Abstract
Background
Patients with familial fibrotic interstitial lung disease (ILD) experience worse survival than patients with sporadic disease. Current guidelines do not consider family aggregation or genetic information in the diagnostic algorithm for idiopathic pulmonary fibrosis or other fibrotic ILDs. Better characterizing familial cases could help in diagnostic and treatment decision-making.
Methods
This retrospective cohort study included 222 patients with fibrotic ILD (104 familial and 118 sporadic) from Bellvitge University Hospital. Clinical, radiological, pulmonary functional tests (PFT), and histological evaluations were performed at diagnosis and follow-up. Telomere shortening and disease-associated variants (DAVs) in telomerase-related genes were analysed in familial patients and sporadic patients with telomeric clinical signs. Primary outcomes were the presence of a UIP histological pattern and disease progression.
Results
Patients with idiopathic pulmonary fibrosis (IPF) (52%), fibrotic hypersensitivity pneumonitis (23%), and other fibrotic ILDs (25%) were included. 42% of patients underwent lung biopsy. Patients with family aggregation were younger and less frequently associated comorbidities, male sex, and smoking history. However, usual interstitial pneumonia (UIP) was more frequent on pathology (p = 0.005; OR 3.37), especially in patients with indeterminate or non-UIP radiological patterns. Despite similar PFT results at diagnosis, familial patients were more likely to present with progressive disease (p = 0.001; OR 3.75). Carrying a DAV increased the risk of fibrotic progression in familial and sporadic patients (p = 0.029, OR 5.01).
Discussion
Familial patients diagnosed with different fibrotic ILDs were more likely to exhibit a histological UIP pattern and disease progression than sporadic patients, independent of radiological findings and pulmonary function at diagnosis.
Conclusion
Considering the diagnostic likelihood of the histological UIP pattern and disease outcome, the presence of family aggregation would be useful in the decision making of multidisciplinary committees.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03063-y.
Keywords: Prognosis; Retrospective studies; Telomere Shortening; Lung diseases, interstitial; Lung; Biopsy; Idiopathic pulmonary fibrosis; Disease Progression; Fibrosis; Family
Background
Fibrotic interstitial lung diseases (ILDs) represent a substantial burden, with a prevalence of 76 cases per 100,000 inhabitants in Europe. These include drug or radiation-induced fibrotic ILDs, inhaled exposure-related fibrotic ILDs (organic or inorganic substances), autoimmune or connective tissue disease(CTD)-associated fibrotic ILDs and idiopathic interstitial pneumonias (IIPs) [idiopathic nonspecific interstitial pneumonia (NSIP) and idiopathic pulmonary fibrosis (IPF)], among others [1]. IPF is the most lethal and frequent fibrotic IIP, with an estimated mean prevalence of 2–10 cases per 100,000 inhabitants [2, 3]. Pulmonary fibrosis is present in many other non-IPF ILDs and may progress despite conventional integral treatment, which is defined as progressive pulmonary fibrosis (PPF) [4–6]. The incidence of PPF in non-IPF patients varies between 13 and 40%, depending on the entity [1, 7]. Pirfenidone and Nintedanib, anti-fibrotic drugs evaluated in clinical trials, have shown to slow functional decline and disease progression [6–10]. Lung transplantation improves survival in eligible patients with PPF [6, 11].
A confident diagnosis has strong implications for patient management and prognosis [12]. The multidisciplinary ILD diagnostic approach includes clinical, functional, radiological and serological assessments, with or without bronchoscopy or surgical lung sampling [1]. In IPF, lung biopsy is not required for patients with radiological consistent or probable UIP patterns on high resolution computed tomography (HRCT) and proper clinical context; due to the high probability (> 90%) of a histological UIP pattern being found in lung biopsy [6, 13, 14]. Therefore, lung biopsy is mostly indicated in young patients with fibrotic ILDs and those with indeterminate UIP or non-UIP radiological patterns, balancing the potential morbidity and mortality of the procedure [6].
Familial pulmonary fibrosis (FPF) is defined as the presence of at least 2 cases of pulmonary fibrosis in the same family [15, 16]. Familial aggregation is the most important risk factor for IPF, and is also associated with earlier onset of the disease [17]. Up to 20% of patients with IPF have a first degree relative with ILD [18]. Family history also predicts worse transplant-free survival and an increased risk of death, both in IPF and non-IPF fibrotic ILD patients [19, 20].
Furthermore, disease-associated variants in telomere-related genes (TRGs) are detected in approximately 30% of the families (TERT, TERC, DKC1, PARN, RTEL1, etc.) [21]. Telomere attrition, a consequence of TRG pathogenic variants, may be found in sporadic and familial fibrotic ILD. IPF patients with telomere shortening more frequently exhibit a decrease in forced vital capacity (FVC) and short transplant-free survival [22]. Similar results have been reported for other non-IPF fibrotic ILDs with telomere shortening such as hypersensitivity pneumonitis, unclassifiable ILD, interstitial pneumonitis with autoimmune features (IPAF) and CTD-ILD [23, 24]. Genetic factors of FPF have already been extensively described [16, 25].
Despite increasing evidence of the impact of family history and the genetic component of the disease, recommendations for clinical practice diagnosis are just starting to emerge. These issues are only discussed in the most recent guidelines as future directions to improve patient diagnosis and management [6, 26]. To assist in the differential diagnosis of fibrotic ILDs; and in the management and prognosis prediction of patients, we aimed to evaluate the relevance of familial aggregation and telomere dysfunction in predicting the UIP histological pattern and progressive fibrotic disease.
