Abstract
Objectives
Although neoadjuvant chemotherapy (NCT) is a standard approach for operable triple negative breast cancer (TNBC), the potential risks brought by it should also be noticed. Is the expanding indication of NCT to T1cN0M0 population appropriate? We conducted an investigation to compare the long-term survival of small tumor TNBC between NCT and adjuvant chemotherapy (ACT).
Methods
For this propensity-matched analysis, we used data from Surveillance, Epidemiology, and End Results (SEER) database. We enrolled 1183 cases with NCT and 2550 cases with ACT who are AJCC clinical T1c–T2 N0–N1, diagnosed with invasive triple-negative breast cancer, from 2016 to 2017. The propensity score matching was utilized to minimize baseline characteristics bias. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated by the Cox proportional hazard regression model.
Results
Compared with patients receiving ACT, patients with NCT in this study presented a higher proportion of younger age, T2 stage, N1 stage, and underwent more mastectomy. Multivariate analysis in matched patients showed that NCT had no significant survival benefit compared with ACT in T1c–2N0–1M0 TNBC patients. Stratified analyses by T stage and N stage demonstrated NCT mainly presented a survival advantage in patients with N1 stage. Further investigation found that NCT didn’t improve BCSS (HR, 0.472; 95% CI 0.135–1.647; P = 0.239) and OS (HR, 0.392; 95% CI 0.147–1.047; P = 0.062) for patients with T1cN0M0 TNBC; however, it was associated with improved OS (HR, 1.951; 95% CI 1.003–3.797; P = 0.049) only for patients with T2N1M0 TNBC.
Conclusions
In this study, we did not find any profit brought by NCT in the stage I and stage IIa cohorts, but even more unfavorable outcomes appeared in the T1cN0M0 cohort. Therefore, whether the candidates of NCT should be extended to T1cN0M0 still need to be cautious.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02139-1.
Keywords: Neoadjuvant, Triple negative, Breast cancer, Survival, Chemotherapy
Background
Neoadjuvant chemotherapy (NCT) is now a standard approach to operable breast cancer, particularly in triple-negative breast cancer (TNBC). As CREATE-X trial presented better outcomes when adding post-surgical capecitabine in non-responders, the candidates for NCT were continuously expanding in TNBC [1]. Meanwhile, a controversy was raised, especially when cT1c and cN0 were included in the indication of NCT according to the ASCO guide-line and NCCN guide-line [2, 3].
The traditional purpose of NCT is to shrink the tumor in local advanced breast cancer and to increase the opportunity of changing inoperable disease to operable disease or to contribute to raising the breast-conserving surgery rate. And now, it has been endowed another important role to monitor response in vivo and tailor follow-up adjuvant treatments. Small tumors, for those with a tumor diameter less than 2 cm and without lymph node involved, the only objective to undergo NCT is to improve overall survival through the “response-adjusted” method. If this target cannot be achieved, it means we cannot get benefit from NCT but have to face additional risks brought by it.
Doubtless, NCT may also be accompanied by increased hazards. To pursue the biggest opportunities for achieving pathological complete remission (PCR), NCT typically involves the use of escalating regimens, such as those including platinum or additional immunotherapy. However, this approach may also increase the incidence of serious adverse events. Although most studies demonstrated benefit from adjuvant chemotherapy (ACT) in TNBC with T1c or higher stage, they also pointed out that less intensive chemotherapy regimens, for example, anthracycline-free or taxane-free, can achieve excellent survival outcomes similar to anthracycline and taxane combination regimens in this group of patients [4–7]. This suggests that overtreatment may exist in them. Not to mention that ineffective pre-operative treatment may bring the possibility of disease progression or may even dally away their best opportunity to receive an operation. The utilization of NCT is not always associated with better results in clinical trials. It was reported a higher risk of local recurrence when compared with traditional adjuvant systemic treatment in a meta-analysis recently [8].
Therefore, re-investigating the value of NCT in early stage TNBC is particularly important under the trend of extending the indication of NCT. Since no previous studies focused on this issue, we conduct a study to explore the impact of neoadjuvant therapy on long-term survival compared with adjuvant therapy based on the SEER database which provides information on neoadjuvant setting from 2016 to 2017 and at least 5 years of survival data of breast cancer-specific survival (BCSS) and overall survival (OS). We especially focused on the population with tumor size smaller than 5 cm and bigger than 1 cm with the number of involved lymph nodes lower than that of three.
Methods
Patient population
This retrospective population-based registry study used the data from Surveillance, Epidemiology, and End Results (SEER) database, a registry for incident cancers in the United States. Patients aged between 20 and 79 years who had triple negative and cT1c–2N0–1M0 invasive breast cancer from January 1, 2016, to December 31, 2017, due to the SEER database reported clinical stage status only in this period, were identified from the SEER database. Patients who had stage M1 disease, without breast surgery, and with unknown surgery, radiotherapy, or chemotherapy treatment data were excluded. Two patient’s groups were defined according to the RX Summ Systemic/Sur Seq code in the SEER database: neoadjuvant systemic therapy group (Systemic therapy before surgery or Systemic therapy both before and after surgery) or traditional adjuvant therapy (Systemic therapy after surgery) group, and the Chemotherapy recode of both groups was defined as YES. We collected data concerning tumor status, nodal status, grade, race, age, marital status, laterality, and HER2 status, and information concerning surgical procedure, chemotherapy (NCT or ACT), and radiotherapy.
