Abstract
Background
In recent years, the increasing incidence of osteoarthritis (OA) has attracted widespread public attention; however, the available effective treatments are limited. As a result, new therapeutic approaches, including stem cell and exosome therapies, have been proposed and are gradually gaining popularity. Because exosomes are immunocompatible, there is thought to be more potential for their use in clinical settings. This study summarizes the efficacy of exosomes in the treatment of OA.
Methods
In total, we conducted a comprehensive search of the PubMed, Web of Science, and Embase databases using medical subject headings terms to identify studies published from their inception until November 2023 that investigated the use of stem cell-derived exosomes in treating OA. We focused on specific outcomes including osteophyte score, chondrocyte count, pain level, qPCR and histological assessments such as the OARSI (Osteoarthritis research society international) score to measure cartilage degeneration. For data extraction, we used GetData Graph Digitizer to retrieve values from graphs, and the meta-analysis was conducted using RevMan 5.3 software. We chose mean difference (MD) as the primary effect measure since all included studies reported the same outcomes. Ultimately, 20 articles met the inclusion criteria and were included in the meta-analysis.
Results
We evaluated 20 studies comprising a total of 400 subjects. Compared with control groups, the exosome-treated groups showed significantly improved histological outcomes, as measured by the OARSI score (n = 400; MD = −3.54; 95% CI = [−4.30, −2.79]; P < 0.00001; I2 = 98%). This indicates a marked reduction in cartilage degeneration and OA severity in the exosome-treated groups. Notably, exosome therapy was more effective when administered during the early stages of OA. Additionally, a once-weekly dosing schedule yielded better results compared to more frequent administrations. Of the three exosome isolation methods assessed, kit-based extraction demonstrated a trend toward superior therapeutic efficacy.
Conclusions
Exosome treatment improved OA compared to placebo treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05227-4.
Keywords: Stem cell, Exosomes, Osteoarthritis, Meta-analysis
Introduction
Osteoarthritis (OA) is a progressive degenerative joint disease that can result in a decline in an individual's quality of life, physical disability, reduced joint function, and chronic joint pain [1]. This prevalent degenerative joint condition affects over 300 million individuals worldwide [2] and is a frequent cause of disability among the elderly population. This burdensome syndrome is expected to become more prevalent with the combined effects of an aging global population, obesity, and an increasing number of joint injuries [3]. Because its pathophysiology is not well understood, there are few effective therapeutic approaches available for treating OA. Currently, there are no disease-modifying osteoarthritis drugs (DMOADs) that can stop or reverse OA progression [4]. Therefore, developing a new therapeutic approach is especially significant.
Cell-based therapies, especially with MSCs, have gained attention for OA treatment [5–7]. While initially valued for tissue repair, MSCs mainly act by secreting cytokines and growth factors. However, most are rapidly cleared before reaching the target tissue [8, 9]. However, recent studies have shown that the consequences on the biology of stem cells are mainly paracrine, especially through the exosomes they produce [10]. Therefore, exosome-based therapy offers the option of "cell-free" therapy and may be a promising substitute for stem cell therapy for cartilage injury/OA. Among them, exosomes, a novel biocarriers, have received increasing attention in recent years for their role and therapeutic potential in OA.
Exosomes typically have diameters between 30 and 150 nm and densities between 1.13 and 1.19 g ml−1 [11]. Trams et al. reported that shedding membrane vesicles may have physiological functions and suggested that these vesicles are exosomes [12]. Exosomes mediate cell‒cell communication and have been demonstrated in an expanding amount of research to play significant physiological and pathological roles [13, 14]. In recent years, the initiative of exosomes in the pathophysiology and function of numerous physiological systems, as well as their potential for clinical therapy and diagnosis, has led to the rapid expansion of their biomedical uses [15]. Exosomes have been identified in OA studies from a variety of sources in joints, including tissue-specific MSCs, chondrocytes, osteoblasts, synovial fibroblasts (SFBs), tendon cells, infrapatellar fat pad adipocytes, and platelet-rich plasma (PRP), and they have been observed to change as OA progresses [16–18].
The study of extracellular vesicles (EVs), including exosomes, has gained significant attention in recent years due to their therapeutic potential. To ensure rigor in the reporting and characterization of exosomes in this field, it is important to adhere to the MISEV2023 (Minimal Information for Studies of Extracellular Vesicles) guidelines [19], which provide a framework for standardizing research on extracellular vesicles, including EV isolation, characterization, and functional analysis. Although there are many researchers working on the use of stem cell-derived exosomes for the treatment of OA, most of these studies are preclinical in nature. To further analyze the effectiveness of this approach, this paper performs a meta-analysis and summarizes and updates in vivo studies using stem cell-derived exosomes to treat OA.
Methods
Data sources and search strategy
This systematic review and meta-analysis is registered in the International Prospective Register of Systematic Reviews (PROSPERO) trial registry (CRD42024503330). The systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. The researcher independently conducted manual searches of publications from their publication until November 2023, using the Embase, PubMed, and Web of Science databases. The searches incorporated MeSH terms such as "extracellular vesicles (EVs)" or "exosomes," along with "osteoarthritis." The literature search strategy also employed free terms and Boolean operators (AND or OR) (Additional files 1: Table S1). Following the removal of duplicates, the initial phase of article selection was conducted by considering the abstract and title. The subsequent phase entailed a comprehensive evaluation of the complete articles, focusing on pertinent information such as sample size, participants, study methodology, and intervention.
Study selection criteria
Inclusion Criteria (1) Studies involving OA animal models treated with stem cell-derived EVs or exosomes; (2) Studies that provided data on relevant outcomes such as OARSI score or osteophyte score; (3) Studies published in English; (4) Controlled trials;
Exclusion criteria (1) Lack of in vivo testing; (2) Absence of an exosome treatment group or control group; (3) Combination therapies involving EVs/exosomes and other drugs or treatments; (4) Review articles or conference abstracts without full-text availability; (5) Studies with no outcome data or incomplete data; (6) Studies for which full publications were not available;
Study selection
To streamline the process, the literature from each database was imported into Endnote, and any remaining duplicates were eliminated. Subsequently, a meticulous examination of titles and abstracts was carried out to exclude literature that did not meet the required criteria, and the selected full-text papers were subsequently assessed for eligibility. The evaluation process involved the consideration of the most recent or extensive study among multiple publications pertaining to the same trial. The authors exercised independent judgment in determining which studies to include in the assessment, and any disagreements were referred to a second party for resolution.
Data extraction and quality assessment
Data from the selected studies were retrieved independently by the authors, adhering to the predefined inclusion criteria. In instances of discordance regarding study selection or data extraction, the second author intervened to facilitate discussion and resolution. The data extracted for this meta-analysis included the authors' names, the nation and year of publication, the kind, quantity, and sex of the animals, the method used to induce osteoarthritis (modeling technique), method of extracting exosomes, stem cell type, experimental and control group protocols, follow-up time of animal experiments, and outcome indicators.
The primary outcomes of interest included the OARSI score, which evaluates the severity of cartilage degeneration in osteoarthritis, with higher scores indicating more extensive damage; the osteophyte score, which assesses the formation of bone spurs in the joint, with higher scores representing greater osteophyte development; and chondrocyte count, which measures the number of viable chondrocytes in the cartilage, reflecting cellular integrity and potential regenerative effects. Additionally, pain scores were used to quantify the level of discomfort in osteoarthritis models, with higher scores indicating more severe pain. qPCR results measured the gene expression of key markers, including COL2A1 (collagen type II), which reflects cartilage matrix integrity, and MMP13 and ADAMTS5, both of which are associated with cartilage degradation. These outcomes were selected to evaluate the therapeutic efficacy of exosome treatment in osteoarthritis models.
The study design encompasses various details, such as the origin of stem cell exosomes, the sample size in both experimental and control groups, the method and frequency of administration. In case any crucial research data or information is omitted from the paper, we will request the corresponding author to provide comprehensive data via email. To assess the quality of the included research studies, the Systematic Review Center for Laboratory Animal Experiments (SYRCLE) risk of bias tool was employed [21, 22].
Statistical analysis
The data in this study were collected and analyzed using GetData Graph Digitizer and Review Manager (RevMan) 5.3. Means and standard deviations (SD) were obtained for key outcomes, including histological scores (such as the OARSI score), osteophyte formation, and chondrocyte count, for both the exosome and control groups. The mean difference (MD) and 95% confidence interval (CI) were determined for continuous data, calculated using the formula: MD = ∑(Xexosome − Xcontrol)/n where X represents the mean values and n the number of studies. Heterogeneity was assessed using the I2 test, with a value greater than 50% indicating the presence of heterogeneity between studies. If sufficient variation was found, a random effects model was utilized.
Random effects models were commonly utilized in animal experiments. Subgroup analyses, sensitivity analyses, or other correlation analyses were conducted to comprehend and elucidate notable heterogeneity among trials. To verify the reliability of the findings, sensitivity analysis was performed using Stata. The evaluation of publication bias was carried out through the creation of funnel plots. A significance level of p < 0.05 was deemed indicative of statistical significance.