Methods
The primary outcomes were the presence of a UIP histopathological pattern and progressive fibrotic disease.
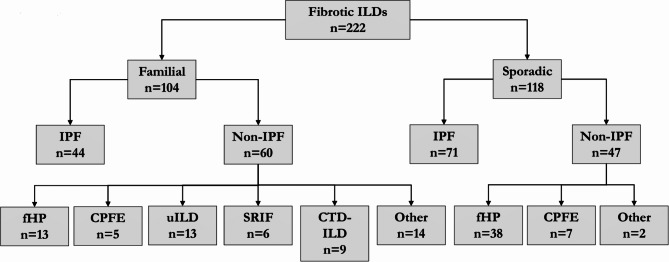
This retrospective observational study included 222 consecutive newly diagnosed patients with fibrotic ILD at the ILD Unit of Bellvitge University Hospital (HUB), in L’Hospitalet de Llobregat, from January 2015 to December 2019. An ILD was considered fibrotic when presented with features of diffuse fibrosing lung disease of > 10% extent on an HRCT scan [27]. ILD diagnoses and classifications were made according to the most recent IPF and ILD guidelines [6, 15, 28]. Four patients were excluded because of a non-ILD diagnosis. The Ethics Committee of HUB approved the study (PR307/16), and all patients provided written informed consent before inclusion, including written informed consent for genetic testing if needed. Clinical data were recorded at the time of patient inclusion. Patients were split into sporadic and familial groups depending on the existence of at least 2 cases of fibrotic ILD in the same family, resulting in 118 patients in the sporadic group and 104 patients in the familial group [15, 18]. A flow chart of the study cohort composition is available in Fig. 1.
Fig. 1.
Flow chart of grouping based on final diagnosis
IPF: idiopathic pulmonary fibrosis, fHP: fibrotic hypersensitivity pneumonitis, uILD: unclassifiable interstitial lung disease, CPFE: combined pulmonary fibrosis and emphysema, SRIF: smoking-related interstitial fibrosis, CTD-ILD: connective tissue disease associated interstitial lung disease. Other include ANCA-associated vasculitis, cocaine-induced pulmonary fibrosis, stage IV sarcoidosis, fibrotic non-specific interstitial pneumonia, radiotherapy induced pulmonary-fibrosis, interstitial pneumonia with autoimmune features and pleuroparenchymal fibroelastosis
Clinical features, including extrapulmonary manifestations of telomere shortening and family history, were recorded [29, 30]. Pulmonary function test (PFT) results were collected and both the FVC and hemoglobin-adjusted diffusing capacity for carbon monoxide (DLCO) were collected at diagnosis and annually during follow-up. Changes were evaluated based on the values at diagnosis and one year after diagnosis. Radiologic fibrotic signs were visually semiquantified by two independent expert radiologists, who compared the diagnostic HRCT with a second HRCT (obtained 24 months after diagnosis or closest available). The need for transplantation or death at any point in follow-up was noted, and an extensive case review was performed. Lung biopsy was performed when required for a final diagnosis based on ATS/ERS guidelines or multidisciplinary committee consensus, through transbronchial cryobiopsy or video-assisted thoracic surgery (VATS). HRCT and histopathological pattern definitions were reviewed by the multidisciplinary committee at the time of diagnosis, according to the IPF guidelines in place [15, 28, 31]. Fibrotic progression was considered when the FVC decline was ≥ 10% of the predicted value (without any other explanation) 1 year after the diagnosis or an increase in the extent of chest HRCT fibrotic signs on the follow-up HRCT, the need for a lung transplant or death due to respiratory disease [4, 5, 8, 32].
DNA samples were isolated from mouth epithelial cells and peripheral blood mononuclear cells with commercial kits (Isohelix, Cell Projects Ltd) as previously described [22]. Telomere length was assessed first by quantitative polymerase chain reaction (qPCR) and, when needed, by Southern blot analysis of terminal restriction fragment lengths (TRFLs) [22]. The ratio of telomere repeat copy number to that of the single-copy gene 36B4 was determined and compared with that of a reference DNA sample. Age-adjustment was made by calculating the z-score as previously described [22]. A telomere shortening was defined by a z-score < 25th percentile, and severe telomere shortening was considered when it was < 10th percentile. TRG sequence variants were identified by panel sequencing or whole-exome sequencing in patients without any identified variant of interest and high suspicion of a genetic cause (strong family aggregation and inheritance or clear clinical conditions associated with telomere dysfunction and severe telomere attrition). The whole-exome sequencing procedure was conducted as described previously [33]. Relevant genetic variants, designated disease-associated variants (DAVs), encompassed those classified as pathogenic, likely pathogenic or variants of uncertain significance possibly associated with diseases identified in the TERT, TERC, PARN, DKC1 or RTEL1 genes [34, 35].
A summary of the relevant collected variables was completed with basic descriptive statistics, using either proportions for qualitative variables or the arithmetic mean and standard deviation for quantitative variables, calculated for overall, the familial group and the sporadic group. P values of differences between these categories for quantitative variables were calculated using independent samples t-test (or paired sample t-test) if both assumptions of homogeneity of variances (with Levene’s test) and normality (Shapiro-Wilk test, and Q-Q plot) were met. Otherwise, Mann-Whitney U test was employed for independent samples. For differences in proportions, the independent samples chi-square test of association was used, unless expected frequencies in at least one level of the variable were less than 5, for which Fisher’s exact test was used.
