Abstract
Background
The necessity of post procedural prophylactic antibiotics following clean surgeries is controversial. While most evidence suggests that there is no benefit from these additional antibiotics and guidelines do not support their use, there is a paucity of evidence as to how often they are still being used and their impact on infection outcomes. The current study assessed the use of prophylactic antibiotics following cardiac implantable electronic device (CIED) implantations in the province of Alberta, and their impact on infection and mortality.
Methods
We conducted a population-based cohort study in the province of Alberta. Administrative data was used to link all patients ≥ 18 who underwent outpatient CIED implantation from January 1, 2011 through December 31, 2019 to antibiotics commonly used for surgical prophylaxis which were prescribed within 48 h of implantation. The primary outcome, explored with an adjusted Poisson model, was incidence of complex surgical site infection within one year of device implantation. All-cause mortality was a secondary outcome.
Results
Post implantation prophylactic antibiotics were used 41% of the time overall, though the rate has been decreasing over time. The most commonly used prophylactic antibiotic was cefalexin (52%). When adjusted analyses were completed, there was no difference in the outcome of infection between those who did and did not receive post implantation prophylactic antibiotics (Relative Risk 0.74, 95% CI 0.46–1.17) and there was no difference in mortality (Relative Risk 0.8, 95% CI 0.63–1.02).
Conclusions
The use of prophylactic antibiotics following CIED implantation does not correlate to a reduced rate of complex surgical site infection or reduced mortality. The widespread use of these antibiotics, which is not guideline concordant, suggests the need for targeted antimicrobial stewardship interventions for surgical prophylaxis to ensure that antibiotic use is being optimized. Further work should explore other adverse outcomes associated with this antibiotic usage and stewardship programs should explore interventions to educate and reduce antibiotic use for this indication.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01512-3.
Keywords: Antibiotic Prophylaxis, CIED infections, Surgical Site infections, Antimicrobial stewardship
Introduction
The use of postoperative prophylactic antibiotics following clean surgical procedures has been a controversial issue for many years. When medical devices are implanted, such as in cardiac and orthopedic procedures, the balance between uncertain benefit versus relative harms of postoperative prophylactic antibiotics becomes an even more important consideration due to difficulty eradicating infections associated with prosthetic material [1]. Surgical site infections (SSIs) associated with hardware and prosthetic materials often require repeated surgeries to remove materials, prolonged courses of antibiotics and repeat hospital admissions [2].
The rate of cardiac implantable electronic device (CIED) infections ranges from approximately 1 to 4% [2, 3]. The most common causative organisms are Staphylococcus aureus and coagulase negative Staphylococci [4, 5]. These pathogens have a propensity to form a biofilm on prosthetic material generally requiring removal of the entire CIED in order to achieve cure [6]. These infections reduce patient quality of life and are very costly to the healthcare system [7, 8]. While prevention of infection is important, there is limited evidence to suggest that prophylactic antibiotics following CIED implantation are linked to improved outcomes. The Prevention of Arrhythmia Device Infection Trial (PADIT) was a randomized controlled trial that compared conventional (i.e., pre-operative antibiotics alone) to incremental (i.e., addition of intraoperative antibiotic washes plus post-operative oral cefalexin for 2 days) periprocedural antibiotics for CIED implantations [9]. Notably, there was no difference between groups in the 1 year rates for device infection [9].
While guidelines do not recommend the routine use of post-CIED implantation antibiotics as a method to prevent infection, evidence suggests antibiotics are often being inappropriately used following surgery [10, 11]. However, antibiotic use is not benign and is associated with increased risk of adverse events including Clostridioides difficile infection, antibiotic -associated diarrhea, drug allergy and perhaps most concerningly selection of antimicrobial resistance in the etiologic organism, rendering infections more difficult to treat and creating substantial patient morbidity and economic burden [12–14]. The objective of the current study was to explore the association between post CIED procedure prophylactic antibiotics outcomes, including SSI and all-cause mortality, in the province of Alberta, Canada. This will help to inform optimal antimicrobial stewardship strategies in this area.
