phase. The focus revolved around several notable compounds such as V2B2, Cr2B2, Mn2B2, Ti2B2, Zr2B2, and Nb2B2. Ab initio molecular dynamics simulations and phonon spectra confirmed the thermodynamic and kinetic stability of monolayer MBenes at room temperature. This stability was pivotal for their application in energy technologies. Notably, MBenes exhibited intrinsic metallic properties. Their mechanical characteristics further revealed high Young's modulus and anisotropy. Additionally, low diffusion potential and open-circuit voltage enhanced their appeal, showcasing remarkable rate performance and robust chemical stability during practical applications.59 However, it's essential to navigate the realm of functionalization cautiously. Research indicates that functionalization can significantly impair electrochemical performance. Thus, it should be minimized in experimental setups to harness the full potential of these materials. These compelling findings illuminate the pathway for utilizing monolayer MBenes as promising anode materials for Li/Na ion batteries.
Fig. 3. The electrochemical activity of the MoB MBene anodes in Li-ion batteries is evaluated through several metrics: (a) the cyclic voltammetry (CV) curves are recorded at a scan rate of 0.1 mV s−1. (b) Charge–discharge profiles are measured at a current density of 50 mA g−1. (c) The cycling stability of both MoAlB and MoB MBene is assessed at 50 mA g−1. (d) The rate capability is analyzed. (e) The corresponding voltage curves for the MoB MBene. (f) The long-term cycling performance is investigated at a current density of 2 A g−1. Reproduced with permission from ref. 54. Copyright 2022 Elsevier.
The journey toward large-scale applications of Li–selenium (Li–Se) batteries has encountered significant hurdles. Predominantly, the dissolution of high-order polyselenides within electrolytes and the sluggish kinetics of low-order polyselenides have thwarted progress. To address these challenges, Gao et al.60 systematically explored the electrocatalysis and anchoring properties of MBene as cathode materials. It was revealed that O/F-functionalized Mo2B2 MBenes showcased suitable binding strengths, crucial for maintaining the structural integrity of polyselenides. This characteristic significantly inhibited the deleterious shuttle effect, a common issue in Li–Se batteries. Notably, both Mo2B2O2 and Mo2B2F2 MBenes could retain notable electronic conductivity even after binding with Li2Se, effectively facilitating redox reactions within the battery system. Moreover, the excellent electrocatalytic activity of these materials could be obtained, characterized by ultra-low kinetic energy barriers. This included both the diffusion and decomposition processes of Li2Se, which are essential for bolstering the coulomb efficiency and overall capacity of Li–Se batteries. The authors proposed that Mo2B2O2 and Mo2B2F2 MBenes could be considered as promising candidates for anchoring and catalytic roles within Li–Se batteries.60 This discovery paves the way for a novel strategy in designing highly efficient cathodes for Li–Se batteries, potentially transforming their performance and broadening their applicability in energy storage solutions.
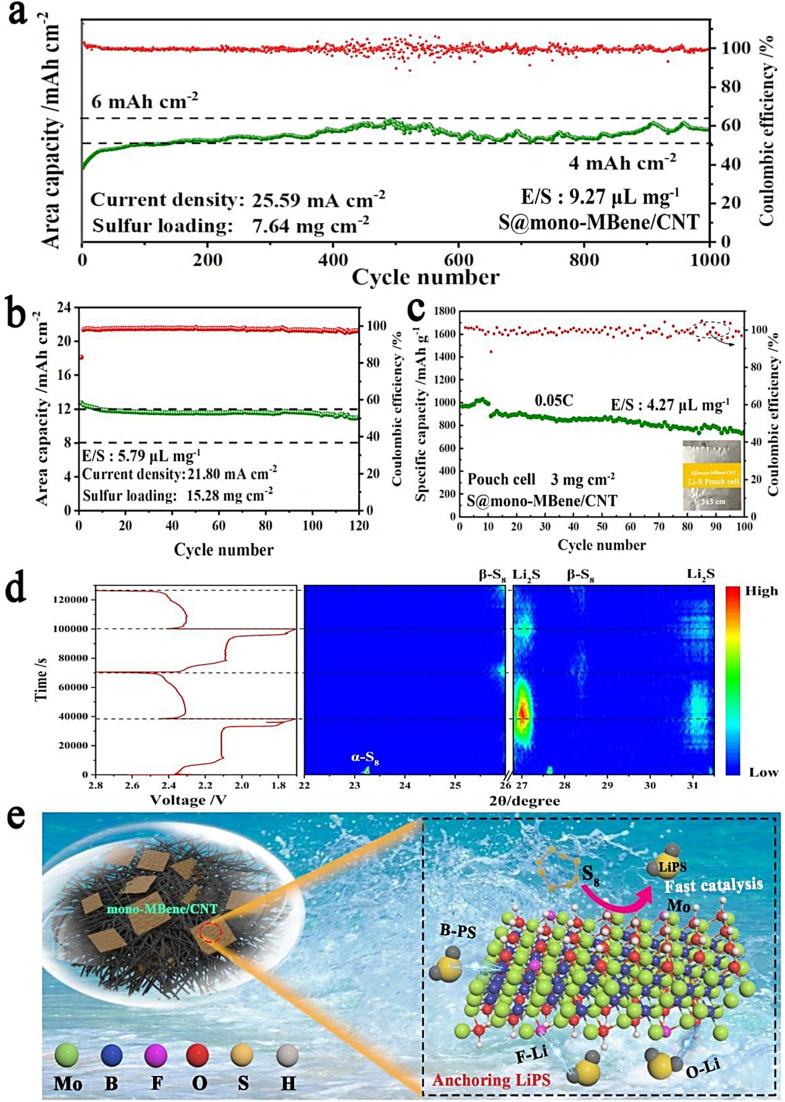
The application of lithium–sulfur (Li–S) batteries encounters substantial challenges. Key issues include the shuttling effect, which complicates performance, alongside the poor electronic conductivity of sulfur. Additionally, the inactive decomposition of low-order lithium polysulfides (LiPS) further complicates the efficiency and stability of these batteries.61 To address these concerns, Xiao et al.61 conducted a comprehensive study on the anchoring and electrocatalytic properties of MBenes, aiming to establish overarching design principles for enhancing electrochemical performance in Li–S batteries. Their research indicated that employing bare Mo2B2 MBene directly in Li–S batteries could result in the undesirable decomposition of high-order LiPSs. This issue stemmed from the excessively strong binding interactions between S atoms and the surface Mo atoms. To address this challenge, the authors highlighted the advantages of surface functionalization on Mo2B2 MBene. This approach significantly improved the anchoring performance and effectively mitigated the shuttling effect. Remarkably, the functionalized MBenes maintained excellent electronic conductivity, even after the adsorption of LiPS, thereby facilitating the essential redox electrochemistry required for the operation of Li–S batteries. Furthermore, with enhanced diffusion of Li atoms, the functionalized MBene demonstrated superior electrocatalytic activity for the decomposition of Li2S. It exhibited ultra-low diffusion (0.191 eV) and decomposition (0.441 eV) energy barriers, leading to improved performance and coulombic efficiency in Li–S batteries.61 Additionally, Li et al.62 introduced a new composite made of monolayer MBene nanosheets combined with carbon nanotubes (referred to as mono-MBene/CNT), prepared for the first time using an ice template technique (Fig. 4). This composite served as a novel cathode host for high-loading Li–S batteries. The unique properties of the mono-MBene/CNT cathode, such as its ability to anchor LiPS, had multiple functional sites, and facilitated rapid Li-ion transport, significantly enhanced the performance of the cells. Notably, the S@mono-MBene/CNT cathode displayed exceptional long-term cycling stability, maintaining performance over 1000 cycles. Remarkably, even with a S loading of 15.28 mg cm−2, the cells with the S@mono-MBene/CNT cathode achieved an initial area capacity of 12.73 mA h cm−2. After 120 cycles at a current density of 21.80 mA cm−2, the capacity remains at 11.07 mA h cm−2, showing an impressive capacity retention of 86.96%. This performance was ∼3 times higher than that of conventional Li-ion batteries.62 Such studies demonstrated the potential of MBenes as both anchoring and electrocatalytic materials in Li–S batteries, providing valuable insights for the design of high-performance cathodes in these energy storage systems.
Fig. 4. (a) Long-term cycling performance of high-S-loading cells utilizing the S@mono-MBene/CNT cathode. (b) Cycling performance data for ultra-high sulfur-loading cells featuring the cathode. (c) Cycling performance of a pouch cell with the cathode tested at a rate of 0.05C. (d) In situ X-ray diffraction analysis (XRD) analysis of the high-loading cell with the cathode, alongside the related charge and discharge curves. The color gradient indicates signal strength, with red representing the highest intensity and blue the lowest. (e) Schematic representation depicting the mechanism of the cathode. Reproduced with permission from ref. 62. Copyright 2022 Elsevier.
3.3.3. Information energy devices
In recent explorations, researchers have delved into the remarkable properties of 2D transition metal borides. These materials, particularly CrB and FeB, exhibit intriguing magnetic characteristics. By conducting first-principles calculations, Dou et al.63 discovered that the CrB monolayer behaved as a ferromagnetic metal. In contrast, FeB displayed antiferromagnetic semiconductor properties. Notably, both monolayers demonstrated ferromagnetic behavior when subjected to specific functional group modifications. This transformation led to an enhancement in magnetic anisotropy energy, making them quite fascinating. Monte Carlo simulations provided significant insights into the Curie temperature (Tc) of these materials. For instance, the CrB monolayer showcased a Tc of approximately 520 K. Remarkably, this temperature escalated to 580 K and 570 K when treated with –F and –OH chemical modifications, respectively. On the other hand, the FeB variants, including FeBF, FeBO, and FeBOH, revealed Curie temperatures of 250 K, 275 K, and 300 K, correspondingly.63 Thus, MBenes hold substantial promise for applications in information storage devices. Their unique magnetic properties and the ability to modify their characteristics through chemical means open avenues for future technological advancements. As researchers continue to investigate these materials, the potential for innovative solutions in data storage becomes increasingly tangible.
3.4. Catalysis
3.4.1. Nitrogen reduction reactions (NRRs)
Traditionally, ammonia (NH3) production is energy-intensive and environmentally unfriendly. Electrochemical nitrogen reduction reactions (NRRs) are crucial for sustainable ammonia production.64 These reactions occur under ambient conditions, making them appealing. However, the quest for high-activity and high-selectivity catalysts remains imperative. This is where the energy change profile and electrical structure come into play. The quest for efficient ammonia production has led to the exploration of low-energy consumption and highly selective NRR catalysts.64,65 In one study, Yang et al.66 conducted an investigation into ten distinct types of MBenes for their capabilities in the electrocatalysis of the NRR. Utilizing comprehensive first-principles calculations, their study demonstrated that MBenes exhibited significant stability in aqueous environments and showed remarkable selectivity for NRR over the HER. Notably, both the surface boron and metal atoms within MBenes served as active sites, attributed to the presence of occupied and unoccupied p (d) states associated with these atoms. Interestingly, the boron reaction centers displayed enhanced catalytic activity compared to the metal atoms, underscoring their critical role in the NRR process. Additionally, the catalytic performance of these 2D borides could be fine-tuned by modifying their work function, enabling optimization tailored to specific applications.66
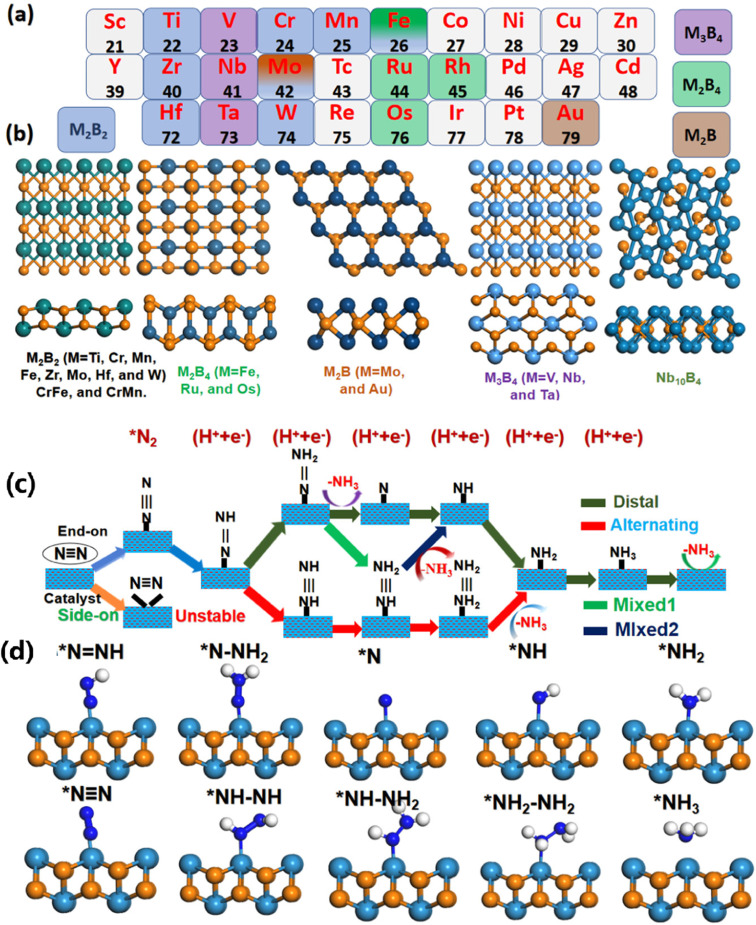
Qi et al.67 concentrated on a range of MBenes showcasing exceptional properties, with elements such as Sc, Ti, V, Y, Zr, Nb, Mo, Hf, Ta, and W included as M. They investigated the high stability and metallic electronic band structures of these materials. Furthermore, the electronic characteristics significantly boosted the catalytic activity for the NRR, with the abundance of active sites playing a vital role in this enhancement. These active sites accelerated the NRR process, fostering a dynamic environment conducive to improved performance. Consequently, the high selectivity towards NRR effectively suppressed the HER, a prevalent obstacle in this domain. Following thorough screening, they identified four promising MBenes: TiB, YB, ZrB, and MoB. Each of these materials exhibited favorable limiting overpotentials of 0.64, 0.68, 0.65, and 0.68 V, respectively, highlighting their potential for efficient nitrogen fixation applications.67 This research not only enriches the MBenes family but also provides a feasible strategy for designing NRR catalysts. In another study, a theoretical screening approach was employed to design MBenes as electrocatalysts for NRRs.68 The results from DFT calculations were enlightening. Several MBenes were discovered, including Ta3B4, Nb3B4, CrMnB2, Mo2B2, Ti2B2, and W2B2, which exhibited significant catalytic performance. These materials effectively reduced N2 to NH3 under ambient conditions (Fig. 5).68 These MBenes exhibited a notable affinity for both N2 and hydrogen (H) around their metal active centers. This unique dual attraction may effectively mitigate the competing HER. In particular, W2B2 showcased an impressive limiting potential of −0.24 V, marking it as a promising candidate catalyst for NRRs. Additionally, other materials such as Nb3B4 (0.50 eV), Ta3B4 (0.39 eV), and Ti2B2 (0.37 V) also revealed low limiting potentials. This characteristic can be linked to strong back-donation interactions between the hybridized d orbital of the metal and the 2p orbital in N2. The enhanced catalytic activity for NRRs, with limiting potentials ranging from −0.7 V to −0.2 V, was based on theoretical evaluation criteria. The rate-determining step was validated through the limiting potential depicted in the volcano plot. The energy barrier for the reduction of O/OH* to *H2O was regarded as redox potential. Furthermore, the difference in UR–UL values provided a means to further investigate the catalytic activity of these materials.68 Overall, this theoretical screening paves the way for investigating reaction mechanisms, thereby aiding in the design of innovative catalysts for the reduction of N2 to NH3 on MBenes.
