Abstract
Background
The extraordinary Galapagos Islands, with an impressive number of endemic and native species, maintain the interest and curiosity for researchers from all over the world. The native species are known to be vulnerable to new pathogens, cointroduced with their invasive hosts. In the case of invasive parasitic arthropods, their evolutionary success is related to the association with other invasive hosts (such as domestic animals). These associations could become a significant driver of change, as occasionally they can seek another hosts and have the capacity to transmit pathogens between domestic and wild animals. The current study aims to identify the distribution and abundance of canine vector-borne parasites in the Galapagos Islands based on the possibility that some of them could spill over to endemic mammals.
Methods
A total of 1221 blood samples were randomly collected from privately owned dogs on San Cristóbal, Isabela, Santa Cruz, and Floreana Islands during the years 2021 and 2022. All samples were examined for vector-borne pathogens using the modified Knott’s test and conventional, nested, and multiplex polymerase chain reactions (PCRs), followed by sequencing.
Results
The PCR and Knott tests confirmed the presence of Dirofilaria immitis (2%, 25/1221) in all islands. While molecular analyses showed heartworm DNA only in dogs from the San Cristóbal (3.3%) and Isabela (2.4%) Islands. Moreover, other pathogens Babesia vogeli (3%, 37/1221) and Hepatozoon canis (0.2%, 2/1221) were detected for the first time by molecular analyses.
Conclusions
Dogs from Galapagos are hosts to various pathogens, of which some are potentially zoonotic while some other could be spill-over to endemic endangered carnivores, such as sea lions. To understand and limit their impact, long-term surveillance, control, and awareness is needed.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06592-z.
Keywords: Domestic dogs, Heartworm, Molecular biology, Invasive species, Endemic species
Background
The Galapagos Islands have an impressive number of endemic species and maintained the interest of researchers from all over the world, being also named by UNESCO “a living museum and showcase of evolution.” Therefore, this archipelago is one of the world’s most strictly protected natural areas, as it has many threatened and endangered species [1, 2]. Despite their remoteness, invasive species have been introduced intentionally or unintentionally to the islands throughout the time. Native species are known to be vulnerable to new pathogens cointroduced with their invasive hosts [2, 3]. For instance, invasive insects constitute 23% of the total insect species [4] in the archipelago.
In the case of invasive parasitic arthropods, their evolutionary success is related not only to abiotic factors but also to the association with other invasive hosts, such as domestic animals [2, 5]. These associations could become a significant driver of change, as occasionally they can switch hosts and have the capacity to transmit pathogens between domestic and wild animals [6]. It is hard to determine when the first invasive parasites were introduced to Galapagos [2]. However, over time, vector-borne disease transmission by introduced or indigenous arthropods in which domestic dogs (i.e., could be hosts to various pathogens) can have negative consequences for various native and endemic species, disrupting the cycle of entire ecosystems [2, 6–8].
Eleven species of hard ticks are present in the Galapagos, three which are introduced and eight are endemic [9–11]. In the case of fleas, there are no data on their diversity, distribution, or hosts. The only flea in Galapagos, Parapsyllus cedei, was recorded in nests of seabirds in Genovesa and Santa Cruz and is considered to be native [12–14]. Mosquitoes (Culicidae) represent another important group of vectors in this fragile ecosystem. Three species of mosquitoes are known in Galapagos: two of them (Aedes aegypti and Culex quinquefasciatus) are introduced and one (Aedes taeniorhynchus) is considered to be native [15–19].
Marine endemic mammals of Galapagos, the Galapagos fur seal (Arctocephalus galapagoensis) and Galapagos sea lion (Zalophus wollebaeki), were isolated from many of the mainland carnivore-associated pathogens due to their remote geographic position [20]. Generally, due to coevolution, native parasites and microbes are not pathogenic to their hosts; however, for introduced pathogens, native species lack an immune response [2, 21]. Few studies are available on the impact of multihost pathogens on endemic fauna, although the same threats can affect native species and humans in different ways. The current study aims to identify the distribution and abundance of canine vector-borne parasites in the Galapagos Islands based on the possibility that some of them could spill over to endemic mammals. Genetic characterization aimed to identify the pathogens that are present in the Galapagos Islands and could have an impact on resident fauna.
Methods
Sample collection
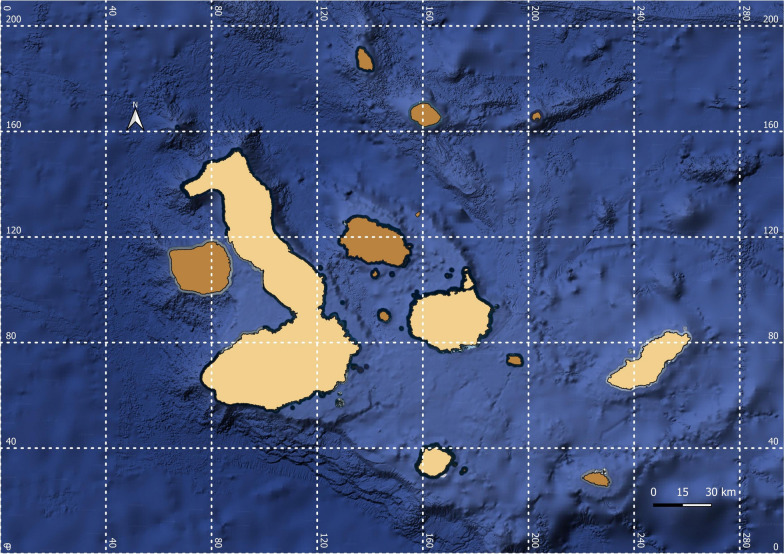
The sampling was conducted over a period of 2 years, between July and September 2021 (San Cristóbal Island) and between July and August 2022 (Isabela, Santa Cruz, and Floreana Islands), corresponding in both cases to the dry season. A total of 1221 dogs (652 males and 569 females) from four human-inhabited islands were examined for vector-borne parasites (Fig. 1 and Table 1).