Methods
Study design and patient cohort
This was a population-based cohort study conducted in Alberta, Canada, a province of approximately 4.4 million people with a single healthcare system, Alberta Health Services (AHS). Using linked administrative data, we identified a cohort of adults (i.e., age ≥ 18 years) who underwent surgery for CIED implantation (including pacemaker (PM), implantable cardioverter defibrillator (ICD), or cardiac resynchronization therapy (CRT)) in an outpatient day surgery setting between January 1, 2011, and December 31, 2019. We excluded any adults who underwent CIED implantation within the inpatient setting or required admission (> 48 h) from their initial outpatient day surgical encounter. The rationale for this exclusion criteria was that prolonged admission following implantation likely had a more complex admission and it would have been difficult to determine if any antibiotics given were for prophylaxis or treatment of an active infection.
Patients were categorized based on whether or not prophylactic antibiotics were prescribed post procedure, and followed for 1 year to ascertain outcomes of all-cause mortality and complex CIED SSI.
Administrative data sources
Paceart™
CIED implantations were identified using the Paceart™ database which contains all device-related clinical encounters for patients followed within the province of Alberta, Canada. Paceart™ contains information regarding indications for device implantation, type of device, date of operation and basic demographic information including sex. To avoid double counting patients that may have multiple revisions in the setting of operative complications, repeat procedures within a two-year period from the index surgical date were censored.
AHS analytics
Index CIED implantation surgeries, identified using the PaceArt database, were linked to administrative data housed within AHS Data & Analytics, including Discharge Abstract Database (DAD) for inpatient and outpatient hospitalization visits, the National Ambulatory Care Reporting System (NACRS) for ambulatory visits including emergency department encounters and the Pharmaceutical Information Network (PIN) for dispensed medications [15]. PIN is a central repository of prescription drugs dispensed from community pharmacies and is inclusive of all drugs regardless of supplementary drug plan coverage; in-hospital dispensations are not included. Currently, at least 95% of all community pharmacies in Alberta submit records to this database. The data is patient identifiable and includes data elements such as patient identification data, drug name, drug identification number, drug dose, quantity dispensed, and number of days supplied [15]. All-cause mortality was ascertained from vital statistics. Elixhauser comorbidities of included patients were obtained from DAD and NACRS using a 2-year look back window from index date [16]. Additionally, specific comorbidities validated using cardiac registry data were looked at individually [17].
Prophylactic antibiotic use
Prophylactic antibiotics were defined as any oral antibiotic that was prescribed for > 1 day, and tracked within PIN within 48 h of CIED implantation. These antibiotics included cefalexin (also commonly known as cephalexin), amoxicillin, clindamycin, amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, doxycycline, ampicillin, penicillin, cefadroxil, and cloxacillin.
Outcomes
The primary outcome was presence of a complex SSI following CIED implantation identified using Discharge Abstract Database (DAD) and International Classification of Disease 10th revision Canada codes (T827, T857, I330, I339, I38, I398, L0330, L0339, L038, L039) that were previously validated as able to identify complex CIED SSIs (i.e. deep and organ space but not superficial SSIs) with sensitivity and specificity > 90% [18]. All SSIs that occurred within one year from the index date of CIED implantation were tracked. Secondary outcomes included one year all-cause mortality also stratified by whether prophylactic antibiotics were used.
Statistical analyses
Descriptive statistics, including frequencies and percentages were used to describe baseline characteristics of patients with and without SSIs and with and without usage of antibiotics within 48 h. For our primary method, we assessed the relationship between prophylactic antibiotic prescription on the rate of SSI for the total cohort using multivariable generalized linear models with a Poisson distribution and a logit/log link function. Adjusted models included the covariates of age, sex, location of CIED implantation, type of CIED device, and medical comorbidities. All statistical analyses were performed using R Statistical Software (Version 1.4.0). This research was approved by the University of Calgary Health Research Ethics Board (REB20-2186).
