Abstract
Background
This study aims to examine the alterations and clinical significance of CD8+ regulatory T cell subsets in the peripheral blood of individuals with type 1 diabetes mellitus (T1DM).
Methods
From January 2020 to December 2023, a study was conducted involving 40 individuals with T1DM, who visited the Department of Endocrinology at the First Affiliated Hospital of Nanjing Medical University (T1DM group). For comparison, 40 healthy individuals who underwent routine physical examinations at the same hospital during this period were selected as the control group. Peripheral blood mononuclear cells were isolated, and CD8+ T cells were labeled with CD3, CD25 and FoxP3 to analyze their subset frequencies using flow cytometry. The study examined differences in subset frequencies between the two groups and explored correlations between subset frequency, disease duration, and age of onset.
Results
The frequencies of CD8+ CD25+, CD8+ FoxP3+, CD8+ CD25+ CD3+ and CD8+ FoxP3+ CD3+ subsets in peripheral blood mononuclear cells did not significantly differ between the healthy control group and the T1DM group (P > 0.05). In the T1DM group, the expression level of CD25 on CD8+ T cells showed no correlation with the age of onset or disease duration, and FoxP3 levels were also unrelated to the age of onset, with no statistical differences (P > 0.05). However, within the T1DM group, FoxP3 levels progressively decreased with longer disease duration, demonstrating a statistically significant negative correlation (Pearson r = -0.331, P < 0.05). In the T1DM group, the level of CD3+ CD8+ T cells expressed CD25, and there was no correlation between Foxp 3 level and age of onset, not statistically significant (P > 0.05), but the level of Foxp 3 in the T1DM group decreased with the duration of the disease, (Pearson r= − 0.363, P < 0.05).
Conclusion
The levels of CD8+FoxP3+ regulatory T cells in peripheral blood mononuclear cells of patients with T1DM show a significant correlation with disease duration, suggesting that these cells may play a critical role in the progression of T1DM.
Keywords: CD8+ regulatory T cells, CD25+, FoxP3+, Type 1 diabetes mellitus
Background
Type 1 diabetes mellitus (T1DM) is an endocrine and metabolic disorder arising from the interaction of susceptibility genes and environmental factors, leading to the destruction of islet β cells by autoantigen-specific and pathogenic T cells, thus necessitating lifelong insulin therapy [1]. Notably, individuals aged 20 years or older constitute 65.3% of newly diagnosed T1DM cases [2]. The pathogenesis of T1DM involves both immune response and immunosuppressive mechanisms. Regulatory T cells (Tregs), a subset of T lymphocytes, are critical in maintaining immune homeostasis by playing a pivotal role in the generation and maintenance of peripheral immune tolerance. Dysregulation in the number and function of Tregs has been implicated in various autoimmune diseases, while an excessive presence of Tregs may hinder the immune response against cancer or pathogens [3, 4]. Historically, the term Treg has typically referred to CD4+CD25High+FOXP3+ T cells, known as conventional Tregs. However, research on CD8+ Tregs has been less common. In recent years, a growing body of research has indicated that CD8+ Tregs play a crucial role in immune regulation in various contexts, including tumors and autoimmune diseases [5]. In the pathogenesis of T1DM, Tregs are not only involved in the suppression of autoreactive T cells, thereby protecting islet β-cells from destruction, but may also influence the natural course of diabetes and response to treatment [6]. Compared to CD4+ Tregs, CD8+ Tregs possess distinct phenotypes and functions; they suppress immune responses through direct cell contact-dependent mechanisms or by secreting cytokines such as Transforming Growth Factor-beta (TGF-β) and Interleukin-10 (IL-10). Importantly, CD8+ Tregs play a crucial role in immune evasion in the tumor microenvironment and immune regulation in autoimmune diseases, suggesting that they may have a vital function in the immune dysregulation associated with T1DM [7]. However, the role of CD8+ Tregs in T1DM remains to be fully elucidated. The aim of this study is to assess the frequency changes of peripheral blood CD8+ Treg cells and their subsets in individuals with T1DM and to explore their clinical significance in the disease’s progression.
