Abstract
Background
The ratio of triglycerides to high-density-lipoprotein cholesterol (TG/HDL-C) is increasingly recognized as a practical marker for insulin resistance and cardiovascular risk assessment. This retrospective study investigates the potential of the TG/HDL-C ratio to predict the development of calcific aortic valve disease (CAVD), thereby extending its applicability in cardiovascular diagnostics.
Methods
Data from 400 individuals, comprising 200 patients with diagnosed CAVD and 200 matched healthy controls, were analyzed. Clinical parameters were compared between groups, and logistic regression was utilized to explore the association of the TG/HDL-C ratio with CAVD. The diagnostic performance of the TG/HDL-C ratio was assessed using receiver operating characteristic (ROC) curves.
Results
The TG/HDL-C ratio was notably higher in the CAVD group than in the controls (Z = -7.98, P < 0.001). Multivariable logistic regression analysis indicated that the TG/HDL-C ratio is an independent predictor of CAVD after adjusting for confounders including gender. The ROC curve analysis revealed that the TG/HDL-C ratio achieved a sensitivity of 80.5%, a specificity of 59.5%, and an area under the curve (AUC) of 0.731 (P < 0.001), confirming its efficacy in predicting CAVD.
Conclusions
High TG/HDL-C ratio was significantly associated with the occurrence of CAVD, and the TG/HDL-C ratio could be used as a potential diagnostic tool and risk assessment indicator for CAVD.
Clinical trial number
Not applicable.
Keywords: TG/HDL-C ratio, Calcific aortic valve disease, Evaluation indicators
Introduction
Calcific aortic valve disease (CAVD) is the predominant form of valvular heart diseases, marked by the thickening of aortic valve leaflets and the transformation of valvular mesenchymal stromal cells into an osteoclast-like phenotype, recognized as a hallmark of CAVD [1]. The pathogenesis of CAVD is complex and initially parallels that of atherosclerosis. Factors such as cytokines and altered mechanical shear stress contribute to valve endothelial cell damage, subendothelial lipid accumulation, and inflammatory cell infiltration, which collectively precipitate fibrosis and osteogenic differentiation of the valve tissue [2]. It is estimated that approximately 25% of individuals over the age of 65 and 50% over the age of 85 exhibit signs of aortic valve calcification [3]. In 2017, the global prevalence of CAVD was approximately 12.6 million with mortality figures reaching 102,700 [4]. Currently, there are no targeted pharmacotherapies for CAVD, and severe aortic stenosis typically necessitates aortic valve replacement [5]. Thus, early detection and intervention in CAVD are crucial for improving patient outcomes.
Insulin resistance (IR), characterized by diminished insulin sensitivity in target organs, is a well-established risk factor for cardiovascular diseases [6, 7]. The hyperinsulinemic euglycemic clamp technique, considered the gold standard for IR assessment, is seldom used in clinical practice due to its complexity [8]. Recently, simpler surrogate markers such as the triglyceride glucose index (TyG), TG/HDL-C ratio, and metabolic score for insulin resistance (METS-IR) have gained prominence for evaluating IR [9]. A cross-sectional study by Hu et al. [10] involving 366 participants assessed the TyG index and its association with calcific aortic stenosis, finding that TyG independently influenced calcific aortic stenosis and was negatively associated with its progression. Compared with other potential markers such as TyG index and METS-IR, the calculation of TG/HDL-C ratio is simple and easily obtained from routine blood lipid testing. Studies show that the TG/HDL-C ratio has independent predictive effects in predicting atherosclerosis and coronary artery calcification, and its clinical significance has been validated in several studies [11–13]. However, studies exploring the association between the TG/HDL-C ratio and CAVD are lacking. Therefore, this study aims to determine whether the TG/HDL-C ratio can aid in identifying the onset of CAVD and serve as a valuable tool for the early detection and management of the disease. Specifically, this study seeks to (1) compare the TG/HDL-C ratios between healthy individuals and patients with CAVD, (2) investigate the independent factors influencing the occurrence of CAVD, and (3) evaluate the sensitivity, specificity, and reliability of the TG/HDL-C ratio in predicting CAVD.