Outcome measurement
The breast cancer-specific survival (BCSS) was defined as primary outcome of this study, which was counted from the time from diagnosis to death due to breast cancer. The date of the last contact, the date of death from other causes, or the end date of the study was used for the BCSS analysis, if the patient died from other causes. The secondary outcome of this study was overall survival (OS), which was counted from the date of diagnosis to the date of death or the last follow-up time.
Statistical analysis
The statistical differences were compared by the chi-square test for the baseline characteristics of the NCT and ACT cases, in the whole groups and matched groups. The annual rate of utilization for NCT in TNBC patients was extracted from SEER database between 2010 and 2019 to examine national trends, and all rates were age-adjusted.
The propensity score-matched (PSM) model was performed to reduce the bias of demographic and clinical characteristics differences on the outcome. Propensity matching between patients treated with NCT and patients treated with ACT was done with the nearest-neighbor matching method. Covariables included in the PSM model were age, race, marital status, grade, laterality, tumor status, nodal status, surgery approach, and radiation status. Univariable and multivariable analyses were used to find the prognosis factors. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated by the Cox proportional hazard regression model. We further did an additional Cox proportional hazard regression subgroup analysis in patients with tumor status, nodal status, and AJCC TNM stage, to establish survival outcomes between the NCT versus ACT groups.
All p values of less than 0.05 were considered as a statistical significance level. These statistical analyses were performed by using IBM SPSS Statistics 26 and R software version 4.0.5. IBM SPSS Statistics 26 was used to generate the graph plots.
Results
Demographics and clinical characteristics of the study population
Between Jan 1, 2016, and Dec 31, 2017, we enrolled 3733 eligible patients in this study, 1183 patients of these patients had received NCT, and 2550 had received ACT. The median follow-up time of these patients was 34 months. The patient baseline characteristics of the NCT group and the ACT group are shown in Table 1. Age varied between these two groups, with age between 20 and 49 being presented in 483 (40.8%) patients treated with NCT, and 628 (24.6%) treated with ACT. In the NCT group, 700 (59.2%) patients aged between 50 and 79 compared with 1922 (75.4%) in the ACT group. The patients treated with NCT presented a higher proportion of larger tumor size (T1c, 22.3% vs. 53.1%; T2, 77.7% vs. 46.9%; p < 0.001), and more lymph node involvement (N0, 70.1% vs. 80.4%; N1, 29.9% vs. 19.6%; p < 0.001). Additionally, mastectomy was more common in the NCT group (585 [49.5%] patients), compared with the ACT group (989 [38.8%] patients).
Table 1.
Baseline characteristics of patients with neoadjuvant chemotherapy and adjuvant chemotherapy
| Characteristics | Neoadjuvant chemotherapy (n = 1183) | Adjuvant chemotherapy (n = 2550) | Total (n = 3733) | Pc | ||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | |||
| Age (years) | 20–49 | 483 | 40.8 | 628 | 24.6 | 1111 | 29.8 | < 0.001 |
| 50–79 | 700 | 59.2 | 1922 | 75.4 | 2622 | 70.2 | ||
| Race | White | 861 | 72.8 | 1772 | 69.5 | 2633 | 70.5 | 0.122 |
| Black | 215 | 18.2 | 521 | 20.4 | 736 | 19.7 | ||
| Othera | 107 | 9.0 | 257 | 10.1 | 364 | 9.8 | ||
| Marital status | Married | 687 | 58.1 | 1461 | 57.3 | 2148 | 57.5 | 0.587 |
| Not marriedb | 455 | 38.5 | 983 | 38.5 | 1438 | 38.5 | ||
| Other/unknown | 41 | 3.5 | 106 | 4.2 | 147 | 3.9 | ||
| Grade | I and II | 153 | 12.9 | 332 | 13.0 | 485 | 13.0 | < 0.001 |
| III and IV | 995 | 84.1 | 2202 | 86.4 | 3197 | 85.6 | ||
| Unknown | 35 | 3.0 | 16 | 0.6 | 51 | 1.4 | ||
| Laterality | Left | 605 | 51.1 | 1295 | 50.8 | 1900 | 50.9 | 0.839 |
| Right | 578 | 48.9 | 1255 | 49.2 | 1833 | 49.1 | ||
| Tumor status | T1c | 264 | 22.3 | 1355 | 53.1 | 1619 | 43.4 | < 0.001 |
| T2 | 919 | 77.7 | 1195 | 46.9 | 2114 | 56.6 | ||
| Nodal status | N0 | 829 | 70.1 | 2051 | 80.4 | 2880 | 77.1 | < 0.001 |
| N1 | 354 | 29.9 | 499 | 19.6 | 853 | 22.9 | ||
| Surgery approach | BCS | 598 | 50.5 | 1561 | 61.2 | 2159 | 57.8 | < 0.001 |
| Mastectomy | 585 | 49.5 | 989 | 38.8 | 1574 | 42.2 | ||
| Radiation status | No | 523 | 44.2 | 1071 | 42.0 | 1594 | 42.7 | 0.204 |