A binomial logistic regression model was built to adjust for potential confounding among three primary variables of interest, which showed significant statistical differences in the univariate analysis. A computed binary categorical variable, “progressive disease”, was created to match the definition of fibrotic progression previously described. The pattern on biopsy was simplified into a binary categorical variable, regrouping “consistent UIP” and “probable UIP” into “UIP” and others into “non-UIP” [6]. For the binomial logistic regression model, telomere shortening was transformed into a binary categorical variable, with a 25% cut-off, since telomere length < 10th percentile is usually associated with the presence of disease-associated TRG variants (pathogenic, probably pathogenic, or uncertain significance favouring pathogenicity), but some of them are not associated with severe shortening [25].
P values < 0.05 were regarded as statistically significant. The magnitude of the difference in proportions was provided as odds ratios (ORs) and 95% confidence intervals (CIs).
Results
A summary of patient characteristics is available in Table 1. Familial patients were younger (p < 0.001) and presented fewer comorbidities and smoking history (p < 0.001). Despite a male sex predominance in both groups, the female frequency was higher among familial cases (p = 0.037). A significantly greater percentage of sporadic had probable or confident UIP radiological patterns (p < 0.001). Familial patients were more likely to have telomere shortening (p < 0.001), extrapulmonary disease manifestations (p < 0.001), blood count abnormalities at diagnosis (p < 0.001), liver abnormalities (p < 0.001), to require a multidisciplinary committee diagnostic approach (p = 0.025) and to carry a disease-associated TRG variant (p < 0.001). There were no significant differences in the occurrence of lung cancer or other cancers. The FVC and DLCO at diagnosis, as well as the proportion of patients who received antifibrotic drugs or prednisone, were similar. More IPF patients were included in the sporadic group (p < 0.001). Most sporadic IPF patients (88.6%) exhibited a probable or consistent UIP HRCT pattern, while only 38.6% of familial IPF patients exhibited this pattern. Patients who experienced worsening of radiological fibrosis after two years were more likely to be in the familial group (OR 3.41, 95% CI 1.86–6.24; p < 0.001).
Table 1.
Baseline characteristics of patients
| Overall n = 222 |
Familial n = 104 |
Sporadic n = 118 |
P value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 66.5 ± 10.0 | 63.2 ± 11.2 | 69.4 ± 7.9 | < 0.001u |
| Gender (Female) | 62 (28) | 36 (35) | 26 (22) | 0.037χ |
| Comorbidities (Yes) | 164 (74) | 60 (58) | 104 (88) | < 0.001χ |
| Smoking history (Yes) | 130 (59) | 47 (45) | 83 (70) | < 0.001χ |
| Antifibrotic drugs (Yes) | 130 (59) | 60 (58) | 70 (59) | 0.806χ |
| Prednisone (Yes) | 80 (36) | 43 (41) | 37 (31) | 0.122χ |
| Idiopathic pulmonary fibrosis | 122 (55) | 44 (42) | 78 (66) | < 0.001χ |
| Radiological Pattern | n = 221 | n = 104 | n = 117 | < 0.001χ |
| Non-UIP | 86 (39) | 52 (50) | 34 (29) | |
| Indeterminate UIP | 27 (12) | 21 (20) | 6 (5) | |
| Probable UIP | 63 (29) | 12 (12) | 51 (44) | |
| UIP | 45 (20) | 19 (18) | 26 (22) | |
| Relative Telomere Length | n = 149 | n = 102 | n = 47 | - |
| p75-p100 | 1 (1) | 1 (1) | 0 (0) | |
| p50-75 | 18 (12) | 13 (13) | 5 (11) | |
| p25-50 | 45 (30) | 18 (18) | 27 (57) | |
| <p25 | 43 (29) | 34 (33) | 9 (19) | |
| <p10 | 17 (11) | 15 (15) | 2 (4) | |
| <p1 | 25 (17) | 21 (20) | 4 (9) | |
| Telomere shortening < p25 (Yes) | 85 (57) | 70 (69) | 15 (32) | < 0.001χ |
| Pathology Pattern | n = 94 | n = 41 | n = 53 | - |
| UIP | 32 (34) | 18 (44) | 14 (26) | |
| Probable UIP | 12 (13) | 8 (20) | 4 (8) | |
| Non-UIP | 50 (53) | 15 (36) | 35 (66) | |
| Associated lung cancer (Yes) | n = 219 | n = 104 | n = 115 | 0.314f |
| 9 (4) | 6 (6) | 3 (3) | ||
| Other cancer (Yes) | n = 219 | n = 104 | n = 115 | 0.634χ |
| 23 (10) | 12 (12) | 11 (10) | ||
| Telomere-related extrapulmonary manifestations (Yes) | n = 221 | n = 104 | n = 117 | < 0.001χ |
| 59 (27) | 43 (41) | 16 (14) | ||
| Blood count abnormality at diagnosis (Yes) | n = 218 | n = 103 | n = 115 | < 0.001χ |
| 55 (25) | 43 (42) | 12 (10) | ||
| Anaemia (Yes) | n = 219 | n = 104 | n = 115 | 0.015χ |
| 24 (11) | 17 (16) | 7 (6) | ||
| Thrombocytopenia (Yes) | n = 220 | n = 104 | n = 118 | 0.021χ |
| 16 (7) | 12 (12) | 4 (3) | ||
| Liver abnormality at diagnosis (Yes) | n = 220 | n = 104 | n = 116 | < 0.001χ |