Fig. 5. (a) The periodic table includes all MBenes with both experimental and theoretical investigations. Different colors represent the stable structural prototypes of these MBenes. (b) Calculation models depicting top and side views for various MBene structural prototypes have been constructed. (c) A schematic representation outlines the NRR mechanisms, highlighting the distal, alternating, and mixed pathways for different MBene structural prototypes. (d) The side view of the initial calculation models for M2B MBene structural prototypes is showcased, based on the proposed NRR intermediate species. Reproduced with permission from ref. 68. Copyright 2021 American Chemical Society.
Cheng et al.69 performed DFT calculations to evaluate the exfoliation properties of 14 MAlB phases. The goal was to assess their potential for water splitting and NRR performances. The results revealed a striking linear relationship between the binding energy and exfoliation energy, achieving a coefficient (R2) of 0.95. This correlation indicated that as the binding energy of aluminum (Al) in MAlB (M2AlB2) decreased, the exfoliation energy required to synthesize monolayer MB from MAlB increased. Among the studied MBenes, NiB (B site) emerged as the top contender, showcasing the remarkable electrocatalytic performance for water splitting. Specifically, it demonstrated remarkable performance in both HER and oxygen evolution reaction (OER). They calculated the overpotentials on the NiB surface at 0.08 V for HER and 0.37 V for OER, affirming its efficiency. The electronic properties, coupled with dynamic simulations, further supported their claim that NiB was the most promising catalyst for water splitting applications. On the other hand, it was indicated that the Fe site on FeB (FeB–Fe) exhibited the highest NRR performance among the examined MBenes, with an overpotential (ηNRR) of 0.11 V. This highlighted FeB's potential in catalyzing nitrogen fixation processes. Additionally, the B site of TaB (TaB–B) was recognized as the most effective NRR catalyst when considering the HER side reaction. This distinction was crucial for optimizing performance in practical applications, where side reactions could significantly impact overall efficiency.69
Zhang et al.70 evaluated the potential of boron centers as active sites, specifically exploring single and double boron atoms supported on defective Mo2B2O2 MBene structures. These configurations, referred to as B@Mo2B2O2 and B2@Mo2B2O2, were systematically analyzed for their ability to capture and reduce nitrogen gas. Their findings revealed that both B@Mo2B2O2 and B2@Mo2B2O2 demonstrated impressive catalytic activity. Notably, they operated through an enzymatic pathway, exhibiting remarkably low limiting potentials of −0.42 V and −0.34 V, respectively. This significant reduction in energy requirement underscored the efficiency of these boron-centered catalysts. Notably, a crucial distinction emerged between the two configurations. The double boron active sites in B2@Mo2B2O2 exhibited a marked preference for suppressing the competitive HER compared to their single boron counterparts. This phenomenon could be attributed to the synergistic effect and the covalent bonding facilitated by the interaction between the two boron atoms. An in-depth analysis of the electronic structures further elucidated the relationship between the limiting potentials of NRR and the p-band center of the boron atom. The overlap position with the nitrogen p orbitals also played a vital role in determining catalytic efficiency.70 This study demonstrates that double boron active sites (B2@Mo2B2O2) possess a unique capability to modulate the adsorption free energy of *NH3 more effectively than single boron sites. This modulation results in a lower limiting potential, enhancing catalytic performance. These insights not only elucidate the superior catalytic abilities of boron-centered catalysts for NRR but also provide valuable guidance for the design of advanced single and double-atom catalysts in future research. Additionally, the potential of ReB2 as an efficient electrocatalyst was evaluated for NRR aimed at sustainable NH3 production.71 Remarkably, ReB2 exhibited a record-low limiting potential of UL = −0.05 V, coupled with a Faraday efficiency of 100%. It was indicated that high pressure and temperature conditions were beneficial for enhancing N2 adsorption and minimizing kinetic barriers. The maximum turnover frequency at 700 K and 100 bar reaches 1.24 × 10−2 per second per site, which was competitive with benchmark catalysts like Fe3/Al2O3, facilitating an extremely rapid reaction rate. Furthermore, the crystal orbital Hamilton population of *N2 was analyzed to elucidate the intrinsic mechanisms behind N2 activation. By examining the d–2π* interactions, it was found that integrated crystal orbital Hamilton population could serve as a quantitative descriptor for evaluating the degree of N2 activation.71
For effective NH3 synthesis through the electrochemical NRR, a suitable catalyst must exhibit both significant specific performance and a large active surface area. Though, achieving this balance within a single material presents significant challenges, particularly in managing multiple reaction intermediates.72 Guo et al.72 demonstrated that MBenes could successfully meet these challenges by providing both high activity and extensive reaction surface areas for NRR. Through extensive DFT calculations involving 16 different MBenes, they identified seven candidates (CrB, MoB, WB, Mo2B, V3B4, CrMnB2, and CrFeB2) that not only displayed intrinsic basal plane activity for NRR—characterized by limiting potentials ranging from −0.22 to −0.82 V-but also exceled at inhibiting the competitive HER. Notably, unlike MXenes, which may experience surface oxidation that blocks active sites, the oxidized forms of these MBenes could still catalyze NRR through a self-activating process. This process involved the reduction of O*/OH* to H2O* under reaction conditions, thereby promoting the electro-reduction of N2. Consequently, the remarkable activity, selectivity, and high active area, along with their antioxidative properties, position these MBenes as pH-universal catalysts for NH3 formation without the need for additional dopants or defects.72
The core principle of single-atom catalysis revolves around establishing robust interactions between individual atoms and their supporting materials, which is crucial for stabilizing electrocatalytic NRR. For instance, Yao et al.73 utilized cutting-edge ab initio calculations to create strong coupling systems between individual atoms and MBenes. This innovative approach highlighted their potential as exceptional electrocatalysts for the electrocatalytic NRR. The research examined a diverse range of transition metal atoms spanning groups IVB to VIII, strategically placed within the Mo vacancies of the MBene nanosheet (Mo2B2O2). As a result, a thorough evaluation of their activities and selectivity in the electrocatalytic NRR process was performed. The computational results demonstrated that rhenium (Re) and osmium (Os), when integrated into the defective Mo2B2O2 layer, showcased remarkable catalytic performance. They achieved low barriers for the potential-determining steps (PDS), measuring at 0.29 eV for Re and 0.32 eV for Os. In comparison, a single ruthenium (Ru) atom on Mo2CO2 exhibited a higher PDS of 0.46 eV. Moreover, the incorporation of Re and Os atoms not only significantly improved the electrocatalytic NRR process but also reduced the HER in the context of oxygen vacancies. The analysis further revealed a direct correlation between the number of d electrons in the single atoms and their respective periods in the periodic table, particularly influencing the initial protonation step of the electrocatalytic NRR. To clarify the relationship between the inherent atomic characteristics and catalytic efficiency, a binary descriptor was introduced. This descriptor encompassed the number of d electrons along with the periodic classification of the elements, thus paving the way for enhanced understanding of catalytic behavior.73
3.4.2. Electrocatalytic conversion of nitrogen oxide
The electrocatalytic conversion of nitrogen oxide (NO) into value-added chemicals presents a thrilling opportunity. This innovative strategy aims to address the significant human-induced disturbances in the global nitrogen cycle. However, despite the promise, controlling product selectivity poses a formidable challenge for researchers.74 In one study, DFT calculations were performed to evaluate the catalytic activity of various transition metal borides, specifically the M2B2 type MBenes.75 The research meticulously screened these compounds, ranging from group IVB to V transition metals of the periodic table. The authors systematically probed the catalytic activity and selectivity associated with the nitric oxide reduction reaction (NORR). Notably, they discovered that Fe2B2, Mn2B2, and Rh2B2 exhibited exceptional function in converting NO to NH3. Remarkably, these catalysts exhibited lower limiting potential when compared to other MBenes. Specifically, Nb2B2 and Hf2B2 revealed limiting potentials of −0.11 V and −0.17 V, respectively, during the generation of NH3 from NO. The binding energy of ΔG*N is a crucial indicator of catalytic efficiency, shaped by the volcano plot associated with the rate-limiting step. Furthermore, the researchers explored the complex reaction mechanisms involved in the reduction of NO. They investigated various pathways that culminate in the production of NH3, N2, and N2O. Their findings indicated that atomic *N could react with another *N or a *NO molecule, eventually resulting in the creation of N2 and N2O through two consecutive hydrogenation steps. Additionally, it was noted that the formation energy for the hydrogenation of *NO to *NOH was considerably lower than that required for *HNO.75 This study highlights the ability of MBenes to selectively promote the NORR while effectively suppressing the competing HER. Thus, these findings underscored the potential of transition metal borides as effective catalysts in nitric oxide reduction processes.
3.4.3. Oxygen evolution reaction (OER), oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER)
MBenes have recently gained attention as promising substrates for single-atom catalysts (SACs) in crucial electrocatalytic procedures, including the OER, ORR, and HER.76,77 Their remarkable attributes, including a high surface area and tunable electronic properties, position them as excellent candidates for improving catalytic performance in renewable energy applications. The high surface area of MBenes allows for greater dispersion of SACs, maximizing the active sites available for reactions.53,73 This enhances the efficiency of catalysis, particularly in OER, where multiple electron transfers are required. Additionally, the tunable electronic characteristics of MBenes enable the optimization of their interaction with SACs, facilitating better charge transfer and improving overall catalytic activity. In the case of OER, MBenes can effectively support SACs, leading to lower overpotentials and higher reaction rates, which are essential for efficient water splitting. Similarly, for ORR, the unique properties of MBenes enhance the stability and performance of SACs, making them viable alternatives to precious metal catalysts. Furthermore, in HER, MBenes provide a conducive environment for SACs, promoting rapid proton reduction and hydrogen production. The combination of high conductivity and large active surface areas ensures that MBenes remain efficient and stable under various reaction conditions.78,79
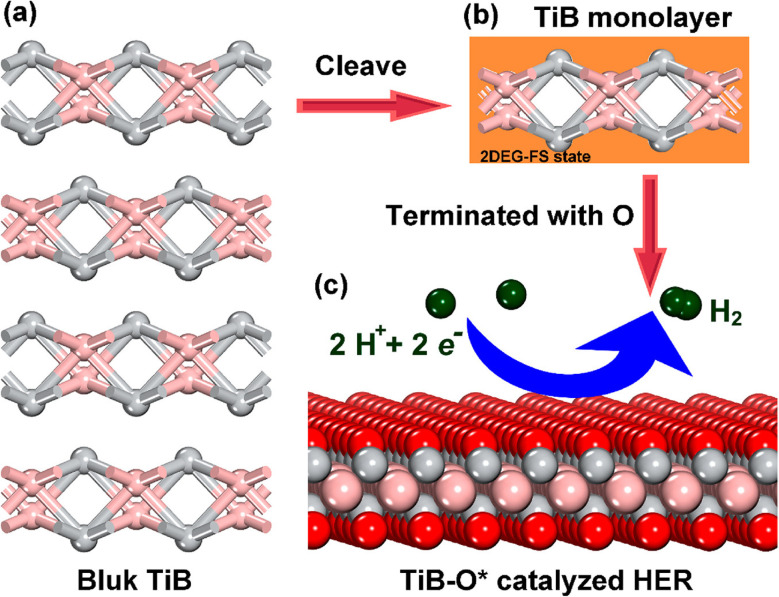
Recent advancements in material science have unveiled the remarkable synthesis of a layered MBene, specifically TiB.80 This breakthrough adds a new member to the MBene family, yet, intriguingly, reports detailing its properties and applications remain scarce. Utilizing first-principles techniques, Li et al.80 evaluated the mechanical and electronic characteristics of this novel MBene. The TiB monolayer exhibited metallic electronic behavior, coupled with outstanding mechanical and dynamical stability. This stability was crucial, as it underscored the material's potential in various applications. Notably, the presence of a slight surface 2D electron gas in free space state suggested exciting opportunities for TiB in electronic devices, particularly as a low-barrier electron transport channel. For catalytic applications, the TiB monolayer, especially in its O-terminated form (TiB–O*), demonstrated promising features. It revealed a thermoneutral Gibbs free energy for atomic hydrogen adsorption 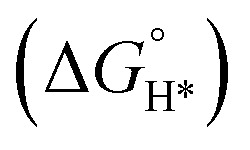 , approximately 0.03 eV. This value was tantalizingly close to the ideal 0 eV, indicating its potential as an effective 2D electrocatalyst for promoting HER. Furthermore, the thermodynamic HER performance of TiB–O* surpasses that of most carbide-based MXenes, marking it as a compelling candidate for future research (Fig. 6).80
, approximately 0.03 eV. This value was tantalizingly close to the ideal 0 eV, indicating its potential as an effective 2D electrocatalyst for promoting HER. Furthermore, the thermodynamic HER performance of TiB–O* surpasses that of most carbide-based MXenes, marking it as a compelling candidate for future research (Fig. 6).80
Fig. 6. The illustration depicts the HER process facilitated by TiB in three distinct forms: (a) the bulk form of TiB; (b) a TiB monolayer extracted from the bulk for investigating its electronic properties and reactivity; and (c) a TiB layer that has undergone surface functionalization to enhance its efficiency in the HER process. Reproduced with permission from ref. 80. Copyright 2019 American Chemical Society.