| Yes | 660 | 55.8 | 1479 | 58.0 | 2139 | 57.3 | ||
BCS breast-conserving surgery
aOther includes American Indian/Alaskan native and Asian/Pacific Islander and Unknown
bNot married includes divorced, separated, single (never married), unmarried or domestic partner, and widowed
cThe p value of the chi-square test was calculated between the neoadjuvant chemotherapy and adjuvant chemotherapy groups, and bold type indicates significance
Status quo of the utilization for neoadjuvant systemic therapy in TNBC patients
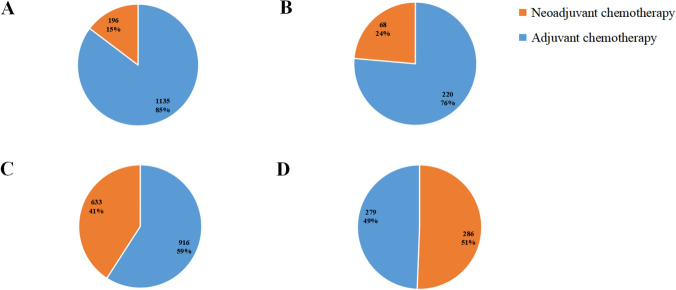
As shown in Fig. 1, the proportion of patients receiving NCT increased with the increase of the AJCC TNM stage. Among the patients, 196 (15%) patients had been treated with NCT in the T1cN0M0 stage, 68 (24%) patients in the T1cN1M0 stage, 633 (41%) patients in the T2N0M0 stage, and 286 (51%) patients in the T2N1M0 stage. The national trends of utilization for NCT in TNBC patients between 2010 and 2019 are presented in Figure S1. From 2010 to 2013 the annual age-adjusted rate of utilization for NCT remain stable about 8.5 to 10, but it began to increase in 2013 and the growth rate was speeding up in recent 2 years. We divided the TNBC patients into different groups according to different stages, we could find that the rate of utilization for NCT in stage I and stage II TNBC was increasing largely, especially after 2015.
Fig. 1.
Proportion of patients receiving neoadjuvant chemotherapy or adjuvant chemotherapy in different AJCC TNM stages: A T1cN0M0; B T1cN1M0; C T2N0M0; D T2N1M0
Comparison of survival between neoadjuvant systemic therapy group and traditional adjuvant therapy group
The univariate Cox regression analysis in all patients for each variable is presented in Table S1. The result of multivariate Cox regression analysis shown in Table 2 indicated NCT didn’t bring benefit compared with the survival of overall patients in the ACT group, according to BCSS and OS (HR = 1.063, 95% CI 0.771–1.465, p = 0.711; HR = 1.101, 95% CI 0.828–1.463, p = 0.507, respectively). In the 1:1 PSM analysis, a total of 1914 patients were successfully matched between the group treated with NCT and ACT group, and finally each group included 957 patients. The baseline characteristics of matched patients are shown in Table 3. The chi-square test indicated that each variable didn’t have a statistical difference, which confirms the propensity score overlapped well between the two groups.
Table 2.
Multivariate cox proportional hazard model of breast cancer-specific survival (BCSS) and overall survival (OS) in all patients
| Variables | BCSS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | 20–49 | Reference | Reference | ||
| 50–79 | 1.551 (1.100–2.189) | 0.012 | 1.772 (1.295–2.424) | < 0.001 | |
| Marital status | Married | Reference | Reference | ||
| Not marrieda | 1.318 (0.979–1.775) | 0.068 | 1.336 (1.031–1.731) | 0.028 | |
| Other/unknown | 1.017 (0.471–2.196) | 0.996 | 0.762 (0.355–1.634) | 0.484 | |
| Race | White | Reference | Reference | ||
| Black | 1.203 (0.856–1.692) | 0.287 | 1.333 (0.994–1.789) | 0.055 | |
| Otherb | 0.587 (0.317–1.087) | 0.090 | 0.777 (0.477–1.265) | 0.309 | |
| Grade | I and II | Reference | Reference | ||
| III and IV | 1.344 (0.834–2.167) | 0.224 | 1.670 (1.065–2.618) | 0.025 | |
| NA | 1.052 (0.243–4.549) | 0.946 | 1.577 (0.467–5.327) | 0.463 | |
| Laterality | Left | Reference | Reference | ||
| Right | 0.966 (0.726–1.286) | 0.814 | 0.895 (0.696–1.149) | 0.383 | |
| Tumor status | T1c | Reference | Reference | ||
| T2 | 1.528 (1.113–2.096) | 0.009 | 1.527 (1.160–2.010) | 0.003 | |
| Nodal status | N0 | Reference | Reference | ||
| N1 | 1.776 (1.299–2.427) | < 0.001 | 1.879 (1.434–2.462) | < 0.001 | |
| Surgery approach | BCS | Reference | Reference | ||
| Mastectomy | 1.103 (0.764–1.592) | 0.602 | 0.790 (0.575–1.085) | 0.145 | |
| Radiation status | No | Reference | Reference | ||
| Yes | 0.728 (0.504–1.053) | 0.092 | 0.563 (0.410–0.772) | < 0.001 | |
| Chemotherapy model | Neoadjuvant | Reference | Reference | ||
| Adjuvant | 1.063 (0.771–1.465) | 0.711 | 1.101 (0.828–1.463) | 0.507 | |
20–49 20–49 years, 50–79 50–79 years, BCS Breast Conserving Surgery, HR hazard ratio
aNot married includes divorced, separated, single (never married), unmarried or domestic partner, and widowed
bOther includes American Indian/Alaskan native and Asian/Pacific Islander and Unknown
Bold type indicates significance
Table 3.