Results
Study selection
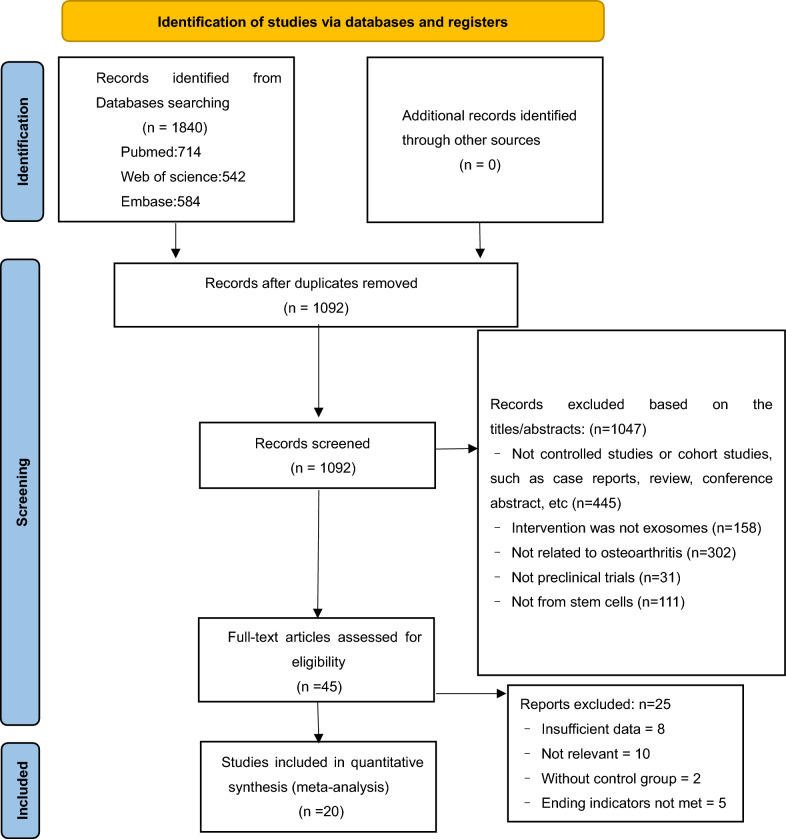
Figure 1 displays the flowchart of the study selection process for the systematic evaluation and meta-analysis following the preferred reporting item. A thorough search of the PubMed, Embase, and Web of Science library databases using MeSH terms and free words yielded 714, 584, and 542 studies, respectively, with no additional records from other sources. After 748 duplicates were eliminated, a total of 1092 records were evaluated, and 1047 were disqualified according to the abstract and title. A thorough evaluation of the complete content of 45 potentially eligible papers was performed. Among these, 10 studies were excluded from the analysis owing to their lack of relevant results, two studies were disqualified for lacking adequate data, two studies were disqualified for lacking a control group, and five were disqualified for not having outcome markers. Ultimately, 20 eligible studies containing 22 comparisons [23–42] were included in the quantitative analyses and meta-analyses.
Fig. 1.
PRISMA flow diagram of the literature search and selection of studies for meta-analysis
Characteristics of included studies
The review included 20 studies reporting 22 comparisons from 2017, 2019, 2020, 2021,to 2022. Characteristics of the included studies are shown in Table 1. Table 2 outlines the adherence of the selected studies to the MISEV2023 guidelines. Each aspect of the guidelines was systematically evaluated, revealing that while many studies provided adequate information on isolation methods, several lacked comprehensive characterization and functional testing data. This highlights the need for standardized practices in exosome research to enhance reproducibility and reliability.
Table 1.
Characteristics of the studies included in the comparisons
| Study | Year | Country | Experimental subject | Sex n | Intervention arms & subjects | Age | Weight | Model | Source of exosomes | Isolation method | Treatment group (method/dose of Exos) | Treatment cycle | Duration of follow-up (weeks) | Sample area | Outcome index | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhu et al | 2017 | China | C57B/L10 mice | Female n = 35 | Normal (n = 5), iMSC-Exos treatment (n = 10), SMMSC-Exos treatment (n = 10), OA (n = 10) | 6 week-old | – | CIOA | iMSC/ SMSC | Ultracentrifugation | Intra-articular injection/8 μl | On days 7, 14, and 21 after OA induction | 4 | knee joints | 1. Macroscopic (ICRS score) 2. Histology (OARSI score) 3. IHC (Collagen I and II) 4. Chondrocyte migration and proliferation assays | [23] |
| Tao et al | 2017 | China | SD rats | Male n = 30 | Normal (n = 10), OA (n = 10), OA + SMSC-Exos group (n = 10) | Approximately 12 weeks old | 300–350 g | Complete transection of the medial collateral ligament, ACLT and DMM | SMSC | Unknown | Intra-articular injection/100 μl | Weekly on weeks 8, 9 and 10 after OA induction | 12 | knee joints | 1. Histology (OARSI score) 2. IHC (Collagen II, ACAN) 3. Chondrocyte counts | [24] |
| Wang et al | 2017 | China | C57BL/6 J mice | Unknown n = 32 | Sham group (n = 12), OA (n = 10), Exo group (n = 10) | 2 month-old | – | DMM | ESC-MSCs | Ultracentrifugation | Intra-articular injection/5 μl | Twice a week at week 4 after OA induction | 8 | knee joints | 1. Histology (OARSI score) 2. IHC (Collagen II and ADAMTS5) | [25] |
| Cosenza et al | 2017 | France | C57BL/6 mice | Unknown n = 45 | Normal group (n = 15), OA (n = 15), Exo group (n = 15) | 3 days old | – | CIOA | BMSC | Ultracentrifugation | Intra-articular injection/5 μl |
Weekly at week 1 after OA induction |
6 | knee joints |
1. Bone parameter 2. Confocal laser scanning microscopy 3. Histology (OARSI score, osteophyte score) 4. qPCR (ACAN, COL2B, COL1, MMP-13, ADAMTS5 and iNOS) |
[26] |
| Wu et al | 2019 | China | C57BL/6 mice | Male n = 39 | Unknown | 9 week-old | – | DMM | IPFP-MSC | ExoQuick ™ reagent kit and ultrafiltration | Intra-articular injection/10 μl |
Twice a week at week 4 after OA induction |
8 | knee joints |
1. Histology (OARSI score) 2. IHC (Collagen II, MMP13 and ADAMTS5) 3. Gait analysis 4. Apoptosis |
[27] |
| Zavatti et al | 2019 | Italy | CD® rats | Unknown n = 12 | OA (n = 4), Exo group (n = 8) | 8 week-old | – | MIA-OA | AFSC | Ultracentrifugation | Intra-articular injection/100 μg | twice(10 days of distance) after OA induction | 3 | knee joints | 1. Histology (OARSI score) 2. IHC (Collagen II, HMIT and Sox9) 3. pain level | [28] |
| Zhou et al | 2020 | China | C57BL/6 J mice | Male n = 23 | Control (n = 5), OA (n = 6), BMSC-EXOs treatment (n = 6), pBMSC-EXOs treatment (n = 6) | 6 week-old | – | CIOA | BMSC | total exosome isolation kit | Intra-articular injection | On days 7, 14, and 21 after OA induction | 4 | knee joints | 1. Histology (OARSI score) 2.Chondrocyte counts 3. Wound-healing assays | [29] |
| Li et al | 2020 | China | C57BL/6 mice | Unknown n = 60 | Control (n = 20), OA (n = 20),BMSCs-exosomes treatment group (n = 20) | – | 25–30 g | Surgically induce instability in the lumbar spine | BMSC | Ultracentrifugation | Tail vein injection/200 μl | weekly for 4 weeks after surgery | 8 | Lumbar facet joint |
1. Pain assessment (vocalization threshold) 2. Bone parameter 3. Histology (OARSI score) 4.Immunofluorescence (MMP13 and ACAN) |
[30] |
| Zhang et al | 2020 | China | SD rats | Male n = 36 | Sham group (n = 12), OA group (n = 12), BMSC-derived exosomes group (n = 12) | Adult | 200 − 250 g | ACLT and DMM | BMSC | Ultracentrifugation | Intra-articular injection/10 μl |
Once every three days at 4 weeks after OA induction |
8 | knee joints |
1. Bone parameters 2. Histology (OARSI score, Osteophyte score) 3. IHC (Collagen II and X) 4. Cytokine determination in synovial fluid |
[31] |
| He et al | 2020 | China | SD rats | Male n = 24 | Normal group (n = 8), OA (n = 8), Exo group (n = 8) | 10 week-old | – | MIA-OA | BMSC | Ultracentrifugation | Intra-articular injection/100 μ | Weekly at week 1 after OA induction | 6 | knee joints |
1. Histology (OARSI score) 2. IHC (Collagen II and MMP13) 3. Pain assessment 4. Chondrocyte migration 5. qPCR (TGFβ1, PCNA, Casp3, COL2A1, ACAN, MMP13 and ADAMTS5) 6. ELISA(IL-1β, IL-6, IL-10 and TNF-α) |
[32] |
| Xu et al | 2020 | China | SD rats | Male n = 40 | Normal group (n = 5), OA (n = 5), Exo group (n = 5) | – | 200–220 g | DMM | SF-MSCs | Ultracentrifugation | Intra-articular injection/100 μl | Weekly at week 1 after OA induction | 5 | knee joints |
1. Histology (OARSI score) 2. IHC (Collagen I, II and ACAN) |
[33] |
| Wang et al | 2020 | China | Specific pathogen-free (SPF) BALB/C mice | Unknown n = 30 | Normal group (n = 10), OA (n = 10), Exo group (n = 10) | – | – |
Low-temperature induction |
SMSC | Unknown | Intra-articular injection/30 μl |
20 days later; continuous injection for 2 weeks, once a day |
7 | knee joints |
1. Histology (OARSI score) 2. IHC (Collagen II and Caspase-3) 3. Chondrocyte counts 4. Chondrocyte migration |
[34] |
| Rong et al | 2020 | China | SD rats | Female n = 60 | Normal group (n = 10), OA (n = 10), Exo group (n = 10) | 7–8 weeks | 200–250 g | ACLT and DMM | BMSC | Ultracentrifugation | Intra-articular injection/200 μl | In the 4th week after operation | 8 | knee joints |
1. Histology (OARSI score and osteophyte score) 2. Bone parameters 3. IHC (Collagen II and PCNA) |
[35] |
| Woo et al | 2020 | Korea | SD rats | Male n = 50 | Four treatment groups (n = 6) | 7 weeks | 200–250 g | MIA-OA(subacute arthritis model) | AD-MSC | TFF | Intra-articular injection/30 μl | Weekly at week 1 after OA induction | 4 | knee joints | 1. Histology (OARSI and Mankin score) 2. IHC (MMP13) 3. chondrocyte proliferation 4. qPCR (MMP-1, MMP-3, MMP-13, COL2A1 and ADAMTS-5) | [36] |
| SD rats | Male n = 50 | Four treatment groups (n = 6) | 7 weeks | 200–250 g | MIA-OA(chronic arthritis model) | AD-MSC | TFF | Intra-articular injection/30 μl |