The discovery of Ti2InB2 has sparked interest in hexagonal MAB (h-MAB) phases and their 2D derivatives, known as h-MBenes, as promising materials for various applications.81 In one study, Feng et al.81 identified Hf2BO2 as a potential h-MBene that served as an promising platform for the electrocatalysis of the HER, utilizing DFT calculations (Fig. 7). To enhance HER performance of Hf2BO2, they proposed two strategies involving transition metal modifications: atom deposition and implantation. It was indicated that a moderate reduction in the charge of surface oxygen, achieved through transition metal atom introduction, significantly improved the catalytic activity. The synergistic interaction between the implanted transition metal atoms and the Hf2BO2 matrix effectively activated the surface by broadening the O-p orbitals and elevating the p-band center. This effect was particularly pronounced with V, Cr, and Mo as dopants, leading to a reduction in Gibbs free energy (ΔGH*) from 0.939 eV to −0.04, 0.05, and −0.04 eV, respectively. Notably, this enhancement occurred within a localized region, and the catalytic activity could also be assessed by examining the bond lengths of Hf–O, in addition to ΔGH*.81 This discovery encourages further exploration into the fabrication of h-MAB phases and the extraction of h-MBenes, potentially advancing the field of electrocatalysis.
Fig. 7. This illustration outlines the preparation process of h-MBenes, which involves the exfoliation of the A layer along with transition metal modification techniques. These modifications are achieved through methods such as atomic deposition and atomic implantation. Reproduced with permission from ref. 81. Copyright 2021 American Chemical Society.
Cr-based MBenes can be employed in advancing the field of electrocatalysis for sustainable hydrogen production. Zhang et al.79 utilized DFT calculations to investigate a series of chromium-based MBenes with varying atomic-layer thicknesses, denoted as Crn+1B2n (where n = 1–3). These materials demonstrate outstanding electrocatalytic performance for HER. It was revealed that these Cr-based MBenes were not only highly stable but also exhibited mechanical anisotropy and intrinsic ferromagnetic conductivity. Notably, their mechanical features and HER catalytic performance were dependent on thickness. For instance, Cr4B6 showcased a remarkable Young's modulus of 335 N m−1, which was comparable to the 342 N m−1 of graphene, with measured values of 247 N m−1 along the x and y axes, respectively. Most importantly, Cr4B6 could be considered as an exceptional HER electrocatalyst, exhibiting an overpotential of merely 0.003 V at a hydrogen coverage of 1 monolayer. This performance positioned it as a promising alternative to traditional platinum catalysts.79
The catalytic performance of Mo2B2 MBene-supported SACs has garnered significant attention.76 By employing first-principles calculations, researchers have meticulously analyzed the OER, ORR, and HER activities of various transition metal atoms embedded within Mo vacancies. These metals—specifically, Ti, V, Cr, Mn, Fe, Co, Ni, and Cu—were scrutinized as potential electrocatalysts. Notably, all TM@Mo2B2 SACs exhibited remarkable metallic conductivity. This characteristic is vital, as it promotes efficient charge transfer during electrocatalytic reactions. Among these catalysts, Ni@Mo2B2 emerged as a standout option, functioning effectively as a bifunctional electrocatalyst for both HER and OER. With a lower |ΔGH| of −0.09 eV under 1/4H coverage, Ni@Mo2B2 demonstrated enhanced performance in HER. Furthermore, its overpotential (ηOER = 0.52 V) surpassed that of conventional IrO2 (ηOER = 0.56 V), showcasing its potential for practical applications. On the other hand, Cu@Mo2B2 distinguished itself as an effective OER/ORR bifunctional electrocatalyst. It boasted an impressive overpotential of 0.31 V for OER, which was lower than those of both IrO2 (ηOER = 0.56 V) and RuO2 (ηOER = 0.42 V). Additionally, it exhibited superior ORR performance with an overpotential of 0.34 V, outperforming platinum (Pt) which has an ηORR of 0.45 V.76 This research unveils a promising pathway for developing non-noble-metal-based bifunctional MBene electrocatalysts, potentially developing the field of electrocatalysis. The search for affordable and abundant electrocatalysts is critical for advancing efficient ORR and OER technologies.82 Such advancements are essential for developing clean and renewable energy storage and conversion systems Wang et al.82 utilized first-principles calculations to investigate the catalytic activity of SACs based on various 2D MBene substrates, designated as TM–M2B2O2, where TM includes Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Pt, and M represents Ti, Mo, and W. It was revealed that several combinations had promising ORR catalytic activity. Specifically, Ni–Ti2B2O2, Cu–Ti2B2O2, Ni–Mo2B2O2, Ni–W2B2O2, and Pt–W2O2B2 demonstrated low overpotentials of 0.47 V, 0.57 V, 0.23 V, 0.43 V, and 0.30 V, respectively, which were comparable to the performance of Pt (0.45 V). In terms of OER catalysts, they identified Fe–Ti2B2O2, Co–Ti2B2O2, Fe–Mo2B2O2, Co–Mo2B2O2, Ni–Mo2B2O2, Fe–W2B2O2, and Co–W2B2O2 as promising candidates with low overpotentials of 0.51 V, 0.46 V, 0.48 V, 0.41 V, 0.54 V, 0.58 V, and 0.30 V, respectively. These values were competitive with those of RuO2 (0.42 V) and IrO2 (0.56 V). Furthermore, they employed the Integrated Crystal Orbital Hamilton Population (ICOHP) as a descriptor for evaluating the catalytic activity of ORR and OER.82 This approach provides valuable insights for the future fabrication of highly active catalysts for both reactions, fostering the development of efficient energy conversion technologies.
3.4.4. Electrochemical synthesis of urea
Urea plays a crucial role as a fundamental raw material in the chemical industry, particularly as a nitrogen source for fertilizers. However, conventional industrial methods for urea synthesis demand harsh reaction conditions and consume significant amounts of ammonia (NH3) generated through artificial processes. In contrast, the electrochemical conversion of nitrogen (N2) and carbon dioxide (CO2) into urea under ambient conditions emerges as a promising green fabrication approach. Nonetheless, the widespread adoption of this innovative method is hindered by the scarcity of promising electrocatalysts. In one study, Zhu et al. employed DFT computations to systematically investigate the catalytic activity of three experimentally accessible MBenes–Mo2B2, Ti2B2, and Cr2B2—for the simultaneous electrocatalytic coupling of N2 and CO2 to synthesize urea at ambient conditions (Fig. 8).83 Their findings revealed that these MBenes had remarkable intrinsic basal activity for urea formation, with limiting potentials ranging between −0.49 and −0.65 eV. Moreover, these materials effectively suppressed the competing reaction of N2 reduction to ammonia, which was critical for enhancing urea yield. Notably, the 2D structures of Mo2B2 and Cr2B2 demonstrated superior capabilities in mitigating surface oxidation and self-corrosion during electrochemical reactions. This characteristic positioned them as particularly promising electrocatalysts for urea preparation. This study lays the foundation for advancing the electrochemical synthesis of urea, highlighting the potential of metal borides as effective catalysts in this transformative process. This could lead to more sustainable urea production methods, aligning with ecological and economic goals in the chemical industry.
Fig. 8. This diagram illustrates the process of urea production via the electrochemical coupling of N2 and (CO2). In the illustration, gray, red, pink, and blue spheres symbolize carbon (C), oxygen (O), nitrogen (N), and hydrogen (H) atoms, respectively. Reproduced from ref. 83 under the terms of the Creative Commons CC BY license. Copyright 2021, Springer Nature.
To facilitate the preparation of urea with efficiency through electrocatalytic C–N coupling, a novel approach was proposed utilizing MBenes based on theoretical predictions.84 Recent advancements in the field of electrocatalysis have unveiled a fascinating approach to convert inert CO2 and N2 molecules into urea. This method, contrasting sharply with traditional industrial processes, holds immense promise. However, the weak adsorption and activation of CO2 and N2 hinder the C–N coupling reaction, complicating matters further. To address these obstacles, researchers are exploring innovative strategies. By leveraging renewable energy sources—such as solar energy—scientists aimed to enhance the electrochemical synthesis of urea. This approach targeted the crucial C–N bond coupling between *N N* and *CO*, resulting in the formation of *NCON* intermediates. These intermediates played a vital role in the overall urea synthesis process. Moreover, computational screening could be considered an invaluable tool. It provided insights into the underlying mechanics of C–N coupling and the necessary protonation steps. This knowledge served as a guide, paving the way for sustainable pathways to synthesize carbon nitride chemicals via C–N coupling. The theoretical strategies developed could help identify efficient catalysts for urea electro-synthesis. The primary focus remained on coupling CO2 and N2 to yield H2NCONH2. By establishing the Gibbs free energy landscape, researchers calculated the limiting potential based on rate-determining steps. This rigorous analysis culminated in the creation of a volcano plot, which illustrated the relationship between ΔG(*NCON*) and the suitability of MBenes for urea preparation. Critical factors such as kinetic stability, adsorption capabilities of CO2 and N2, and overall catalytic activity were meticulously considered. Interestingly, findings suggested that Mo2B2 and Ru2B4 could be applied as promising catalysts for urea electro-synthesis. They exhibited remarkable activity and selectivity, making them strong contenders in the quest for efficient urea production. This research not only sheds light on the potential of inert molecules but also paves the path towards sustainable chemical synthesis.84
3.4.5. Photocatalytic hydrogen (H2) production
The quest for sustainable energy sources has led to significant interest in photocatalytic hydrogen production. This innovative process harnesses sunlight to drive chemical reactions, producing hydrogen as a clean fuel. The implications are enormous, especially in the context of renewable energy and environmental sustainability. At the heart of this process lies photocatalysts materials that absorb light and facilitate chemical reactions. Typically, semiconductor materials, such as titanium dioxide (TiO2) and, increasingly, MBenes and MXenes, are employed.85 When these photocatalysts are exposed to sunlight, they generate electron–hole pairs. This phenomenon enables the splitting of water molecules into hydrogen and oxygen, a process that can be optimized through material selection and environmental conditions. One of the primary advantages of photocatalytic hydrogen production is its renewable aspect. Utilizing solar energy directly addresses the global reliance on fossil fuels. Moreover, hydrogen serves as a versatile energy carrier; it can be stored and transported easily, making it an attractive alternative to traditional energy sources. However, despite its potential, several challenges impede the widespread adoption of photocatalytic hydrogen production. For instance, the efficiency of current photocatalysts is often low. Researchers are continually exploring ways to enhance light absorption and improve charge separation to maximize hydrogen output. Additionally, the scalability remains a critical issue. While laboratory results may show promise, translating these findings into large-scale applications involves numerous complexities. The design of reactors, optimization of reaction conditions, and integration into existing energy systems must all be carefully considered. Furthermore, the stability and sustainability of photocatalysts are crucial. Many materials degrade over time or under varying environmental conditions, which can reduce their effectiveness. Developing stable catalysts that maintain their performance over prolonged periods is essential for commercial viability.86,87
In the pursuit of efficient solar hydrogen production, the role of co-catalysts cannot be overstated. Highly active and low-cost co-catalysts significantly enhance photocatalytic reactions, providing a pathway to sustainable energy solutions. In one study, MoB MBene was employed as a noble-metal-free co-catalyst to improve semiconductor performance in photocatalytic hydrogen formation.27 Consequently, MoB MBene was synthesized through a straightforward hydrothermal etching technique followed by manual scraping from MoAlB. The result was a high-purity material, exceeding 95 wt%, which undergoes ultrasonic cell pulverization to yield ultrathin nanosheets, measuring ∼0.61 nm in thickness. This meticulous process not only preserved the integrity of the material but also maximized its surface area, enhancing its catalytic properties. When integrated with cadmium sulfide (CdS) through an electrostatic interaction strategy, the CdS/MoB composites demonstrated remarkable photocatalytic hydrogen production capabilities. With an astonishing activity of 16 892 μmol g−1 h−1 under visible light, these composites outperformed pure CdS by an impressive factor of ∼1135%. Such exceptional performance underscored the potential of MoB MBene as a transformative co-catalyst in the field of photocatalysis. The underlying mechanisms contributing to this high performance were elucidated through theoretical calculations and various experimental measurements. Factors such as Gibbs free energy, work functions, and photoelectrochemical features were critical in understanding the efficiency of the CdS/MoB system.27
3.5. Biomedical applications
3.5.1. (Bio)sensing
MBenes and their composites represent a new frontier in the field of biosensing applications, where their special features can be harnessed to enhance the detection and quantification of biomolecules.52 The high surface area and remarkable electrical conductivity of MBenes facilitate efficient electron transfer, which is crucial for improving the sensitivity and response time of biosensors. When combined with various organic or inorganic materials, MBenes can form composites that exhibit synergistic effects, further enhancing their performance. For instance, integrating MBenes with nanomaterials such as graphene or metal nanoparticles can significantly amplify signal transduction, permitting for the recognition of low-abundance biomarkers in complex biological samples. In one study, researchers developed copper nanoclusters with a superlative structure, referred to as Cu NCSL.88 These nanoclusters served as luminescence probes for detecting miRNA-221, a crucial biomarker in cancer diagnostics. The tightly aligned superlattice structure of Cu NCSL exhibited remarkable advantages. It effectively mitigated the disordered accumulation of nanoparticles. This reduction in chaos limited the intramolecular vibrations and internal rotations of the copper nanoclusters. Consequently, a more stable environment fostered enhanced performance. Moreover, the compact stacking of particles within the superlattice greatly boosted electron transport efficiency. This efficiency was pivotal, as it directly correlated with the luminescence. As the radiation energy transfer rate increases, so does the luminescence of the Cu NCSL, leading to brighter signals for detection. Simultaneously, the research team prepared a 2D MBene material. This material exhibited excellent electrical conductivity and impressive electrochemical activity. When employed as an electrode alteration, it significantly amplified the electrochemiluminescence (ECL) signal of Cu NCSL. The constructed ECL sensor, designed for the detection of miRNA-221, showcased the synergy between Cu NCSL and MBene nanosheets. The results from actual tumor tissue analyses indicated that this Cu NCSL/MBene-based biosensor was not only highly selective but also stable, achieving a low detection limit.88 This advancement holds great promise for clinical cancer detection, paving the way for more effective diagnostic tools.