Baseline characteristics of patients with neoadjuvant chemotherapy and adjuvant chemotherapy in PSM group
| Characteristics | Neoadjuvant chemotherapy (n = 957) | Adjuvant chemotherapy (n = 957) | Total (n = 1914) | Pc | ||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | |||
| Age (years) | 20–49 | 338 | 35.3 | 344 | 35.9 | 682 | 35.6 | 0.775 |
| 50–79 | 619 | 64.7 | 613 | 64.1 | 1232 | 64.4 | ||
| Race | White | 721 | 75.3 | 720 | 75.2 | 1441 | 75.3 | 0.962 |
| Black | 168 | 17.6 | 166 | 17.3 | 334 | 17.5 | ||
| Othera | 68 | 7.1 | 71 | 7.4 | 139 | 7.3 | ||
| Marital status | Married | 570 | 59.6 | 570 | 59.6 | 1140 | 59.6 | 0.989 |
| Not marriedb | 363 | 37.9 | 364 | 38.0 | 727 | 38.0 | ||
| Other/unknown | 24 | 2.5 | 23 | 2.4 | 47 | 2.5 | ||
| Grade | I and II | 93 | 9.7 | 92 | 9.6 | 185 | 9.7 | 0.261 |
| III and IV | 859 | 89.8 | 864 | 90.3 | 1723 | 90.0 | ||
| Unknown | 5 | 0.5 | 1 | 0.1 | 6 | 0.3 | ||
| Laterality | Left | 494 | 51.6 | 495 | 51.7 | 989 | 51.7 | 0.964 |
| Right | 463 | 48.4 | 462 | 48.3 | 925 | 48.3 | ||
| Tumor status | T1c | 234 | 24.5 | 238 | 24.9 | 472 | 24.7 | 0.832 |
| T2 | 723 | 75.5 | 719 | 75.1 | 1442 | 75.3 | ||
| Nodal status | N0 | 723 | 75.5 | 721 | 75.3 | 1444 | 75.4 | 0.915 |
| N1 | 234 | 24.5 | 236 | 24.7 | 470 | 24.6 | ||
| Surgery approach | BCS | 515 | 53.8 | 509 | 53.2 | 1024 | 53.5 | 0.783 |
| Mastectomy | 442 | 46.2 | 448 | 46.8 | 890 | 46.5 | ||
| Radiation status | No | 417 | 43.6 | 420 | 43.9 | 837 | 43.7 | 0.890 |
| Yes | 540 | 56.4 | 537 | 56.1 | 1077 | 56.3 | ||
BCS breast-conserving surgery
aOther includes American Indian/Alaskan native and Asian/Pacific Islander and Unknown
bNot married includes divorced, separated, single (never married), unmarried or domestic partner, and widowed
cThe p value of the chi-square test was calculated between the neoadjuvant chemotherapy and adjuvant chemotherapy groups, and bold type indicates significance
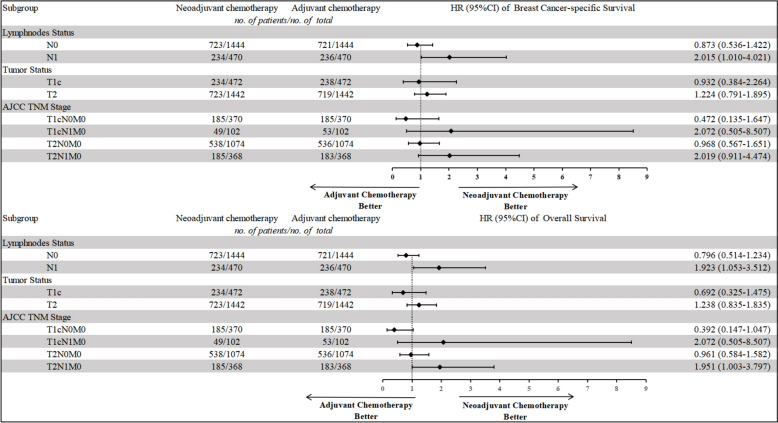
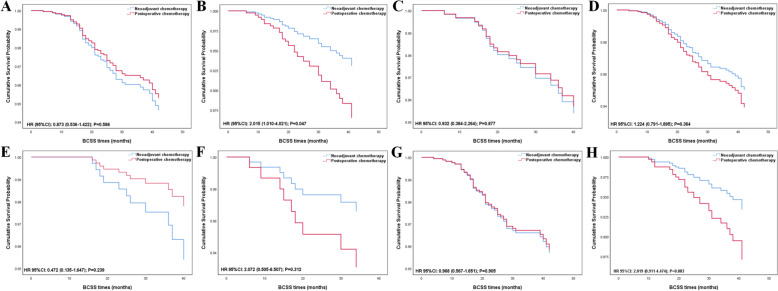
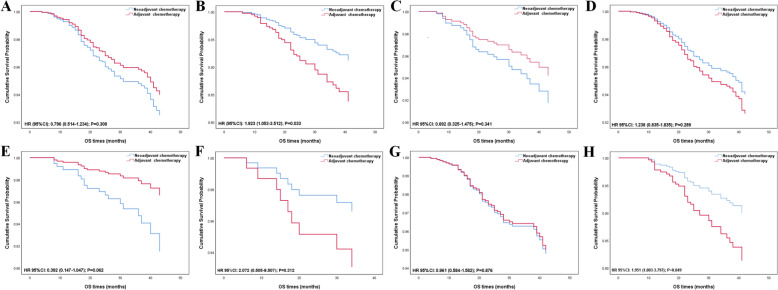
In the matched patients, patients still couldn’t benefit from NCT in both BCSS and OS (BCSS, HR = 1.164, 95% CI 0.788–1.721, p = 0.445; OS, HR = 1.099, 95% CI 0.777–1.554, p = 0.594, shown in Table 4). We stratified the patients by different clinical features or AJCC TNM stages to investigate the effect of NCT on patients with different subgroups (Table 5, Figs. 2, 3, 4). Nodal status is an important predictor of NCT administration. In adjusted Cox regression subgroup analysis, N1 status was associated with significantly improved BCSS and OS in the NCT group versus the ACT group (BCSS, HR = 2.015, 95% CI 1.010–4.021, p = 0.047; OS, HR = 1.923, 95% CI 1.053–3.512, p = 0.033, shown in Table 5, Figs. 2, 3, 4), but N0 status didn’t show any benefit from NCT (BCSS, HR = 0.873, 95% CI 0.536–1.422, p = 0.586; OS, HR = 0.796, 95% CI 0.514–1.234, p = 0.308). Larger tumor size always tends to be more selective for NCT in clinical practice. However, NCT group didn’t improve outcome in both T1c and T2 status (T1c