Twice a week for 40 days at week 2 after OA induction |
8 | ||||||
| C57BL/6 mice | Male n = 18 | Unknown | 9 weeks | 20–25 g | DMM | AD-MSC | TFF | Intra-articular injection/6 μl | Weekly at week 5 after OA induction | 11 | ||||||
| Wang et al | 2020 | China | C57 mice | Male n = 20 | Sham group (n = 5), OA (n = 5), OA + SMSC-EV group (n = 5) | 8 weeks old | 25–30 g | Complete transection of the medial collateral ligament, ACLT and DMM | SMSC | Ultracentrifugation | Intra-articular injection/5 μl | every 3 days for 4 weeks after OA induction | 12 | knee joints |
1. Histology (OARSI score) 2. Cytokine determination in synovial fluid 3. Chondrocyte counts |
[37] |
| Tang et al | 2021 | China | SD rats | male n = 24 | Normal group (n = 6), treatment with hUC-MSCs (n = 6), treatment with hUC-MSC-sEVs (n = 6), control (n = 6) | 8 weeks old | – | ACLT | hUC-MSCs | commercial kit | intra-articular injection/200 μL | once a week for 4 weeks | 9 | Knee joint | 1. Histology (OARSI) 2. IHC (Collagen II, ADAMTS5 and MMP13) 3. Chondrocyte migration | [38] |
| Hoda Fazaeli et al | 2021.9 | Qom, Iran | BALB/C mice | Female n = 40 | normal (n = 5), OA (n = 5), AD-Exo (n = 10), BMM-Exo (n = 10), sham (n = 10) | 3 weeks | – | Ciprofloxacin | BM-MSC/AD-MSC | Exocib exosome extraction kit | Intra-articular injection/25 μl | On the 15th, 22nd, and 29th day after OA induction | 9 | tibia | 1. Histology (OARSI score) 2. IHC (Collagen I and II) 3.qPCR (collagen type I, Sox9, collagen type II, and aggrecan) | [39] |
| Jin et al | 2021.1 | China | SD rats | male n = 20 | Control (n = 5), OA (n = 5),EXOs treatment group (n = 5) | – | 358 ± 5 g | ACLT and DMM | BM-MSC | Ultracentrifugation | Intra-articular injection/100 μl | Weekly after OA induction | 8 | knee joints | 1. Histology (OARSI score, Mankin score) 2.Bone parameter analyses 3. qPCR (ADAMTS5, MMP‐13 and COL2A1) | [40] |
| Liu et al | 2022 | China | SD rats | Female n = 40 | Sham (n = 10), PBS (n = 10), hUSC-Exos (n = 10) | 12 week-old | 250 ± 20 g | ACLT and DMM | hUSCs | Ultracentrifugation | Intra-articular injection/100 mL | 30 days after OA surgery, once a week | 4 | knee joints | 1.Behavioral and Macroscopic Evaluation 2. Histology (OARSI score) 3. IHC (Collagen I and II, VEGFA) | [41] |
| Li et al | 2022 | China | SD rats | Unknown n = 15 | Normal group (n = 5), OA (n = 5), Exo group (n = 5) | – | 230–280 g | MIA-OA | AD-MSC | Ultracentrifugation | Intra-articular injection/250 μl | Once after 2 weeks of MIA injection | 2 | knee joints | 1.Histology (OARSI score) 2.IHC (Collagen II) 3.Chondrocyte counts | [42] |
AD-MSC, adipose tissue-derived mesenchymal stem cell; BMSC, bone marrow-derived mesenchymal stem cell; CIOA, collagenase-induced osteoarthritis; DMM, destabilization of the medial meniscus; ESC-MSC, embryonic stem cell-derived mesenchymal stem cell; ICRS, international cartilage repair society; IHC, immunohistochemistry; iMSC, induced pluripotent stem cell-derived mesenchymal stem cell; MIA-OA, monosodium iodoacetate induces osteoarthritis; MSC, mesenchymal stem cell; OA, osteoarthritis; OARSI, osteoarthritis research society international; SF-MSC, synovial fluid-derived mesenchymal stem cell; SMSC, synovium-derived mesenchymal stem cell; TFF, T angential flow filtratio; hUSC, human urine–derived stem cells; ACLT, anterior cruciate ligament transection; IPFP-MSC, infrapatellar fat pad mesenchymal stem cell; AFSC, amniotic fluid stem cell; hUC-MSCs, human umbilical cord-derived mesenchymal stem cells
Table 2.
MISEV2023 guidelines adherence table
| Study | Isolation method | Characterization method | Size distribution | Surface marker analysis | Functional testing | Reporting clarity | Statistical methods | Adherence status |
|---|---|---|---|---|---|---|---|---|
| Zhu et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Tao et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Wang et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Cosenza et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Wu et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Zavatti et al | Yes | Yes | Yes | Yes | No | Yes | Yes | Partially adhered |
| Zhou et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Li et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Zhang et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| He et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Xu et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully Adhered |
| Wang et al | No | Yes | Yes | Yes | Yes | Yes | Yes | Fully Adhered |
| Rong et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Woo et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully Adhered |
| Wang et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully Adhered |
| Tang et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully Adhered |
| Hoda Fazaeli et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Jin et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Liu et al | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Fully adhered |
| Li et al | Yes | Yes | No | Yes | Yes | Yes | Yes | Partially adhered |
16 of the trials were conducted in China, and the rest were in France, South Korea, Italy, and Iran. The total sample size had 726 animals, 399 rats (54.96%) and 327 mice (45.04%), included Sprague–Dawley (SD) rats (n = 379, 52%), C57 mice (n = 272, 37%), CD rats (n = 20, 3%), and BALB/C mice (n = 55, 8%). Characteristics of the subjects and all 22 experiments were collected and listed (Table 1), including (but not restricted to) the type of exosome, injection technique, age, sex, number, species, and sampling area of the animal model utilized. Twenty studies [23–42] modeled OA, and 12 studies [24, 25, 27, 30, 31, 33, 35–38, 40, 41] modeled cartilage defects by surgery. Eight studies [23, 26, 28, 29, 32, 36, 42] modeled OA by injection of collagenase or sodium iodoacetate. One study modeled OA by injection of ciprofloxacin [39]. One study induced OA by cryotherapy [34]. Most studies used intra-articular injections for EV implantation, but one study used caudal vein injection in a lumbar OA model [30].
MSCs are derived from a variety of tissues. Table 3 summarizes the characterization of exosomes derived from various studies. MSCs in eight studies were obtained from bone marrow [26, 29–32, 35, 39, 40]. One study used MSCs generated from embryonic stem cells [25]. Four studies used MSCs derived from synovial tissue [24, 34, 37] and one study used MSCs derived from synovial fluid [33]. One study used urine-derived stem cells (USC) [41]. One study used human umbilical cord MSCs [38]. One study used amniotic fluid-derived MSCs [28]. One study used adipose tissue-derived MSCs [27, 36, 42]; in one study, MSCs were derived from the infrapatellar fat pad [27]; in another study, commercial adipose-derived MSCs [36]. One study used induced pluripotent stem cell-derived MSCs (iMSCs) and compared them to synovial-derived MSCs (SMSCs) [23]. Another study used MSCs from bone marrow and compared them to adipose-derived MSCs [39]. Three studies used extracellular vesicles and the rest used exosomes. Despite the fact that exosomes originate from the cells of various species, every study's finding separately showed how well they worked to treat osteoarthritis in animal models. In every trial, the exosome-treated group and the placebo group were directly compared. Ultracentrifugation (n = 13, 59%) was the most commonly used method for exosome/EVs separation. Four studies used commercially available kits (n = 4, 18%) [27, 29, 38, 39] and three studies used tangential flow filtration (TFF) (n = 3, 14%) [36] to isolate exosomes. Two research did not provide detailed instructions on how to isolate exosomes (n = 2, 9%) [24, 34]. Exosomes were injected using two methods, intra-articular injection (n = 21, 95%) and tail vein injection (n = 1, 5%).