Results
Baseline cohort characteristics
There were 14,718 CIED implantations occurring in the outpatient setting that met inclusion criteria. Of these 14,718 surgeries, 69% (n = 10,148) were for pacemakers, 17% (n = 2,525) for implantable cardioverter defibrillators, and 13% (n = 1,962) for cardiac resynchronization therapy (Table 1). The average age of the cohort was 73.6 (standard deviation 13.4), 36% were female, and 79% had 2 or greater Elixhauser comorbidities. The most common comorbidities included hypertension (56%), heart failure (40%), and diabetes (26%).
Table 1.
Baseline characteristics for entire cohort stratified by those who did and did not receive prophylactic antibiotics within 48 h of CIED implantation
| Factors | ABX within 48 h N = 5978 |
Non-ABX within 48 h N = 8740 |
All N = 14,718 |
|---|---|---|---|
| Age | |||
| 18–29 | 72 (1.2) | 57 (0.65) | 129 (0.88) |
| 30–39 | 120 (2.01) | 90 (1.03) | 210 (1.43) |
| 40–49 | 218 (3.65) | 220 (2.52) | 438 (2.98) |
| 50–59 | 637 (10.66) | 734 (8.4) | 1371 (9.32) |
| 60–69 | 1275 (21.33) | 1679 (19.21) | 2954 (20.07) |
| 70–79 | 1747 (29.22) | 2469 (28.25) | 4216 (28.65) |
| 80+ | 1909 (31.93) | 3491 (39.94) | 5400 (36.69) |
| Sex | |||
| Female | 2071 (34.64) | 3283 (37.56) | 5354 (36.38) |
| Male | 3889 (65.06) | 5440 (62.24) | 9329 (63.38) |
| Unknown | 18 (0.3) | 17 (0.19) | 35 (0.24) |
| Device Type | |||
| PM | 3798 (63.53) | 6350 (72.65) | 10,148 (68.95) |
| CRT | 1144 (19.14) | 818 (9.36) | 1962 (13.33) |
| ICD | 1023 (17.11) | 1502 (17.19) | 2525 (17.16) |
| LPM | 0 (0) | 43 (0.49) | 43 (0.29) |
| S-ICD | 13 (0.22) | 27 (0.31) | 40 (0.27) |
| Generator Replacement | 2281 (38.16) | 3157 (36.12) | 5438 (36.95) |
| Site/Location of CIED Care | |||
| Site 1 | 575 (9.62) | 4538 (51.92) | 5113 (34.74) |
| Site 2 | 5126 (85.75) | 2708 (30.98) | 7834 (53.23) |
| Site 3 | 149 (2.49) | 159 (1.82) | 308 (2.09) |
| Site 4 | 65 (1.09) | 855 (9.78) | 920 (6.25) |
| Site 5 | 61 (1.02) | 478 (5.47) | 539 (3.66) |
| Number of Elixhauser Comorbidities | |||
| 0–1 | 1531 (25.61) | 1576 (18.03) | 3107 (21.11) |
| 2–3 | 2216 (37.07) | 3138 (35.9) | 5354 (36.38) |
| 4–5 | 1308 (21.88) | 2109 (24.13) | 3417 (23.22) |
| 6+ | 923 (15.44) | 1917 (21.93) | 2840 (19.3) |
| Cardiac Comorbidities | |||
| Cerebrovascular Diseases | 433 (7.24) | 931 (10.65) | 1364 (9.27) |
| Chronic Obstructive Pulmonary Disease | 996 (16.66) | 1524 (17.44) | 2520 (17.12) |
| Congestive Heart Failure | 2335 (39.06) | 3568 (40.82) | 5903 (40.11) |