Materials and methods
Study subjects
Forty individuals with T1DM who visited the Department of Endocrinology at the First Affiliated Hospital of Nanjing Medical University between January 2020 and December 2023 were selected for this study (T1DM group). The group consisted of 18 males and 22 females, aged between 19 and 62 years, with a mean age of 30.4 ± 11.3 years, and an average disease duration of 1.5 to 16.8 years. The inclusion criteria were: (1) meeting the 1999 WHO diagnostic criteria for diabetes mellitus; (2) having at least one positive antibody among glutamate acid decarboxylase antibody (GADA), protein tyrosine phosphatase antibody (IA − 2 A), zinc transporter 8 antibody (ZnT8A), or insulin autoantibody (IAA), with ZnT8A, GADA, and IA-2 detected using the radiobinding assay, and IAA detected using the enzyme-linked immunosorbent assay; (3) continuous reliance on insulin replacement therapy since onset, with fasting C-peptide levels below 300 pmol/L at initial diagnosis. Exclusion criteria were: (1) presence of microvascular complications or acute and chronic inflammatory diseases; (2) history of smoking and alcohol consumption; (3) comorbid acute and chronic infections, malignant tumors, or thyroid diseases. A control group comprising 40 healthy individuals who underwent physical examinations during the same period, with 18 males and 22 females, aged 18 to 65 years, and a mean age of 30.8 ± 11.6 years was used for this study. The inclusion criteria for the control group are as follows: Healthy individuals were from the same geographic area, without age restriction, and have no diabetes or evident autoimmune diseases or any chronic conditions. The basic demographic data of the two groups are shown in Table 1. The study protocol was reviewed and approved by the hospital ethics committee, and informed consent was obtained from all participants.
Table 1.
Baseline characteristics of the type 1 diabetes group and normal control group
| T1D | HD | |
|---|---|---|
| n | 40 | 40 |
| Age at draw | 30.4 ± 11.3 | 30.8 ± 11.6 |
| Gender (M/F) | 18/22 | 18/22 |
| Age at diagnosis | 19.8 ± 8.8 | – |
| Duration | 4.8 (1.5–16.8) | – |
| Autoantibody positive | ||
| ZnT8A (%) | 16 (40.0) | – |
| GADA (%) | 24 (60.0) | – |
| IA-2 A (%) | 23 (57.5) | – |
| IAA (%) | 35 (87.5) | – |
Age at draw and age at T1D diagnosis are shown as mean ± SD. T1D disease duration is shown as median and quartile
M male, F female, HD, healthy donor
Serum samples and antibody detection
All patients with T1DM and healthy controls underwent a fasting period of over 8 h before blood collection. In the morning, 10 ml of blood was drawn from the cubital vein. The samples were centrifuged at 2500 rpm for 20 min within 2 h to separate the serum, which was subsequently stored at −80 °C. GADA, ZnT8A, and IA-2 were measured using a standardized radiobinding assay. Specifically, 5 µl of serum was incubated overnight at 4 °C with sulfur-35 isotope-labeled antigen. Following incubation, sulfur-35-labeled antibodies were isolated via protein A precipitation (GE Healthcare, USA), and the radioactivity was quantified using a multifunctional liquid scintillation luminescence analyzer (Perkin Elmer, USA). IAA levels were determined using an enzyme-linked immunosorbent assay (Biomerica, USA).
Multi-color flow antibody labeling
Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll density gradient centrifugation. The cells were then surface-labeled with anti-human CD8 antibody (PerCP-Cy5.5, clone SK1, BD Biosciences, USA), anti-human CD25 antibody (PE-Cy7, clone M-A251, BD Biosciences, USA) and anti-human CD3 antibody (BV 605, clone SK7, Biolegend, USA) at room temperature, protected from light, for 30 min. Following two washes with PBS, the cells were fixed with Fix/Perm buffer at room temperature for 45 min. After two additional washes with PBS, intracellular labeling was performed using anti-human FoxP3 antibody (PE-CF594, clone 259D/C7, BD Biosciences, USA) at room temperature and shielded from light for 30 min. The labeled cells were then analyzed using a FACSAria II flow cytometer (BD Biosciences, USA), with data processed using FlowJo V10 software.