| 59 (27) | 43 (41) | 16 (14) | ||
| Pulmonary Function Tests | ||||
| FVC (L) at diagnosis | n = 215 | n = 102 | n = 113 | 0.633t |
| 2.82 ± 0.88 | 2.85 ± 0.98 | 2.79 ± 0.784 | ||
| FVC (%) at diagnosis | n = 214 | n = 102 | n = 112 | 0.147t |
| 84.2 ± 20.1 | 86.3 ± 21.6 | 82.3 ± 18.4 | ||
| DLCO (%) at diagnosis | n = 213 | n = 101 | n = 112 | 0.726t |
| 59.4 ± 22.2 | 60.0 ± 22.5 | 58.9 ± 22.1 | ||
| Multidisciplinary committee (Yes) | n = 222 | n = 104 | n = 118 | 0.025χ |
| 187 (83) | 94 (90) | 93 (79) | ||
| Telomere-related gene variants (Yes) | n = 182 | n = 103 | n = 79 | < 0.001χ |
| 83 (46) | 66 (64) | 17 (22) | ||
| Family History | - | |||
| 1st degree relative (Yes) | - | 98 (94) | - | |
| 2nd degree relative (Yes) | - | 18 (17) | - | |
| More than 1 relative (Yes) | - | 49 (47) | - |
The results are given with 3 decimals: mean ± standard deviation if numerical and n (%) if categorical. tt-test, uMann-Whitney U test, χchi-square test, fFisher’s exact test
There were no statistically significant differences in the frequency of biopsies obtained through VATS or transbronchial cryobiopsy (p = 0.863). A histological pattern of UIP (including both UIP and probable UIP) was more frequently present in the familial group (OR 3.37, 95% CI 1.44–7.91; p = 0.005). When restricted to patients with radiologically indeterminate UIP or non-UIP patterns on HRCT, this difference was greater (OR 5.11, 95% CI 1.88–13.93; p = 0.001). All patients with an indeterminate UIP pattern on HRCT showed a UIP pattern on biopsy. Among those with a non-UIP radiological pattern, familial patients were more likely to have a UIP histological pattern (OR 10.67; 95% CI 2.66–42.83; p < 0.001). The results are described in Table 2.
Table 2.
Unadjusted outcomes of pathology pattern differences
| Overall N = 222 |
Familial N = 104 |
Sporadic N = 118 |
p | OR | 95%CI | |
|---|---|---|---|---|---|---|
| Overall population | n = 94 | n = 41 | n = 53 | |||
|
UIP Non-UIP |
44 (47) 50 (53) |
26 (63) 15 (37) |
18 (34) 35 (66) |
0.005χ | 3.37 | 1.44–7.91 |
| iUIP and non-UIP on HRCT | n = 74 | n = 37 | n = 37 | |||
|
UIP Non-UIP |
32 (43) 42 (57) |
23 (62) 14 (38) |
9 (24) 28 (76) |
0.001χ | 5.11 | 1.88–13.93 |
| non-UIP on HRCT | n = 61 | n = 30 | n = 31 | |||
|
UIP Non-UIP |
19 (31) 42 (69) |
16 (53) 14 (47) |
3 (10) 28 (90) |
< 0.001χ | 10.67 | 2.66 − 42.83 |
The results are given with N (%) if categorical. χchi-square. OR odds ratio. 95% CI 95% confidence interval. Probable UIP and UIP are included in UIP. iUIP indeterminate UIP
Familial patients were more likely to present with progressive pulmonary fibrosis (OR 4.53, 95% CI 2.51–8.18; p < 0.001) and to require lung transplantation (OR 2.51, 95% CI 1.15–5.48; p = 0.018). No statistically significant difference in mortality was found between the groups (Table 3). The presence of telomere shortening was associated with fibrotic progression (OR 2.59, 95% CI 1.29–5.18; p = 0.007), as was the presence of TRG variants (OR 4.01, 95% CI 2.11–7.62; p < 0.001), see Supplementary Table 1. The frequencies of progressive fibrotic disease depending on the entity are available in Supplementary Table 2.
Table 3.
Unadjusted outcomes of relevant clinical status
| Overall N = 222 |
Familial N = 104 |
Sporadic N = 118 |
P value | OR | 95%CI | |
|---|---|---|---|---|---|---|
| Progressive disease (Yes) |
n = 204 96 (47) |
n = 93 62 (67) |
n = 111 34 (31) |
< 0.001χ | 4.53 | 2.51–8.18 |
| FVC (%) change |
n = 204 -2.07 ± 12.1 |
n = 95 -3.24 ± 15.9 |
n = 109 -1.05 ± 7.08 |
0.116u | - | - |
| DLCO (%) change |
n = 198 -2.55 ± 10.6 |
n = 92 -2.70 ± 12.0 |
n = 106 -2.00 ± 9.26 |
0.524u | - | - |
| FVC (L) change |
n = 204 -0.095 ± 0.31 |
n = 95 -0.12 ± 0.39 |
n = 109 -0.072 ± 0.22 |
0.134u | - | - |
| Lung transplantation (Yes) |
n = 218 33 (15) |
n = 104 22 (21) |
n = 114 11 (10) |
0.018χ | 2.51 | 1.15–5.48 |
| Death (Yes) |
n = 216 43 (20) |
n = 104 24 (23) |
n = 112 19 (17) |
0.261χ | 1.47 | 0.75–2.88 |
|
Radiological Worsening 2y Yes No |
n = 207 69 (33) 138 (67) |
n = 94 45 (48) 49 (52) |
n = 113 24 (21) 89 (79) |
< 0.001X | 3.41 | 1.86–6.24 |
Results are presented as the means ± standard deviations if numerical and as N (%) if categorical. uMann-Whitney U, χChi-Square. OR odds ratio. 95%CI 95% confidence interval
The binomial logistic regression shows the adjusted ORs for the three statistically significant variables in determining a progressive fibrotic disease: familial or sporadic, telomere shortening < 25th percentile, and carrying TRG variants; see Table 4.