Methods
Study population
This investigation included 200 patients diagnosed with CAVD who were treated at the First Affiliated Hospital of Henan University of Chinese Medicine between January 2020 and June 2023. All patients met the following inclusion criteria: (1) ≥ 18 years and (2) met the assessment criteria for aortic valve calcification: aortic leaflet thickening, echo enhancement, with or without acoustic [14]. All patients underwent transthoracic echocardiography performed by two experienced echocardiographers. Additionally, 200 healthy individuals who demonstrated no signs of CAVD via transthoracic echocardiography during the same period were recruited as controls. Among the included patients, those with complicated malignancies, congenital heart disease, hepatic and renal insufficiency, severe infectious disease, autoimmune disease, cardiomyopathy, pulmonary heart disease, rheumatic valvular heart disease, and incomplete data were excluded from the final list (Fig. 1). This study received approval from the Ethics Committee of the First Affiliated Hospital of Henan University of Chinese Medicine, adhering to the ethical principles outlined in the Declaration of Helsinki. The retrospective nature of the study allowed for a waiver of informed consent by the review committee.
Fig. 1.
Study Participant flow diagram. The flow diagram illustrates the selection process of study participants. A total of 235 CAVD (calcific aortic valve disease) patients were initially identified. After excluding 35 patients due to various reasons (autoimmune disease, N = 7; hypothyroidism, N = 1; hepatic and renal insufficiency, N = 3; incomplete data, N = 24), 200 CAVD patients were included for statistical analysis. A control group consisting of 200 non-CAVD individuals (normal control) was also included. Both groups underwent statistical analysis to explore associations with CAVD
Clinical parameters
Clinical data were extracted from the electronic medical records at the First Affiliated Hospital of Henan University of Chinese Medicine. The parameters included demographic information (sex, age), anthropometric measurements (height, weight, body mass index [BMI]), and vital signs (systolic blood pressures and diastolic blood pressures [SBP and DBP]). Laboratory values such as total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), serum creatinine (SCR), and blood urea nitrogen (BUN) were obtained from blood samples collected after an overnight fast and analyzed by the hospital’s Laboratory Department. Diabetes was diagnosed according to the American Diabetes Association (ADA) guidelines [15], which define diabetes as fasting plasma glucose (FPG) ≥ 126 mg/dL (7.0 mmol/L), HbA1c ≥ 6.5%, or 2-hour plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT). The TG/HDL-C ratio was calculated as the quotient of triglyceride concentration to high-density lipoprotein cholesterol concentration.
Statistical analysis
Data distribution was assessed using the Shapiro-Wilk test. Continuous variables with normal distribution are presented as mean ± standard deviation, while those with a non-normal distribution are shown as median with interquartile range. Categorical variables are reported as frequency and percentage. Differences between groups were analyzed using the t-test for normally distributed data and the Kruskal-Wallis test for non-normally distributed data. Categorical data were compared using the χ2 test. Univariate logistic regression analysis was used to explore the potential influencing factors of the presence of CAVD, and then the independent variables with P < 0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis to determine the independent influencing factors and their efficacy. The receiver operating characteristic curve (ROC) was used to evaluate the predictive power of the TG/HDL-C ratio for the presence of CAVD. To further evaluate the predictive value of TG/HDL-C ratio distribution width for CAVD, we segmented them by the median (low TG/HDL-C ratio and high TG/HDL-C ratio groups) and analyzed the differences between the two intervals to determine their predictive power for CAVD. All statistical analyses were conducted using SPSS version 26.0 (SPSS Inc., Chicago, USA). A P-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
As detailed in Table 1, the study analyzed 200 patients in the CAVD group and 200 in the control group. The CAVD group consisted of 121 males and 79 females, whereas the control group included 94 males and 106 females. Comparative analysis of the groups revealed no statistically significant differences in age, height, weight, BMI, DBP, TC, LDL-C, and BUN levels (P > 0.05).
Table 1.