The tunable surface chemistry of MBenes enables functionalization with specific recognition elements, such as antibodies or aptamers, which are vital for selective biosensing. This adaptability allows for developing highly sensitive and selective biosensors for various applications, including disease diagnosis, environmental monitoring, and food safety. Despite their potential, challenges remain in optimizing the stability and reproducibility of MBenes-based biosensors, as well as ensuring their compatibility with biological systems. Nonetheless, ongoing research and innovation in this area hold great promise for advancing the capabilities of biosensing technologies, paving the way for more effective and accessible diagnostic tools.25,52 In the field of optoelectronic applications, MBenes are gaining recognition for their promising attributes.89 Despite their prevalent use in optoelectronics, the utilization of MBenes in molecular sensing, particularly as surface-enhanced Raman scattering (SERS) active materials, remains largely uncharted. For instance, a novel and high-performance MBene SERS platform has been developed, marking a significant advancement in the field.89 The innovative SERS platform was distinguished by its ordered vacancy-triggered design, showcasing a heightened sensitivity to Raman signals and exceptional signal uniformity. Leveraging the unique properties of a 2D Mo4/3B2 MBene material, this platform exhibited superior SERS activity compared to traditional semiconductor substrates, boasting an impressive Raman enhancement factor of 3.88 × 106 and an exceptionally low detection limit of 1 × 10−9 M. Through a meticulous integration of systematic experiments and DFT calculations, the research elucidates the intricate SERS mechanism underpinning the exceptional sensitivity of the 2D Mo4/3B2 MBene. The remarkable Raman enhancement capabilities of this material stemmed from the efficient photo-induced charge transfer process between the MBene substrates and the adsorbed molecules. Notably, the abundant electronic density of stated near the Fermi level of the MBene enabled a Raman enhancement surpassing that of bulk MoB by 100 000 times. The introduction of ordered metal vacancies in the MBene engendered uniform charge transfer sites, culminating in exceptional signal uniformity with a relative standard deviation as low as 6.0%.89 This research paves the way for a new era in SERS platforms, harnessing the potential of MBene materials for advanced chemical sensing applications.
The evolution of sensitive point-of-care testing (POCT) for circulating tumor DNA (ctDNA) marks a significant leap in the realm of non-invasive cancer diagnostics. This advancement proves especially crucial in low-resource settings where traditional methods may falter. Among various innovative approaches, the electrochemical paper-based analytical device (ePAD) emerges as a promising frontier, yet it remains underexplored in the context of ctDNA assays.90,91 In one study, Yang et al.91 evaluated the capabilities of a magnetic clutch probe to effectively pre-enrich ctDNA, thereby enhancing the overall detection performance of our ePAD. The integration of MBene/Au as sensing substrates not only amplified signals but also provided a greater number of binding sites for probes (Fig. 9). Notably, MBene exhibited commendable electronic properties, making it a superior candidate for electrode interface modification. Comparative studies with MXene further underscored its potential, supported by both DFT and experimental findings. The introduction of MBene/Au in this assay design showcased a synergistic effect, significantly improving electron transfer and expanding the effective detection area. They employed two distinct electrochemical assay strategies—turn-off and turn-on—to validate detection accuracy. Remarkably, their methods achieved a linear detection range spanning from 1 to 50 pM, with limits of detection marked at 178 fM and 216 fM, respectively, after a mere 30 minutes incubation with extracted ctDNA. Further evaluations delved into the biosensor's selectivity, reproducibility, and stability, yielding favorable outcomes across the board. In assessing the reliability of our detection method, they compared ctDNA assay results from tumor cell samples and mouse serum against conventional qPCR methods. The correlation coefficients were strikingly high, at R2 values of 0.993 and 0.995, respectively.91 The implications of this study could significantly influence cancer diagnostics, particularly in resource-limited environments, ultimately enhancing patient outcomes.
Fig. 9. The initial step involves the preparation of the sample (A). Following that, the paper-based electrochemical device (B) is utilized for analysis. Finally, the detection process is carried out using the proposed biosensor (C). Reproduced with permission from ref. 91. Copyright 2024 Elsevier.
Thyroid cancer stands out as the most common type of endocrine cancer. Consequently, there is an urgent need for the development of sensitive and dependable detection methods for this malignancy. In response to this need, Gao et al.92 created an ECL biosensor that leveraged MBene derivative quantum dots (MoB QDs) alongside a nanocavity structure composed of Ag nanoparticles on a mirror (NPoM). These MBene QDs were employed as a novel luminescent agent during the ECL process, where they interacted with H2O2, serving as a co-reactant. Additionally, the nanostructure of NPoM was prepared by integrating an Ag mirror with Ag nanoparticles. This design provided highly localized hot spots, which were crucial for enhancing signal detection. The NPoM structure exhibited a remarkable capacity for light field confinement and electromagnetic field enhancement, acting as an effective signal modulator. As a result, the synergistic interaction between the nanocavity and localized surface plasmon resonance (LSPR) mode could lead to an impressive amplification of the ECL signal from MoB QDs, achieving a boost of over 21.7 times. As a result, this innovative ECL biosensing system was applied for measuring the expression levels of miRNA-222-3p in exosomes derived from thyroid cancer. The findings revealed a significant correlation between miRNA-222-3p and the BRAFV600E mutation. This MoB QDs/NPoM biosensor demonstrated substantial potential in evaluating thyroid cancer progression, paving the way for enhanced clinical diagnostic applications.92 In another study, a multifunctional paper-based ECL biosensor emerges as a groundbreaking advancement.93 This biosensor showcased exceptional photothermal performance, which enabled precise detection of interleukin-6. Interestingly, it connected visualized distance and temperature readouts seamlessly with an ultrasensitive ECL response. The innovative design employed a multipurpose MBene and TaSe2 composite. Prepared through a self-assembly method, this composite served as a target-associated photothermal element within the paper-based analytical device (PAD). Its function as a multi-signals trigger was particularly noteworthy. Under laser irradiation, the MBene@TaSe2 probe generated heat, leading to rapid temperature output. This process also triggered a phase transition in the thermoresponsive poly(N-isopropylacrylamide) (pNIPAM) hydrogel. The phase transition facilitated the release of malachite green (MG) dye, enhancing distance-based visual readouts. At the same time, the released MG dye acted as an effective quencher, which reduced the ECL signal of luminol. This dual functionality significantly boosted the analytical capabilities of the device. Thanks to this clever architecture, the platform allowed for swift preliminary screening and precise quantitative analysis using just a single-drop sample. The one-step sandwich immunoreaction approach eliminated the need for additional separation operations. Consequently, this significantly simplified the analysis process, making it more efficient and user-friendly.93 Undeniably, this study offers ingenious insights into the evolution of convenient and rapid multifunction-integrated PADs. These advancements hold great promises for family surveillance and intelligent diagnosis, potentially transforming how health monitoring is conducted in everyday settings. By bridging the gap between complex analytical procedures and user-friendly devices, we move closer to achieving effective point-of-care testing solutions for all.
3.5.2. Targeted tumor/cancer therapy
Tumor-targeted drug delivery is crucial for improving the effectiveness of tumor therapies while reducing toxic side effects. However, existing methods often fall short in efficiency. A recent study introduced a novel MBene, zirconium boride nanosheet (ZBN), as a versatile nanoplatform.34 This cutting-edge platform enabled NIR-controlled intratumoral retention and drug release. ZBN exhibited an impressive NIR-photothermal conversion efficiency of 76.8%. Moreover, surface modification with hyaluronic acid (HA) via polyol–borate esterification granted ZBN-HA excellent dispersion. This modification played a critical role in enhancing the stability and bioavailability of ZBN-HA in various environments. Notably, the photopyrolysis of borate ester-initiated HA detachment, which resulted in ZBN aggregation. This distinctive mechanism promoted NIR-controlled intratumoral retention and could lead to considerable intratumoral accumulation. Thanks to surface borate esterification, chemotherapeutic agents like doxorubicin (DOX) and NO prodrugs such as β-galactosyl-diazeniumdiolate (Gal-NO) could efficiently and stably conjugate to the ZBN-HA surface. Impressively, this method permitted the conjugation of 1.21 g DOX or 0.57 g Gal-NO per gram of ZBN, with no visible drug leakage. The efficiency of this conjugation was remarkable, ensuring that therapeutic agents remain intact until they reach their target. The photopyrolysis of borate ester not only facilitated drug release but also ensured high responsiveness and controllability. This means that the therapy can be fine-tuned to respond to specific NIR signals, leading to optimized treatment outcomes. The combination of chemothermal and gasothermal therapies utilizing ZBN-HA/DOX and ZBN-HA/NO nanomedicines could be applied as a powerful strategy.34 This multifaceted approach may develop cancer treatment, offering a targeted, efficient, and highly controlled therapeutic option. Such synergistic approaches effectively eliminate primary tumors while inhibiting metastasis by modifying the tumor microenvironment and directly targeting cancer cells within the primary tumors.