status: BCSS, HR = 0.932, 95% CI 0.384–2.264, p = 0.877; OS, HR = 0.692, 95% CI 0.325–1.475, p = 0.341; T2 status: BCSS, HR = 1.224, 95% CI 0.791–1.895, p = 0.364; OS, HR = 1.238, 95% CI 0.835–1.835, p = 0.289). To explore the effects of NCT on patients with different AJCC TNM stages, we categorized the patients into T1cN0M0, T1cN1M0, T2N0M0, and T2N1M0. From the results, we did not find NCT could lower the risk of cancer specific mortality and all-cause mortality in the T1cN1M0 and T2N0M0 cohort (T1cN1M0: BCSS, HR = 2.072, 95% CI 0.505–8.507, p = 0.312; OS, HR = 2.072, 95% CI 0.505–8.507, p = 0.312; T2N0M0: BCSS, HR = 0.968, 95% CI 0.567–1.651, p = 0.905; OS, HR = 0.961, 95% CI 0.584–1.582, p = 0.876), but even more unfavorable outcomes appeared in T1cN0M0 cohort (BCSS, HR = 0.472, 95% CI 0.135–1.647, p = 0.239; OS, HR = 0.392, 95% CI 0.147–1.047, p = 0.062). NCT can lower the risk of all-cause mortality in patients diagnosed with T2N1M0 (OS, HR = 1.951, 95% CI 1.003–3.797, p = 0.049), however, there is no significant difference in BCSS level was detected (BCSS, HR = 2.019, 95% CI 0.911–4.474, p = 0.083).
Table 4.
Multivariate Cox proportional hazard model of breast cancer-specific survival (BCSS) and overall survival (OS) in the propensity score matched group
| Variables | BCSS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | 20–49 | Reference | Reference | ||
| 50–79 | 1.756 (1.113–2.770) | 0.015 | 1.839 (1.217–2.779) | 0.004 | |
| Marital status | Married | Reference | Reference | ||
| Not marrieda | 1.528 (1.019–2.293) | 0.040 | 1.599 (1.117–2.289) | 0.010 | |
| Other/unknown | 0.962 (0.232–3.980) | 0.957 | 0.714 (0.174–2.932) | 0.640 | |
| Race | White | Reference | Reference | ||
| Black | 1.255 (0.782–2.015) | 0.364 | 1.160 (0.753–1.789) | 0.501 | |
| Otherb | 0.595 (0.217–1.631) | 0.313 | 0.945 (0.458–1.952) | 0.879 | |
| Grade | I and II | Reference | Reference | ||
| III and IV | 1.295 (0.623–2.693) | 0.488 | 1.720 (0.835–3.545) | 0.141 | |
| Laterality | Left | Reference | Reference | ||
| Right | 1.077 (0.729–1.590) | 0.710 | 0.919 (0.650–1.300) | 0.634 | |
| Tumor status | T1c | Reference | Reference | ||
| T2 | 1.320 (0.806–2.161) | 0.270 | 1.157 (0.759–1.764) | 0.498 | |
| Nodal status | N0 | Reference | Reference | ||
| N1 | 1.761 (1.147–2.704) | 0.010 | 1.924 (1.322–2.801) | 0.001 | |
| Surgery approach | BCS | Reference | Reference | ||
| Mastectomy | 1.204 (0.706–2.054) | 0.495 | 0.916 (0.571–1.472) | 0.718 | |
| Radiation status | No | Reference | Reference | ||
| Yes | 0.895 (0.518–1.549) | 0.693 | 0.744 (0.458–1.209) | 0.233 | |
| Chemotherapy model | Neoadjuvant | Reference | Reference | ||
| Adjuvant | 1.164 (0.788–1.721) | 0.445 | 1.099 (0.777–1.554) | 0.594 | |
20–49 20–49 years, 50–79 50–79 years, BCS Breast Conserving Surgery, HR hazard ratio
aNot married includes divorced, separated, single (never married), unmarried or domestic partner, and widowed
bOther includes American Indian/Alaskan native and Asian/Pacific Islander and Unknown
Bold type indicates significance
Table 5.