Comparison of baseline characteristics of participants in the normal control and CAVD groups
| Clinical parameters | Total (n = 400) | Control (n = 200) | CAVD (n = 200) | Statistic | P |
|---|---|---|---|---|---|
| Age, year | 66 (62, 71) | 65 (62, 69) | 68 (63, 72) | Z=-1.83 | 0.067 |
| Male, n(%) | 215 (53.75) | 94 (47.00) | 121 (60.50) | χ²=7.33 | 0.007 |
| Height, cm | 166.28 ± 7.35 | 165.49 ± 7.76 | 167.24 ± 6.79 | t=-1.05 | 0.297 |
| Weight, kg | 67.49 ± 11.30 | 65.27 ± 12.33 | 70.21 ± 9.36 | t=-1.95 | 0.055 |
| BMI, kg/cm2 | 24.31 ± 3.09 | 23.70 ± 3.27 | 25.06 ± 2.72 | t=-1.96 | 0.053 |
| SBP, mmHg | 136.02 ± 19.11 | 130.56 ± 18.42 | 142.40 ± 17.97 | t=-5.35 | < 0.001 |
| DBP, mmHg | 76.89 ± 10.92 | 75.86 ± 10.95 | 78.08 ± 10.81 | t=-1.68 | 0.095 |
| TC, mmol/L | 5.02 ± 1.14 | 5.10 ± 1.07 | 4.94 ± 1.20 | t = 1.42 | 0.155 |
| HDL-C, mmol/L | 1.35 ± 0.29 | 1.44 ± 0.30 | 1.26 ± 0.26 | t = 6.54 | < 0.001 |
| LDL-C, mmol/L | 3.31 ± 0.85 | 3.33 ± 0.79 | 3.29 ± 0.90 | t = 0.47 | 0.635 |
| TG, mmol/L | 1.54 (1.14, 2.07) | 1.25 (0.95, 1.84) | 1.75 (1.36, 2.23) | Z=-6.70 | < 0.001 |
| FPG, mmol/L | 5.43 (5.09, 6.28) | 5.32 (4.91, 6.01) | 5.52 (5.27, 6.57) | Z=-4.53 | < 0.001 |
| SCR, µmmol/L | 63.90 (53.30, 75.50) | 62.30 (52.20, 73.00) | 65.10 (54.55, 77.40) | Z=-2.17 | 0.030 |
| BUN, mmol/L | 5.23 (4.56, 6.12) | 5.17 (4.53, 6.04) | 5.30 (4.56, 6.12) | Z=-1.43 | 0.153 |
| TG/HDL-C | 1.18 (0.80, 1.72) | 0.88 (0.65, 1.36) | 1.41 (1.10, 1.99) | Z=-7.98 | < 0.001 |
| Diabetes, n(%) | 63 (15.75) | 18 (9.00) | 45 (22.50) | χ²=13.73 | < 0.001 |
Data are expressed as Mean ± SD, frequency (percentage), or median (interquartile range)
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, TC: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TG: triglyceride, FPG: fasting plasma glucose, SCR: serum creatinine, BUN: blood urea nitrogen
Significantly, SBP, TG, FPG, SCR, and the TG/HDL-C ratio were higher in the CAVD group compared to the control group (P < 0.05). Conversely, HDL-C levels were significantly lower in the CAVD group (P < 0.05).
Correlation analysis of TG/HDL-C ratio and CAVD
Univariate and multivariate logistic regression analysis was used to determine the factors influencing the occurrence of CAVD. The results of the univariate logistic regression analysis showed that sex, diabetes, SBP, TG, HDL-C, FPG, and the TG/HDL-C ratio were associated with the occurrence of CAVD (P < 0.05). (Table 2).
Table 2.