Table 3.
Exosome characterization table
| Study | Size range (nm) | Surface marker expression (CD63) | Surface marker expression (CD9) | Surface marker expression (CD81) | Exosome concentration (µg/mL) | Morphology (TEM/SEM) |
|---|---|---|---|---|---|---|
| Zhu et al | 50–200 nm | ✔ | ✔ | Unknown | ✔ | TEM |
| Tao et al | 30–150 nm | ✔ | ✔ | ✔ | ✔ | TEM |
| Wang et al | 38–169 nm | ✔ | ✔ | Unknown | 176.2 μg/mL | TEM |
| Cosenza et al | 223 ± 15.6 nm | Unknown | ✔ | ✔ | ✔ | TEM |
| Wu et al | 50–250 nm | ✔ | ✔ | ✔ | 2 × 10^9 particles/mL | TEM |
| Zavatti et al | Unknown | ✔ | ✔ | ✔ | Unknown | Unknown |
| Zhou et al | 30–150 nm | ✔ | ✔ | Unknown | Unknown | TEM |
| Li et al | 120.31 ± 15.28 nm | ✔ | ✔ | Unknown | ✔ | TEM |
| Zhang et al | about 140 nm | ✔ | ✔ | Unknown | 4 × 10^6/ml | TEM |
| He et al | about 153 nm | ✔ | Unknown | Unknown | 7.5 × 10^6/ml | TEM |
| Xu et al | 100–300 nm | ✔ | Unknown | ✔ | 1.8 × 10^6/ml | TEM |
| Wang et al | Unknown | ✔ | Unknown | ✔ | 10 × 10^11/ml | TEM |
| Rong et al | 50–150 nm | ✔ | ✔ | ✔ | 5 × 10^6/ml | TEM |
| Woo et al | about 86.46 nm | ✔ | ✔ | ✔ | ✔ | TEM |
| Wang et al | 95.01 ± 35.91 nm | ✔ | ✔ | ✔ | ✔ | TEM |
| Tang et al | about 80.48 nm | ✔ | ✔ | ✔ | 2.301 × 10^7 ± 1.774^7 particles/mL | TEM |
| Hoda Fazaeli et al | 30–150 nm | ✔ | Unknown | ✔ | Unknown | TEM |
| Jin et al | 50–150 nm | ✔ | ✔ | Unknown | Unknown | TEM |
| Liu et al | about 135.5 nm | ✔ | ✔ | ✔ | 5.1 × 10^10 particles/mL | TEM |
| Li et al | Unknown | ✔ | ✔ | Unknown | Unknown | TEM |
The included studies assessed three main outcomes to evaluate the efficacy of stem cell-derived exosomes in OA. First, histology—20 studies used the OARSI score [43] as an outcome of histologic assessment [28–47]. Second, immunohistochemistry—12 studies used immunohistochemistry to assess the tissue expression of different proteins, including collagen type I, collagen type II, collagen type X, IL-1, CD86, PCNA, VEGFA, Caspase-3, MMP-13, ACAN and ADAMTS5 [23–25, 27, 28, 30–36, 38, 39, 41, 42]. Third, imaging assessment—Five studies analyzed bone parameters using microcomputed tomography [26, 30, 31, 35, 40]. In addition to the above three outcome measures, osteophyte score [26, 31, 35], synovial cytokine assay [37], pain assessment [30, 32], chondrocyte counts [24, 34, 37], and behavioral assessment [41] were also used to assess the efficacy of MSC-EVs. The follow-up period was 4–12 weeks. No significant side effects were observed in all subjects.
Methodological quality and risk of bias in research
The inclusion of studies was assessed based on the SYRCLE risk of bias tool. The evaluation of the quality of each study is presented in Table 4. The findings of the studies were classified into three categories: 'Yes' denoting a low risk of bias, 'No' indicating a high risk of bias, and 'U' indicating an uncertain risk of bias. Following the assessment of the 10 criteria, a composite quality score was assigned to each piece of literature. Despite ensuring balanced and comparable animals across all animal studies, the descriptions of selection bias and measurement bias remained inconclusive. Only three studies provided comprehensive details regarding the allocation of hidden factors, while the randomized rearing methods were inadequately described. Furthermore, the methods of performance blinding and detection blinding were not sufficiently elucidated. Studies were considered to be free from bias of incomplete outcome data. In addition, six articles were unclear about other sources of bias. However, the combined assessment indicated that all of these studies shown little bias.
Table 4.
Results of the assessment of bias risk in animal studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhu 2017 | Y | Y | U | U | U | Y | Y | Y | Y | N |
| Tao 2017 | Y | Y | U | U | U | Y | Y | Y | Y | N |
| Wang 2017 | Y | Y | U | U | U | U | U | Y | Y | N |
| Cosenza 2017 | U | Y | Y | U | U | U | U | Y | U | N |
| Wu 2019 | Y | Y | U | U | U | Y | Y | Y | U | N |
| Zhou 2020 | Y | Y | U | U | U | U | U | Y | U | U |
| Zavatti 2019 | U | Y | U | U | U | U | U | Y | U | U |
| Li 2020 | Y | Y | U | U | U | Y | Y | Y | U | N |
| Zhang 2020 | U | Y | U | U | U | U | U | Y | U | U |
| He 2020 | U | Y | Y | U | U | U | U | Y | U | N |
| Xu 2020 | U | Y | U | U | U | Y | Y | Y | Y | N |
| Wang 2020 | Y | Y | U | U | U | Y | Y | Y | U | U |
| Rong 2020 | U | Y | U | U | U | U | U | Y | U | N |
| Woo 2020 | Y | Y | U | U | U | U | U | Y | U | U |
| Wang 2020(2) | Y | Y | U | U | U | U | U | Y | U | N |
| Tang 2021 | Y | Y | U | U | U | U | U | Y | U | U |
| Hoda Fazaeli 2021 | Y | Y | Y | U | U | U | U | Y | Y | N |
| Jin 2021 | U | Y | U | U | Y | Y | Y | Y | U | N |
| Liu 2022 | U | Y | U | U | Y | U | U | Y | U | N |
| Li 2022 | Y | Y | U | U | Y | U | U | Y | U | N |
1. If the allocation sequence adequately generated and applied or not; 2. If the groups similar at baseline or were they adjusted for confounders in the analysis or not; 3. the allocation to the different groups adequately concealed during or not; 4. If the animals randomly housed during the experiment or not; 5.If the caregivers and/or investigators blinded or not; 6.If animals selected at random for outcome assessment or not; 7. the outcome assessor blinded or not; 8. incomplete outcome data adequately addressed or not; 9.reports of the study free of selective outcome reporting or not; 10. the study apparently free of other problems that could result in high risk of bias or not
Standard meta-analysis
Histological evaluation
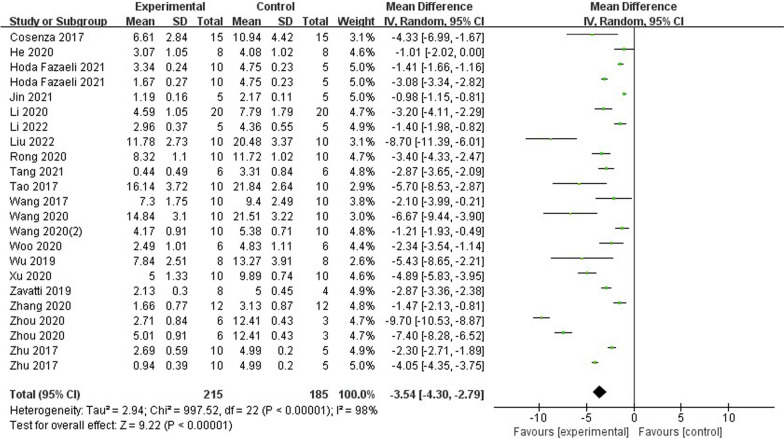
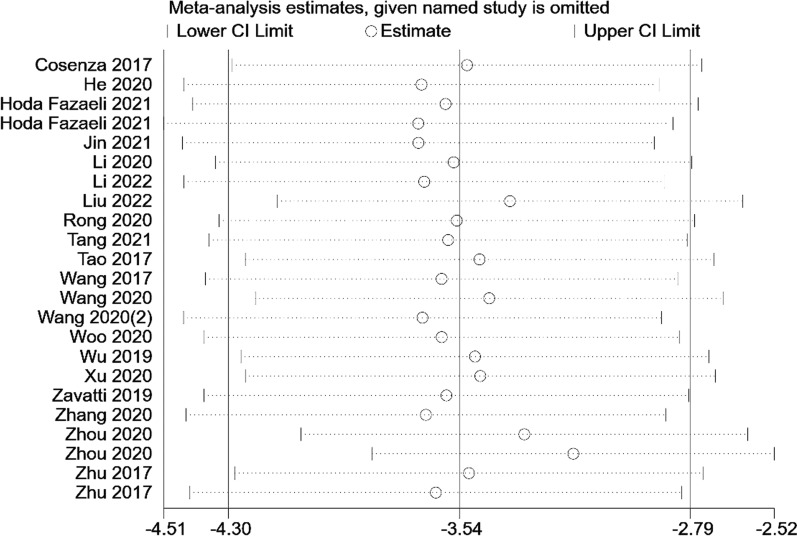
The OARSI histological score was utilized in twenty studies to evaluate the histological quality of newly formed cartilage. These studies were based on the OA model and included a total of 22 comparisons. The statistical analysis revealed a significant difference in OARSI histological scores between the exosomes group and the control group (n = 400; MD = −3.54; 95% CI = [−4.30, −2.79]; P < 0.00001; I2 = 98%) (Fig. 2). Furthermore, the funnel plot displayed asymmetry, indicating the presence of publication bias (Fig. 3). Sensitivity analyses were conducted to assess the robustness of the aforementioned findings (Fig. 4).