Fig. 1.
Islands from where samples were collected (San Cristóbal, Isabela, Santa Cruz, and Floreana), in light brown
Table 1.
Distribution of sampled dogs according to the location and category
| Category | Isabela | Floreana | Santa Cruz | San Cristóbal | Total |
|---|---|---|---|---|---|
| Owned dogs | 168 | 22 | 341 | 603 | 1134 |
| Animal shelters | 0 | 0 | 16 | 0 | 16 |
| Veterinary clinics | 44 | 0 | 27 | 0 | 71 |
| Urban | 158 | 17 | 315 | 547 | 1037 |
| Rural | 54 | 5 | 69 | 56 | 184 |
| Total | 212 | 22 | 384 | 603 | 1221 |
The blood samples were collected from the cephalic vein of the foreleg or the saphenous vein of each dog using S-Monovette an EDTA K2E system (SARSTEDT AG and Co., Germany). All blood samples were stored at 4 °C until further analysis but for no longer than 12 h. In parallel, 0.5 mL of whole blood was taken, mixed with 96% ethanol, and was stored at −20 °C until further molecular processing.
Blood examination
All blood samples collected in Isabela, Santa Cruz, and Floreana Islands were analyzed for the presence of microfilariae in circulating blood using the modified Knott’s test [22–26], while the results of the Knott’s test from San Cristóbal Island are already published [26].
DNA extraction and PCR amplification
The DNA was extracted from the ethanol preserved blood using the DNeasy Blood and Tissue Kit (Qiagen, Germany), according to the manufacturer’s instructions. The DNA concentration and purity (260/280 ratio) were assessed in duplicates using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE) on 5% of randomly selected samples.
Conventional polymerase chain reaction (PCR) protocols were used to assess genomic DNA of vector-borne pathogens in blood samples using the techniques described in Supplementary File 1. The DNA extracted from blood samples was processed by multiplex PCR (mPCR) targeting several filaroid species that infect dogs (D. immitis, 170 bp; D. repens, 480 bp; and A. reconditum, 590 bp; [27]). For improved specificity, the positive samples obtained from the mPCR assay were further tested using NTF/NTR primers combination, following the techniques outlined in [28]. This molecular test amplifies a partial sequence of the cytochrome c oxidase subunit 1 (cox1) gene of spirurid nematodes, generating products with longer sequences. To assess the possible presence of other filariae that infect dogs, a partial fragment of the ITS2 region was amplified. The amplification procedure was conducted using conventional PCR with pan-filarial primers (DIDR-F1/DIDR-R1) described by ref. [29].
To detect blood Apicomplexan hemoparasites (Babesia spp., Theileria spp., and Hepatozoon spp.), a nested PCR protocol was used for initial screening with the primers BTH-1F and BTH-1R and GF2 and GR2 [30–32]. All positive or doubtful samples were subsequently screened by specific nested PCR assays targeting the 18S rDNA of Babesia spp. [33] and the mitochondrial cytochrome b (cytb) and cox1 genes of Babesia spp. [34] and Hepatozoon spp. [35] (Supplementary File 1).
For each reaction, a positive control with DNA and a negative control without DNA were added to evaluate the reaction’s specificity and determine the presence of any contaminants. Positive control consisted of DNA that had been confirmed to be positive for the targeted pathogens. Thus, the positive controls for Dirofilaria spp. genes are available in GenBank under the following accession numbers: KT716014 for D. immitis, and MW656250, and MW656251 for Dirofilaria repens. Furthermore, for Babesia spp., the positive control accession numbers are MW939359 (18S rDNA), MW938761 (cytb gene), and MW938763 (cox1 gene). The positive control for Hepatozoon spp. (18S) was derived from Hepatozoon sp. obtained from a spleen sample of a wild cat in Romania. Although this sequence has not been submitted to GenBank, it is 100% similar to accession number OM256568, with 99% query coverage. PCR products were visualized on 1.5% agarose gels using ECO Safe Nucleic Acid Staining Solution (PacificImage Electronics, New Taipei City, Taiwan).
The amplicons obtained were purified with the Gel/PCR DNA Fragments Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan) according to the manufacturer’s instructions and sequenced bidirectionally (Macrogen Europe, Amsterdam, the Netherlands). Subsequently, all sequences were subjected to analyses and edited using Geneious® 4.85 software [36]. The sequences underwent comparison with those present in GenBank™ using the local Basic Local Alignment Search Tool (BLASTn) analyses.