Relationship between various clinical parameters and CAVD
| Variables | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | S.E | P | OR (95% CI) | β | S.E | P | OR (95% CI) | ||
| Age, year | 0 | 0.01 | 0.886 | 1.00 (0.98 to 1.02) | |||||
| Sex | |||||||||
| Female | — | — | — | — | — | — | — | — | |
| Male | 0.55 | 0.20 | 0.007 | 1.73 (1.16 to 2.57) | 0.41 | 0.28 | 0.140 | 1.51 (0.87 to 2.63) | |
| Height, cm | 0.03 | 0.03 | 0.294 | 1.03 (0.97 to 1.10) | |||||
| Weight, kg | 0.04 | 0.02 | 0.060 | 1.04 (1.00 to 1.09) | |||||
| BMI, kg/cm2 | 0.15 | 0.08 | 0.060 | 1.16 (0.99 to 1.36) | |||||
| SBP, mmHg | 0.04 | 0.01 | < 0.001 | 1.04 (1.02 to 1.05) | 0.03 | 0.01 | < 0.001 | 1.03 (1.02 to 1.05) | |
| DBP, mmHg | 0.02 | 0.01 | 0.096 | 1.02 (1.00 to 1.04) | |||||
| TC, mmol/L | -0.13 | 0.09 | 0.155 | 0.88 (0.74 to 1.05) | |||||
| TG, mmol/L | 0.47 | 0.12 | < 0.001 | 1.61 (1.26 to 2.05) | |||||
| HDL-C, mmol/L | 2.38 | 0.40 | < 0.001 | 0.09 (0.04 to 0.20) | |||||
| LDL-C, mmol/L | -0.06 | 0.12 | 0.634 | 0.95 (0.75 to 1.19) | |||||
| FPG, mmol/L | 0.38 | 0.09 | < 0.001 | 1.46 (1.23 to 1.74) | 0.24 | 0.10 | 0.019 | 1.27 (1.04 to 1.55) | |
| SCR, µmmol/L | 0 | 0 | 0.599 | 1.00 (0.99 to 1.01) | |||||
| BUN, mmol/L | 0.07 | 0.07 | 0.283 | 1.08 (0.94 to 1.23) | |||||
| TG/HDL-C | |||||||||
|
Low (< 1.18) |
— | — | — | — | — | — | — | — | |
|
High (≥ 1.18) |
1.46 | 0.21 | < 0.001 | 4.31 (2.84 to 6.56) | 1.08 | 0.28 | < 0.001 | 2.96 (1.71 to 5.72) | |
| Diabetes | |||||||||
| No | — | — | — | — | |||||
| Yes | 1.08 | 0.3 | < 0.001 | 2.94 (1.63 to 5.28) | |||||
OR: Odds Ratio, CI: Confidence Interval, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, TC: total cholesterol, TG: triglyceride, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, FPG: fasting plasma glucose, SCR: serum creatinine, BUN: blood urea nitrogen
Independent variables with P < 0.05 in the univariate logistic regression analysis were included in the further multivariate logistic regression analysis. To avoid interference by collinearity, TG, HDL-C, and diabetes were not included at this time. The results of the multivariate logistic regression analysis showed that SBP, FPG, and the TG/HDL-C ratio were independently associated with the occurrence of CAVD (P < 0.05). (Table 2).
Sensitivity and specificity of CAVD assessed by TG, HDL-C, and TG/HDL-C ratio
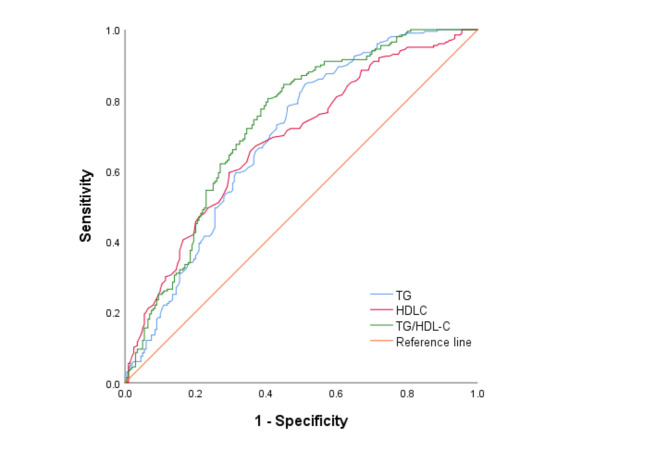
ROC curves were constructed to evaluate the effectiveness of TG, HDL-C, and the TG/HDL-C ratio in diagnosing CAVD. The analyses are presented in Fig. 2.
Fig. 2.