Fig. 2.
Forest plot of mean change in OARSI score after exosome and placebo treatment (mean ± standard deviation)
Fig. 3.
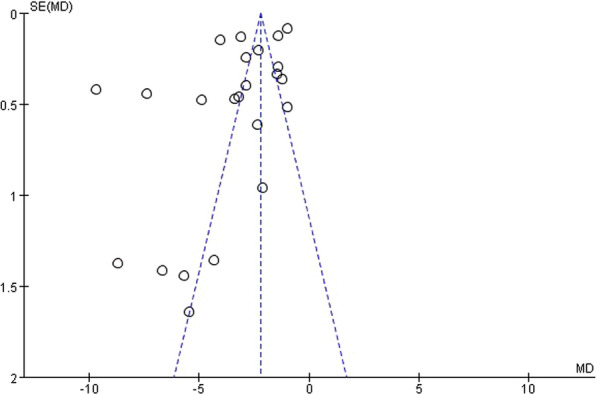
Funnel plot with pseudo-95% confidence limits
Fig. 4.
Sensitivity analysis of studies in OARSI score
Osteophyte score
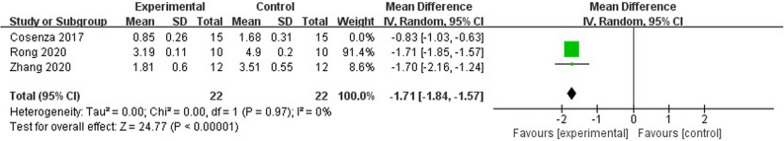
Three studies have employed the osteophyte score as a means to evaluate the extent of articular cartilage damage. Among these investigations, two utilized models simulating cartilage defects, while the remaining study employed a model replicating osteoarthritis induced by collagenase. Given the substantial heterogeneity observed (P < 0.00001, I2 = 96%), a randomized model was employed for our analysis. We conducted a comparison between the MD of the exosome group and the control group. Notably, the exosome group exhibited significant superiority over the control group, resulting in a reduction in the number of periarticular osteophytes when compared to the control group (n = 74; MD = −1.40; 95% CI = [−2.06, −0.75]; P < 0.00001) (Additional files 2: Fig. S1).
Sensitivity analyses revealed that the observed high level of heterogeneity may be attributed to [26]. The exclusion of this factor resulted in a reduction in heterogeneity (p < 0.00001, I2 = 0%) (Fig. 5). However, given the limited amount of available data, further investigations are necessary to establish definitive conclusions.
Fig. 5.
Forest plot of mean change in osteophyte score after exosome and placebo treatment (mean ± standard deviation)
Chondrocyte counts
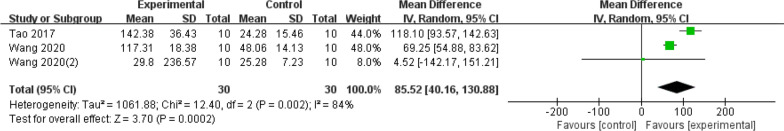
Chondrocytes play a crucial role in the synthesis and secretion of matrix and fibres, which contribute to the maintenance of weight-bearing and cushioning functions in cartilage tissue on joint surfaces. Five studies utilized chondrocyte counts as a measure to assess the extent of articular cartilage damage. Three of the studies included in the analysis solely reported chondrocyte counts, while the remaining two studies reported counts of both chondrocytes and stem cells after co-culture. Given the substantial heterogeneity observed (I2 = 84%), a randomized model was employed for the analysis. The results indicate a significant increase in chondrocyte counts in the exosome group compared to the control group (MD = 85.52; 95% CI = [40.16, 130.88]; p = 0.0002) (Fig. 6). However, given the limited amount of available data, further studies are necessary to establish conclusive findings.
Fig. 6.
Forest plot of mean change in Chondrocyte counts after exosome and placebo treatment (mean ± standard deviation)
Pain tolerance level
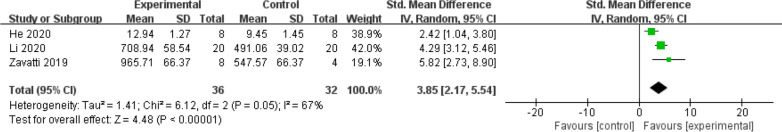
The meta-analysis of pain tolerance levels included data from 3 studies. A pooled analysis revealed a significant improvement in pain tolerance levels in the exosome treatment group compared to the control group (SMD = 3.85; 95% CI = [2.17, 5.54]; p < 0.00001) (Fig. 7). Heterogeneity was moderate, with an I2 value of 67%. These results suggest that stem cell-derived exosome therapy may alleviate pain sensitivity in animal models of osteoarthritis.
Fig. 7.
Forest plot of mean change in Pain Tolerance Level after exosome and placebo treatment (mean ± standard deviation)
qPCR
In this study, we used qPCR to analyze the expression of genes related to cartilage formation and degradation, including COL2A1, MMP13, and ADAMTS5. The results were visualized using a forest plot, showing the relative expression level of each gene in different experiments.
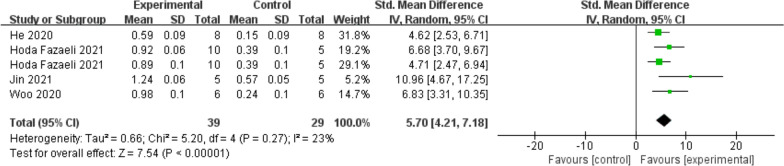
COL2A1 expression was notably higher in the exosome treatment group compared to the control group (SMD = 5.70; 95% CI = [4.21, 7.18]; p < 0.00001) (Fig. 8). These results suggest that exosome treatment is associated with sustained upregulation of COL2A1, which is essential for cartilage regeneration.
Fig. 8.
Forest plot of mean change in qPCR (COL2A1) after exosome and placebo treatment (mean ± standard deviation)
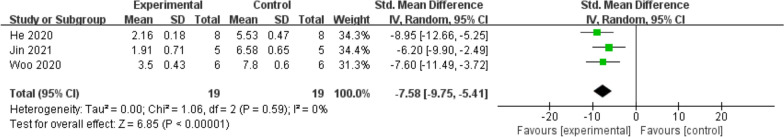
Conversely, MMP13 expression was significantly lower in the exosome treatment group (SMD = −7.58; 95% CI = [−9.75, −5.41]; p < 0.00001) (Fig. 9), suggesting that exosome therapy effectively inhibits MMP13, a key matrix metalloproteinase involved in collagen degradation.
Fig. 9.
Forest plot of mean change in qPCR (MMP13) after exosome and placebo treatment (mean ± standard deviation)
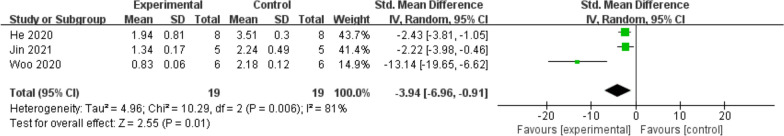
Additionally, ADAMTS5 expression showed some heterogeneity across studies, but overall analysis indicated a significant decrease in the treatment group (SMD = −3.94; 95% CI = [−6.96, −0.91]; p = 0.01) (Fig. 10). ADAMTS5 is an enzyme involved in cartilage matrix degradation, and its inhibition may help protect cartilage and improve the pathological state of OA.
Fig. 10.
Forest plot of mean change in qPCR (ADAMTS5) after exosome and placebo treatment (mean ± standard deviation)
Subgroup analysis
Based on what came out of these analyses, we analyzed OARSI scores in different subgroups according to animal species, cell type, mode of exosome extraction and frequency of administration variables.