The rapid progress in biomedicine has catalyzed the creation of multifunctional 2D nanomaterials aimed at cancer theranostics. Nevertheless, crafting innovative strategies to synthesize novel 2D nanomaterials with adaptable structures and functions poses significant challenges. In one study, a new type of MBene, monolayer Bi-anchored manganese boride nanosheets (MBBN), was introduced.94 Accordingly, their exceptional NIR-photothermal and photoacoustic properties, along with X-ray absorption and MRI imaging capabilities, were unveiled. Consequently, MBBN was developed as a new nanotheranostic agent for multimodal imaging-guided photothermal cancer therapy. This innovative approach signified a step forward in the realm of cancer treatment, providing not only therapeutic benefits but also comprehensive imaging solutions. Utilizing a microwave-assisted chemical etching process, bulk manganese boride was exfoliated into flower-like manganese boride nanoparticles (MBN). A subsequent coordination-induced exfoliation technique further separated MBN into dispersive monolayer MBBN through the coordination between Bi and B on the surface. This intricate process highlighted the sophistication involved in nanoparticle synthesis, ensuring optimal characteristics for therapeutic applications. The presence of the B–OH group on MBBN's surface facilitated seamless modification with HA via borate esterification. This modification not only enhanced monodispersion but also promoted active tumor targeting. Such targeting is crucial in maximizing therapeutic efficacy while minimizing off-target effects, a common challenge in conventional cancer therapies. The synthesized MBBN exhibited an impressive NIR-photothermal conversion efficiency (η = 59.4%), with remarkable photothermal stability. This efficiency is vital for the effectiveness of photothermal therapy, ensuring that the nanoparticles generate sufficient heat to ablate tumors effectively. Moreover, it showed versatile imaging functionalities, encompassing photoacoustic, photothermal, computed tomography (CT), and T1-weighted magnetic resonance imaging (MRI) imaging. Both in vitro and in vivo assessments revealed that MBBN could offer high photothermal ablation and multimodal imaging performance. This combination achieves substantial efficacy in imaging-guided cancer therapy, making MBBN a promising candidate in the ongoing battle against cancer.94
The advancement of NIR-photothermal nanomaterials with biosafety is crucial for effective photothermal therapy of tumors as well as photoacoustic imaging in high-performance theranostics, though significant challenges remain. In this context, Chen et al.95 introduced a novel ultrasound-assisted chemical etching method. This method leverages the Fenton reaction to synthesize 2D iron boride nanosheets (FBN) as promising NIR-photothermal nanomaterials. The ingenuity behind this synthesis process highlights the potential of using ultrasound to enhance chemical reactions, paving the way for the creation of advanced nanomaterials. The resulting FBN theranostic agent demonstrated impressive NIR adsorption along with high photothermal conversion efficiencies. These properties are essential for maximizing therapeutic effects. The ability to absorb NIR light effectively translates into better heat generation, crucial for photothermal therapy. This innovative agent enabled biosafe and highly effective photoacoustic imaging-guided tumor-targeted NIR-photothermal therapy in tumor-bearing mice. Such applications underscore the potential of FBN not only in treatment but also in real-time imaging.95 The successful application of these FBN nanosheets not only underscores their potential in improving therapeutic outcomes but also paves the way for enhanced approaches in cancer treatment and imaging, highlighting the significance of developing advanced nanomaterials in modern medicine.
3.5.3. Neurodegenerative diseases
Alzheimer's disease, a formidable neurodegenerative condition, primarily stems from the accumulation of amyloid-β (Aβ) peptides within brain neurons.96 This accumulation triggers a cascade of detrimental effects, leading to cognitive decline and memory loss. While current medications may alleviate some symptoms, they fall short of offering a definitive cure. Consequently, prevention emerges as the most viable strategy to combat this insidious disease. In one study, researchers proposed innovative structures derived from MBenes—specifically Cd2B, Mo2B, Cu2B, and Ta2B—as potential deterrents against amyloid-β accumulation.96 The intrinsic properties of MBenes are nothing short of remarkable. Their tunability, biocompatibility, and low manufacturing costs make them attractive candidates for further exploration. Molecular dynamics simulations played a crucial role in this investigation. The effect of these MBenes on the deformation of amyloid-β was meticulously examined. Through atomic-level analysis, researchers probed the compaction, contact area, and stability of beta-amyloid in the existence of these structures. The results were promising, showcasing the considerable destabilization of amyloid-β structures when interacting with MBenes. Among the structures examined, Cd2B exhibited the most significant interactions with amyloid-β. This interaction led to a pronounced instability in the amyloid-β particles, affecting their compaction and the overall contact area. The findings from this investigation underscored Cd2B's potential as the leading candidate in preventing amyloid-β accumulation.96 These findings illuminate a pathway for developing MBenes as medicinal agents in the fight against Alzheimer's disease.
3.5.4. Metabolic reprogramming of amino acid metabolism
As research progresses, understanding how these nanosheets interact with biological systems becomes paramount. Building upon previous work, the concept of nanometabolomics emerges as a promising approach. This innovative methodology merges nanoscience with metabolomics, allowing researchers to delve into the metabolic reprogramming induced by nanomaterials.97 By employing aqueous metabolomics and lipidomics, Zhang et al.97 provided invaluable bioinformation regarding Mo4/3B2−x nanosheets. They identified a safe concentration threshold of less than 50 mg L−1 for human umbilical vein endothelial cells (HUVECs) (Fig. 10). This discovery was pivotal, ensuring biosafety while exploring the nanosheets' cellular interactions. Interestingly, a range of concentrations, including low (5 mg L−1) and high (50 mg L−1), were utilized to investigate Mo4/3B2−x-cell interactions. Nanometabolomics revealed significant insights into the biocompatibility of Mo4/3B2−x nanosheets. The metabolic shifts observed in HUVECs were particularly noteworthy. At higher concentrations, specifically 50 mg L−1, a pronounced alteration in amino acid metabolism was detected. This shift disrupted various amino acid-related pathways, including degradation, fatty acid biosynthesis, and lipid metabolism. Moreover, these metabolic disturbances correlated closely with oxidative stress and an increase in reactive oxygen species (ROS).97 The integration of nanometabolomics provided a robust framework for future research, paving the way for advancements in biochemical, medical, and industrial applications.
Fig. 10. This illustration outlines the various biological processes occurring in human umbilical vein endothelial cells (HUVECs) when treated with Mo4/3B2−x nanosheets. The interaction between the nanosheets and HUVECs triggers several cellular responses, leading to distinct metabolic changes. Understanding these pathways is crucial for elucidating the effects and potential applications of Mo4/3B2−x nanosheets in biomedical contexts. Reproduced with permission from ref. 97. Copyright 2024 American Chemical Society.
4. Challenges
While both MXenes and MBenes are promising 2D materials, their differences in composition, synthesis methods, electronic properties, and stability highlight their unique advantages and potential applications. Understanding these distinctions paves the way for innovative uses in various fields.98 The theoretical predictions surrounding the stability of many MBenes spark significant interest.99 Yet, a notable gap exists: corresponding precursors have not been discovered or produced. This presents an intriguing challenge for researchers. Interestingly, all currently identified stable MBenes consist of early transition metal boron. However, there is a promising opportunity to synthesize and explore MBenes made from intermediate or late transition metals. Such advancements could broaden the scope of applications, leading to enhanced performance in energy storage and conversion technologies.100 In the preparation of MBene nanosheets, one of the primary challenges is ensuring consistency in morphology and physicochemical properties. This consistency is crucial, especially when scaling up production for practical applications. Moreover, researchers have concentrated their efforts on the electronic structure, elasticity, and magnetism of the M2AlB2 phase. However, there remains a vast, uncharted territory in studying other groups of MAB phases, such as MAlB and M3AlB4, along with their related MBenes. This gap indicates the necessity for systematic investigations into the structure and properties of MAlB, M2AlB2, M3AlB4, and their derived MBenes.99
The application of MBenes as catalysts in HER, OER, and ORR has garnered significant attention.101 Investigating their electrocatalytic activities reveals that MBenes possess promising characteristics that can be further enhanced. One of the most effective strategies for improving the electrocatalytic performance of MBenes involves modifying these materials by incorporating various dopants. Such modifications can drastically influence their catalytic activities, leading to optimized performance in specific applications. This adaptability is key, as it allows researchers to tailor the properties of MBenes to meet the demands of different catalytic reactions. The conductivity of MBenes significantly influences their efficacy in numerous electronic and catalytic scenarios. High conductivity acts as a catalyst for efficient electron transfer, which is vital for both catalyst and electrode materials. Consequently, these properties are paramount for rapid charge and discharge cycles in energy storage devices like batteries and supercapacitors. In the realm of electrocatalysts, the importance of efficient electron transfer cannot be overstated. It is indispensable for driving reactions at the electrode interface. Moreover, the electronic structure of MBenes intricately connects to their conductivity. This relationship subsequently impacts their overall catalytic activity. Researchers have discovered that by modulating the electronic structure, they can enhance the material's performance in targeted catalytic reactions. Thus, high conductivity leads to low resistance, swift charge transport, and improved electrochemical performance. These characteristics are crucial for the successful incorporation of MBenes in various energy conversion processes, including HER, ORR, and OER. All these factors combine to create a compelling case for the ongoing investigations of MBenes in advanced electronic and catalytic applications.101,102
Despite their promising characteristics, the implementation of MBenes in energy storage and conversion applications faces several significant challenges. Addressing these obstacles is crucial for maximizing their potential in this rapidly evolving field. One primary challenge lies in synthesis scalability. While laboratory-scale production of MBenes has been successful, scaling up synthesis methods to meet industrial demands remains difficult. The intricate processes required for synthesizing high-purity MBenes often involve costly and time-consuming techniques, hindering their commercialization. Researchers must focus on developing more efficient and cost-effective synthesis routes to facilitate broader application. Another critical issue is stability under operational conditions. MBenes may exhibit instability when exposed to varying temperature and humidity levels, which can lead to degradation over time. This stability issue is particularly concerning energy storage systems, where materials must endure repeated charge–discharge cycles without significant performance loss. Enhancing the stability of MBenes in real-world environments is essential for their successful integration into energy technologies. Moreover, the electrochemical performance of MBenes requires further optimization. While they show promise as electrode materials, their conductivity and overall electrochemical properties need improvement. Enhancing charge transfer and ion diffusion processes within the material can significantly boost performance in energy storage devices. Research into doping strategies or hybrid material systems could offer pathways to enhance their electrochemical characteristics. Furthermore, limited knowledge of the interactions between MBenes and other materials poses a challenge. The mechanisms that govern their performance in composite systems, such as batteries or supercapacitors, are not yet fully understood. Comprehensive studies are necessary to elucidate these interactions and optimize the design of MBenes for specific applications. Additionally, regulatory and safety concerns associated with the use of certain transition metals in MBenes must be addressed. Potential toxicity and the environmental impact of materials can hinder their acceptance in the market. Rigorous testing and adherence to safety standards are vital to ensure the responsible use of MBenes in energy applications.16,29,44,99
Biocompatibility is crucial when assessing materials for biomedical applications.103 In the context of MBenes, understanding their interaction with biological systems is paramount. As researchers explore the fascinating properties of MBenes, the implications for human health and safety come into play. MBenes exhibit unique structural and electronic characteristics, which influence their interactions with cells and tissues. When introduced into biological environments, their surface properties, such as charge and morphology, can dictate how they interact with cellular components. This interaction often determines the extent of cytotoxicity or biocompatibility. For instance, studies have shown that MBenes can promote cellular proliferation at certain concentrations. This is particularly evident in endothelial cells, where MBenes potentially enhance vascularization. However, the specifics of these interactions depend on various factors, including particle size, shape, and concentration. Identifying safe concentration ranges is essential. Research indicates that lower concentrations of MBenes may exhibit favorable biocompatibility, while higher concentrations can lead to toxic effects. For example, concentrations below 50 mg L−1 have been observed to maintain cellular health, whereas elevated levels can trigger oxidative stress and inflammatory responses.97 This concentration-dependent behavior highlights the need for careful optimization when considering MBenes for biomedical use. To gauge biocompatibility, it's vital to assess both toxicity and bioactivity. Various assays, including cell viability tests, oxidative stress measurements, and cytokine profiles, can provide insights into the safety and efficacy of MBenes. These assessments help in understanding how these materials may influence cellular functions, such as growth, differentiation, and apoptosis. Moreover, it is important to consider the long-term effects of MBene exposure. Chronic exposure studies can reveal whether these materials induce any adverse reactions over time, shedding light on their suitability for sustained biomedical applications. The integration of advanced characterization techniques and clinical translation studies will provide deeper insights into their biological interactions.
Overall, MBenes present a plethora of challenges that researchers must navigate. Firstly, synthesizing these materials often proves to be a complex endeavor due to their sensitive nature and the specific conditions required for their formation. The variability in chemical compositions can lead to inconsistencies in properties, complicating their characterization. The lack of standardized characterization techniques also poses a challenge, making it difficult to compare results across studies and fully understand the performance of MBenes in specific applications. Moreover, understanding their electronic and thermal conductivity poses another hurdle; variations in structure can significantly alter these key attributes. Additionally, the scalability of production methods remains a pressing issue, limiting the practical applications of MBenes in industries such as electronics and energy storage. Finally, environmental stability is a concern, as these materials may degrade under certain atmospheric conditions, impacting their longevity and performance. Thus, while MBenes hold great potential, overcoming these challenges is essential for unlocking their capabilities in real-world applications.
5. Future perspectives
The future of MBenes appears incredibly promising across various fields, including energy storage and conversion, catalysis, water treatment/pollutant removal, and biomedical applications. As research continues to declare their unique properties, MBenes could play a pivotal role in advancing technologies that address some of the world's most pressing challenges. For energy storage, MBenes are ready for the advancement of battery and supercapacitor technologies. Their suitable conductivity, high surface area, and tunable electronic properties make them ideal candidates for enhancing charge storage capacity and rate capability. Future research should focus on optimizing their electrochemical performance through innovative composite formulations or hybrid structures. By integrating MBenes with other materials, researchers can develop high-performance electrodes that enhance energy density and cycling stability.
The potential of MBenes in photocatalytic hydrogen production may lead to breakthroughs in sustainable energy solutions, contributing to a cleaner energy landscape. MBenes exhibit unique optical and electronic properties that facilitate efficient light absorption and charge separation—processes essential for effective photocatalysis. By harnessing solar energy, MBenes can facilitate the conversion of water into hydrogen, a clean fuel source that can be used in various energy applications. Furthermore, the versatility of MBenes allows for the development of composites with other photocatalytic materials, enhancing their stability and overall performance. This synergy can lead to improved photocatalytic efficiencies and increased hydrogen generation rates, making the process more viable for large-scale applications. However, challenges such as optimizing the fabrication strategies, enhancing the stability of MBenes under operating conditions, and improving their light absorption capabilities must be addressed to fully realize their potential in photocatalytic hydrogen production.