ROC Curves for TG, HDL-C, and TG/HDL-C in Predicting CAVD. The receiver operating characteristic (ROC) curves illustrate the predictive performance of triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and the TG/HDL-C ratio for calcific aortic valve disease (CAVD). The area under the curve (AUC) is highest for the TG/HDL-C ratio (green line), indicating its superior predictive ability compared to TG (blue line) and HDL-C (red line). The reference line (orange) represents a non-informative classifier. Sensitivity is plotted against 1-specificity to assess the diagnostic accuracy of each biomarker
The sensitivity and specificity of TG as a diagnostic marker for CAVD were 84.5% and 49%, respectively, with an area under the curve (AUC) of 0.694 (95% confidence interval [CI]: 0.642 to 0.745, P < 0.001). HDL-C demonstrated a sensitivity of 67% and a specificity of 63%, with an AUC of 0.684 (95% CI: 0.632 to 0.735, P < 0.001). The TG/HDL-C ratio showed a higher diagnostic performance, with a sensitivity of 80.5%, a specificity of 59.5%, and an AUC of 0.731 (95% CI: 0.681 to 0.78, P < 0.001).
TG/HDL-C ratio median grouping analysis results
The median TG/HDL-C ratio was 1.18, and the TG/HDL-C ratio was divided into low TG/HDL-C ratios (TG/HDL-C ratio < 1.18) and high TG/HDL-C ratios (TG/HDL-C ratio ≥ 1.18). Patients with a high TG/HDL-C ratio were more likely to develop CAVD (P < 0.05). Weight, BMI, SBP, DBP, TG, FPG, SCR increased in higher TG/HDL-C ratio, while HDL-C decreased (P < 0.05). (Table 3).
Table 3.
Clinical characteristics of subjects based on the TG/HDL-C ratio median quartiles
| variables | low TG/HDL-C ratio (n = 200) | high TG/HDL-C ratio (n = 200) | P |
|---|---|---|---|
| Age, year | 65.50 (62.00, 70.00) | 67.00 (62.00, 72.00) | 0.319 |
| Male, n(%) | 87 (43.50) | 128 (64.00) | < 0.001 |
| Height, cm | 165.49 ± 6.42 | 167.24 ± 8.35 | 0.297 |
| Weight, kg | 63.48 ± 11.03 | 72.40 ± 9.68 | < 0.001 |
| BMI, kg/cm2 | 23.08 ± 3.04 | 25.82 ± 2.44 | < 0.001 |
| SBP, mmHg | 133.17 ± 20.18 | 139.16 ± 17.42 | 0.009 |
| DBP, mmHg | 74.26 ± 10.54 | 79.78 ± 10.63 | < 0.001 |
| TC, mmol/L | 5.10 ± 1.07 | 4.94 ± 1.20 | 0.171 |
| HDL-C, mmol/L | 1.50 ± 0.28 | 1.20 ± 0.22 | < 0.001 |
| LDL-C, mmol/L | 3.32 ± 0.79 | 3.30 ± 0.90 | 0.805 |
| TG, mmol/L | 1.13 (0.93, 1.36) | 2.07 (1.74, 2.76) | < 0.001 |
| FPG, mmol/L | 5.29 (4.90, 5.88) | 5.63 (5.27, 6.69) | < 0.001 |
| SCR, µmmol/L | 60.05 (51.92, 71.60) | 67.70 (55.90, 79.05) | < 0.001 |
| BUN, mmol/L | 5.20 (4.44, 6.08) | 5.29 (4.64, 6.19) | 0.14 |
| CAVD presence | 65 (32.50) | 135 (67.50) | < 0.001 |
| Diabetes, n(%) | 17 (8.50) | 46 (23.00) | < 0.001 |
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, TC: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TG: triglyceride, FPG: fasting plasma glucose, SCR: serum creatinine, BUN: blood urea nitrogen
Discussion
This study has highlighted the TG/HDL-C ratio as a significant biomarker for CAVD, revealing its correlation with established risk factors akin to those of atherosclerosis, including advanced age, gender, smoking, hypertension, diabetes, and dyslipidemia [16]. In this study, the incidence of CAVD is higher in the male population and may be associated with androgen levels, metabolic abnormalities, and lifestyle. Notably, in comparison to the control group, the present study observed a significant elevation in FBG levels among patients in the CAVD group, consistent with prior research [17–19]. Multivariable logistic regression analysis revealed that elevated FBG independently influenced CAVD, likely due to its role in triggering an inflammatory response in valve endothelial cells, accelerating the production of cell adhesion molecules, and promoting the exudation of blood mononuclear cells, facilitating platelet adhesion. Moreover, it increases the presence of osteopontin, thereby fostering the growth of molecules associated with vascular calcification, consequently heightening the risk of heart valve calcification [20]. In addition, this study found that the prevalence of diabetes was significantly higher in the CAVD group. Due to the long-term hyperglycemic status, diabetic patients may aggravate vascular endothelial dysfunction, promote inflammatory response and lipid metabolism disorders, thus accelerating the calcification process of the aortic valve. In addition, hyperglycemia can further worsen the pathophysiology of CAVD through oxidative stress and inflammatory pathways.