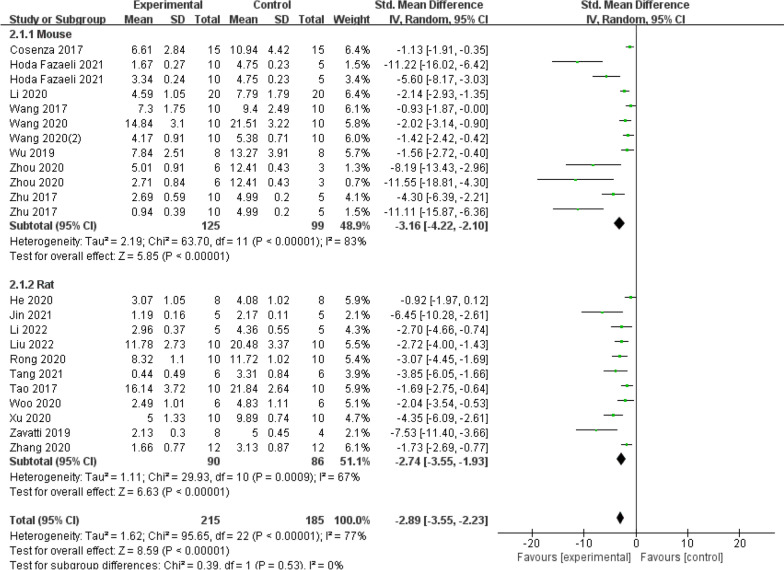
The subgroup analysis of OARSI scores included a total of twenty studies. Firstly, the trials were further divided into two subgroups based on different animal models, including rats and mice (Fig. 11). The early OA animal model saw a substantial impact (SMD = −3.16, 95% CI = [−4.22, −2.10], P < 0.00001, I2 = 83% versus SMD = −2.74, 95% CI = [−3.55, −1.93]; P = 0.0009, I2 = 67%). Meanwhile, the results of the SMD from both subgroups showed that the exosome therapy increased the effectiveness of treatment for osteoarthritis. There was no significant change in the pooled size effect after excluding individual trials from the sensitivity analyses, suggesting that the findings are relatively robust and reliable.
Fig. 11.
Subgroup analysis of the OARSI score (animal model)
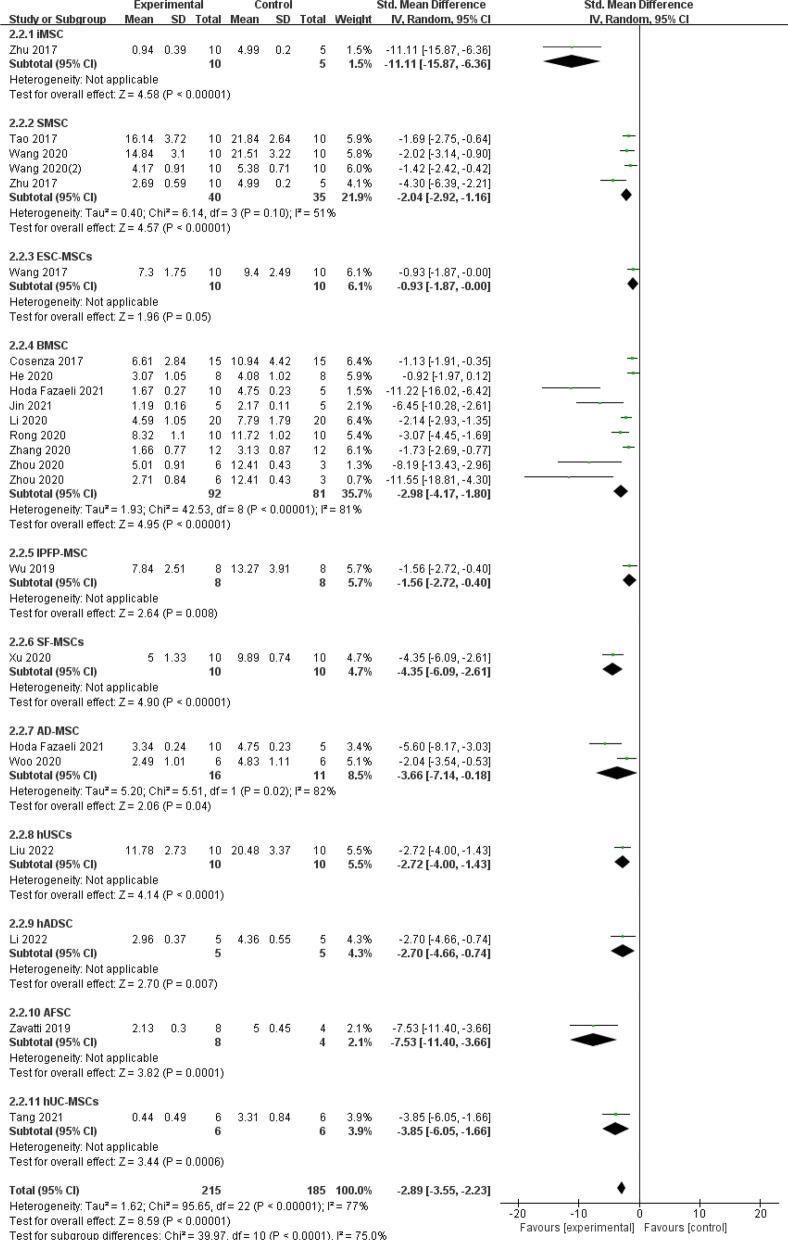
Secondly, the subgroup analysis of cell types involved in the study (Fig. 12), there were 11 subgroups (iMSC: SMD = −11.11, 95% CI [−15.87, −6.36], P < 0.00001; SMSC: SMD =−2.04, 95% CI [−2.92, −1.16], P < 0.00001; ESC-MSCs: SMD = −0.93, 95% CI [−1.87, −0.00], P = 0.05; BMSC: SMD = −2.98, 95% CI [−4.17, −1.80], P < 0.00001; IPFP-MSC: SMD = −1.56, 95% CI [−2.72, −0.40], P = 0.008; SF-MSC: SMD = −4.35, 95% CI [−6.09, −2.61], P < 0.00001; AD-MSC: SMD = −3.66, 95% CI [−7.14, −0.18], P = 0.04; hUSCs: SMD = −2.72, 95% CI [−4.00, −1.43], P < 0.0001; hADSC: SMD = −2.70, 95% CI [−4.66, −0.74], P = 0.007; AFSC: SMD = −7.53, 95% CI [−11.40, −3.66], P = 0.0001; hUC-MSCs: SMD = −3.85, 95% CI [−6.05, −1.66], P = 0.0006). It can be seen that EXOs of different cellular origins produce similar therapeutic outcomes, with iMSC-derived exosomes being the most effective in treating osteoarthritis. The pooled impact size was unaffected significantly by excluding specific trials from the sensitivity analysis, indicating that the findings are relatively robust and dependable.
Fig. 12.
Subgroup analysis of the OARSI score (cell types)
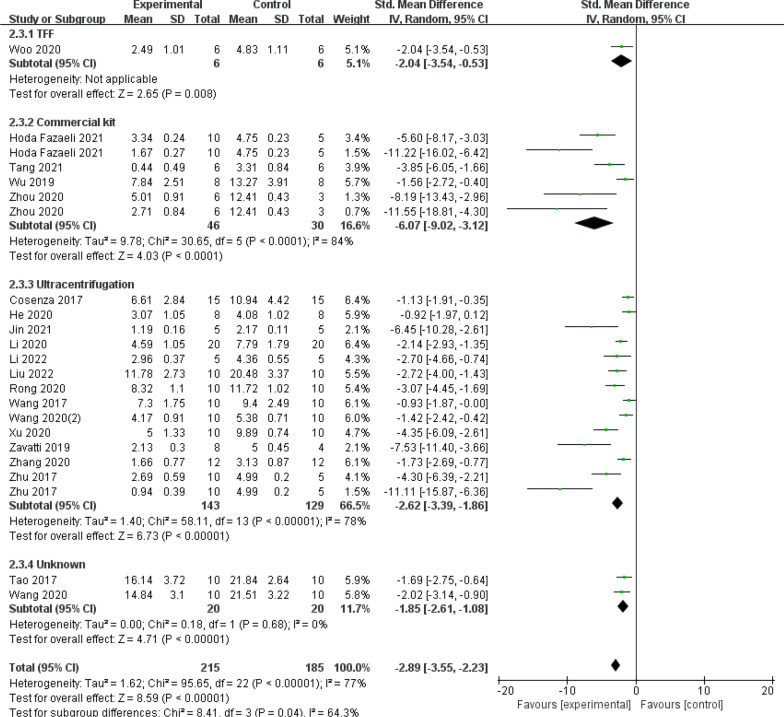
Thirdly, in the subgroup analysis of the way exosomes were extracted (Fig. 13), there were four subgroups (TFF: SMD = −2.04, 95% CI [−3.54, −0.53], P = 0.008; Commercial kit: SMD = −6.07, 95% CI [−9.02, −3.12], P < 0.0001. Ultracentrifugation: SMD = −2.62, 95% CI [−3.39, −1.86], P < 0.00001; Unknown: SMD = −1.85, 95% CI [−2.61, −1.08], P = 0.68;). The data illustrates that there were no observable changes between subgroups in the stratified subgroup analyses of the separation method, but the commercially available kits showed a trend towards better efficacy. The pooled effect size was not significantly altered by the removal of specific trials from the sensitivity analysis, indicating that the findings are relatively robust and reliable.
Fig. 13.