Comparison of breast cancer-specific survival (BCSS) and overall survival (OS) between patients with neoadjuvant chemotherapy and adjuvant chemotherapy in clinical variables using a multivariate Cox proportional hazard model in the propensity score matched group
| Variables | BCSS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | Pa | HR (95% CI) | Pa | |
| N0 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 0.873 (0.536–1.422) | 0.586 | 0.796 (0.514–1.234) | 0.308 |
| N1 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 2.015 (1.010–4.021) | 0.047 | 1.923 (1.053–3.512) | 0.033 |
| T1c | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 0.932 (0.384–2.264) | 0.877 | 0.692 (0.325–1.475) | 0.341 |
| T2 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 1.224 (0.791–1.895) | 0.364 | 1.238 (0.835–1.835) | 0.289 |
| T1cN0M0 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 0.472 (0.135–1.647) | 0.239 | 0.392 (0.147–1.047) | 0.062 |
| T1cN1M0 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 2.072 (0.505–8.507) | 0.312 | 2.072 (0.505–8.507) | 0.312 |
| T2N0M0 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 0.968 (0.567–1.651) | 0.905 | 0.961 (0.584–1.582) | 0.876 |
| T2N1M0 | ||||
| Neoadjuvant chemotherapy | Reference | Reference | ||
| Adjuvant chemotherapy | 2.019 (0.911–4.474) | 0.083 | 1.951 (1.003–3.797) | 0.049 |
HR hazard ratio, CI confidence interval, BCSS breast cancer-specific survival, OS overall survival
ap value was adjusted by a multivariate Cox proportional hazard regression model
Bold type indicates significance
Fig. 2.
Treatment effect on breast cancer-specific survival (BCSS) and overall survival (OS) by subgroup. HR hazard ratio, 95% CIs 95% confidence intervals
Fig. 3.
Kaplan–Meier survival curves of the effect of neoadjuvant chemotherapy and postoperative chemotherapy on breast cancer-specific survival (BCSS) by clinical variables: A N0 status; B N1 status; C T1c status; D T2 status; E T1cN0M0; F T1cN1M0; G T2N0M0; H T2N1M0
Fig. 4.
Kaplan–Meier survival curves of the effect of neoadjuvant chemotherapy and postoperative chemotherapy on overall survival (OS) by clinical variables: A N0 status; B N1 status; C T1c status; D T2 status; E T1cN0M0; F T1cN1M0; G T2N0M0; H T2N1M0
Discussion
TNBC is considered a highly aggressive subtype with a high risk of early recurrence and distant metastasis. Meanwhile, high heterogeneity of it always induces therapeutic resistance. Therefore, the administration of chemotherapy before rather than after surgery is regarded as an effective tool for testing drug-sensitivity in vivo which may help physicians to monitor the therapeutic efficacy and avoid futile treatment as far as possible. This may be one of the reasons why the rate of neoadjuvant in TNBC kept on raising in recent years. As shown in Figure S1, from 2010 to 2013 the annual age-adjusted rate of utilization for NCT remain stable about 8.5–10, but it began to increase in 2013 and the growth rate was speeding up in recent 2 years. If we divided the TNBC patients into different groups according to different stages, we could easily find that the rapid increasing rate was largely due to more and more TNBC in stage I and stage II being enrolled in NCT, especially after 2015. We suggested that the benefit evidence from CTREATX trial which presented on SABCS2015 promoted the occurrence of this phenomenon. Between 2016 and 2017, nearly 41% of T2N0M0 and 15% of T1N0M0 TNBC were arranged to receive systemic therapy before surgery. Although CREATEX trial showed us post operation intensive treatment for non-PCR cohort may improve the prognosis in TNBC. Does this strategy really suit the whole group of patients? As we could see from the subgroup analysis of DFS in CREATEX, those patients with T1 or N0 did not acquire significant benefit from the potentiate treatment strategy [1]. Thus, considering those potential risks which may be brought by NCT, we really need to re-evaluate the necessity of performing neoadjuvant in small tumor of TNBC.
We enrolled the patients with T1c and T2 and N0 or N1. In this population, the only purpose of NCT is to evaluate the drug sensitivity in vivo and guide the treatment in an adjuvant period to improve long-term outcomes. However, we did not see any difference in survival outcomes whatever in BCSS or OS between NCT and ACT groups in the analysis of the multivariate Cox proportional hazard model. To eliminate the interference of the unbalance baseline characteristics, we analyzed the survival difference between the two groups after PSM and we got the same conclusion again that NCT could not improve the prognosis in these patients. This phenomenon suggests that the use of NCT should not be excessive in low-stage TNBC. The ubiquity of over-treatment in NCT needs to pay attention to. Anthracycline combined with taxane could bring about 35–45% PCR rates and be used as a routine regimen in NCT, however, it could lead to 30–40% of grade 3–4 side effects at the same time [9–12]. Adding platinum to the regimen could increase the PCR rate by 25–30% but also double the incidence of severe toxicity [9, 10]. As recent studies showed that a combination of PD-L1 inhibitor with anthracycline and taxane or even adding platinum should be the most efficient NCT regimen [13, 14]. The patients who receive this prescription also need to face up to 77% of side effects higher than grade 3 [15, 16]. This is obviously unreasonable to low tumor burden TNBC.