Definition of positive results
For the epidemiological statistical analysis, the final status for the D. immitis infection was determined based on the outcomes of microscopic (Knott’s test) and molecular testing performed for common filarial nematodes. The molecular processing involved both mPCR and conventional PCR to obtain a comprehensive profile of the common filarial nematodes (panfilarial—nine species could be detected). We retested positive amplicons from the mPCR for sequencing using the mitochondrial cox1 gene. Therefore, the Knott’s test defined the positive results for D. immitis as the presence of microfilaria in the circulating blood under microscopic examination, even if the PCR results were negative. Similarly, samples that tested positive in one, two, or all three molecular tests without microscopic detection of microfilaria were considered positive results.
The positive results for Babesia vogeli were determined by either two positive molecular tests out of the three species-specific tests or by one test with a positive result that was confirmed by sequencing. Regarding the positive Hepatozoon canis result, it was concluded by the use of a specific molecular target gene, followed by sequencing. The positive molecular results indicate dogs that tested positive for the target pathogens.
Statistical analysis
Statistical analysis was performed using RStudio 2023.09.1. The prevalence values were compared through the Fisher’s exact test on the islands where positive animals were found and the free roam condition on each of these islands. A generalized linear model (GLM) from the stats “R package” was applied to determine if the variables: housing, island, location, sex, and free roam are correlated with the positivity in dogs. Odds ratios (ORs), 95% confidence intervals (CI), and P values were calculated for epidemiological statistical analysis. When the value of OR is equal to 1, it indicates no association between the exposure and the outcome. Statistical significance was established by a P value of less than 5% (0.05) and a confidence level of 95%.
The prevalence values were compared through the Fisher’s test on the islands where positive animals were found and the free roam condition on each of these islands. The predictive values of the tests used (Knott test and cox1 gene filarial nematodes) were calculated using mPCR as the gold standard. To evaluate the usefulness and applicability of these tests, we calculated the sensitivity and specificity [37]. Finally, a probability model was developed using the PROC package and the receiver operating characteristic (ROC) curve was plotted, with the area under the curve (AOC) calculated.
A generalized linear model (GLM) from the “Stats” package was applied to determine if the variables such as housing, island, location, sex, and free roam are correlated with the positivity to D. immitis and B. vogeli in dogs.
Results
Roaming behavior
In our study, 25.2% of dogs with owners roam freely. The Floreana, Isabela, and San Cristóbal Islands were the ones that mostly showed this behavior, 40% (9/22), 30.6% (65/212), and 29.7% (179/603), respectively, while on Santa Cruz Island only 14.3% (35/384) of owned dogs roamed freely. Although Santa Cruz Island has the largest human and dog population, the tendency of dogs to roam unrestricted was statistically lower than on San Cristobal Island (P = 0.001, OR 0.001) and Isabela Island (P = 0.001, OR 5.29), where positive cases were documented.
D. immitis
Overall, 25 out of 1221 blood samples (2.0%) were positive for D. immitis (Table 2). The DNA sequences from the 25 positive samples revealed similarity (97–100%) with the available DNA sequences of D. immitis at NCBI GenBank (Supplementary File 2). The high-quality sequences obtained were submitted to the GenBank international database under the accession number (PQ044876-PQ044877, Q > 20 value higher then 90; Supplementary File 3).
Table 2.
Comparative analysis of D. immitis prevalence in dogs
| Variable | Category | No. of samples | Positive (%) | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Total | 1221 | 25 (2.0) | 1.3–2.9 | |||
| Sex | Males | 652 | 16 (2.5) | 0.3837 |
1.5653 NA |
1.3–3.8 |
| Females | 569 | 9 (1.6) | 0.6–2.6 | |||
| Age | <1 Year | 149 | 2 (1.4) | 0.1441 | NA | 0–3.2 |
| 1–4 Years | 648 | 9 (1.4) | 0.5–2.3 | |||
| 4–10 Years | 345 | 12 (3.5) | 1.6–5.4 | |||
| >10 Years | 50 | 2 (4) | 0–9.4 | |||
| Unknown | 29 | 0 | 0 | |||
| Breed | Pure breed | 385 | 1 (0.3) | 0.0055* | 0.0881 | 0–0.8 |
| Mixed breed | 836 | 24 (2.9) | 1.7–4 | |||
| Environment | Urban | 1013 | 21 (2.1) | 1 |
1.0796 NA |
1.2–3.0 |
| Rural | 208 | 4 (1.9) | 0.1–3.8 | |||
| Housing | Outdoor | 560 | 18 (3.2) | 0.0284* | NA | 1.8–4.7 |
| Indoor | 231 | 3 (1.3) | 0–2.8 | |||
| Outdoor and indoor | 430 | 4 (1) | 0.1–1.9 | |||
| Free roaming | Yes | 308 | 7 (2.3) | 0.9282 |
1.1563 NA |
0.6–4 |
| No | 913 | 18 (2) | 1.1–2.9 |
*Statistically significant, OR odds ratio, 95% CI 95% confidence interval, NA not applicable
The prevalence of heartworm in domestic dogs by island was as follows: Isabela 2.4%, Floreana 0%, Santa Cruz 0%, and San Cristóbal 3.3% (Figs. 2 and 3). The sample analysis revealed that 19 of the examined dogs tested positive (1.6%) using mPCR. Out of these, 14 dogs also showed positive results (1.2%) when their cox1 gene sequences were analyzed. These sequences were found to be 97–100% similar to different sequences of D. immitis in the GenBank sequence database. Furthermore, we confirmed the presence of this species by amplifying and analyzing the 5.8S-ITS2-28S rDNA gene fragment.