The catalytic properties of MBenes present exciting opportunities for various chemical processes. Their ability to facilitate reactions without the need for noble metals can significantly reduce costs, while maintaining efficiency. In the future, MBenes could become integral components in catalytic converters, CO2 reduction systems, and other green chemistry applications. The exploration of their bifunctional catalytic activity-where they can simultaneously promote multiple reactions—may further enhance their utility. Tailoring the surface chemistry of MBenes through functionalization or alloying could open new catalytic pathways and improve selectivity for desired products. Notably, MBenes demonstrate significant potential for sustainable environmental applications, particularly in water purification, photocatalytic remediation, and pollutant removal. Their high surface area and special adsorption features make them effective in capturing heavy metals and organic pollutants from wastewater, while their photocatalytic activity allows for the breakdown of harmful substances under sunlight. Additionally, MBenes can facilitate the conversion of CO2 into valuable chemicals and fuels, supporting climate change mitigation efforts. By replacing traditional noble metal catalysts with low-cost MBenes in industrial processes, industries can achieve greener production methods. Furthermore, integrating MBenes into biodegradable polymer composites enhances their properties, contributing to the reduction of plastic waste.
Air pollution stands as a formidable global challenge, demanding immediate and effective mitigation strategies. MBenes and their composites have emerged as promising materials for addressing air pollution through various innovative approaches. One of the primary strategies involves utilizing MBenes as catalysts in the decomposition of harmful pollutants, including volatile organic compounds (VOCs) and nitrogen oxides (NOx), resulted from their high surface area and unique electronic properties that allow for efficient catalytic activity, promoting the breakdown of these pollutants into less harmful substances. By integrating MBenes into existing catalytic converters or air purification systems, researchers can enhance the efficiency of pollutant removal, contributing to cleaner air. In addition to catalytic applications, MBene-based composites can be employed in adsorption technologies for air pollution control. Their ability to adsorb harmful gases, including carbon dioxide and particulate matter, positions them as effective materials for developing advanced filtration systems. By functionalizing the surfaces of MBenes, researchers can tailor their properties to selectively capture specific pollutants, improving the overall effectiveness of air purification systems. Furthermore, combining MBenes with other materials in hybrid systems could lead to enhanced performance and broader applicability in various environmental settings.
In the biomedical field, MBenes and their composites hold great potential for cancer therapy, drug delivery systems, and biosensing applications. Their biocompatibility, coupled with their ability to be engineered at the nanoscale, opens avenues for targeted therapies that minimize side effects. Future advancements may involve the development of multifunctional MBenes that can simultaneously deliver drugs and monitor therapeutic efficacy through real-time biosensing capabilities. Furthermore, their unique optical properties could lead to innovations in imaging techniques or photothermal therapies, allowing for precise treatment of diseases such as cancer. The integration of MBenes along with their composites into biosensing technologies opens up thrilling possibilities. This advancement paves the way for sensitive and selective detection of biomolecules, pathogens, and disease markers. With their unique properties, MBenes enhance the accuracy and efficiency of biosensors, making them crucial in the realm of diagnostics. MBene-based composites possess a high surface area that enhances interaction with target analytes, improving sensor sensitivity. By functionalizing their surfaces with specific receptors or antibodies, researchers can create tailored biosensors capable of detecting substances like glucose or proteins. Their electrochemical properties enable real-time monitoring through changes in current or voltage, while their photoluminescent characteristics facilitate fluorescence-based assays for early disease detection. It appears that future explorations should focus on the application of MBenes in biosensing, including advancements in diagnostics and personalized medicine.
One of the most important things that should be considered in this context is the effect of MBenes on the environment. MBenes are gaining attention for their environmental friendliness, as they are made from abundant, low-toxicity elements such as boron and metals like titanium, nickel, and iron. These materials are not only earth-abundant but also pose less environmental risk compared to rare or toxic metals used in many advanced nanomaterials. The synthesis of MBenes often employs eco-friendly methods, such as using mild reaction conditions and non-toxic solvents, which helps reduce energy consumption and minimizes the generation of harmful by-products. Furthermore, MBenes can be produced using green chemistry principles, such as low-temperature processing or solvent-free reactions, which make the entire lifecycle of these materials more sustainable and align well with environmentally conscious industrial practices.
In terms of economic feasibility and scalability, MBenes offer significant promise due to their low-cost raw materials and the simplicity of their synthesis. Compared to other advanced nanomaterials, the production of MBenes requires less expensive components, making their integration into various industries more affordable. Their synthesis can also be scaled up efficiently, using techniques such as chemical vapor deposition or liquid-phase exfoliation, which are compatible with large-scale production without significantly increasing energy requirements. This scalability ensures that MBenes could be widely adopted for applications in energy storage, catalysis, sensors, and environmental remediation, all of which have growing market demand. As research continues to optimize their synthesis methods, the commercial viability of MBenes becomes even more apparent, making them a sustainable and economically attractive material for diverse industrial applications.
6. Conclusion
Recent years have witnessed remarkable advancements in the environmental and biomedical applications of MBenes. These developments highlight the growing versatility and effectiveness of MBenes, showcasing their potential to address pressing challenges in both fields. Their innovative uses are transforming how we approach environmental sustainability and health diagnostics. Researchers have successfully demonstrated the potential of MBenes in water purification, where their unique adsorption capabilities and high surface area enable efficient elimination of organic pollutants and toxic heavy metals. Notably, MBenes offer promising air pollution mitigation strategies through their catalytic properties for decomposing harmful pollutants and their ability to adsorb gases, making them effective materials for enhancing air purification systems and improving overall air quality. MBenes exhibit significant potential in batteries and supercapacitors by enhancing electrochemical performance through their high surface area, conductivity, and tunable properties, thus contributing to improved energy storage capacity and cycling stability. Additionally, MBenes present a promising approach for NRRs by acting as efficient catalysts that facilitate the conversion of atmospheric nitrogen into ammonia, thereby contributing to sustainable fertilizer production and addressing global nitrogen fixation challenges. Notably, MBenes serve as effective electrocatalysts for the conversion of nitrogen oxides, enabling the reduction of these harmful pollutants into less toxic substances, thereby providing a sustainable solution for mitigating air pollution and improving environmental quality. These 2D materials demonstrate remarkable potential in facilitating the OER, ORR, and HER, acting as efficient electrocatalysts that enhance overall energy conversion procedures in fuel cells and water-splitting applications, ultimately promoting sustainable energy technologies. Their ability to serve as effective co-catalysts in photocatalytic hydrogen production positions them as key players in sustainable energy solutions. In the biomedical field, MBenes have shown promise in biosensing applications, offering enhanced sensitivity for detecting biomolecules and disease markers.
Despite their promising potential, MBenes face several challenges that hinder their widespread adoption in various applications. One significant challenge is the scalability of synthesis methods, as producing high-quality MBenes in large quantities remains difficult and often involves complex processes that may not be economically feasible for industrial applications. Additionally, the stability of MBenes under operational conditions is a concern; they may degrade or lose their functional properties when exposed to harsh environments, which can limit their effectiveness in long-term applications. Another challenge lies in the understanding of their interactions with other materials and components in composite systems. Without a comprehensive understanding of how MBenes behave in different contexts, optimizing their performance in applications like catalysis or energy storage becomes challenging. Furthermore, there is a need for standardized characterization techniques to assess the properties and performance of MBenes consistently across various studies. Continued research will focus on optimizing their synthesis methods to ensure scalability and cost-effectiveness, making MBenes more accessible for practical applications. Enhancements in their stability and performance will be crucial for their successful integration into real-world systems, particularly in energy storage and conversion technologies. Furthermore, the exploration of novel hybrid structures that combine MBenes with other materials could lead to breakthroughs in both environmental remediation and biomedical diagnostics. As the understanding of MBenes deepens, their multifunctional capabilities could pave the way for innovative solutions that address critical challenges in sustainability and healthcare, ultimately contributing to a cleaner environment and improved health outcomes.
Data availability
No data was used for the research described in the article.
Author contributions
S. Iravani: supervision, conceptualization, writing – review & editing; A. Zarepour: writing – review & editing; A. Khosravi: visualization, writing – review & editing; A. Zarrabi: supervision, writing – review & editing.
Conflicts of interest
Author(s) declared no conflict of interest.
References
- Khaledialidusti R. Khazaei M. Wang V. Miao N. Si C. Wang J. Wang J. Exploring structural, electronic, and mechanical properties of 2D hexagonal MBenes. J. Phys. Condens. 2021;33:155503. doi: 10.1088/1361-648X/abbb0e. [DOI] [PubMed] [Google Scholar]
- Iravani S. MXenes and MXene-based (nano)structures: A perspective on greener synthesis and biomedical prospects. Ceram. Int. 2022;48:24144–24156. doi: 10.1016/j.ceramint.2022.05.137. [DOI] [Google Scholar]
- Mostafavi E. Iravani S. MXene-Graphene Composites: A Perspective on Biomedical Potentials. Nano–Micro Lett. 2022;14:130. doi: 10.1007/s40820-022-00880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R. Maktedar S. S. MXenes: A comprehensive review of synthesis, properties, and progress in supercapacitor applications. J Materiomics. 2023;9:1196–1241. doi: 10.1016/j.jmat.2023.08.011. [DOI] [Google Scholar]
- Gogotsi Y. The Future of MXenes. Chem. Mater. 2023;35:8767–8770. doi: 10.1021/acs.chemmater.3c02491. [DOI] [Google Scholar]