| Peripheral Vascular Disease | 298 (4.98) | 642 (7.35) | 940 (6.39) |
| Liver Disease | 183 (3.06) | 371 (4.24) | 554 (3.76) |
| Renal Disease | 494 (8.26) | 876 (10.02) | 1370 (9.31) |
| Malignancy | 399 (6.67) | 789 (9.03) | 1188 (8.07) |
| Hypertension | 3035 (50.77) | 5217 (59.69) | 8252 (56.07) |
| Hyperlipidemia | 648 (10.84) | 744 (8.51) | 1392 (9.46) |
| Diabetes | 1484 (24.82) | 2321 (26.56) | 3805 (25.85) |
| Myocardial Infarction | 724 (12.11) | 820 (9.38) | 1544 (10.49) |
| Coronary Artery Bypass Grafting | 462 (7.73) | 537 (6.14) | 999 (6.79) |
| Percutaneous Coronary Intervention | 479 (8.01) | 633 (7.24) | 1112 (7.56) |
Prophylactic antibiotic use
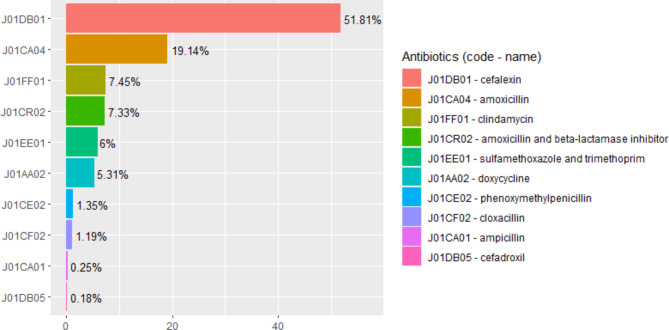
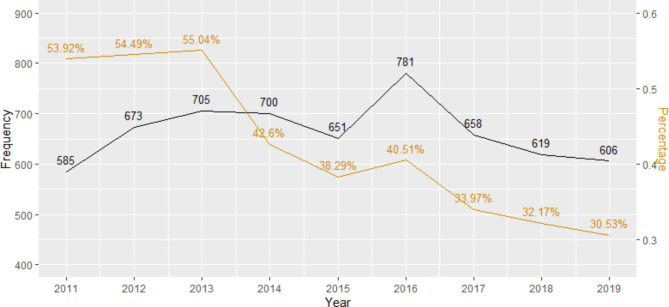
Of the 14,718 CIED implantations, 5,978 (41%) had a prophylactic antibiotic prescription that was dispensed within 48 h of CIED implantation. The most prescribed antibiotic for prophylaxis was cefalexin at 52% (Fig. 1), which was prescribed for an average of 7 days (standard deviation 5.4, see Table 2). The percentage of prophylactic antibiotics prescribed annually following CIED implantation has been decreasing with 54% of patients with CIED implantation receiving them in 2011 and 31% receiving them in 2019 (Fig. 2).
Fig. 1.
Relative percentages of prophylactic antibiotics prescribed within 48 h of CIED implantation
Table 2.
Average duration of antibiotics (days prescribed) with standard deviation
| Antibiotic | Mean days of therapy prescribed | Standard deviation |
|---|---|---|
| Cefalexin | 7 | 5.4 |
| Amoxicillin | 6 | 6.1 |
| Clindamycin | 6 | 3.5 |
| Amoxicillin-clavulante | 9 | 8.5 |
| Sulfamethoxazole-Trimethoprim | 21 | 25.4 |
| Doxycycline | 14 | 17.0 |
| Penicillin | 15 | 21.0 |
| Cloxacillin | 9 | 4.0 |
| Ampicillin | 6 | 6.9 |
| Cefadroxil | 13 | 8.2 |
Fig. 2.
The frequency and percentage of prophylactic antibiotics prescribed within 48 h after CIED implantation by year
Clinical endpoints
Of the 5,978 CIED implantations associated with prophylactic antibiotics within 48 h post CIED implantation, 0.8% (n = 46) developed a complex SSI at 1 year. In the group that did not receive post procedural prophylactic antibiotics within 48 h, 1.1% (n = 99) developed a complex SSI (p < 0.05). One year mortality in the group that received prophylactic antibiotics was 2.0% (n = 122). In the group that did not receive prophylactic antibiotics one year all-cause mortality was 3.4% (n = 293) (p < 0.05).