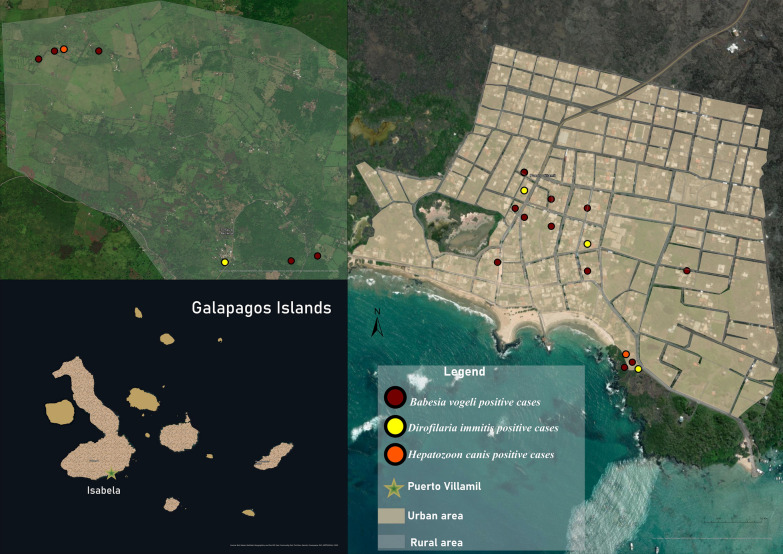
Fig. 2.
The spatial distribution of positive dogs on Isabela Island
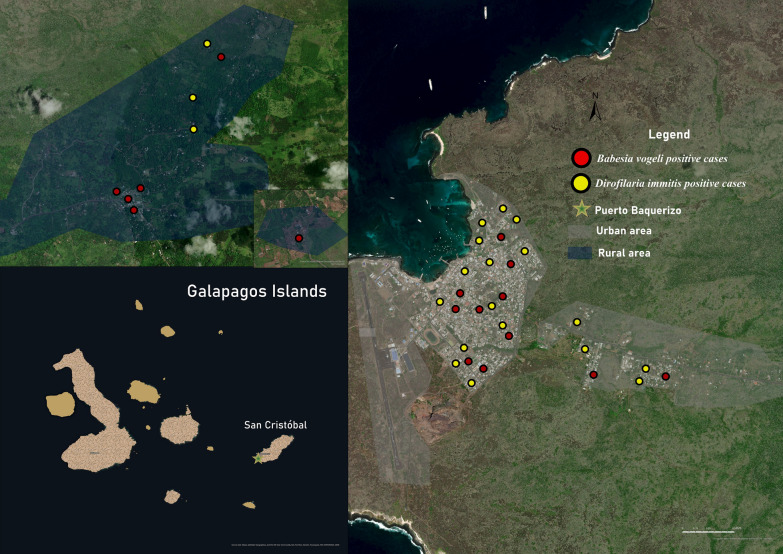
Fig. 3.
The spatial distribution of positive dogs on San Cristóbal Island
The cox1 gene filarial nematodes test showed greater sensitivity than the Knott’s test, while the Knott’s test showed greater specificity than the cox1 gene filarial nematodes test (Table 3).
Table 3.
Sensitivity and specificity of the Knott versus molecular diagnosis of D. immitis using mPCR as the gold standard
| Method | TP | TN | FN | FP | Se (%) | Sp (%) | AUC |
|---|---|---|---|---|---|---|---|
| Knott | 8 | 1160 | 10 | 6 | 57 | 99.1 | 99.7 |
| cox1NTF/NTR | 14 | 4 | 7 | 2 | 87.5 | 36.3 | 69.5 |
TP true positives, TN true negatives, FN false negatives, FP false positives, Se sensitivity, Sp specificity, AUC Area under the curve
However, when applying a GLM, no significant relationship was found between variables examined and positivity. The influence of the variables housing, island, and sex could not be related to the presence of D. immitis (Supplementary File 4).
Apicomplexan hemoparasites
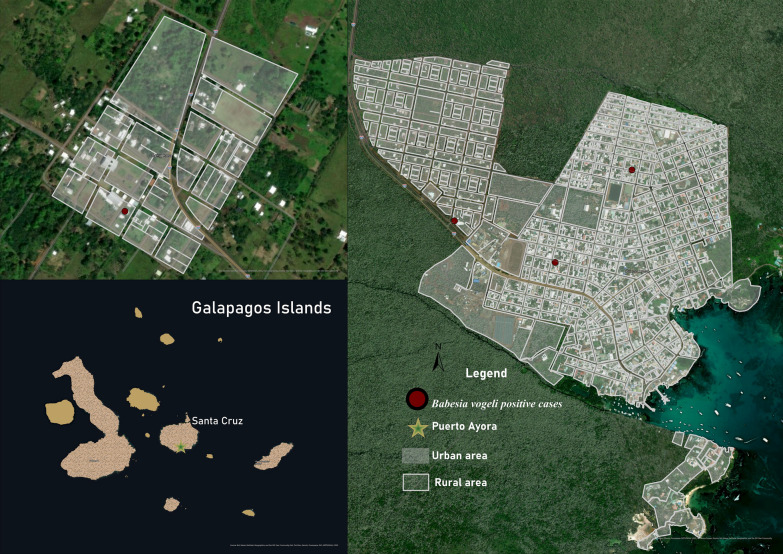
The BLAST analysis of the 18S rDNA sequences showed a 97–100% identity with different sequences of Babesia spp., Hepatozoon spp., and Theileria spp. in 67 dogs (5.5%, n = 1221). To further validate the positive results, species-specific nPCR assays were performed targeting the 18S rDNA of Babesia spp. (Supplementary File 5). The sequence analysis showed that 37 dogs tested positive for B. vogeli according to the sequences (95–100%) (Table 4 and Figs. 2, 3 and 4). The high-quality sequences obtained are available online under the accession numbers PQ136446–PQ136456; Q > 20 higher then 95%.