- Downes M. Shuck C. E. Lord R. W. Anayee M. Shekhirev M. Wang R. J. Hryhorchuk T. Dahlqvist M. Rosen J. Gogotsi Y. M5X4: A Family of MXenes. ACS Nano. 2023;17:17158–17168. doi: 10.1021/acsnano.3c04967. [DOI] [PubMed] [Google Scholar]
- Oyehan T. A. Salami B. A. Abdulrasheed A. A. Hambali H. U. Gbadamosi A. Valsami-Jones E. Saleh T. A. MXenes: Synthesis, properties, and applications for sustainable energy and environment. Appl. Mater. Today. 2023;35:101993. doi: 10.1016/j.apmt.2023.101993. [DOI] [Google Scholar]
- Guo Z. Zhou J. Sun Z. New two-dimensional transition metal borides for Li ion batteries and electrocatalysis. J. Mater. Chem. A. 2017;5:23530–23535. doi: 10.1039/C7TA08665B. [DOI] [Google Scholar]
- Zhang B. Zhou J. Sun Z. MBenes: progress, challenges and future. J. Mater. Chem. A. 2022;10:15865–15880. doi: 10.1039/D2TA03482D. [DOI] [Google Scholar]
- Zhang H. Xiang H. Dai F.-z. Zhang Z. Zhou Y. First demonstration of possible two-dimensional MBene CrB derived from MAB phase Cr2AlB2. J. Mater. Sci. Technol. 2018;34:2022–2026. doi: 10.1016/j.jmst.2018.02.024. [DOI] [Google Scholar]
- Alameda L. T. Moradifar P. Metzger Z. P. Alem N. Schaak R. E. Topochemical Deintercalation of Al from MoAlB: Stepwise Etching Pathway, Layered Intergrowth Structures, and Two-Dimensional MBene. J. Am. Chem. Soc. 2018;140:8833–8840. doi: 10.1021/jacs.8b04705. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wang H. Wang C. Liu J. Wu H. Liu N. Wang Q. Shang Y. Zheng J. Novel 2D Material of MBenes: Structures, Synthesis, Properties, and Applications in Energy Conversion and Storage. Small. 2024:2405870. doi: 10.1002/smll.202405870. [DOI] [PubMed] [Google Scholar]
- Guo Z. Zhang L. Azam T. Wu Z.-S. Recent advances and key challenges of the emerging MBenes from synthesis to applications. Metal Met. 2024;1:e12. [Google Scholar]
- Javed M. S. Zhang X. Ahmad T. Usman M. Shah S. S. A. Ahmad A. Hussain I. Majeed S. Khawar M. R. Choi D. Xia C. Al Zoubi W. Assiri M. A. Hassan A. M. Ali S. Han W. MXenes to MBenes: Latest development and opportunities for energy storage devices. Mater. Today. 2024;74:121–148. doi: 10.1016/j.mattod.2024.01.001. [DOI] [Google Scholar]
- Khan A. J. Shah S. S. Khan S. Mateen A. Iqbal B. Naseem M. He L. Zhang Y. Che Y. Tang Y. Xu M. Gao L. Zhao G. 2D metal borides (MBenes): Synthesis methods for energy storage applications. Chem. Eng. J. 2024;497:154429. doi: 10.1016/j.cej.2024.154429. [DOI] [Google Scholar]
- Seetharaman A. Kandasamy M. Khanolkar H. Manickavasakam K. Chakraborty B. MBenes: Powering the future of energy storage and electrocatalysis. J. Energy Storage. 2024;100:113310. doi: 10.1016/j.est.2024.113310. [DOI] [Google Scholar]
- Wang J. Ye T.-N. Gong Y. Wu J. Miao N. Tada T. Hosono H. Discovery of hexagonal ternary phase Ti2InB2 and its evolution to layered boride TiB. Nat. Commun. 2019;10:2284. doi: 10.1038/s41467-019-10297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao N. Wang J. Gong Y. Wu J. Niu H. Wang S. Li K. Oganov A. R. Tada T. Hosono H. Computational Prediction of Boron-Based MAX Phases and MXene Derivatives. Chem. Mater. 2020;32:6947–6957. doi: 10.1021/acs.chemmater.0c02139. [DOI] [Google Scholar]
- Zhou J. J. Palisaitis J. J. Halim J. J. Dahlqvist M. M. Tao Q. Q. Persson I. I. Hultman L. L. Persso P. O. Å. Rosen J. Boridene: Two-dimensional Mo4/3B2-x with ordered metal vacancies obtained by chemical exfoliation. Science. 2021;373:801–805. doi: 10.1126/science.abf6239. [DOI] [PubMed] [Google Scholar]
- Gorkan T. Vatansever E. Akıncı Ü. Gökoglu G. Aktürk E. Ciraci S. Above Room Temperature Ferromagnetism in Gd2B2 Monolayer with High Magnetic Anisotropy. J. Phys. Chem. C. 2020;124:12816–12823. doi: 10.1021/acs.jpcc.0c03304. [DOI] [Google Scholar]
- Yao Y. Miao N. Gong Y. Wang J. Theoretical exploration of quaternary hexagonal MAB phases and two-dimensional derivatives. Nanoscale. 2021;13:13208–13214. doi: 10.1039/D1NR02882K. [DOI] [PubMed] [Google Scholar]
- Dahlqvist M. Tao Q. Zhou J. Palisaitis J. Rosen J. Theoretical Prediction and Synthesis of a Family of Atomic Laminate Metal Borides with In-Plane Chemical Ordering. J. Am. Chem. Soc. 2020;142:18583–18591. doi: 10.1021/jacs.0c08113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T. Zhang S. Guo Y. Wang Q. TiC2: a new two-dimensional sheet beyond MXenes. Nanoscale. 2016;8:233–242. doi: 10.1039/C5NR04472C. [DOI] [PubMed] [Google Scholar]
- Yu Y. Guo Z. Peng Q. Zhou J. Sun Z. Novel two-dimensional molybdenum carbides as high capacity anodes for lithium/sodium-ion batteries. J. Mater. Chem. A. 2019;7:12145–12153. doi: 10.1039/C9TA02650A. [DOI] [Google Scholar]
- Jakubczak M. Szuplewska A. Rozmysłowska-Wojciechowska A. Rosenkranz A. Jastrzębska A. M. Novel 2D MBenes—Synthesis, Structure, and Biotechnological Potential. Adv. Funct. Mater. 2021;31:2103048. doi: 10.1002/adfm.202103048. [DOI] [Google Scholar]
- Chang M. Ai L. Yang R. Wang X. Xu Y. Jiang J. Two-dimensional layered MBene membrane towards sustainable freshwater production from solar interfacial evaporation. Chem. Eng. J. 2024;486:150078. doi: 10.1016/j.cej.2024.150078. [DOI] [Google Scholar]
- Jin S. Shi Z. Wang R. Guo Y. Wang L. Hu Q. Liu K. Li N. Zhou A. 2D MoB MBene: An Efficient Co-Catalyst for Photocatalytic Hydrogen Production under Visible Light. ACS Nano. 2024;18:12524–12536. doi: 10.1021/acsnano.4c02642. [DOI] [PubMed] [Google Scholar]
- Lin X. Chen J. Yuan Z. Yang M. Chen G. Yu D. Zhang M. Hong W. Chen X. Integrative solar absorbers for highly efficient solar steam generation. J. Mater. Chem. A. 2018;6:4642–4648. doi: 10.1039/C7TA08256H. [DOI] [Google Scholar]
- Akgul E. T. Altıncı O. C. Umay A. Aghamohammadi P. Farghaly A. A. Ma P. Chen Y. Demir M. Nanoengineering of 2D MBenes for energy storage applications: A review. J. Energy Storage. 2024;84:110882. doi: 10.1016/j.est.2024.110882. [DOI] [Google Scholar]
- Shah S. S. Aziz M. A. Al Marzooqi M. Khan A. Z. Yamani Z. H. Enhanced light-responsive supercapacitor utilizing BiVO4 and date leaves-derived carbon: a leap towards sustainable energy harvesting and storage. J. Power Sources. 2024;602:234334. doi: 10.1016/j.jpowsour.2024.234334. [DOI] [Google Scholar]
- Han Y. Wang L. Zheng B. Wang J. Zhang L. Xiao B. Exploring the potential of MB2 MBene family as promising anodes for Li-ion batteries. RSC Adv. 2024;14:11112–11120. doi: 10.1039/D4RA00287C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao N. Gong Y. Zhang H. Shen Q. Yang R. Zhou J. Hosono H. Wang J. Discovery of Two-dimensional Hexagonal MBene HfBO and Exploration on its Potential for Lithium-Ion Storage. Angew. Chem. 2023;135:e202308436. doi: 10.1002/ange.202308436. [DOI] [PubMed] [Google Scholar]
- Khaledialidusti R. Khazaei M. Wang V. Miao N. Si C. Wang J. Wang J. Exploring structural, electronic, and mechanical properties of 2D hexagonal MBenes. J. Phys.: Condens. Matter. 2021;33:155503. doi: 10.1088/1361-648X/abbb0e. [DOI] [PubMed] [Google Scholar]
- Chen D. Jin Z. Zhao B. Wang Y. He Q. MBene as a Theranostic Nanoplatform for Photocontrolled Intratumoral Retention and Drug Release. Adv. Mater. 2021;33:2008089. doi: 10.1002/adma.202008089. [DOI] [PubMed] [Google Scholar]
- Xu T. Wang Y. Xiong Z. Wang Y. Zhou Y. Li X. A Rising 2D Star: Novel MBenes with Excellent Performance in Energy Conversion and Storage. Nano–Micro Lett. 2022;15:6. doi: 10.1007/s40820-022-00976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed M. S. Zhang X. Ahmad T. Usman M. Shah S. S. A. Ahmad A. Hussain I. Majeed S. Khawar M. R. Choi D. MXenes to MBenes: latest development and opportunities for energy storage devices. Mater. Today. 2024;74:121–148. doi: 10.1016/j.mattod.2024.01.001. [DOI] [Google Scholar]
- Guo Z. Zhang L. Azam T. Wu Z. S. Recent advances and key challenges of the emerging MBenes from synthesis to applications. Metal Met. 2024;1:e12. [Google Scholar]
- Rout C. S. Shinde P. V. Patra A. Jeong S. M. Recent Developments and Future Perspectives of Molybdenum Borides and MBenes. Advanced Science. 2024:2308178. doi: 10.1002/advs.202308178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R. Bogdanovski D. Achenbach J.-O. Zhang S. Hans M. Primetzhofer D. Schneider J. M. Scheu C. Direct MoB MBene domain formation in magnetron sputtered MoAlB thin films. Nanoscale. 2021;13:18077–18083. doi: 10.1039/D1NR05712J. [DOI] [PubMed] [Google Scholar]
- González-Poggini S. Rosenkranz A. Colet-Lagrille M. Two-Dimensional Nanomaterials for the Removal of Pharmaceuticals from Wastewater: A Critical Review. Processes. 2021;9:2160. doi: 10.3390/pr9122160. [DOI] [Google Scholar]
- Tao J. Arshad N. Maqsood G. Asghar M. S. Zhu F. Lin L. Irshad M. S. Wang X. The Quest for Two-Dimensional MBenes: From Structural Evolution to Solar-Driven Hybrid Systems for Water-Fuel-Energy Generation and Phototherapy. Small. 2024;20:2401603. doi: 10.1002/smll.202401603. [DOI] [PubMed] [Google Scholar]
- Liu F. Lou Y. Xia F. Hu B. Immobilizing nZVI particles on MBenes to enhance the removal of U(VI) and Cr(VI) by adsorption-reduction synergistic effect. Chem. Eng. J. 2023;454:140318. doi: 10.1016/j.cej.2022.140318. [DOI] [Google Scholar]
- Liu X. Liu Z. Deng H. Theoretical Evaluation of MBenes as Catalysts for the CO2 Reduction Reaction. J. Phys. Chem. C. 2021;125:19183–19189. doi: 10.1021/acs.jpcc.1c02749. [DOI] [Google Scholar]
- Ozkan M. MXenes vs MBenes: Demystifying the materials of tomorrow's carbon capture revolution. MRS Energy Sustain. 2024;11:181–190. doi: 10.1557/s43581-024-00082-6. [DOI] [Google Scholar]
- Li M. Zhang Y. Gao D. Li Y. Yu C. Fang Y. Huang Y. Tang C. Guo Z. Prediction of M3B4-type MBenes as Promising Catalysts for CO2 Capture and Reduction. Chemphyschem. 2024;25:e202300837. doi: 10.1002/cphc.202300837. [DOI] [PubMed] [Google Scholar]
- Mir S. H. Yadav V. K. Singh J. K. Efficient CO2 Capture and Activation on Novel Two-Dimensional Transition Metal Borides. ACS Appl. Mater. Interfaces. 2022;14:29703–29710. doi: 10.1021/acsami.2c02469. [DOI] [PubMed] [Google Scholar]
- Xiao Y. Shen C. Hadaeghi N. Quantum Mechanical Screening of 2D MBenes for the Electroreduction of CO2 to C1 Hydrocarbon Fuels. J. Phys. Chem. Lett. 2021;12:6370–6382. doi: 10.1021/acs.jpclett.1c01499. [DOI] [PubMed] [Google Scholar]
- Zhang B. Zhou J. Sun Z. New horizons of MBenes: highly active catalysts for the CO oxidation reaction. Nanoscale. 2023;15:483–489. doi: 10.1039/D2NR05705K. [DOI] [PubMed] [Google Scholar]
- Shukla A. Sharma G. Krishnamurty S. Functionalized Mo2BX2 (X = H, OH, O) MBenes as a promising sensor, capturer and storage material for environmentally toxic gases: A case study of 1T and 2H phase. Appl. Surf. Sci. 2023;615:156299. doi: 10.1016/j.apsusc.2022.156299. [DOI] [Google Scholar]
- Chen C. Xu B. Zhang J. Ma S. Wang Y. Li J. Yi L. Gas sensing potential of monolayer MoB: A first principles study. Vacuum. 2023;210:111883. doi: 10.1016/j.vacuum.2023.111883. [DOI] [Google Scholar]
- Thakur A. Rasyotra A. Jasuja K. Review and Perspectives of Electrochemical Energy Conversion and Storage in Metal Diborides and XBenes. Energy Fuels. 2023;37:18310–18329. doi: 10.1021/acs.energyfuels.3c02785. [DOI] [Google Scholar]
- Tripathy D. B. Novel Two-Dimensional MBenes: Synthesis, Properties, and Energy Storage and Electrocatalytic Applications of Two-Dimensional Metal Borides. ACS Appl. Eng. Mater. 2024;2:1209–1224. doi: 10.1021/acsaenm.4c00211. [DOI] [Google Scholar]
- Li B. Wu Y. Li N. Chen X. Zeng X. Arramel A. Zhao X. Jiang J. Single-Metal Atoms Supported on MBenes for Robust Electrochemical Hydrogen Evolution. ACS Appl. Mater. Interfaces. 2020;12:9261–9267. doi: 10.1021/acsami.9b20552. [DOI] [PubMed] [Google Scholar]
- Xiong W. Feng X. Xiao Y. Huang T. Li X. Huang Z. Ye S. Li Y. Ren X. Wang X. Ouyang X. Zhang Q. Liu J. Fluorine-free prepared two-dimensional molybdenum boride (MBene) as a promising anode for lithium-ion batteries with superior electrochemical performance. Chem. Eng. J. 2022;446:137466. doi: 10.1016/j.cej.2022.137466. [DOI] [Google Scholar]
- Gao S. Hao J. Zhang X. Li L. Zhang C. Wu L. Ma X. Lu P. Liu G. Two dimension transition metal boride Y2B2 as a promising anode in Li-ion and Na-ion batteries. Comput. Mater. Sci. 2021;200:110776. doi: 10.1016/j.commatsci.2021.110776. [DOI] [Google Scholar]