Table 4.
Binomial logistic regression: progressive disease, family history and carrier of DAV
| Predictor | Z | P value | OR | 95%CI |
|---|---|---|---|---|
|
Familial or Sporadic: Familial – Sporadic |
3.22 | < 0.001 | 3.75 | 1.68–8.38 |
|
Telomere Shortening < 25 Yes – No |
-1.01 | 0.311 | 0.47 | 0.11–2.04 |
|
Carrier of TRG variant: Yes – No |
2.18 | 0.029 | 5.01 | 1.17–21.36 |
The estimates represent the log odds of “progressive disease = yes” vs. “progressive disease = no”. Z value = estimate/ standard error. OR adjusted odds ratio. 95% CI 95% confidence interval. Reference levels in the model were set to not progressive, sporadic, no telomere shortening and no genetic variant of interest
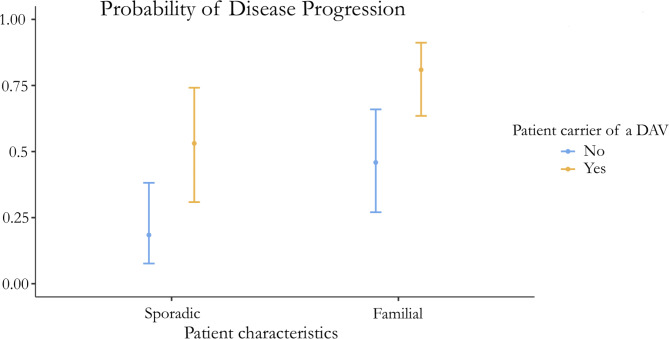
There was a statistically significant difference in the proportion of patients with progressive disease between familial and sporadic patients (adjusted OR 3.75, 95% CI 1.68–8.38; p = 0.001) and between patients who were carriers of a genetic variant of interest (adjusted OR 5.01, 95% CI 1.17–21.36; p = 0.029), but there was no statistically significant difference in the proportion of patients with telomere shortening < 25th percentile (OR 0.47, 95% CI 0.11–2.04; p = 0.311). A graphic representation of these results, with numeric detail, is available through the estimated marginal means presented in Fig. 2; Table 5.
Fig. 2.
Estimated marginal means of the probability of progressive disease
Estimated marginal means of the probability of progressive disease according to familial vs. sporadic and carrying a disease-associated gene variant yes/no based on the binomial logistic regression model, with 95% confidence intervals of the probability and the numeric values. SE standard error 95% CI 95% confidence interval
Table 5.
Probability of progressive disease, according to the logistic regression model
| ILD form | Carrier of DAV | Probability | SE | 95% CI |
|---|---|---|---|---|
| Sporadic | No | 0.184 | 0.077 | 0.08–0.38 |
| Sporadic | Yes | 0.531 | 0.118 | 0.31–0.74 |
| Familial | No | 0.459 | 0.105 | 0.27–0.66 |
| Familial | Yes | 0.809 | 0.070 | 0.63–0.91 |
DAV: Disease-associated gene variant, SE: standard error 95% CI 95% confidence interval
Discussion
Our study suggests that the presence of familial aggregation in patients with fibrotic ILDs increases the likelihood of a histological UIP pattern, which is especially relevant in patients with indeterminate or non-UIP HRCT patterns, even with a higher number of sporadic patients with an IPF diagnosis. Furthermore, despite the lower percentage of IPF patients, familial patients exhibited a greater risk of fibrotic progression, particularly compared with sporadic patients without TRG variants.
IPF diagnosis remains challenging for patients with indeterminate and non-UIP patterns on HRCT or those without a clinical context (mainly in young adults). Chung et al. reported that only 55% of indeterminate UIP and 60% of non-UIP HRCT patterns correlated with histological UIP [13]. Therefore, the likelihood of an IPF diagnosis in fibrotic ILD patients with indeterminate or non-UIP HRCT patterns is lower than 70%, and a lung biopsy is recommended. However, previous studies analysing the UIP HRCT-histological correlation did not consider family history [13]. Based on our findings, family aggregation increases the probability of histological UIP. The inclusion of family history in the analysis of IPF likelihood based on the HRCT radiological pattern could optimize lung biopsy decision-making.
Similar to previous reports, familial IPF cases showed clinical peculiarities compared with sporadic IPF cases, including a younger age at onset, frequent indeterminate or non-UIP HRCT at diagnosis, and a lower prevalence of male patients and individuals with a smoking history [19, 36]. The younger age at diagnosis in familial IPF patients could be due to the effect of genetic anticipation. This could also be explained by the earlier referral for diagnosis due to family history and patient awareness [36]. Interestingly, sporadic patients who were carriers of a disease-associated gene variant had a similar age at diagnosis and risk for fibrotic progression as familial patients without a detected genetic variant. Therefore, incorporating the use of genetic tests in sporadic fibrotic ILD patients with clinical suspicion of a genetic component could identify the first potential “familial” case, and the inheritance context would be relevant for the relatives.