Table 4.
Comparative analysis of B. vogeli prevalence in dogs
| Variable | Category | No. of samples | Positive (%) | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Total | 1221 | 37 (3) | 2.1–4 | |||
| Sex | Males | 652 | 19 (2.9) | 0.9313 | 0.9188 | 1.7–4.2 |
| Females | 569 | 18 (3.2) | 1.8–4.6 | |||
| Age | <1 Year | 149 | 12 (8.1) | 0.0002* | NA | 3.7–12.4 |
| 1–4 Years | 648 | 21 (3.3) | 1.9–4.6 | |||
| 4–10 Years | 345 | 1 (0.3) | 0–0.9 | |||
| >10 Years | 50 | 2 (4) | 0–9.4 | |||
| Unknown | 29 | 1 (3.5) | 0–10.1 | |||
| Breed | Pure breed | 385 | 9 (2.4) | 0.4363 | 0.6907 | 1–3.9 |
| Mixed breed | 836 | 28 (3.4) | 2.1–4.6 | |||
| Environment | Urban | 1013 | 26 (2.6) | 0.0624 | 0.4718 | 1.6–3.5 |
| Rural | 208 | 11 (5.3) | 2.3–8.3 | |||
| Housing | Outdoor | 560 | 27 (4.8) | 0.0034* | NA | 3.1–6.6 |
| Indoor | 231 | 4 (1.7) | 0.1–3.4 | |||
| Outdoor and indoor | 430 | 6 (1.4) | 0.2–2.5 | |||
| Free roaming | Yes | 308 | 11 (3.6) | 0.6538 |
1.2635 NA |
1.5–5.6 |
| No | 913 | 26 (2.8) | 1.8–3.9 |
*Statistically significant, OR odds ratio, 95% CI 95% confidence interval, NA not applicable
Fig. 4.
The spatial distribution of positive dogs on Santa Cruz Island
However, further confirmation revealed that only 28 samples showed the presence of this species via cytb gene amplification and analysis and only 24 samples through cox1 gene amplification and analyses. The sequences obtained from domestic dogs matched 99–100% with B. vogeli sequences from GenBank (available in Supplementary File 5). The sequences obtained from the cytb and cox1 gene are available in Supplementary File 3.
The GLM analysis revealed that none of the variables examined were significantly associated with the presence B. vogeli (Supplementary File 4).
Two samples from Isabela Island tested positive for H. canis (0.2%) (Fig. 2). One case was from the rural area, and the other was from the urban area. Both sequences matched 99.70% with the H. canis sequences stored in GenBank (MN393911, Cuba). All sequences obtained from the positive samples were deposited in GenBank international database under the accession numbers: PQ136654–55 and PQ136656–57; Q > 20 higher than 95%.
Discussion
Our work extends completes the data of the previous studies about the pathogens introduced in the Galapagos archipelago (Table 5). As reviewed by [38], most of the invasive parasites tend to infect and to be more virulent in native hosts, due to the lack of natural resistance.
Table 5.
Literature review of vector-borne pathogens reported in dogs in Galapagos
| Island | Pathogen | Prevalence (%) | Method | References |
|---|---|---|---|---|
| San Cristóbal | D. immitis | 1.7 | Knott test | [26] |
| 3.3 | PCR | Present study | ||
| Babesia vogeli | 2.8 | PCR | Present study | |
| H. canis | – | PCR | Present study | |
| Floreana | D. immitis | 77 | Antibody | [39] |
| 87 | Knott test | [39] | ||
| 14.8 | Necropsy | [39] | ||
| – | Knott test | Present study | ||
| – | PCR | Present study | ||
| Babesia vogeli | – | PCR | Present study | |
| H. canis | – | PCR | Present study | |
| Santa Cruz | D. immitis | – | Antigen | [40] |
| – | Antigen | [41] | ||
| 6.9 | Antigen | [11] | ||
| Not given | PCR | [11] | ||
| Not given | Diff-Quik | [11] | ||
| – | Knott test | Present study | ||
| – | PCR | Present study | ||
| Babesia vogeli | 1.05 | PCR | Present study | |
| H. canis | – | PCR | Present study | |
| Isabela | D. immitis | Not given | Necropsy1 | [42] |
| 34 | Antigen | [43] | ||
| 1.9 | Knott test | Present study | ||
| 2.4 | PCR | Present study | ||
| Babesia spp. | – | Antibody | [43] | |
| Babesia vogeli | 7.6 | PCR | Present study | |
| H. canis | 1.0 | PCR | Present study |
1Feral dogs
The canine heartworm, D. immitis is particularly important, as it can cause disease in dogs and other wild carnivores and also infect humans [44, 45]. The heartworm was reported in the Galapagos Islands for the first time in the 1980s [39, 42] and occasionally also later (Table 5). Furthermore, a recent study [8] reported the presence of 20 heartworms in a male sea lion on Santa Cruz Island. On Floreana Island, local people tested positive for antibodies, and it was shown that the sea lions that inhabit this island had circulating microfilariae [39]. The different results obtained on Floreana Island reflect changes over time. Several factors could explain the absence of D. immitis, such as lower mosquito densities, lower movement of dogs from other islands, and mainly the low population size of dogs. Additionally, they tested 25 young sea lions on San Cristóbal Island for D. immitis antigen, where 8% tested positive [8].