In this study, SBP was significantly higher in the CAVD group compared to controls. Adjusted multivariate logistic regression analysis identified elevated SBP as an independent predictor of CAVD, likely due to increased transvalvular pressure gradients exacerbating shear stress and valve annulus damage. This mechanistic pathway suggests that high SBP contributes to endothelial dysfunction, chronic inflammation, and extracellular matrix remodeling, all of which accelerate aortic valve calcification [21].
This study demonstrated that a TG/HDL-C ratio was significantly associated with the development of CAVD, especially when this ratio is high (P < 0.05). We divided the TG/HDL-C ratio into low and high ratio groups according to the median of 1.18. The results showed that patients in the high TG/HDL-C ratio group (the TG/HDL-C ratio ≥ 1.18) not only had a higher incidence of CAVD, but also accompanied by a series of metabolic index abnormalities. Specifically, patients in the high TG/HDL-C ratio group showed higher weight, BMI, SBP, DBP, TG, FPG and SCR, but lower HDL-C levels (P < 0.05). Abnormalities of these metabolic parameters may be closely associated with insulin resistance, atherosclerosis, as well as increased cardiovascular risk, further supporting the role of TG/HDL-C ratio as an important predictor of cardiovascular metabolic health. Studies have shown that the elevated TG/HDL-C ratio is closely associated with insulin resistance, metabolic syndrome, and the development of atherosclerosis [11, 12]. Our findings are consistent with these previous studies, indicating that the TG/HDL-C ratio can not only reflect the metabolic disorder status, but can also serve as an important predictor of the risk for CAVD. In particular, patients in the high TG/HDL-C ratio group showed both higher blood pressure and SCR, which may imply the association of a high TG/HDL-C ratio with renal function impairment and hypertension. These abnormalities in metabolic and vascular function indicators further aggravate the risk of developing cardiovascular disease, suggesting that patients with elevated TG/HDL-C ratio should be concerned in clinical management with more aggressive cardiovascular prevention measures. Moreover, a high TG/HDL-C ratio is also strongly associated with decreased HDL-C levels, and HDL-C is usually seen as a protective factor against atherosclerosis. The protective role of HDL-C in cardiovascular health, specifically its ability to extract cholesterol from arterial walls and modulate cytokine-induced inflammatory responses, is well-documented [22]. HDL-C’s impact on reducing alkaline phosphatase activity and inhibiting osteogenic differentiation further underscores its relevance in CAVD pathology [23]. The decrease in HDL-C further suggests that abnormal lipid metabolism may be more significant in the high TG/HDL-C group, thus increasing the risk of cardiovascular events.
The TG/HDL-C ratio, as a composite indicator, has an advantage over other markers in predicting metabolic abnormalities as well as the progression of CAVD. ROC curve analysis showed that TG/HDL-C ratio had higher sensitivity and specificity in predicting CAVD than TG or HDL-C alone, and that TG/HDL-C ratio was able to reflect lipid-related risk factors. This ratio reflects the two states of increased TG levels (suggesting a disturbance of lipid metabolism) and decreased HDL-C levels (HDL-C has a protective effect against lipid deposition and inflammation) [24]. It has been shown that elevated TG/HDL-C ratio is associated with vascular calcification and atherosclerosis [12] and these mechanisms are closely associated with the development of CAVD. Our findings are consistent with previous studies, further validated the close association of TG/HDL-C ratio with cardiovascular disease (including CAVD). For example, studies showed that TyG index predicts the occurrence and severity of aortic valve calcification, which further illustrates the potential predictive role of markers of metabolic syndrome in valvular disease [25]. Furthermore, the TG/HDL-C ratio with the risk of cardiovascular disease was also validated by other large population studies, for example, in an analysis of UK Biobank (UK Biobank) based data, elevated TG/HDL-C ratio was associated with increased CVD risk, mainly mediated by increased prevalence of dyslipidemia, type 2 diabetes, and hypertension [13]. These studies are consistent with our findings showing that an elevated TG/HDL-C ratio is associated with more severe metabolic abnormalities in CAVD patients.