Relationship between prophylactic antibiotic prescription and clinical endpoints
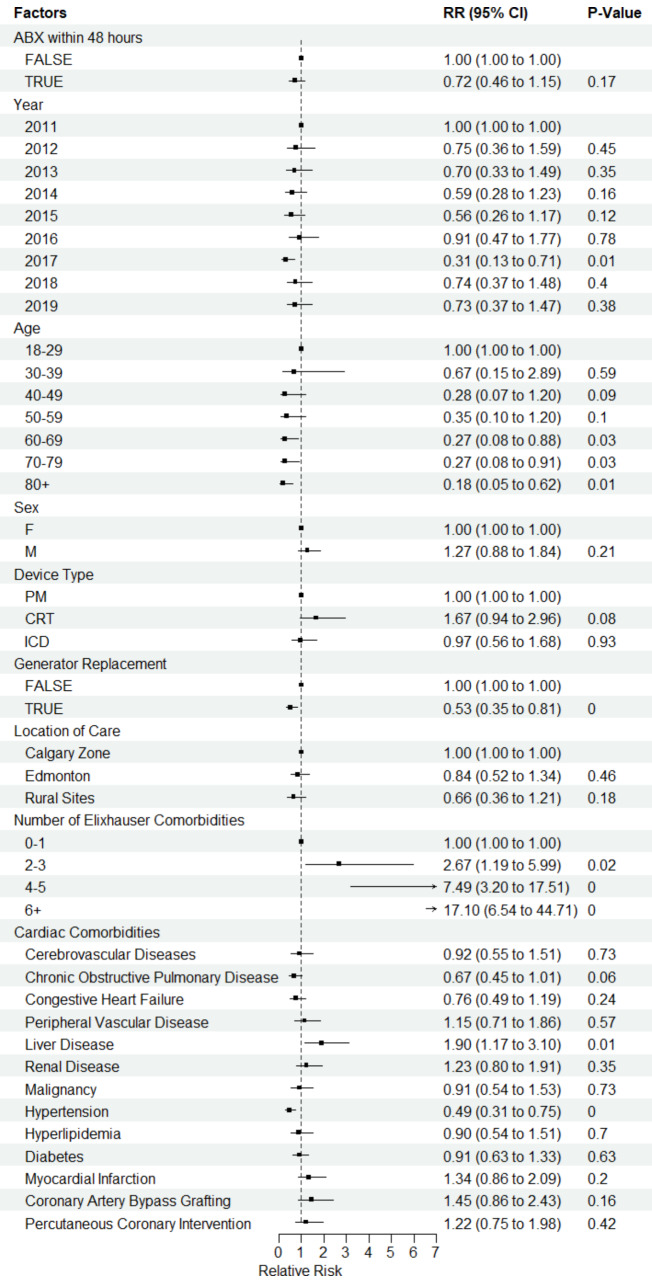
Two tables demonstrating the results of the univariable and multivariable analyses can be found in additional file 1. In the adjusted multivariable models, there was no difference in SSI rates between the group that received prophylactic antibiotics compared to the group that did not receive antibiotics (relative risk (RR) 0.74, 95% confidence interval (CI) 0.46–1.17, p = 0.20) (Fig. 3). In the adjusted model for all-cause mortality, there was no significant difference in mortality between those who did and did not receive antibiotics (RR 0.80, CI 0.63–1.02, p = 0.07). It was noted that trimethoprim-sulfamethoxazole was prescribed for a mean duration longer than anticipated for post procedural prophylaxis. Therefore, the adjusted analyses were also completed excluding this antibiotic as a sensitivity analyses. However, this did not impact the clinical outcomes or p values as it accounted only for a small percentage of prescribed antibiotics.
Fig. 3.
Forest Plot demonstrating the relative risk of infection using an adjusted Poisson model with location of care as a fixed factor
Discussion
This work demonstrated that approximately 40% of patients received postoperative antibiotic prophylaxis following CIED implantation between 2011 and 2019. The most common antibiotic used for prophylaxis was cefalexin. Antibiotic prophylaxis was neither associated with a lower risk of complex SSI nor all-cause mortality at 1 year, after adjusting for medical comorbidities, device type, or implanting site. Although absolute rates of antibiotic prophylaxis are substantial, the rate of prescribing has been decreased overall since 2011. Reasons for the decreased use of post-operative antibiotic prophylaxis is unclear and there were no widely accepted guidelines published during the study period that would have accounted for the change in trajectory in antibiotic use. The results of the PADIT trial were published in 2018 demonstrating that an enhanced antibiotic strategy including post procedural use did not reduce the risk of CIED infections significantly [9]. The decreasing use of antibiotic prophylaxis in our work was observed well before the completion of PADIT.