Concerning the risk factors, the prevalence of D. immitis showed that males had a slightly higher prevalence (2.5%) compared with females (1.6%), although this difference was not statistically significant (P = 0.3837), suggesting that sex may not be a determinant for the presence of the parasite. In contrast, age significantly influenced positivity, with dogs aged 4–10 years showing the highest prevalence (3.5%), possibly due to the longer time of exposure.
However, the GLM results did not establish whether there was a significant interaction between these variables in relation to positivity. A study conducted in Bucaramanga, Colombia, found a prevalence of 10.82% of D. immitis in dogs [46], highlighting the importance of demographic and environmental factors in the spread of the disease [46–48].
Molecular methods have a higher sensitivity to detect microfilariemia in canine blood samples, even at low levels [49–54]. Therefore, the difference between the results obtained via the Knott test and molecular test could be due to low microfilariemia. An important aspect of the results from combining microscopic and molecular methods is that together they can improve the accuracy of the diagnosis [53]. The molecular methods employed in this study were chosen to enhance results sensitivity, as well as the characterization and differentiation of filarial species. Two molecular tools targeting the cox1 gene (mPCR and conventional PCR) were utilized concurrently to enhance the quality of the obtained positive sequences. The third method was employed to detect the presence of other filarial species (A. dracunculoides; B. pahangi; B. malayi; B. timori; M. ozzardi; O. volvulus) in the extracted DNA, targeting the ITS2 gene [54].
The presence of vectors from the Culicidae family favors a complete cycle of D. immitis [26, 55–57]. Given that canine heartworm is a mosquito-borne filarial nematode and three species of mosquitoes capable of transmitting the parasite are found on these islands [11, 15, 16, 18, 19, 39, 58], we should consider the risk posed to pinnipeds, which often live close to dogs and mosquitoes.
Human settlements on the San Cristóbal, Santa Cruz, and Isabela Islands present a great risk of disease transmission from domestic carnivores to Galapagos sea lions [8, 59–61]. On each island, domestic dogs are present and they are often allowed to roam the streets and beaches [62]. This behavior can increase the risk of infection transmission, added to the fact that dogs probably do not receive regular antiparasitic treatments (Mihalca and Culda, personal observation).
Another essential aspect of this study was to provide the first demonstration of B. vogeli and H. canis in dogs in Galapagos. Ticks represent the second disease vector group after mosquitoes. They are frequently considered as a growing threat to human and animal health on worldwide [63]. It is known that they have the ability to transmit Apicomplexa (Babesia, Theileria, and Hepatozoon), which can cause pathogenicity in the affected host [64, 65]. Previous studies showed that ticks collected from dogs in Galapagos were Rhipicephalus sanguineus sensu lato [11, 40, 41, 43]. However, all these studies were done before the recent taxonomic agreement [66], which concluded that the so called “tropical linage” of R. sanguineus s.l. should be regarded as R. linnaei.
The 18S rDNA gene is a highly conserved region that plays an essential role in the molecular diagnosis of piroplasmids [67–75]. Nevertheless, several studies suggested that detecting multiple pathogens in a single sample may have some limitations and variables in the target size gene’s amplification efficiency [74, 76, 77].
B. vogeli is an emerging pathogen of dogs, with a worldwide distribution, particularly in tropical and subtropical countries [72, 78–80]. In our case, the highest prevalence was obtained in dogs younger than 1 year old (8.1%; 12/1,221) and in those aged 1–4 years (3.3%; 21/1,221). This may be related to higher exposure to ticks and mainly to the lack of immunity, as known for Babesia spp. in animals following multiple exposure [80, 81]. Several studies evaluated the prevalence of this pathogen in dogs across different continents, and its presence seems to be more common in warm climate areas. Australia, Cambodia, Thailand, Egypt, and Costa Rica have the highest prevalence of B. vogeli [72, 80]. Moreover, it is interesting that B. vogeli was detected also in wild carnivores in other regions, such as Brazil [79, 82], Thailand [80, 83], Zimbabwe [80, 84], South Africa [80, 84], and Tanzania [80, 85].
Regarding B. vogeli risk factors, the study revealed a slightly lower prevalence in males (2.9%) compared with females (3.2%), although this difference was not statistically significant (P = 0.9313), suggesting that sex is not a determinant factor for the presence of the parasite. Conversely, age had a significant influence on positivity, with dogs under 1 year of age showing the highest prevalence (8.1%) (P = 0.0002), possibly due to a higher susceptibility to infection.