Before the establishment of the PPF guidelines, no clear definition of progressive fibrotic disease was established [4–6, 8, 29, 32]. The INBUILD trial criteria considered clinical worsening, FVC decline, and HRCT fibrotic changes within 24 months before screening, similar to the criteria identified by Hambly et al., while other studies also considered DLCO decline, among other criteria [4, 5, 8, 32]. This study employed criteria for progression similar to those previously reported, with the inclusion of death and lung transplant. Although no predictive factors for PPF at ILD diagnosis have been validated, the type of ILD could be associated with a greater risk of progression. Similar to previous results, the proportions of PPF in patients with CPFE, fHP, CTD-ILD, and uILD were greater [32]. Interestingly, our cohort also included familial PPF patients with SRIF, fibrotic sarcoidosis, and other ILDs previously considered to be at lower risk for fibrotic progression [32]. Furthermore, for fHP, the proportion of patients with PPF in the familial cohort was three times greater than that in the sporadic cohort. Therefore, our results suggest that family history may be informative when assessing the risk of progression at diagnosis for a wide variety of patients with fibrotic ILD.
Cutting et al. reported a statistically significant increase in the short- and long-term risk of death or transplantation in familial IPF and non-IPF patients compared with sporadic patients [19]. However, other factors analysed in our study, such as the presence of the UIP patterns, the FVC (%) at diagnosis, and the presence of TRG variants, may impact patient prognosis [12]. A significantly greater proportion of probable or consistent UIP HRCT patterns was found in sporadic IPF and fHP patients, but the prevalence of fibrotic progression and lung transplantation was greater among familial cases with the same diagnosis. Finally, a few different genetic variants have been associated with a worse prognosis [37]. FPF patients with no identified gene variants of interest also had a worse prognosis than sporadic patients without such a genetic component, probably due to the challenge in identifying relevant gene variants even in patients with strong familial aggregation. Our study revealed a high percentage of disease-associated TRG variants, probably due to different factors. Since PARN variants are not always associated with severe telomere shortening, TRG variants were analysed when the percentile of telomere length was < 25th and not only in those with a percentile < 10th [25]. Moreover, some patients with phenotypical suspicion of pathogenic genetic inheritance were tested despite having no telomeric attrition. Since genetic studies are currently available only in a minority of ILD centres worldwide, the clinical information of family aggregation could be more broadly and easily included in clinical practice than genetic testing.
In recent years, clinical trials have been performed to evaluate the efficacy of antifibrotic drugs in treating PF-ILD [8, 9]. No randomized clinical trials have directly assessed the response of patients with FPF to pharmacological treatment. Several retrospective and post-hoc analyses have evaluated the antifibrotic effect of pirfenidone or nintedanib in patients with familial aggregation and/or genetic variants [38–40]. Studies have shown a worsening pulmonary function without treatment and have shown an inhibitory effect of antifibrotic drugs [38–40]. Therefore, early treatment in patients with non-IPF FPF could be considered in future settings and clinical trials to slow the disease from earlier stages rather than waiting to meet the PPF criteria [8, 9]. However, evidence is still limited, clinical trials are scarce, and additional therapies would be helpful in both familial IPF and PPF [41].
Some limitations of the present work are intrinsic to the observational and retrospective nature of this study [42]. However, a relatively large number of newly diagnosed patients with a variety of fibrotic ILDs were included, and confounding bias was partially limited by the logistic regression model. The familial fibrotic ILD group exhibited greater diversity of pulmonary diseases than did the sporadic group, which may be attributed to differences in referral patterns for sporadic versus familial patients, as all kind of patients with familial ILD may be more likely to be referred to our tertiary centre. Some of the diagnoses absent in the sporadic group, such as CTD-ILD, do not typically require biopsies, which should minimize their impact on the biopsy findings. Since our goal was not to develop a full predictive model, the logistic regression model was built using only three variables of interest with statistically significant differences in the univariate analysis. Further works could look into building a full predictive model with all relevant variables and possible confounders in fibrotic ILDs and validate it in a different cohort. Environmental factors have been linked to the development of fibrotic-ILDs, and these factors can vary among populations and countries at various levels, which may reduce the generalizability of the results [1]. However, genetic variants, which are strongly associated with the development of fibrotic-ILDs, were also considered in this study [1]. More studies are needed to confirm this findings in different populations.
Conclusion
Fibrotic ILD patients with family aggregation that present indeterminate UIP or non-UIP patterns on HRCT are more likely to show UIP patterns on lung biopsy than sporadic fibrotic ILD patients. Additionally, patients with familial aggregation and/or disease-associated TRG variants are at a significantly greater risk of disease progression. These results suggest that family history and genetic testing could be considered by the multidisciplinary committee in the diagnostic approach and in predicting the probability of fibrotic progression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1: Adding further analysis of notable variables for progressive fibrotic disease
Supplementary Table 2: Adding frequencies of progressive disease stratified by final diagnosis and then by familial or sporadic cohort
Acknowledgements
None.