Statistical methods
The data were analyzed using SPSS version 20.0. Quantitative data are presented as means ± standard deviations (x̄ ± s). Inter-group comparisons were conducted using a two-sided unpaired t-test. Pearson’s correlation coefficients were employed to evaluate relationships between quantitative variables. Statistical significance was set at a P < 0.05.
Results
Frequency of CD8+regulatory T cell subset in peripheral blood mononuclear cells of patients with TIDM
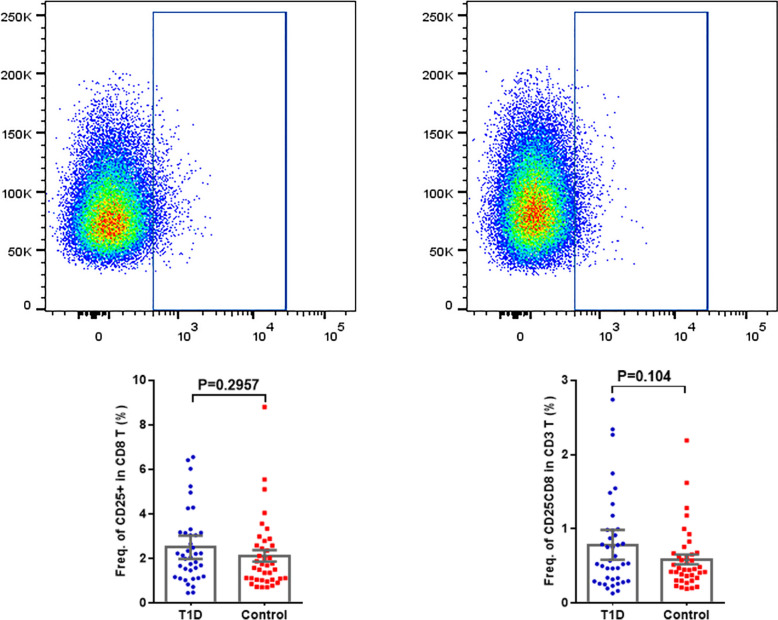
Figure 1 displays the frequency of CD8+CD25+ regulatory T cell subsets. The percentage of CD25+ subsets among total CD8+ regulatory T cells was 2.51 ± 0.26 in the normal control group and 2.13 ± 0.26 in the T1DM group, with a P = 0.2957, indicating no statistical difference (P > 0.05). No significant difference in the frequency of CD25+ CD8+ subset for CD3+ T cells (P = 0.104 > 0.05).
Fig. 1.