As the result still cannot tell us the definitive cohort who should not receive NCT. We continue to do further analysis in subgroups. Firstly, we separated the population according to the status of the lymph node. Lymph node metastasis is considered an indication for performing NCT in TNBC according to NCCN and ASCO guidelines [2, 3]. As might be expected, the patients with lymph node positive in this study could obtain significantly better survival when receiving NCT. The hazards of breast cancer-specific mortality and all causes of mortality were both reduced by nearly 50%. As lymph node metastasis is associated with poorer prognosis and suggests that stronger treatment is needed [3, 17–19]. This principle should also be applied in a neoadjuvant setting. In addition, there is always a lower PCR rate in lymph node positive patients which means more opportunity for this group of patients to be put into receiving post-operative intensified treatment schedule in current clinical practice [12]. This may improve the prognosis as well. Hence, we believed nodal status is an important predictive factor of NCT in small tumor TNBC. On the other hand, we divided the patients into two groups according to the size of the tumor and took 2 cm as the boundary. There was not a significant difference in BCSS and OS between NCT and ACT in both two groups. Interestingly, we found the survival curves between the two treatment strategies were reversed in the two groups. It hinted NCT may bring better efficacy in larger tumors but the advantage is not significant in patients whose tumor is smaller than 2 cm. Tumor size may be another important considering factor for NCT, but the cutoff value is uncertain. Is 2 cm or 5 cm the reasonable threshold for choosing NCT in TNBC? It requires in-depth discussion. As showed by EBCTCG in their meta-analysis, NCT led to worse outcomes in distant recurrence and breast cancer mortality if the clinical tumor size is between 1 and 2 cm, but trends leading to better outcomes if the tumor size is larger than 2 cm [8]. Does this mean 2 cm may be the more appropriate minimum threshold to differentiate the applicable group of receiving NCT? We need more evidence from prospective studies based on the current treatment strategy.
Without a doubt, we cannot separate the influence of the status of tumor size and lymph node on treatment selection. Next, we further subdivided the population by combining both the factors of T and N. We found that overall survival was significantly higher with NCT than with ACT in the T2N1M0 group, but not in the other groups. NCT did not show survival advantages in the stage I and stage IIa cohorts and even result in unfavorable outcomes in the T1cN0M0 cohort. The numerically worse outcomes associated with NCT warrant our attention. The NCCN guidelines regarding lymph node-negative T1c TNBC are ambiguous, merely recommending that NCT be 'considered.' Previous studies have indicated that women with clinical T1N0M0 tumors generally have an excellent prognosis and may derive limited benefit from NCT [20, 21]. Moreover, they may experience adverse effects related to NCT, including overtreatment and delays in necessary treatment. Therefore, we should cautiously consider recommending NCT for the T1c population, as the evidence suggests potential negative impacts of NCT in this patient group.
It is undeniable that NCT has already changed our clinical strategy. Relying on this treatment strategy, we could distinguish the population who need to enhance their treatment intensity by in vivo drug sensitivity evaluation and make further improvements in overall efficacy. We also believe that in the near future, depending on this platform, we could even perform de-escalation treatment in selected populations as the evidence showed by ADAPT trials [22]. NCT may be an effective way to progressively realize precision medicine. However, the advantages of traditional treatment strategies should not be overlooked. Several studies have shown that the long-term prognosis of clinical T1N0M0 TNBC patients receiving ACT is similar to that of those treated with NCT [6, 23–25]. On one hand, T1 tumors are relatively small and may exhibit favorable biological characteristics, leading to diminished sensitivity to chemotherapy. As a result, they may not respond as effectively to NCT compared to larger or higher-grade tumors. On the other hand, for patients with low tumor burden, accurate pathological assessment of the initial tumor status after surgery can inform a reasonable treatment strategy that offers an excellent prognosis and mitigates the risks associated with NCT. This study suggests that the eligibility criteria for NCT should not be extended to the T1cN0M0 population. However, to date, limited data exist comparing the outcomes of NCT and ACT in clinical T1N0M0 disease. Further prospective trials are needed.
Due to inherent limitations of the SEER database, this study has several deficiencies. First, the lack of detailed information on specific chemotherapy regimens restricts our ability to balance potential differences in therapeutic intensity between NCT and adjuvant treatments. However, our results align with those of the EBCTCG meta-analysis, which provides comprehensive treatment details and enhances the credibility of our conclusions [8]. Second, the absence of data on PCR rates is significant. PCR is often used as a surrogate marker to evaluate the immediate efficacy of neoadjuvant treatment. However, as previous studies have noted, PCR rates are generally easier to obtain in smaller tumors, which does not necessarily indicate that neoadjuvant therapy is the preferred choice for those tumors. Ultimately, long-term outcomes are the only standard for evaluating the value of a treatment method. This study, using BCSS and OS as endpoints, more accurately reflects the advantages of both treatment strategies and provides more reliable conclusions. Third, we acknowledge that the retrospective nature of this study may introduce selection bias, limiting causal inferences. However, after PSM, we were able to reduce inter-group bias associated with the retrospective design. We are the first study to exclusively focus on the indication of NCT in small tumors, specifically enrolling patients who received NCT after 2015, which aligns with current treatment principles. Given the large patient cohort and adequate follow-up period provided by the SEER database, we believe that our research offers credible evidence for evaluating the benefits of NCT in small tumor TNBC.
Conclusion
In this study, we did not find any profit brought by NCT in the stage I and stage IIa cohorts, but even more unfavorable outcomes appeared in the T1cN0M0 cohort. Therefore, whether the candidates of NCT should be extended to T1cN0M0 still need to be cautious.