Our findings are in keeping with prior studies describing the use of prophylactic post-operative antibiotics [10]. The WRAP-IT (Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial) trial assessing the efficacy of an intraoperative antimicrobial envelope at time of CIED implantation, found that 30% of patients in both trial arms received post-operative antibiotics [3]. Although not specific to CIED implantation surgeries, a global point prevalence survey including 53 countries found that prophylaxis post operatively (prescribed for > 1 day) was very common in all regions ranging from 40.6 to 86.3%.19 However, they found that sulfamethoxazole-trimethoprim was the most commonly prescribed antibiotic globally at 63.4% compared to our finding of cefalexin being used the most frequently [19]. This is potentially due to greater rates of methicillin resistant Staphylococcus aureus colonization globally compared to Alberta.
A notable finding of our study is that post-operative antibiotic prophylaxis did not reduce the risk of developing a complex CIED infection at 1 year. This finding is despite probable selection bias as to who received prophylactic antibiotics. That is, implanting operators may prescribe antibiotics due to perceived increased risk of developing infection, which may be related to length of CIED surgery and patient comorbidities. Indeed, higher comorbidity burden was one of the strongest predictors of increased risk of infection. For those with greater than 6 comorbidities, there was a 17-fold increased risk of developing a complex CIED SSI at 1 year. Validated risk scores, which incorporate patient risks (such as comorbidities) and operative factors (such as redo intervention and device type), can help identify patients at greatest risk of post-operative infection complications and provide opportunities for implementation of evidence-based infection prevention strategies, such as intraoperative antibiotic envelopes [20, 21] or use of novel device technologies associated with lower risk of infection, such as leadless pacemakers or non-transvenous defibrillators [21].
Nevertheless, our study suggests that post-operative antibiotics are not one of these evidence-based strategies that prevent CIED infection. Our findings are in keeping with prior work comparing use of pre-procedural antibiotics alone to a strategy of incremental antibiotics consisting of added intraoperative antibiotic washes and post-operative antibiotics. In the Prevention of Arrhythmia Device Infection (PADIT) study of 19,603 patients undergoing CIED implantation, there was no difference in the group treated with peri-procedural antibiotics alone compared to those treated with the incremental antibiotic strategy [9].
Finally, previous work done by our group in Alberta has demonstrated that overall annual complex SSI rates following CIED implantation have decreased from 2011 to 2019 [22]. The current work has shown a reduction in prophylactic antibiotic prescribing over this same time frame further underscoring the lack of relationship between prophylactic antibiotics and SSI rates. Consistent with our prior work as well as the PADIT trial, our multivariable analysis did highlight that older age had a lower relative risk of infection [9, 22]. The exact explanation for this is unclear but reduced immune response with age has been postulated as one mechanism [23]. We also found that generator replacement was associated with lower risk of infection. This is also concordant with our previous work and may be related to the shorter procedural time for generator replacement as well as enhanced vigilance due to the risks with a revision procedure [22].
These findings highlight the importance of antimicrobial stewardship programs targeted at surgery, specifically postoperative prophylaxis. Prior work has shown that even when guidelines and protocols exist around postoperative prophylaxis there is poor adherence [24]. However, when antibiotic stewardship programs are implemented for surgery specific indications antibiotic appropriateness improves demonstrating the success of such programs [24]. One study found that implementation of an antibiotic stewardship program targeting perioperative surgical prophylaxis resulted in significant improvements (p < 0.001) in appropriate antibiotic prescribing, including for indication, antibiotic selection and dosing, timing and duration [25].