Schematic diagrams of flow cytometry dot plots and expression frequency for CD8+ CD25+ T Cells in the T1DM and normal control groups
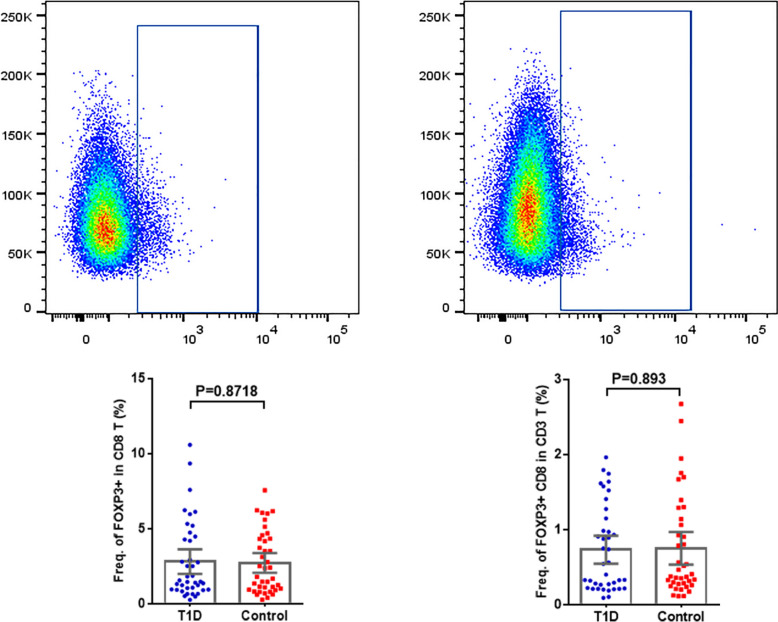
Figure 2 provides a schematic representation of the frequency of CD8+FoxP3+ regulatory T cell subsets. The percentage of FoxP3+ subsets among total CD8+ regulatory T cells was 2.83 ± 0.41 in the normal control group and 2.74 ± 0.32 in the T1DM group, with a P = 0.8718, indicating no statistical difference (P > 0.05). There was no significant difference in the frequency of CD3+ CD8+ T cells (P = 0.893 > 0.05).
Fig. 2.
Schematic diagrams of flow cytometry dot plots and expression frequency for CD8+ FoxP3+ T cells in the T1DM and normal control groups
Relationship between the frequency of CD8+regulatory T cell subsets and age of onset and disease duration
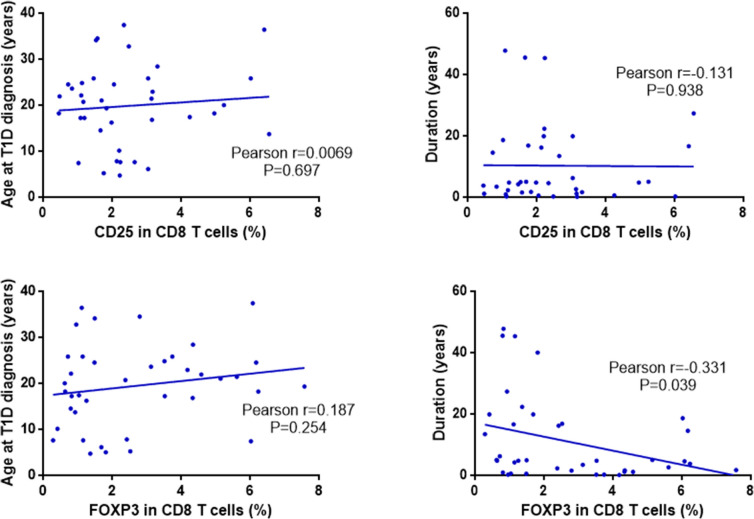
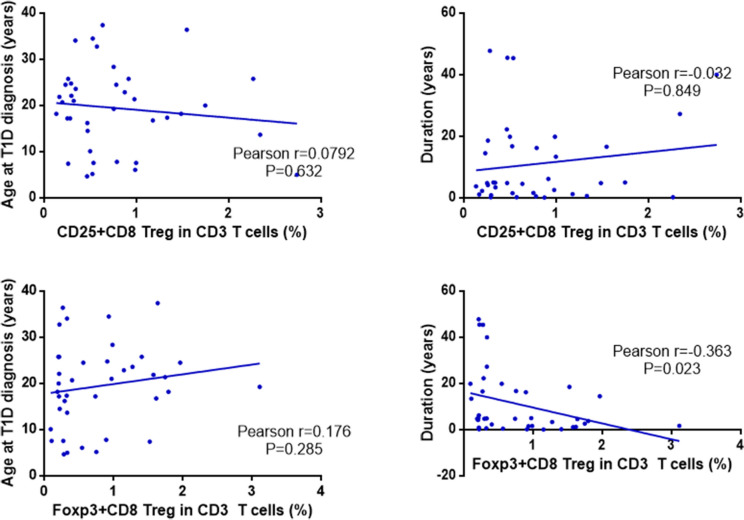
The Pearson’s correlation coefficient was utilized to assess the relationship between the frequency of CD8+ regulatory T cell subsets and both the age of onset and disease duration in the T1DM group. The frequency of CD25+CD8+ regulatory T cell subsets did not show a significant association with the age of onset (Pearson’s r = 0.0069, P = 0.697) or disease duration (Pearson’s r = 0.131, P = 0.938), with no statistically significant differences (P > 0.05). Conversely, the frequency of FoxP3+CD8+ regulatory T cell subsets did not correlate with the age of onset (Pearson’s r = 0.187, P = 0.254). However, it exhibited a significant negative correlation with disease duration (Pearson’s r = − 0.331, P = 0.039), indicating a statistical difference (P < 0.05). See Fig. 3. The frequency of CD25+ expression in CD3+ CD8+ regulatory T cell was not associated with age of onset (Pearson r = 0.0792, P = 0.632), but also not associated with disease duration (Pearson r= − 0.032, P = 0.849) and not statistically significant (P > 0.05). The frequency of FoxP 3+ expression in CD3+ CD8+ regulatory T cell was not associated with age at onset (Pearson r = 0.176, P = 0.285), not statistically significant (P > 0.05), but negatively correlated with disease duration (Pearson r= − 0.363, P = 0.023), statistically significant (P < 0.05). See Fig. 4 for details.
Fig. 3.
Correlation analysis between CD8+ regulatory T cell subset frequencies and age of onset, disease duration in T1DM
Fig. 4.