Supplementary Information
Supplementary material 1. Figure S1. Trend in utilization for neoadjuvant chemotherapy in TNBC patients between 2010 and 2019:overall;different stages
Acknowledgements
None.
Author contributions
The first 3 authors contributed equally to this article. Jie Zhang: Writing original draft and formal analysis. Ruiliang Chen: paper revision and submission. Yushuai Yu, Weiwei Chen: Writing review and editing. Wenfen Fu, Ruiliang Chen: Data acquisition and editing. Wenfen Fu: Figure editing and typesetting. Chuangui Song, Jie Zhang: Study design and supervision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruiliang Chen, Yushuai Yu, and Weiwei Chen have contributed equally to this work.
Contributor Information
Chuangui Song, Email: Songcg1971@hotmail.com.
Jie Zhang, Email: zjie1979@fjmu.edu.cn.
References
- 1.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59. [DOI] [PubMed] [Google Scholar]
- 2.Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. [DOI] [PubMed] [Google Scholar]
- 4.Fasano GA, Bayard S, Chen Y, Varella L, Cigler T, Bensenhaver J, Simmons R, Swistel A, Marti J, Moore A, et al. Benefit of adjuvant chemotherapy in node-negative T1a versus T1b and T1c triple-negative breast cancer. Breast Cancer Res Treat. 2022;192(1):163–73. [DOI] [PubMed] [Google Scholar]
- 5.Ren YX, Hao S, Jin X, Ye FG, Gong Y, Jiang YZ, Shao ZM. Effects of adjuvant chemotherapy in T1N0M0 triple-negative breast cancer. Breast. 2019;43:97–104. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Z, Zheng Y, Yao J, Liu Y, Ruan J, Deng Y, Zhou L, Zhao P, Yang S, Hu J, et al. Evaluation of adjuvant treatments for T1 N0 M0 triple-negative breast cancer. JAMA Netw Open. 2020;3(11):e2021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wang W, Wang J, Luo Y, Chen S, Ma F, Xu B, Fan Y. Survival outcome and impact of chemotherapy in T1 node-negative triple-negative breast cancer: a SEER database analysis. J Oncol. 2020;2020:8880727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19(1):27–39. 10.1016/S1470-2045(17)30777-5 [DOI] [PMC free article] [PubMed]
- 9.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. [DOI] [PubMed] [Google Scholar]
- 10.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, Carrasco E, Calvo L, Segui MA, Ribelles N, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006–03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487–93. [DOI] [PubMed] [Google Scholar]
- 12.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag D, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. [DOI] [PubMed] [Google Scholar]
- 13.Lin YY, Gao HF, Yang X, Zhu T, Zheng XX, Ji F, Zhang LL, Yang CQ, Yang M, Li JQ, et al. Neoadjuvant therapy in triple-negative breast cancer: A systematic review and network meta-analysis. Breast. 2022;66:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Zhang J, Lin Y, Kang S, Lv X, Song C. Efficacy and safety of neoadjuvant therapy for triple-negative breast cancer: a Bayesian network meta-analysis. Expert Rev Anticancer Ther. 2022;22(10):1141–51. [DOI] [PubMed] [Google Scholar]
- 15.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. [DOI] [PubMed] [Google Scholar]
- 16.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. [DOI] [PubMed] [Google Scholar]
- 17.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VC. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102(6):410–25. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, Liu Q, Yuan P, Feng X. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer. 2014;21(1):1–9. [DOI] [PubMed] [Google Scholar]
- 20.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, Giordano SH, Hunt KK, Mittendorf EA. Validation study of the American joint committee on cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4(2):203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavez-MacGregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating tumor characteristics to the American joint committee on cancer breast cancer staging system. Oncologist. 2017;22(11):1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluz O, Nitz U, Kolberg-Liedtke C, Prat A, Christgen M, Kuemmel S, Mohammadian MP, Gebauer D, Kates R, Paré L, et al. De-escalated neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): impact of molecular markers and final survival analysis of the WSG-ADAPT-TN trial. Clin Cancer Res. 2022;28(22):4995–5003. [DOI] [PubMed] [Google Scholar]
- 23.Tarantino P, Leone J, Vallejo CT, Freedman RA, Waks AG, Martínez-Sáez O, Garrido-Castro A, Lynce F, Tayob N, Lin NU, et al. Prognosis and treatment outcomes for patients with stage IA triple-negative breast cancer. NPJ breast cancer. 2024;10(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbajal-Ochoa W, Bravo-Solarte DC, Bernal AM, Anampa JD. Benefit of adjuvant chemotherapy in lymph node-negative, T1b and T1c triple-negative breast cancer. Breast Cancer Res Treat. 2024;203(2):257–69. [DOI] [PubMed] [Google Scholar]
- 25.An X, Lei X, Huang R, Luo R, Li H, Xu F, Yuan Z, Wang S, de Nonneville A, Gonçalves A, et al. Adjuvant chemotherapy for small, lymph node-negative, triple-negative breast cancer: a single-center study and a meta-analysis of the published literature. Cancer. 2020;126(Suppl 16):3837–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1. Figure S1. Trend in utilization for neoadjuvant chemotherapy in TNBC patients between 2010 and 2019:overall;different stages
Data Availability Statement
No datasets were generated or analysed during the current study.