Abbreviations
- AAV
ANCA-associated vasculitis
- CPFE
Combined pulmonary fibrosis and emphysema
- CTD-ILD
Connective tissue disease associated interstitial lung disease
- DAV
Disease-associated variant
- DLCO
Diffusing capacity for carbon monoxide
- DNA
Deoxyribonucleic acid
- fHP
Fibrotic hypersensitivity pneumonitis
- fNSIP
Fibrotic non-specific interstitial pneumonia
- FPF
Familial pulmonary fibrosis
- FVC
Forced vital capacity
- HRCT
High resolution chest computed tomography
- HUB
Bellvitge university hospital
- IIP
Idiopathic interstitial pneumonias
- ILD
Interstitial lung disease
- Induced PF (cocaine)
Cocaine-induced pulmonary fibrosis
- IPAF
Interstitial pneumonia with autoimmune features
- IPF
Idiopathic pulmonary fibrosis
- OR
Odds ratio
- PFT
Pulmonary function test
- PPF
Progressive pulmonary fibrosis
- PPFE
Pleuroparenchymal fibroelastosis
- PR-Radiotherapy
Radiotherapy induced pulmonary fibrosis
- qPCR
Quantitative polymerase chain reaction
- SRIF
Smoking-related interstitial fibrosis
- TRFL
Terminal restriction fragment lengths
- TRG
Telomere-related gene
- uILD
Unclassifiable interstitial lung disease
- UIP
Usual interstitial pneumonia
Author contributions
D.D.-L. contributed to data collection, statistical analysis, writing and extensive reviewing. A.A.-A. contributed to data collection and extensive reviewing. M.M.-M., L.P.-C., G.B., V.V.-Z., P.L., B.D.-C., R.L., L.P., I.E., F.R., A.M.-W., Y.G.-R., D.R.-P., A.P.-M., A.E.-G., B.F.-V., C.F., M.F., R.P., contributed to data collection, patient follow-up, and extensive reviewing. J.D., J.S., S.S. and A.G. contributed to extensive reviewing.
Funding
Part of this study was funded by Instituto de Salud Carlos III and the European Joint Programme on Rare Diseases (EJP RD) (AC19/00006), Ministerio de Ciencia e Innovación (grant RTC-2017-6471-1; AEI/FEDER, UE), the agreements with Instituto Tecnológico y de Energías Renovables to strengthen scientific and technological education, training, research, development and innovation in genomics, epidemiological surveillance based on massive sequencing, Personalized Medicine and Biotechnology (OA17/008 and OA23/043), Cabildo Insular de Tenerife (CGIEU0000219140), and the funds of Boehringer Ingelheim through Fundación Catalana de Pneumologia (FUCAP). IDIBELL receives funding from the CERCA Program.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of HUB approved the study (PR307/16), and all patients provided written informed consent before inclusion, including written informed consent for genetic testing if needed.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
MMM has received grants for research purposes and fees for scientific advice outside this work from Boehringer Ing, Ferrer, Roche, Veracyte and Chiesi. The other authors of this work have no conflicts of interest to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. Duminy-Luppi, Email: duminy.diego@gmail.com
M. Molina-Molina, Email: mariamolinamolina@hotmail.com
References
- 1.Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung diseases. N Engl J Med. 2020;383(10):958–68. PMID: 32877584. [DOI] [PubMed] [Google Scholar]
- 2.Shull JG, Pay MT, Lara Compte C, Olid M, Bermudo G, Portillo K, et al. Mapping IPF helps identify geographic regions at higher risk for disease development and potential triggers. Respirology. 2021;26(4):352–9. PMID: 33167075. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806. PMID: 25976683. [DOI] [PubMed] [Google Scholar]
- 4.George PM, Spagnolo P, Kreuter M, Altinisik G, Bonifazi M, Martinez FJ, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020;8(9):925–34. PMID: 32890499. [DOI] [PubMed] [Google Scholar]
- 5.Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150). PMID: 30578335. [DOI] [PMC free article] [PubMed]
- 6.Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Antoniou KM, Bissell BD, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):E18–47. PMID: 35486072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijsenbeek M, Kreuter M, Olson A, Fischer A, Bendstrup E, Wells CD, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin. 2019;35(11):2015–24. PMID: 31328965. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in Progressive Fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27. PMID: 31566307. [DOI] [PubMed] [Google Scholar]
- 9.Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9(5):479–86. PMID: 33798455. [DOI] [PubMed] [Google Scholar]
- 10.Petnak T, Lertjitbanjong P, Thongprayoon C, Moua T. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: a systematic review and Meta-analysis. Chest. 2021;160(5):1751–63. PMID: 34217681. [DOI] [PubMed] [Google Scholar]
- 11.Kapnadak SG, Raghu G. Lung transplantation for interstitial lung disease. Eur Respir Rev. 2021;30(161):210017. PMID: 34348979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020;21(1):1–10. PMID: 31996266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung JH, Oldham JM, Montner SM, Vij R, Adegunsoye A, Husain AN, et al. CT-pathologic correlation of major types of pulmonary fibrosis: insights for revisions to current guidelines. Am J Roentgenol. 2018;210(5):1034–41. PMID: 29547052. [DOI] [PubMed] [Google Scholar]
- 14.Fukihara J, Kondoh Y, Brown KK, Kimura T, Kataoka K, Matsuda T et al. Probable usual interstitial pneumonia pattern on chest CT: Is it sufficient for a diagnosis of idiopathic pulmonary fibrosis? Eur Respir J. 2020;55(4). PMID: 32029448. [DOI] [PubMed]
- 15.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. PMID: 21471066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borie R, Kannengiesser C, Antoniou K, Bonella F, Crestani B, Fabre A, et al. European Respiratory Society statement on familial pulmonary fibrosis. Eur Respir J. 2023;61(3):2201383. PMID: 36549714. [DOI] [PubMed] [Google Scholar]