Correlation analysis between CD3+ CD8+ regulatory T cell subset frequencies and age of onset, disease duration in T1DM
Discussion
Studies have established that the immune system significantly influences the onset and progression of T1DM. The destruction of islet β cells, which characterizes the disease, involves interactions between autoantigens within the islets and various immune cells, including CD4+ and CD8+ T lymphocytes, B lymphocytes, natural killer cells, and dendritic cells. In the detection of T cells, CD3 is one of the most commonly used markers. CD3 initially appears in the cytoplasm of anterior thymocytes. As T cells mature, CD3 in the cytoplasm gradually decreases and begins to be expressed in the cell membrane, so CD3+ is a marker of mature T cells. Regulatory T cells (Tregs), a subtype of T lymphocytes, are crucial for maintaining immune homeostasis due to their potent immunosuppressive functions. These cells mediate anti-inflammatory effects through the production of cytokines such as IL-10 and TGF-β, which are vital for normal immune regulation [3, 4, 8].
Furthermore, predominant types of CD8+ Tregs, based on surface markers, include CD8+CD103+, CD8+CD122+, CD8+CD28−, and CD8α+HLA-E+TCRαβ+ cells [9]. Other regulatory CD8+ populations include CD8+CD25+ and CD8+CD25+FoxP3+ cells. Churlaud et al. [10] identified CD8+ T cells expressing CD25high+FoxP3+ as immunosuppressive cells in both murine and human models, associating them with immunosuppressive functions. CD8+ Tregs display similar inhibitory capabilities to CD4+ Tregs, exerting their effects through direct cell-to-cell contact or cytokine secretion pathways [7, 11].
Zhang et al. [12] isolated CD8+CD25+ Tregs with high expression of FoxP3, CD62L, CTLA-4, and CD103 from HHD mice. These cells demonstrated significantly greater immunosuppressive abilities compared to CD4+CD25+ Tregs from the same mouse strain. Treatment with H6F led to the expansion of a subset of CD8+CD25+FoxP3+ T cells, which required peptide-specific stimulation for their immunosuppressive activity, suggesting that CD8+ Tregs possess stronger immunosuppressive capabilities than CD4+ Tregs. [12] Additionally, studies indicate that CD8+CD25+ Tregs exert immunosuppressive effects not only peripherally but also locally within the pancreas, potentially inhibiting the autoimmune processes in T1DM through mechanisms involving CTLA-4, CD103, IL-17, and IFN-γ [13].