- Liang B. Ma N. Wang Y. Wang T. Fan J. N-functionalized Ti2B MBene as high-performance anode materials for sodium-ion batteries: A DFT study. Appl. Surf. Sci. 2022;599:153927. doi: 10.1016/j.apsusc.2022.153927. [DOI] [Google Scholar]
- Jin J. Schwingenschlögl U. Exploration of two-dimensional molybdenum-borides and potential applications. npj 2D Mater. Appl. 2022;6:49. doi: 10.1038/s41699-022-00319-3. [DOI] [Google Scholar]
- Xie M. Pang J. Jin W. Kuang X. Theoretical Prediction of Two-Dimensional Metal Boride Mg4B6 as a High-Capacity Electrode Material for Lithium-Ion Batteries. J. Phys. Chem. C. 2022;126:17474–17481. doi: 10.1021/acs.jpcc.2c04448. [DOI] [Google Scholar]
- Jia J. Li B. Duan S. Cui Z. Gao H. Monolayer MBenes: prediction of anode materials for high-performance lithium/sodium ion batteries. Nanoscale. 2019;11:20307–20314. doi: 10.1039/C9NR05708K. [DOI] [PubMed] [Google Scholar]
- Gao D. Zhong J. Li Y. Zhang Y. Zhao S. Guo Z. Lin J. Fang Y. Tang C. Efficient polyselenides anchoring and conversion by functionalized Mo2B2 MBene for advanced Li-Se battery. J. Alloys Compd. 2023;932:167722. doi: 10.1016/j.jallcom.2022.167722. [DOI] [Google Scholar]
- Xiao Y. Li Y. Guo Z. Tang C. Sa B. Miao N. Zhou J. Sun Z. Functionalized Mo2B2 MBenes: promising anchoring and electrocatalysis materials for lithium-sulfur battery. Appl. Surf. Sci. 2021;566:150634. doi: 10.1016/j.apsusc.2021.150634. [DOI] [Google Scholar]
- Li Z. Zeng Q. Yu Y. Liu Y. Chen A. Guan J. Wang H. Liu W. Liu X. Liu X. Zhang L. Application of transition metal boride nanosheet as sulfur host in high loading Li-S batteries. Chem. Eng. J. 2023;452:139366. doi: 10.1016/j.cej.2022.139366. [DOI] [Google Scholar]
- Dou M. Li H. Yao Q. Wang J. Liu Y. Wu F. Room-temperature ferromagnetism in two-dimensional transition metal borides: a first-principles investigation. Phys. Chem. Chem. Phys. 2021;23:10615–10620. doi: 10.1039/D1CP00052G. [DOI] [PubMed] [Google Scholar]
- Iravani S. Zarepour A. Khosravi A. Varma R. S. Zarrabi A. Next-generation nitrogen fixation strategy: empowering electrocatalysis with MXenes. Green Chem. 2024;26:8942–8968. doi: 10.1039/D4GC01566E. [DOI] [Google Scholar]
- Biswas A. Bhardwaj S. Boruah T. Sundar Dey R. Electrochemical ammonia synthesis: fundamental practices and recent developments in transition metal boride, carbide and nitride-class of catalysts. Mater. Adv. 2022;3:5207–5233. doi: 10.1039/D2MA00279E. [DOI] [Google Scholar]
- Yang X. Shang C. Zhou S. Zhao J. MBenes: emerging 2D materials as efficient electrocatalysts for the nitrogen reduction reaction. Nanoscale Horiz. 2020;5:1106–1115. doi: 10.1039/D0NH00242A. [DOI] [PubMed] [Google Scholar]
- Qi S. Fan Y. Zhao L. Li W. Zhao M. Two-dimensional transition metal borides as highly efficient N2 fixation catalysts. Appl. Surf. Sci. 2021;536:147742. doi: 10.1016/j.apsusc.2020.147742. [DOI] [Google Scholar]
- Xiao Y. Shen C. Long T. Theoretical Establishment and Screening of an Efficient Catalyst for N2 Electroreduction on Two-Dimensional Transition-Metal Borides (MBenes) Chem. Mater. 2021;33:4023–4034. doi: 10.1021/acs.chemmater.1c00424. [DOI] [Google Scholar]
- Cheng Y. Mo J. Li Y. Zhang Y. Song Y. A systematic computational investigation of the water splitting and N2 reduction reaction performances of monolayer MBenes. Phys. Chem. Chem. Phys. 2021;23:6613–6622. doi: 10.1039/D0CP06405J. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Li Y. Gao D. Zhao S. Guo Z. Huang Y. Fang Y. Meng F. Tang C. Boosting nitrogen reduction reaction with boron sites supported by defective Mo2B2O2 MBene. Appl. Catal., A. 2022;646:118866. doi: 10.1016/j.apcata.2022.118866. [DOI] [Google Scholar]
- Zhang H. Wang S. Wang H. Huang B. Dong S. Dai Y. Wei W. Two-dimensional transition metal borides as high activity and selectivity catalysts for ammonia synthesis. Nanoscale. 2021;13:17331–17339. doi: 10.1039/D1NR05774J. [DOI] [PubMed] [Google Scholar]
- Guo X. Lin S. Gu J. Zhang S. Chen Z. Huang S. Establishing a Theoretical Landscape for Identifying Basal Plane Active 2D Metal Borides (MBenes) toward Nitrogen Electroreduction. Adv. Funct. Mater. 2021;31:2008056. doi: 10.1002/adfm.202008056. [DOI] [Google Scholar]
- Yao M. Shi Z. Zhang P. Ong W.-J. Jiang J. Ching W.-Y. Li N. Density Functional Theory Study of Single Metal Atoms Embedded into MBene for Electrocatalytic Conversion of N2 to NH3. ACS Appl. Nano Mater. 2020;3:9870–9879. doi: 10.1021/acsanm.0c01922. [DOI] [Google Scholar]
- Kim D. H. Ringe S. Kim H. Kim S. Kim B. Bae G. Oh H.-S. Jaouen F. Kim W. Kim H. Choi C. H. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 2021;12:1856. doi: 10.1038/s41467-021-22147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. Shen C. Transition-Metal Borides (MBenes) as New High-Efficiency Catalysts for Nitric Oxide Electroreduction to Ammonia by a High-Throughput Approach. Small. 2021;17:2100776. doi: 10.1002/smll.202100776. [DOI] [PubMed] [Google Scholar]
- Zhang T. Zhang B. Peng Q. Zhou J. Sun Z. Mo2B2 MBene-supported single-atom catalysts as bifunctional HER/OER and OER/ORR electrocatalysts. J. Mater. Chem. A. 2021;9:433–441. doi: 10.1039/D0TA08630D. [DOI] [Google Scholar]
- Park S. J. Nguyen T. H. Tran D. T. Dinh V. A. Lee J. H. Kim N. H. Delaminated MBene sheets beyond usual 2D transition metal materials for securing Pt single atoms to boost hydrogen evolution. Energy Environ. Sci. 2023;16:4093–4104. doi: 10.1039/D3EE01314F. [DOI] [Google Scholar]
- Liu X. Ge X. Dong Y. Fu K. Meng F. Si R. Zhang M. Xu X. First-principle calculations on the structure, electronic property and catalytic activity for hydrogen evolution reaction of 2D transition-metal borides. Mater. Chem. Phys. 2020;253:123334. doi: 10.1016/j.matchemphys.2020.123334. [DOI] [Google Scholar]
- Zhang B. Zhou J. Guo Z. Peng Q. Sun Z. Two-dimensional chromium boride MBenes with high HER catalytic activity. Appl. Surf. Sci. 2020;500:144248. doi: 10.1016/j.apsusc.2019.144248. [DOI] [Google Scholar]
- Li F. Tang Q. First-Principles Calculations of TiB MBene Monolayers for Hydrogen Evolution. ACS Appl. Nano Mater. 2019;2:7220–7229. doi: 10.1021/acsanm.9b01718. [DOI] [Google Scholar]
- Feng S. Miao N. Wang J. Hexagonal MBene (Hf2BO2): A Promising Platform for the Electrocatalysis of Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces. 2021;13:56131–56139. doi: 10.1021/acsami.1c16449. [DOI] [PubMed] [Google Scholar]
- Wang E. Zhang B. Zhou J. Sun Z. High catalytic activity of MBenes-supported single atom catalysts for oxygen reduction and oxygen evolution reaction. Appl. Surf. Sci. 2022;604:154522. doi: 10.1016/j.apsusc.2022.154522. [DOI] [Google Scholar]
- Zhu X. Zhou X. Jing Y. Li Y. Electrochemical synthesis of urea on MBenes. Nat. Commun. 2021;12:4080. doi: 10.1038/s41467-021-24400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. Shen C. Xiong Z. Li J. Zhang W. A strategy to address the challenge of electrochemical CO2 and N2 coupling to synthesis urea on two-dimensional metal borides (MBenes) by computational screening. Mater. Today Phys. 2022;26:100726. doi: 10.1016/j.mtphys.2022.100726. [DOI] [Google Scholar]
- Biswal L. Mohanty R. Nayak S. Parida K. Review on MXene/TiO2 nanohybrids for photocatalytic hydrogen production and pollutant degradations. J. Environ. Chem. Eng. 2022;10:107211. doi: 10.1016/j.jece.2022.107211. [DOI] [Google Scholar]
- Christoforidis K. C. Fornasiero P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem. 2017;9:1523–1544. doi: 10.1002/cctc.201601659. [DOI] [Google Scholar]
- Rafique M. Hajra S. Irshad M. Usman M. Imran M. Assiri M. A. Ashraf W. M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega. 2023;8:25640–25648. doi: 10.1021/acsomega.3c00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Wang P. Liang Z. Li Z. Liu N. Ma Q. Novel Cu nanocluster superlattice/MBene-induced ECL enhancement strategy for miRNA-221 detection. Chem. Eng. J. 2023;478:147512. doi: 10.1016/j.cej.2023.147512. [DOI] [Google Scholar]
- Lan L. Fan X. Zhao C. Gao J. Qu Z. Song W. Yao H. Li M. Qiu T. Two-dimensional MBenes with ordered metal vacancies for surface-enhanced Raman scattering. Nanoscale. 2023;15:2779–2787. doi: 10.1039/D2NR06280A. [DOI] [PubMed] [Google Scholar]
- Yang S. Zhao L. Yang X. Yang L. Fa H. Wang Y. Huo D. Hou C. Zhong D. Yang M. A portable smartphone detection of ctDNA using MnB2 nanozyme and paper-based analytical device. Talanta. 2024;278:126523. doi: 10.1016/j.talanta.2024.126523. [DOI] [PubMed] [Google Scholar]
- Yang S. Zhao L. Yang X. Yang L. Fa H. Wang Y. Huo D. Hou C. Zhong D. Yang M. MBene as novel and effective electrochemical material used to enhance paper-based biosensor for point-of-care testing ctDNA from mice serum. Chem. Eng. J. 2024;498:155345. doi: 10.1016/j.cej.2024.155345. [DOI] [Google Scholar]
- Gao Y. Wang P. Liang Z. Li Z. Li W. Ma Q. The ECL enhancement of MBene QDs with nanoparticle-on-mirror structure for sensitive detection of exosomal miRNA. Anal. Chim. Acta. 2024;1314:342792. doi: 10.1016/j.aca.2024.342792. [DOI] [PubMed] [Google Scholar]
- Zhang S. Huang Y. Ren H. Chen Y. Yan S. Dai H. Lv L. Facile and portable multimodal sensing platform driven by photothermal-controlled release system for biomarker detection. Biosens. Bioelectron. 2023;235:115413. doi: 10.1016/j.bios.2023.115413. [DOI] [PubMed] [Google Scholar]
- Jin Z. Chen D. Zhao P. Wen Y. Fan M. Zhou G. Wang Y. He Q. Coordination-induced exfoliation to monolayer Bi-anchored MnB2 nanosheets for multimodal imaging-guided photothermal therapy of cancer. Theranostics. 2020;10:1861–1872. doi: 10.7150/thno.39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Wang Y. Zhao P. Jin Z. Yang Q. He Q. Novel two-dimensional FeB nanosheets for photoacoustic imaging-guided NIR-photothermal therapy. Mater. Lett. 2024;376:137306. doi: 10.1016/j.matlet.2024.137306. [DOI] [Google Scholar]
- Dahri M. Jahromi A. M. Nikzad A. Mohammadgholian M. Rahmanian M. Abolmaali S. S. Maleki R. Novel bioengineered MBenes for the treatment of Alzheimer's disease: An in-Sillico study. J. Biomol. Struct. Dyn. 2022;40:12268–12276. doi: 10.1080/07391102.2021.1969288. [DOI] [PubMed] [Google Scholar]
- Zhang D. Liang G. Gui L. Zheng W. Zeng Y. Liu Y. Li X. Yang Y. Fan R. Lu Y. Hu X. Guan J. Li T. Yang H. Cheng J. Gong M. Nanometabolomics Elucidated Biological Prospective of Mo4/3B2–x Nanosheets: Toward Metabolic Reprogramming of Amino Acid Metabolism. ACS Appl. Mater. Interfaces. 2024;16:30622–30635. doi: 10.1021/acsami.4c02018. [DOI] [PubMed] [Google Scholar]
- Gunda H. Klebanoff L. E. Sharma P. A. Varma A. K. Dolia V. Jasuja K. Stavila V. Progress, Challenges, and Opportunities in the Synthesis, Characterization, and Application of Metal-Boride-Derived Two-Dimensional Nanostructures. ACS Mater. Lett. 2021;3:535–556. doi: 10.1021/acsmaterialslett.1c00086. [DOI] [Google Scholar]
- Xu T. Wang Y. Xiong Z. Wang Y. Zhou Y. Li X. A Rising 2D Star: Novel MBenes with Excellent Performance in Energy Conversion and Storage. Nano–Micro Lett. 2023;15:6. doi: 10.1007/s40820-022-00976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. A. Ahmad T. MBenes for Energy Conversion: Advances, Bottlenecks, and Prospects. Langmuir. 2024;40:10835–10846. doi: 10.1021/acs.langmuir.4c00031. [DOI] [PubMed] [Google Scholar]
- Aghamohammadi P. Hüner B. Altıncı O. C. Akgul E. T. Teymur B. Simsek U. B. Demir M. Recent advances in the electrocatalytic applications (HER, OER, ORR, water splitting) of transition metal borides (MBenes) materials. Int. J. Hydrogen Energy. 2024;87:179–198. doi: 10.1016/j.ijhydene.2024.08.412. [DOI] [Google Scholar]
- Rout C. S. Shinde P. V. Patra A. Jeong S. M. Recent Developments and Future Perspectives of Molybdenum Borides and MBenes. Advanced Science. 2024;11:2308178. doi: 10.1002/advs.202308178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagadevan S. Oh W.-C. Comprehensive utilization and biomedical application of MXenes - A systematic review of cytotoxicity and biocompatibility. J. Drug Delivery Sci. Technol. 2023;85:104569. doi: 10.1016/j.jddst.2023.104569. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.