Strengths of this work include that it was population based and a large dataset over an eight-year period, and that we had access to outpatient antibiotic prescriptions for the entire population as frequently this data is only available in select populations based on age.
We recognize limitations in our findings. Antibiotics were presumed to be prophylactic based on choice of class and duration, from expert opinion. However, no chart review was performed to confirm this as this work was based on administrative data. However, these antibiotics were all started within 48 h of CIED implantation which would be too early for infection and the choices are ones commonly associated with postoperative prophylaxis. Additionally, our data did not contain information on adverse antimicrobial associated events such as allergic reaction or antibiotic associated diarrhea and so these outcomes could not be assessed. Finally, we did not have data on the use of intraoperative antibiotic impregnated envelopes which may be utilized in certain patient populations. However, the data on the effectiveness of these envelopes was not published until 20193 and therefore less likely to have impact the findings of the current study.
Conclusion
Our findings are important as they highlight that despite lack of evidence that they reduce SSIs, antibiotics are still commonly being used for postoperative prophylaxis. While their use does appear to be decreasing with time, over a quarter of patients still receive them post CIED implantation. Further work should endeavor to look at all adverse antibiotic related outcomes, and antimicrobial stewardship programs should consider exploring and targeting of postoperative antibiotic prophylaxis, not only in CIED procedures but in all clean surgical procedures. Eliminating unnecessary post procedural prophylaxis has the potential to improve optimization of antibiotic utilization, and importantly, from a global and population perspective, reduce selection pressure on the development of antibiotic resistance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge Alberta Health Services Analytics for their support in providing data.
Abbreviations
- CIED
Cardiac Implantable Electronic Devices
- PM
Pacemaker
- ICD
Implantable Cardioverter Defibrillator
- CRT
Cardiac Resynchronization Therapy
- SSI
Surgical Site Infection
- AHS
Alberta Health Services
- DAD
Discharge Abstract Database
- NACRS
National Ambulatory Care Reporting System
- RR
Relative Risk
- CI
Confidence Interval
Author contributions
The original project was conceived by authors ER, DC and JL. Authors JL, DC, and ER completed data collection and validation. All statistical analyses were completed by author ZZ. ER completed the manuscript with input and revision from authors DC, SS, JC, DE, VK, JL, IR and ZZ.
Funding
The authors received no funding to support this work.
Data availability
The data that support the findings of this study are available from Alberta Health Services but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Alberta Health Services and appropriate ethics.
Declarations
Ethics approval
This study was approved by the University of Calgary Health Research Ethics Board (REB20-2186).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Vavasseur B, Zeller V. Antibiotic therapy for prosthetic joint infections: an overview. Antibiotics. 2022;11(4):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rennert-May E, Chew D, Lu S, Chu A, Kuriachan V, Somayaji R. Epidemiology of cardiac implantable electronic device infections in the United States: a population-based cohort study. Heart Rhythm. 2020;17(7):1125–31. [DOI] [PubMed] [Google Scholar]
- 3.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole J, Stromberg K, et al. Worldwide randomized antibiotic enveloPe infection prevenTion trial (WRAP-IT). Am Heart J. 2016;180:12–21. [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga M, Goya M, Nagashima M, Hiroshima K, Yamada T, An Y, et al. Identification of causative organism in cardiac implantable electronic device infections. J Cardiol. 2017;70(5):411–5. [DOI] [PubMed] [Google Scholar]
- 5.King T, Chew DS, Leal J, Cannon K, Zhang Z, Rennert-May E. Microbiology of cardiac implantable electronic device infections in Calgary, Canada. Antimicrob Steward Healthc Epidemiol. 2024;4(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddour LM, Esquer Garrigos Z, Rizwan Sohail M, Havers-Bordersen E, Krahn A, Chu V, et al. Update on cardiovascular implantable electronic device infections and their prevention, diagnosis, and management: a scientific statement from the American Heart Association. Circulation. 2024;149(2):e201–16. [DOI] [PubMed] [Google Scholar]