Additionally, CD25, a receptor for interleukin-2 (IL-2), is a key marker of T-cell activation and plays a significant role in IL-2’s biological effects. Elevated levels of CD25 indicate T-cell activation and reflect the body’s immune status. CD8+CD25+ regulatory T cells (Tregs), a subset of Tregs, are critical for maintaining immune tolerance and regulating immune responses, akin to CD4+CD25+ Tregs. Despite their lower proportion in peripheral blood, CD8+CD25+ Tregs exhibit greater regulatory effects compared to CD4+CD25+ Tregs [14]. Recent studies have shown that CD8+CD25high+FoxP3+ T cells have even stronger inhibitory properties, particularly under IL-2 stimulation [10].
FoxP3, a member of the forkhead family of transcription factors encoded by the X-chromosome, is essential for the development and function of Tregs. It regulates Treg expression and supports their immunoregulatory functions through sustained expression [15]. FoxP3 is increasingly recognized as a robust indicator of true inhibitory function [10]. Elevated levels of CD8+CD25+FoxP3+ Tregs have been observed in patients with colorectal cancer, where they contribute to tumor progression by modulating TGF-β1 levels. FoxP3+ colon cancer cells are likely to cooperate with Tregs in facilitating tumor immune escape [16]. In individuals with ovarian cancer, increased levels of CD8+CD25+FoxP3+ Tregs are associated with disease progression, and FoxP3 levels positively correlate with tumor staging.
Research by Agle et al. [17] indicates that the Bim apoptosis gene affects the persistence of CD8+FoxP3+ Tregs in graft-versus-host disease (GVHD) target tissues post-transplantation. FoxP3 expression is more stable in CD8+ Tregs lacking Bim, suggesting that variations in FoxP3 levels may be related to the Bim apoptosis gene. From an immunological standpoint, the infusion of Tregs may enhance regulatory functions and mitigate autoimmune attacks on islet cells [18]. Studies in mouse models of T1DM show a decrease in FoxP3+ Tregs and increased apoptosis, with delayed diabetes onset observed when the survival of FoxP3+ Tregs is prolonged [19, 20]. Trzonkowski et al. demonstrated that infusing FoxP3+ Tregs could potentially benefit pediatric patients with T1DM [21]. Additionally, elevated frequencies of CD8+CD25high+ T cells and higher FoxP3 levels have been noted in individuals with liver cancer, mirroring changes observed in patients with systemic lupus, multiple sclerosis, other autoimmune diseases, asthma, and various malignancies [11].
Pellegrino et al. reported a significant reduction in CD8+CD25+ T cell frequency in patients with T1DM with a disease duration of ≥ 10 years [22]. However, Our study revealed no significant difference in the frequency of peripheral blood CD8+CD25+ T cells between the T1DM and healthy control groups. The observed differences may be attributed to the relatively short average disease duration of the patients included in this study. Our study similarly found that the surface expression level of CD25 on CD8+ T cells was not associated with the age of onset or disease duration. Additionally, the FoxP3 expression level did not correlate with the age of onset, although it did show a gradual decrease with increasing disease duration in individuals with T1DM. This finding contrasts with observations in individuals diagnosed with malignant tumors, where Treg levels increase with disease progression, potentially enhancing immunosuppressive effects and contributing to immune evasion mediated by FoxP3+ Tregs. In contrast, those with T1DM experience a decline in CD8+FoxP3+ Tregs over time, leading to reduced immunosuppressive activity. The reduction in FoxP3 expression may be associated with the potential loss of Treg suppressive function, as FoxP3 is a key transcription factor that maintains the suppressive activity of Tregs. This diminished regulatory function could lead to an exacerbation of autoimmune responses in patients with T1DM, thereby accelerating the destruction of islet β-cells. Consequently, our findings underscore the need for novel therapeutic strategies in T1DM treatment to enhance or replace Treg functions, protecting islet β-cells from autoimmune attacks. It is noteworthy that regulatory Tregs exhibit a dual role in cancer patients and autoimmune diseases. In the tumor environment, an increased number of Tregs is often associated with poorer prognosis, as they can suppress antitumor immune responses and aid in the evasion of immune surveillance by tumor cells. However, in certain contexts, Tregs may also play a protective role by limiting excessive inflammation and tissue damage. In autoimmune diseases, Tregs can suppress immune responses to self-antigens, preventing tissue damage caused by autoimmune reactions, but excessive Treg function may also lead to overly robust immune responses that harm healthy tissues.