Similar to the case of D. immitis, differences in prevalence were found according to breed, with a lower prevalence in pure breeds (2.4%) compared with mixed breeds (3.4%), although this difference was not statistically significant (P = 0.4363). The higher prevalence observed in dogs living outdoors (4.8%) compared with those living indoors (1.7%) or having access to both indoor and outdoor environments (1.4%) (P = 0.0034) is possibly due to greater exposure to ticks. A study by Zygner et al. [80] highlighted that the most important risk factors for Babesia infection include living in rural areas, in kennels or animal shelters, or in regions endemic for the infection, as well as tick infestation and lack of acaricide treatment. The characteristics of populated areas in the Galapagos Islands resemble a rural environment, which would be related to what was mentioned by this author. Regarding the GLM, as in the case of D. immitis, the results did not allow the establishment of any significant interaction between these variables in relation to positivity, likely requiring a larger sample size or the investigation of other variables.
Finally, it was also possible to identify H. canis, another tick-borne parasite, which has been recognized in South America as a significant threat to the well-being of dogs, especially in rural areas of Brazil and Costa Rica [86–90].
Conclusions
Our study demonstrated the presence, although with low prevalence, of canine vector-borne parasites in Galapagos. Among the tested pathogens, D. immitis is particularly relevant from a conservation medicine perspective, as it can be transmitted and be pathogenic to the endangered Galapagos sea lion. Our study also brings new data on the global distribution of B. vogeli and H. canis, demonstrating for the first time their presence in Galapagos.
Supplementary Information
Supplementary Material 1. Primers sets used for the amplification of DNA.
Supplementary Material 2. BLAST comparisons between the obtained sequences and the GenBank sequences.
Supplementary Material 3. Consensus sequences derived from the obtained sequences.
Supplementary Material 4. GLM analysis results for Dirofilaria immitis and Babesia vogeli in dogs.
Supplementary Material 5. BLAST comparisons between the obtained sequences and the GenBank sequences.
Acknowledgements
The authors are grateful to the staff of the Galápagos Science Centre, the Galápagos National Park, the Agency for the Regulation and Control of Biosecurity and Quarantine for Galápagos, and the International Union for Conservation of Nature.
Abbreviations
- DNA
Deoxyribonucleic acid
- PCR
Polymerase chain reaction
- mPCR
Multiplex PCR
- nPCR
Nested PCR
- rDNA
Ribosomal RNA
- NCBI
National Center for Biotechnology Information
- BLAST
Basic Local Alignment Search Tool
Author contributions
C.A.C., N.G.T.R., P.F., R.M.P.S., G.D., R.L., R.L.V., and A.D.M. performed the sampling in the field. C.A.C., N.G.T.R., P.F., R.M.P.S., and G.D. worked in the laboratory in Galapagos. Molecular analyses were performed by C.A.C., L.C.P., and C.D.P. C.A.C., R.L., R.L.V., D.P.-R., and A.D.M. obtained the research permits. A.D.M. secured the funding. C.A.C. and A.D.M. wrote the main manuscript; C.A.C., N.G.T.R., P.F., R.M.P.S., L.C.P., C.D.P., G.D., R.L., R.L.V., D.P.-R., and A.D.M. edited the manuscript, and all authors reviewed it.
Funding
This study was funded by the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and Erasmus+.
Availability of data and materials
Data are provided within the manuscript and in the Supplementary Information files. Our accesion numbers include: PQ044876-PQ044877 for D. immitis, PQ136446–PQ136456 for B. vogeli, PQ136654–PQ136655 and PQ136656–PQ136657 for H. canis.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carla Andreea Culda, Email: carla-andreea.culda@usamvcluj.ro.
Andrei Daniel Mihalca, Email: amihalca@usamvcluj.ro.
References
- 1.Campbell NA, Reece JB. Biology. 7th ed. Campbell Reece; 2005. [Google Scholar]
- 2.Torres MDL, Mena CF. Understanding invasive species in the Galapagos Islands: from the molecular to the landscape. Social and ecological interactions in the Galapagos Islands; 2018.