- 7.Rennert-May E, Chew D, Cannon K, Zhang Z, Smith S, King T, et al. The economic burden of cardiac implantable electronic device infections in Alberta, Canada: a population-based study using validated administrative data. Antimicrob Resist Infect Control. 2023;12(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneman N, Homenauth E, Saskin R, Ng R, Ha A, Wijeysundera HC. The predictors and economic burden of early-, mid- and late-onset cardiac implantable electronic device infections: a retrospective cohort study in Ontario, Canada. Clin Microbiol Infect. 2020;26:55. [DOI] [PubMed] [Google Scholar]
- 9.Krahn AD, Longtin Y, Philippon F, Birnie D, Manlucu J, Angaran P, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018;72(24):3098–109. [DOI] [PubMed] [Google Scholar]
- 10.Lee C, Walker SA, Daneman N, Elligsen M, Palmay L, Coburn B, et al. Point prevalence survey of antimicrobial utilization in a Canadian tertiary-care teaching hospital. J Epidemiol Glob Health. 2015;5(2):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourbeti I, Kamiliou A, Samarkos M. Antibiotic stewardship in surgical departments. Antibiotics. 2024;13(4). 10.3390/antibiotics13040329. [DOI] [PMC free article] [PubMed]
- 12.Naylor NR, Atun R, Zhu N, Kulasabanathan K, Silva S, Chatterjee A, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafey A, Jahan S, Farooq U, Akhtarm F, Irshad M, Nizamuddin, et al. Antibiotics associated with Clostridium difficile infection. Cureus. 2023;15(5):e39029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourdan A, Sangha B, Kim E, Nawaz S, Malik V, Vij R, et al. Antibiotic hypersensitivity and adverse reactions: management and implications in clinical practice. Allergy Asthma Clin Immunol. 2020;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Government of Alberta. Overview of administrative health datasets. 2017. https://open.alberta.ca/dataset/overview-of-administrative-health-datasets [Accessed Nov 29 2024].
- 16.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 17.Southern DA, Norris CM, Quan H, ShriveF, Galbraith D, Humphries K, et al. An administrative data merging solution for dealing with missing data in a clinical registry: adaptation from ICD-9 to ICD-10. BMC Med Res Methodol. 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennert-May E, Leal J, MacDonald M, Cannon K, Smith S, Exner D, et al. Validating administrative data to identify surgical site infections following cardiac implantable electronic device implantation: a comparison of traditional methods and machine learning. Antimicrob Resist Infect Contrrol. 2022;11(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versporten A, Zarb P, Caniaux I, Gros M, Drapier N, Miller M, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Global Health. 2018;6(6):e619–29. [DOI] [PubMed] [Google Scholar]
- 20.Rennert-May E, Raj SR, Leal J, Exner DV, Manns BJ, Chew DS. Economic evaluation of an absorbable antibiotic envelope for prevention of cardiac implantable electronic device infection. Europace. 2021;23(5):767–74. [DOI] [PubMed] [Google Scholar]
- 21.Phillips P, Krahn AD, Andrade JG, Chakrabarti S, Thompson C, Harris D, et al. Treatment and prevention of cardiovascular implantable electronic device (CIED) infections. CJC Open. 2022;4(11):946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King T, Chew D, Leal J, Cannon K, Exner D, Smith S, et al. Complex cardiac implantable electronic device infections in Alberta, Canada: an epidemiologic cohort study of validated administrative data. Infect Control Hosp Epidemiol. 2023;44(10):1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32(8):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menz BD, Charani E, Gordon DL, Leather AJM, Moonesinghe SR, Phillips CJ. Surgical antibiotic prophylaxis in an era of antibiotic resistance: common resistant bacteria and wider considerations for practice. Infect Drug Resist. 2021;14:5235–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segala FV, Murri R, Taddei E, Giovannenze F, De Vecchio P, Birocchi E, et al. Antibiotic appropriateness and adherence to local guidelines in perioperative prophylaxis: results from an antimicrobial stewardship intervention. Antimicrob Resist Infect Control. 2020;9(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Alberta Health Services but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Alberta Health Services and appropriate ethics.