This study indicates that FoxP3 expression in peripheral blood CD8+FoxP3+ Tregs diminishes as the disease progresses, suggesting a decline in their inhibitory function. Research has demonstrated that CD8+CD25High+FoxP3+ T cells exhibit enhanced suppressive capacity upon IL-2 stimulation compared to conventional Tregs [10]. Therefore, IL-2-targeted therapy for CD8+CD25High+FoxP3+ T cells presents a novel therapeutic approach for liver cancer. Given these insights, CD8+CD25High+FoxP3+ T cell therapy may offer a promising new treatment strategy for individuals with T1DM.
In summary, this study indicates that FoxP3 levels in the T1DM group decrease progressively with disease duration, showing a negative correlation. This finding may offer new insights into the pathogenesis of T1DM and the potential for CD8+ Treg-targeted immunotherapy. However, due to the limited sample size, this study did not include subgroup stratification analysis for T1DM. Meanwhile, there is a lack of correlation studies with relevant clinical manifestations, such as glycemic control (HbA1c), incidence of complications, or insulin sensitivity. Future research should involve a larger cohort to enable more detailed exploration. Additionally, further functional investigations are required to fully elucidate the role of CD8+ T regulatory cells in T1DM progression. Due to the very low expression of the CD8+FoxP3+ T cell subset within the total lymphocyte population, we are actively seeking new approaches to achieve more precise functional experimental outcomes. In our subsequent studies, we will conduct a broader range of functional assays, such as cytokine production and suppression tests, as well as longitudinal clinical observations to track changes in patient conditions. We believe that future studies delving further into the role of CD8+ Tregs in T1DM will have a profound impact on our understanding of the disease’s immune mechanisms and the development of novel therapeutic strategies. As our comprehension of the functions and regulatory networks of Tregs in T1DM deepens, we can more precisely identify key nodes in the disease process, offering new perspectives for the development of targeted treatments. For instance, enhancing or mimicking the immunomodulatory functions of Tregs may help protect islet β-cells from autoimmune attacks, potentially slowing disease progression. Furthermore, a deeper understanding of the complex interplay between Tregs and T1DM pathology may uncover new biomarkers, thereby improving disease surveillance and management. These studies will not only enhance our knowledge of the etiology of T1DM but also may lead to more effective and personalized treatment options for patients.
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Abbreviations
- CD
Cluster of differentiation
- T1DM
Diabetes mellitus type 1
- Tregs
Regulatory T cell
- PBMC
Peripheral blood mononuclear cells
- Foxp3
Forkhead box P3
- GVHD
Graft versus-host disease
- SPSS
Statistical Package for the Social Sciences
Author contributions
Conception and design of the research: Shu Chen, Chunhua Wang, Xueqin WangAcquisition of data: Hemin Jiang, Min ShenAnalysis and interpretation of the data: Gaoqiang Meng, Xing WangStatistical analysis: Hemin Jiang, Min Shen, Xing WangObtaining financing: Chunhua WangWriting of the manuscript: Shu Chen, Gaoqiang MengCritical revision of the manuscript for intellectual content: Shu Chen, Xueqin Wang, Chunhua WangAll authors read and approved the final draft.
Funding
Social and People’s Livelihood Project of Nantong (MS22022001), Clinical Research Program of Nantong University (2022LZ005).
Data availability
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki(as was revised in 2013). The study was approved by Ethics Committee of the Nantong First People’s Hospital (2024KT358). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shu Chen and Gaoqiang Meng have contributed equally to this work.