Subgroup analysis of the OARSI score (Exosome extraction method)
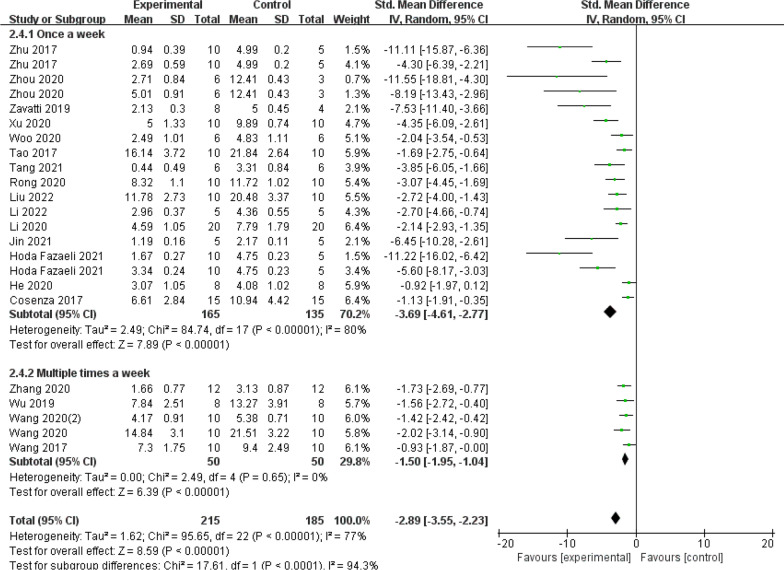
In subgroup analyses assessing the frequency of exosome administration (Fig. 14), there were 2 subgroups (Once a week: SMD = −3.69, 95% CI [−4.61, −2.77], P < 0.00001; Multiple times a week: SMD = −1.50, 95% CI [−1.95, −1.04], P = 0.65). When comparing the frequency of dose, once per week performed better than numerous times per week. Excluding individual trials from the sensitivity analyses did not bring about a significant change in the pooled effect size, indicating that the findings are relatively robust and reliable.
Fig. 14.
Subgroup analysis of the OARSI score (exosome administration frequency)
Discussion
The objective of this meta-analysis and systematic review was to evaluate the efficacy of stem cell-derived exosomes in the treatment of OA in animal models. The analysis encompassed a total of 20 studies, comprising 22 comparisons and involving 726 animals. The results revealed that therapy utilizing exosomes derived from stem cells demonstrated a substantial improvement in OA compared to placebo treatment, as evidenced by the overall OARSI score and Osteophyte score. Furthermore, the group receiving exosomes exhibited significant enhancements in macroscopic and histological scores for OA and cartilage damage, in contrast to the control group. Our findings demonstrate that stem cell-derived exosome therapy significantly improves pain tolerance levels in OA animal models. This is an important aspect of osteoarthritis treatment, as pain is a primary symptom that severely impacts patients' quality of life. The improvement in pain tolerance suggests that exosomes may exert analgesic effects, possibly by modulating inflammation and cartilage repair processes. qPCR results showed exosomes may exert their therapeutic effects by regulating the expression of these key genes and affecting the metabolic balance of cartilage. The upregulation of COL2A1 and the downregulation of MMP13 and ADAMTS5 may jointly promote the repair and regeneration of cartilage. This suggests the potential application of exosomes in the treatment of OA, especially in improving cartilage structure and function. These findings imply that exosomes made from stem cells have a promising future as a cutting-edge OA therapeutic option.
In recent years, there has been a notable increase in the number of scholarly articles discussing the utilization of exosomes derived from various stem cell sources for the management of OA. For instance, the study of EVs in regenerative medicine, such as myocardial repair and wound healing, has laid the foundation for understanding their therapeutic potential in various fields beyond osteoarthritis. Researchers have explored the immunomodulatory and tissue regeneration capabilities of EVs, highlighting their potential therapeutic applications in the treatment of inflammatory diseases such as COVID-19 [44]. Gupta et al. presented a novel cell-free stem cell-derived extract (CCM) from human progenitor endothelial stem cells, containing growth factors, cytokines, and exosomes, which significantly enhances fibroblast proliferation and stem cell migration, suggesting potential benefits in reducing inflammation, alleviating pain, and promoting tissue repair [45]. Other researchers reviewed animal models for osteoarthritis research, emphasizing the advantages of genetically engineered models and surgically induced joint instability for understanding disease progression [46]. Liu et al. conducted a comprehensive review that encompassed the multifaceted role of exosomes in OA, addressing the associated challenges and limitations, with a particular focus on pathophysiology, diagnostics, and therapeutic interventions [47]. Similarly, Yu et al. elucidated the potential of exosomes derived from different types of MSCs in the prevention and treatment of OA, offering novel insights and strategies for OA management [48]. The findings of their study demonstrated the promising therapeutic efficacy of exosomes in the treatment of OA. Nevertheless, prior assessments have exhibited deficiencies in terms of comprehensive outcomes and restricted cell varieties. To evaluate the efficacy of exosomes derived from diverse stem cell origins for OA treatment, we have chosen one of the extensively employed outcome measures for meta-analyses.
Stem cells are undifferentiated cells within the body that exhibit the ability to undergo self-renewal and differentiate into various types of cellular entities. Among these, MSCs represent a subset of pluripotent stem cells primarily found in the umbilical cord veins, adipose tissue, and bone marrow. Stem cells serve as the origin of exosomes, which share comparable characteristics such as inflammation reduction and promotion of angiogenesis. Still unresolved are safety concerns related to stem cell use, such as the possibility of thrombosis and cancer, limited engraftment effectiveness (high apoptosis rate, poor stem cell homing capacity), and moral dilemmas with stem cell transplantation (donor source) [49–51]. Exosomes exhibit a superior capacity to undertake a broader spectrum of functions, such as facilitating osteogenic differentiation and repairing impaired MSCs, thereby circumventing the aforementioned concerns. Additionally, the absence of MHCI and MHCII proteins in exosomes renders them non-immunogenic [49, 50]. Exosomes, or extracellular vesicles, play a crucial role in bone regeneration through intercellular communication, whereas MSCs primarily operate through paracrine mechanisms [52, 53]. By triggering differentiation, osteoblast proliferation, blocking apoptosis, encouraging angiogenesis, and immunomodulation, diverse nucleic acids (such as miRNAs, lncRNAs, and piRNAs), proteins, lipids, and other active substances in exosomes can promote bone repair [52, 54].
The majority of the 20 studies focused on a singular source of MSCs, with only two studies conducting comparisons. One study compared exosomes from iMSC and sMSC, demonstrating the superior efficacy of iMSC-exosomes over sMSC-exosomes [23]. The other study compared exosomes from BM-MSC and AD-MSC, finding that exosomes from BM-MSC exhibited greater efficacy than those from AD-MSC [44]. Additionally, a separate study evaluated the effectiveness of EVs derived from normal bone marrow MSCs and polydactyl tissue-derived bone marrow MSCs. In vivo investigations indicated that the latter possessed superior therapeutic efficacy post-treatment [34]. Different approaches to exosome isolation can affect the outcome in addition to the source of MSCs. The most popular isolation technique, ultracentrifugation, has the benefit of separating a comparatively high number of exosomes but comes with more contaminants and necessitates the use of an ultracentrifuge. Exosomes can be isolated using a variety of commercially available kits; these kits are costly but have the benefit of high purity and minimal equipment needed. Four research employed commercially available kits, three studies used tangential flow filtering, while the majority of the included investigations (13/20) used ultracentrifugation. Subgroup analyses showed a trend toward better efficacy of kit extraction of exosomes among the three isolation methods. Importantly, the quantity of exosomes administered in these studies is a critical factor that should be addressed as part of the dosing issue. A deeper exploration of this limitation could provide valuable insights into the mechanisms of action of orthobiologics and help to clarify how variations in exosome quantity influence therapeutic outcomes.
Selecting an appropriate animal model is essential when researching the pathophysiologic development of an illness. Rats and mice were used as models in all of the investigations. Chemical and surgical modeling techniques were the most often employed modeling techniques. One study used cryopreservation. The primary modeling site was the knee joint. In addition to the knee joint, the lumbar spine was used for surgical modeling in another study. Subgroup analyses showed no significant differences in the efficacy of exosomes between species or modeling methods. Specific methods of exosome administration in animal OA models have not been standardized. One time per week or several times per week were the two primary administration frequencies used in the studies mentioned above. Our findings indicate that once-weekly injections have a superior therapeutic effect. Despite the high heterogeneity, we hypothesize that the efficacy of exosome therapy may vary with these influences. However, in order to increase the uniformity and dependability of the data, this hypothesis needs to be validated by more carefully planned research using high-quality data. Similarly, a lack of studies, a small sample size, and uneven study design (injection volume, injection frequency, and treatment duration) have prevented numerous parameters linked to exosome efficacy from being examined or debated.
Overall, there are limitations to existing treatment options for OA. Current findings suggest that stem cell-derived exosomes have favorable therapeutic effects in small animal models. More studies in large animal models are needed before applying exosomes to the clinical treatment of OA. We conducted a sensitivity analysis because the results of the studies that were part of this analysis were very heterogeneous. The outcome and heterogeneity of the data were rather steady after one study was eliminated. Considering different animal models, cell types, and animal species, there might be a lot of heterogeneity. Although subgroup analyses were also carried out, there was still some degree of study heterogeneity. The validity of the results could be somewhat impacted by excessive heterogeneity. We think that the absence of standardized experimental procedures is the primary cause of heterogeneity. To prevent this, the analysis of experimental results needs to be standardized in addition to more thorough documenting of the experimental procedures.
To mitigate this issue, it is crucial to standardize the analysis of experimental results and ensure thorough documentation of experimental procedures. Future animal studies could utilize our findings as a reference, but additional research is needed to validate them. Specifically, direct comparisons of the effectiveness of exosomes derived from various cell types and investigations into different dosing frequencies are warranted. In conclusion, our analysis suggests that exosome therapy has the potential to reduce OA symptoms, but further research is required to bridge the gap between preclinical and clinical applications.