- 17.García-Sancho C, Buendía-Roldán I, Fernández-Plata MR, Navarro C, Pérez-Padilla R, Vargas MH, et al. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105(12):1902–7. PMID: 21917441. [DOI] [PubMed] [Google Scholar]
- 18.Borie R, Kannengiesser C, Nathan N, Tabèze L, Pradère P, Crestani B. Familial pulmonary fibrosis. Rev Mal Respir. 2015;32(4):413–34. PMID: 25596800. [DOI] [PubMed] [Google Scholar]
- 19.Cutting CC, Bowman WS, Dao N, Pugashetti JV, Garcia CK, Oldham JM, et al. Family History of Pulmonary Fibrosis predicts worse survival in patients with interstitial lung disease. Chest. 2021;159(5):1913–21. PMID: 33484728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholand MB, Coon H, Wolff R, Cannon-Albright L. Use of a genealogical database demonstrates heritability of pulmonary fibrosis. Lung. 2013;191(5):475–81. PMID: 23867963. [DOI] [PubMed] [Google Scholar]
- 21.Borie R, Crestani B. Familial pulmonary fibrosis: a world without frontiers. J Bras Pneumol. 2019;45(5):45–7. PMID: 31596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planas-Cerezales L, Arias-Salgado EG, Buendia-Roldán I, Montes-Worboys A, López CE, Vicens-Zygmunt V, et al. Predictive factors and prognostic effect of telomere shortening in pulmonary fibrosis. Respirology. 2019;24(2):146–53. PMID: 30320420. [DOI] [PubMed] [Google Scholar]
- 23.Adegunsoye A, Vij R, Noth I. Integrating Genomics Into Management of Fibrotic interstitial lung disease. Chest. 2019;155(5):1026–40. PMID: 30660786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock CJW, Renzoni EA. Telomeres in Interstitial Lung Disease. J Clin Med. 2021, Vol 10, Page 1384. 2021;10(7):1384. PMID: 33808277. [DOI] [PMC free article] [PubMed]
- 25.Zhang D, Newton CA. Familial pulmonary fibrosis: genetic features and clinical implications. Chest. 2021;160(5):1764–73. PMID: 34186035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton CA, Oldham JM, Applegate C, Carmichael N, Powell K, Dilling D et al. The Role of Genetic Testing in Pulmonary Fibrosis: A Perspective from the Pulmonary Fibrosis Foundation Genetic Testing Work Group. Chest. 2022;0(0). PMID: 35337808. [DOI] [PMC free article] [PubMed]
- 27.Cottin V, Wollin L, Fischer A, Quaresma M, Stowasser S, Harari S. Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev. 2019;28(151). PMID: 30814139. [DOI] [PMC free article] [PubMed]
- 28.Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. 10.1164/rccm201308-1483ST. 2013;188(6):733–48. PMID: 24032382. [DOI] [PMC free article] [PubMed]
- 29.Kam MLW, Nguyen TTT, Ngeow JYY. Telomere biology disorders. npj Genomic Med. 2021;6(1):36. PMID: 34050178. [DOI] [PMC free article] [PubMed]
- 30.van der Hoffman TW, Biesma DH, Grutters JC, van Moorsel CHM. Extrapulmonary manifestations of a telomere syndrome in patients with idiopathic pulmonary fibrosis are associated with decreased survival. Respirology. 2022;27(11):959–65. PMID: 35419815. [DOI] [PubMed] [Google Scholar]
- 31.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68. PMID: 30168753. [DOI] [PubMed] [Google Scholar]
- 32.Hambly N, Farooqi MM, Dvorkin-Gheva A, Donohoe K, Garlick K, Scallan C et al. Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur Respir J. 2022;2102571. PMID: 35273032. [DOI] [PubMed]
- 33.Planas-Cerezales L, Arias-Salgado EG, Berastegui C, Montes-Worboys A, González-Montelongo R, Lorenzo-Salazar JM, et al. Lung transplant improves survival and quality of life regardless of Telomere Dysfunction. Front Med. 2021;8:695919. PMID: 34395476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borie R, Tabèze L, Thabut G, Nunes H, Cottin V, Marchand-Adam S, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48(6):1721–31. PMID: 27836952. [DOI] [PubMed] [Google Scholar]
- 35.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015. 2015;17(5):175. PMID: 25741868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.n der Vis JJ, Hennekam FAM, Grutters JC, van Moorsel CHM. Pulmonary fibrosis and a TERT founder mutation with a latency period of 300 years. Chest. 2020;158(2):612–9. PMID: 32315675. [DOI] [PubMed] [Google Scholar]
- 37.Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48(6):1710–20. PMID: 27540018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett D, Refini RM, Valentini ML, Fui A, Fossi A, Pieroni M, et al. Pirfenidone Therapy for Familial Pulmonary Fibrosis: a real-life study. Lung. 2019;197(2):147–53. [DOI] [PubMed] [Google Scholar]
- 39.Justet A, Klay D, Porcher R, Cottin V, Ahmad K, Molina MM et al. Safety and efficacy of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis and carrying a telomere-related gene mutation. Eur Respir J. 2021;57(2). PMID: 33214205. [DOI] [PubMed]
- 40.Dressen A, Abbas AR, Cabanski C, Reeder J, Ramalingam TR, Neighbors M, et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med. 2018;6(8):603–14. PMID: 29891356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.M Mura. Use of Nintedanib in interstitial lung disease other than idiopathic pulmonary fibrosis: much caution is warranted. Pulm Pharmacol Ther. 2021;66:101987. PMID: 33387612. [DOI] [PubMed]
- 42.Boyko EJ. Observational Research Opportunities and limitations. J Diabetes Complicat. 2013;27(6):642–8. PMID: 24055326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Adding further analysis of notable variables for progressive fibrotic disease
Supplementary Table 2: Adding frequencies of progressive disease stratified by final diagnosis and then by familial or sporadic cohort
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.