References
- 1.Zhang J, Xiao Y, Xie L, et al. Lipid metabolism in type 1 diabetes mellitus: Pathogenetic and therapeutic implications. Front Immunol. 2022;13:999108. 10.3389/fimmu.2022.999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng J, Zhou Z, Jia W, T1D China Study Group, et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360:j5295. 10.1136/bmj.j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30:465–74. 10.1038/s41422-020-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plitas G, Rudensky AY. Regulatory T cells: differentiation and function. Cancer Immunol Res. 2016;4:721–5. 10.1158/2326-6066.CIR-16-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillonneau C, Hill M, Li XL, et al. CD40Ig treatment results in allograft acceptance mediated by CD8+CD45RClow T cells, IFN-γ, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1096–106. 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer JR, Racine JJ, Chapman HD, Quinlan A, Presa M, Stafford GA, Schmitz I, Serreze DV. Nfkbid overexpression in nonobese diabetic mice elicits complete type 1 diabetes resistance in part associated with enhanced thymic deletion of pathogenic CD8 T cells and increased numbers and activity of regulatory T cells. J Immunol. 2022;209(2):227–37. 10.4049/jimmunol.2100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra S, Srinivasan S, Ma C, Zhang N. CD8 + Regulatory T cell - A mystery to be revealed. Front Immunol. 2021;12:708874. 10.3389/fimmu.2021.708874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao YC, Shen J, Zhao HL, et al. Changes of regulatory T cells and of proinflammatory and Immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and Meta-analysis. J Diabetes Res. 2016;2016:3694957. 10.1155/2016/3694957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy-Batalla R, Acevedo D, Alsina L, et al. Treg in inborn errors of immunity: gaps, knowns and future perspectives. Front Immunol. 2024;14:1278759. 10.3389/fimmu.2023.1278759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churlaud G, Pitoiset F, Klatzmann D, et al. Human and mouse CD8(+)CD25(+)FOXP3(+) regulatory T cells at steady state and during interleukin-2 therapy. Front Immunol. 2015;6:171. 10.3389/fimmu.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahran AM, Nafady-Hego H, Hetta HF, et al. Increased frequency and FOXP3 expression of human CD8+CD25High+ T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80(7):510–6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Wang S, Wang L, et al. An altered CD8+ T cell epitope of insulin prevents type 1 diabetes in humanized NOD mice. Cell Mol Immunol. 2019;16(6):590–601. 10.1038/s41423-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai S, Shameli A, Santamaria P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32(4):568–80. 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Cosmi L, Liotta F, Annunziato F, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102(12):4107–14. 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Yamaguchi T, Ono M, et al. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Grimmig T, Kim M, Waaga-Gasser AM, et al. The role of FOXP3 in disease progression in colorectal cancer patients. Oncoimmunology. 2013;2(6):e24521. 10.4161/onci.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agle K, Vincent BG, Drobyski WR, et al. Bim regulates the survival and suppressive capability of CD8+ FOXP3+ regulatory T cells during murine GVHD. Blood. 2018;132(4):435–47. 10.1182/blood-2017-09-807156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P, et al. Proinsulin-specific T regulatory cells may control immune responses in type 1 diabetes: implications for adoptive therapy. BMJ Open Diabetes Res Care. 2020;8(1):e000873. 10.1136/bmjdrc-2019-000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Adams JY, Bluestone JA, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–97. 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinberg-Bleyer Y, Baeyens A, Piaggio E, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–8. 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Skowronek I, Sieniawska J, Rosolowska I, et al. Potential therapeutic application of regulatory T cells in diabetes mellitus type 1. Int J Mol Sci. 2021;23(1):390. 10.3390/ijms23010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrino M, Crinò A, Fierabracci A, et al. Identification and functional characterization of CD8 + T regulatory cells in type 1 diabetes patients. PLoS ONE. 2019;14(1):e0210839. 10.1371/journal.pone.0210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.