This study is the first to explore the independent association of TG / HDL-C ratio with calcified aortic valve disease (CAVD) and validated its diagnostic value in CAVD. This finding complements the research gap of the TG / HDL-C ratio in metabolic and cardiovascular diseases, highlighting its potential role in the pathophysiological mechanisms of CAVD. Unlike previous literature which mainly focused on metabolic syndrome or coronary heart disease, our study found that patients with elevated TG / HDL-C ratio were more likely to develop CAVD and for the first time determined the sensitivity and specificity of this index in the diagnosis of CAVD by ROC curves. Furthermore, we noted that the increased diabetic status and FPG levels in CAVD patients may have some effect on their lipid metabolism and TG / HDL-C ratio. This suggests that the elevated TG / HDL-C ratio may be the result of the common effects of multiple metabolic abnormalities. The study has several limitations that warrant consideration. Firstly, its observational nature restricts our ability to establish a causal link between the TG/HDL-C ratio and CAVD. Thus, the findings might reflect associations rather than direct causative effects. Secondly, potential confounding variables not accounted for in this study could influence the observed correlations between the TG/HDL-C ratio and CAVD, suggesting the need for cautious interpretation of the results. Thirdly, we did not collect the medication history of the patients, which may affect the lipid concentration and potentially interfere with the results. The use of drugs, such as lipid-lowering drugs and other drugs that affect lipid metabolism, may lead to changes in TG or HDL-C levels, thereby affecting the TG / HDL-C ratio and its relationship with CAVD. Finally, the use of single-center retrospective data and a relatively small sample size may limit the generalizability of our findings.
In response to these limitations, Future studies should consider collecting detailed medication information to assess the effect of medication more comprehensively on TG / HDL-C ratio and risk of CAVD. This will help to further confirm the validity of the TG/HDL-C ratio in CAVD prediction and to improve the reliability of the study findings. Meanwhile, future studies should also consider expanding the sample size to enhance our understanding through a broader and more diverse dataset. These efforts aim to substantiate the predictive value of the TG/HDL-C ratio for CAVD and refine our strategies for early detection and management.
Conclusion
The TG/HDL-C ratio was significantly associated with the development of calcified aortic valve disease (CAVD). A higher TG/HDL-C ratio was an independent risk factor for CAVD and was associated with higher metabolic indicators such as weight, BMI, SBP, FPG, and SCR. The TG/HDL-C ratio had a good predictive value for CAVD and can be used as a potential diagnostic tool and risk assessment indicator for CAVD.
Acknowledgements
We are grateful to the participants in this study.
Abbreviations
- TG/HDL-C
The triglyceride to high-density-lipoprotein cholesterol
- CAVD
Calcific aortic valve disease
- IR
Insulin resistance
- TyG
Triglyceride glucose index
- METS-IR
Metabolic score for insulin resistance
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- FPG
Fasting blood glucose
- SCR
Serum creatinine
- BUN
Blood urea nitrogen
- ROC
Receiver operating characteristic
Author contributions
Z.C. participated in the study design, data collection, performed the statistical analysis, and drafted the manuscript. Z.L. and X.L. contributed to study design, and contributed to preparation, editing and review of the manuscript, and approved the final version of the manuscript. F.W. participated in the patient’s echocardiogram and made the diagnosis. L.L. and X.J. participated in data collection. Y.C. and Y.Z. contributed to quality control of data. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82374269), Henan Provincial Young Talent Support Project Program (2023HYTP038).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study received approval from the Ethics Committee of the First Affiliated Hospital of Henan University of Chinese Medicine (2024HL-217-01), adhering to the ethical principles outlined in the Declaration of Helsinki. The retrospective nature of the study allowed for a waiver of informed consent by the Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhenzhen Lan, Email: 18101829103@163.com.