Limitations
These meta-analyses could have several limitations. First, there was unavoidably some bias in this evaluation because the majority of the studies were carried out in China. Consequently, in order to update the analysis and lessen outcome bias, pertinent studies should be regularly tracked in the future. Second, there was no discernible decrease in the heterogeneity among studies even with our subgroup and sensitivity analysis. The stability of the outcomes could be impacted by this. In addition, some indicators were included in only 2 to 4 studies, and more animal experiments of higher quality need to be included in the future. Third, very few clinical trials have been reported, despite a wealth of preclinical evidence demonstrating the advantageous effect of exosomes produced from stem cells in preclinical models of osteoarthritis. Further standardized investigations are required to address these concerns, as these constraints may have some effect on the results.
Conclusion
This meta-analysis aimed to evaluate the effectiveness of stem cell-derived exosomes in treating OA compared to placebo, utilizing the OARSI score. The findings provide initial preclinical evidence that exosomes can potentially reverse osteoarthritis in animal models. Overall, exosome therapy shows promise for OA treatment in mouse models. However, further investigations are crucial for translating these results to human applications, particularly through studies in larger and more biologically relevant animal models.It is essential to explore the underlying mechanisms of exosome therapy's therapeutic effects. Achieving consensus on research methodologies within the scientific community will enhance data homogeneity and improve the reliability of findings. Furthermore, determining optimal therapeutic conditions—such as dosage, concentration, and treatment duration—is vital for optimizing exosome therapy.
In conclusion, while this meta-analysis presents promising evidence, additional standardized studies are necessary to bridge the gap between preclinical findings and clinical applications of exosome therapy for osteoarthritis. Future research will be crucial in establishing exosomes as a viable therapeutic approach for OA.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1: Fig. S1. Forest plot of exosome-treated versus control group osteophyte score in the mouse study. Heterogeneity was high until the exclusion of the study by Rong et al. Fig. S2. Evaluation of publication bias. Funnel plots for Osteophyte score. Fig. S3. Forest plot of chondrocyte counts in the exosome group versus the control group in the mouse study. Heterogeneity was high until the exclusion of the study by wang et al.
Supplementary file 2: Table S1. The detailed search strategy.
Abbreviations
- OA
Osteoarthritis
- MeSH
Medical subject headings
- DMOADs
Disease-modifying osteoarthritis drugs
- MSC
Mesenchymal stem cell
- EVs
Extracellular vesicles
- SFBs
Synovial fibroblasts
- PRP
Platelet-rich plasma
- iPSC
Induced pluripotent stem cell
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- OARSI
Osteoarthritis research society international
- SYRCLE
Systematic review centre for laboratory animal experiments
- SD
Standard deviation
- MD
Mean difference
- CI
Confidence interval
- SMD
Standardized mean difference
- SD rats
Sprague–dawley rats
- CCM
Cell-free stem cell-derived extract
Author contributions
YZS, YJK and YZW conceived and designed the study. YJK and YJY selected the articles for inclusion and extracted and checked the data. YJK, WYZ, YJY, YH, JJY, MLL and SYX were involved in the analysis of the data. YJK wrote the first draft of the manuscript. YJK, WYZ, and YH revised the final draft. All authors read and approved the final manuscript.
Funding
The work was supported by grants from Hebei Province Key R&D Plan Project (No.22377752D), Medical Science Research Project of Hebei Province (No.20230486), Scientific Research Fund of the Second Hospital of Hebei Medical University (No.2HC202210), and Hebei Provincial Medical Research Project (NO.20210934).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent to publish this manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yajie Kong and Yuzhong Wang have contributed equally.
References
- 1.He Y, Makarczyk MJ, Lin H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020;263:118602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis. JAMA. 2021;325:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Spil WE, Kubassova O, Boesen M, Bay-Jensen A-C, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem Pharmacol. 2019;165:41. [DOI] [PubMed] [Google Scholar]
- 5.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem cells. 2014;32:1254–66. [DOI] [PubMed] [Google Scholar]
- 6.Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review. BMC Musculoskelet Disord. 2016. 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–90. [DOI] [PubMed] [Google Scholar]
- 8.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva FS. Fate of culture-expanded mesenchymal stem cells in the microvasculature. Circ Res. 2009;104:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharm Bull. 2016;6:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 12.Trams EG, Lauter CJ, Norman SalemHeine JRU. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica Biophys Acta BBA - Biomembr. 1981;645:63–70. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. [DOI] [PubMed] [Google Scholar]
- 14.Pluchino S, Smith JA. Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell. 2019;177:225–7. [DOI] [PubMed] [Google Scholar]
- 15.Gurunathan S, Kang M-H, Kim J-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomed. 2021;16:1281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Wang L, Ma C, Wang G, Zhang Y, Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24:10855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020. 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu Q-L, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Medicine [Internet]. 2009;6. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097 [PMC free article] [PubMed]
- 21.Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–8. [DOI] [PubMed] [Google Scholar]
- 22.Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Method. 2014;221:92–102. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Therapy. 2017. 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao S-C, Yuan T, Zhang Y-L, Yin W-J, Guo S-C, Zhang C-Q. Exosomes derived from miR-140–5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Therapy. 2017;8:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017. 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Kuang L, Chen C, Yang J, Zeng W-N, Li T, et al. miR-100–5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. [DOI] [PubMed] [Google Scholar]
- 28.Zavatti M, Beretti F, Casciaro F, Bertucci E, Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors. 2020;46:106–17. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Liang H, Hu X, An J, Ding S, Yu S, et al. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Discov. 2020;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Ding Z, Li Y, Wang W, Wang J, Yu H, et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J Orthop Res. 2019;38:670–9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging. 2020;12:25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020. 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Yan K, Ge G, Zhang D, Bai J, Guo X, et al. Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2020;37:85–96. [DOI] [PubMed] [Google Scholar]
- 35.Rong Y, Zhang J, Jiang D, Ji C, Liu W, Wang J, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325–42. [DOI] [PubMed] [Google Scholar]
- 36.Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9:1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, et al. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol Ther - Nucleic Acids. 2020;22:1078–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang S, Chen P, Zhang H, Weng H, Fang Z, Chen C, et al. Comparison of curative effect of human umbilical cord-derived mesenchymal stem cells and their small extracellular vesicles in treating osteoarthritis. Int J Nanomed. 2021;16:8185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoda Fazaeli, Naser Kalhor, Naserpour L, Davoodi F, Mohsen Sheykhhasan, Seyed Ali Hosseini, et al. A Comparative Study on the Effect of Exosomes Secreted by Mesenchymal Stem Cells Derived from Adipose and Bone Marrow Tissues in the Treatment of Osteoarthritis-Induced Mouse Model. 2021;2021:1–13 [DOI] [PMC free article] [PubMed]
- 40.Jin Y, Xu M, Zhu H, Dong C, Ji J, Liu Y, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25:9281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Zeng Y, Si H-B, Tang L, Xie H-Q, Shen B. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am J Sports Med. 2022;50:1088–105. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Xu Z, Xie Z, Sun X, Li C, Chen Y, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2022;28:362. [DOI] [PubMed] [Google Scholar]
- 43.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18:S17-23. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A, Shivaji K, Kadam S, Gupta M, Rodriguez HC, Potty AG, El-Amin SF, Maffulli N. Immunomodulatory extracellular vesicles: an alternative to cell therapy for COVID-19. Expert Opin Biol Ther. 2021;21(12):1551–60. 10.1080/14712598.2021.1921141. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Cady C, Fauser A-M, Rodriguez HC, Justin Mistovich R, Potty AG, Maffulli N. Cell-free stem cell-derived extract formulation for regenerative medicine applications. Int J Mol Sci. 2020;21(24):9364–9364. 10.3390/ijms21249364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longo UG, Loppini M, Fumo C, Rizzello G, Khan WS, Maffulli N, Denaro V. Osteoarthritis: new insights in animal models. Open Orthopaed J. 2012;6(1):558–63. 10.2174/1874325001206010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Zhuang Y, Fang L, Yuan C, Wang X, Lin K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact Mater. 2023;22:423–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Huang Y, Yang L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res Rev. 2022;80:101684. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Y, Zhang P, Zhang X, Lv L, Zhou Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2020. 10.1111/cpr.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He X-Y, Yu H-M, Lin S, Li Y-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell Mol Biol Lett. 2021. 10.1186/s11658-021-00291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Y, Hu JF, Wu H, Huang ZL, Yan H, Shi Z. Bone marrow stem cells derived exosomes improve osteoporosis by promoting osteoblast proliferation and inhibiting cell apoptosis. Eur Rev Med Pharmacol Sci. 2019;23:1214–20. [DOI] [PubMed] [Google Scholar]
- 54.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015. 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: Fig. S1. Forest plot of exosome-treated versus control group osteophyte score in the mouse study. Heterogeneity was high until the exclusion of the study by Rong et al. Fig. S2. Evaluation of publication bias. Funnel plots for Osteophyte score. Fig. S3. Forest plot of chondrocyte counts in the exosome group versus the control group in the mouse study. Heterogeneity was high until the exclusion of the study by wang et al.
Supplementary file 2: Table S1. The detailed search strategy.
Data Availability Statement
No datasets were generated or analysed during the current study.