Xincan Liu, Email: liuxincan103@163.com.
References
- 1.Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from Basic mechanisms to Novel therapeutic targets. Arterioscler Thromb Vasc Biol. 2020;40(4):885–900. [DOI] [PubMed] [Google Scholar]
- 2.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108(11):1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, et al. Global, Regional, and National Burden of Calcific Aortic Valve and degenerative mitral valve diseases, 1990–2017. Circulation. 2020;141(21):1670–80. [DOI] [PubMed] [Google Scholar]
- 5.The E, de Graaf DM, Zhai Y, Yao Q, Ao L, Fullerton DA, et al. Interleukin 38 alleviates aortic valve calcification by inhibition of NLRP3. Proc Natl Acad Sci U S A. 2022;119(36):e2202577119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between non-insulin-based insulin resistance indices and cardiovascular events in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Xiong T, Chen C, Chen T, Li M, Liang J et al. Association between the triglyceride-glucose index and calcified aortic stenosis in elderly patients: a cross-sectional study. Sci Rep. 2023;13(1). [DOI] [PMC free article] [PubMed]
- 11.Shao QY, Ma XT, Yang ZQ, Li QX, Wang YF, Liang J, et al. Prognostic significance of multiple triglycerides-derived metabolic indices in patients with acute coronary syndrome. J Geriatr Cardiol. 2022;19(6):456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Hua J, Ma L. Triglyceride to high-density lipoprotein ratio can predict coronary artery calcification. Pak J Med Sci. 2022;38(3Part–I):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023;22(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner HC, Hung JC-C, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18(3):254–75. [DOI] [PubMed] [Google Scholar]
- 15.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinauskienė R, Jonkaitienė R. Degenerative aortic stenosis, dyslipidemia and possibilities of Medical Treatment. Med (Kaunas). 2018;54(2). [DOI] [PMC free article] [PubMed]
- 17.Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial Surgical Versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66(25):2827–38. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Greenwood JP, Berry C, Dawson DK, Hogrefe K, Kelly DJ, et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic importance of MIcrovascular dysfunction in aortic stenosis (PRIMID AS) study. Eur Heart J. 2017;38(16):1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-de-Andrés A, Perez-Farinos N, de Miguel-Díez J, Hernández-Barrera V, Méndez-Bailón M, de Miguel-Yanes JM, et al. Impact of type 2 diabetes mellitus in the utilization and in-hospital outcomes of surgical aortic valve replacement in Spain (2001–2015). Cardiovasc Diabetol. 2018;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín-Núñez E, Goñi-Olóriz M, Matilla L, Garaikoetxea M, Mourino-Alvarez L, Navarro A, et al. Influence of diabetes mellitus on the pathological profile of aortic stenosis: a sex-based approach. Cardiovasc Diabetol. 2023;22(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moncla LM, Briend M, Bossé Y, Mathieu P. Calcific aortic valve disease: mechanisms, prevention and treatment. Nat Rev Cardiol. 2023;20(8):546–59. [DOI] [PubMed] [Google Scholar]
- 22.Hofmanis J, Hofmane D, Svirskis S, Mackevics V, Tretjakovs P, Lejnieks A et al. HDL-C role in acquired aortic valve stenosis patients and its relationship with oxidative stress. Med (Kaunas). 2019;55(8). [DOI] [PMC free article] [PubMed]
- 23.Neels JG, Leftheriotis G, Chinetti G. Atherosclerosis calcification: focus on lipoproteins. Metabolites. 2023;13(3). [DOI] [PMC free article] [PubMed]
- 24.Bonacina F, Pirillo A, Catapano AL, Norata GD. HDL in Immune-inflammatory responses: implications beyond Cardiovascular diseases. Cells. 2021;10(5). [DOI] [PMC free article] [PubMed]
- 25.Wang P, Zeng Y, Wang L, Jiang Y, Shen J, Jin F et al. Association of TyG index with aortic valve calcification in valvular heart disease patients. Postgrad Med J. 2024. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.