- 3.Urquía D, Gutierrez B, Pozo G, Pozo MJ, Espín A, Torres MDL. Psidium guajava in the Galapagos Islands: population genetics and history of an invasive species. PLoS ONE. 2019;14:e0203737. 10.1371/journal.pone.0203737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, Landry B. Alien insects: threats and implications for conservation of Galapagos Islands. Ann Entomol Soc Am. 2006;99:121–43. 10.1603/0013-8746(2006)099[0121:AITAIF]2.0.CO;2. [Google Scholar]
- 5.Simberloff D, Von Holle B. Positive interactions of nonindigenous species: invasional melt-down? Biol Invasions. 1999;1:21–32. [Google Scholar]
- 6.Bauer JT. Invasive species: “back-seat drivers” of ecosystem change? Biol Invasions. 2012;14:1295–304. 10.1007/s10530-011-0165-x. [Google Scholar]
- 7.Hughes J, Macdonald DW. A review of the interactions between free-roaming domestic dogs and wildlife. Biol Cons. 2013;157:341–51. 10.1016/j.biocon.2012.07.005. [Google Scholar]
- 8.Gregory TM, Livingston I, Hawkins EC, Loyola A, Cave A, Vaden S, et al. Dirofilaria immitis identified in Galapagos sea lions (Zalophus wollebaeki): A wildlife health and conservation concern. The Journal of Wildlife Diseases. 2023;59:487–94. 10.7589/JWD-D-22-00119. [DOI] [PubMed] [Google Scholar]
- 9.Schatz H. Catalogue of known species of Acari from the Galápagos Islands (Ecuador, Pacific Ocean). Int J Acarol. 1991;17:213–25. 10.1080/01647959108683909. [Google Scholar]
- 10.Baert LL. CDF checklist of Galápagos arachnids. In: Bungartz F, Herrera H, Jaramillo P, Tirado N, Jiménez-Uzcátegui G, Ruiz D, et al., editors. Charles Darwin Foundation Galápagos Species Checklist. Puerto Ayora: Charles Darwin Foundation; 2018. [Google Scholar]
- 11.Jimenez IA, Mariño PAV, Stapleton GS, Prieto JB, Bowman DD. Canine vector-borne disease in domestic dogs on Isla Santa Cruz, Galápagos. Vet Parasitol Reg Stud Rep. 2020;19:100373. 10.1016/j.vprsr.2020.100373. [DOI] [PubMed] [Google Scholar]
- 12.Smit FGAM. A new species of flea from the Galápagos Islands. Entomologische Berichten. 1970;30:244–7. [Google Scholar]
- 13.Linsley EG. Insects of the Galapagos. Occas pap Calif Acad Sci 1977;125:1–50. [Google Scholar]
- 14.Peck SB. Smaller orders of insects of the Galápagos Islands, Ecuador: evolution, ecology, and diversity. NRC Research Press; 2001. [Google Scholar]
- 15.Bataille A, Cunningham AA, Cedeno V, Cruz M, Eastwood G, Fonseca DM, et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galápagos Islands. Proc R Soc Lond B Biol Sci. 2009;276:3769–75. 10.1098/rspb.2009.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bataille A, Cunningham AA, Cedeno V, Patino L, Constantinou A, Kramer LD, et al. Natural colonization and adaptation of a mosquito species in Galápagos and its implications for disease threats to endemic wildlife. Proc Natl Acad Sci. 2009;106:10230–5. 10.1073/pnas.0901308106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bataille A, Cunningham AA, Cruz M, Cedeno V, Goodman SJ. Seasonal effects and fine-scale population dynamics of Aedes taeniorhynchus, a major disease vector in the Galápagos Islands. Mol Ecol. 2010;19:4491–504. 10.1111/j.1365-294X.2010.04843.x. [DOI] [PubMed] [Google Scholar]
- 18.León R, Ortega-Lopez L, Molina C, Waters WF. Mosquitoes of the Galapagos Islands: the risk for arboviruses transmission and the need for a better vector surveillance and control program. In: Water, food and human health in the Galapagos, Ecuador: “a little world within itself.” Cham: Springer International Publishing; 2022. p. 187–208. [Google Scholar]
- 19.Sinclair BJ. An annotated checklist of the Diptera of the Galápagos Archipelago (Ecuador). Zootaxa. 2023;5283:1–102. [DOI] [PubMed] [Google Scholar]
- 20.Riofrío-Lazo M, Páez-Rosas D. Galapagos Pinnipeds, challenges to their survival. In: Endangered species-present status. IntechOpen; 2023. 10.5772/intechopen.113366. [Google Scholar]
- 21.Sinclair BJ. CDF checklist of Galápagos flies. In: Bungartz F, Herrera H, Jaramillo P, Tirado N, Jiménez-Uzcátegui G, Ruiz D, et al., editors. Charles Darwin Foundation Galápagos Species Checklist. Puerto Ayora: Charles Darwin Foundation; 2017. p. 45. [Google Scholar]
- 22.Knott J, Earle K. A method for making microfilarial surveys on day blood. Trans R Soc Trop Med Hyg. 1939;33:191–6. [Google Scholar]
- 23.Newton WL, Wright WH. The occurrence of a Dog Filariid other than Dirofilaria immitis in the United States. J Parasitol Res. 1956;42:246. [PubMed] [Google Scholar]
- 24.Georgi JR, Georgi ME, Theodorides VJ. Parasitology for veterinarians. 5th ed. Philadelphia: W. B. Saunders; 1990. p. 412. [Google Scholar]
- 25.Magnis J, Lorentz S, Guardone L, Grimm F, Magi M, Naucke TJ, et al. Morphometric analyses of canine blood microfilariae isolated by the Knott’s test enables Dirofilariaimmitis and D.repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasites Vectors. 2013;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Culda CA, Dionnet R, Barbu AC, Cârstolovean AS, Dan T, Grijalva J, et al. The presence of Dirofilaria immitis in domestic dogs on San Cristobal Island, Galapagos. Pathogens. 2022;11:1287. 10.3390/pathogens11111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latrofa MS, Weigl S, Dantas-Torres F, Annoscia G, Traversa D, Brianti E, et al. A multiplex PCR for the simultaneous detection of species of filarioids infesting dogs. Acta Trop. 2012;122:150–4. 10.1016/j.actatropica.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. 10.1017/S0031182000007149. [DOI] [PubMed] [Google Scholar]
- 29.Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006;135:303–14. 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, Hostis LM, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207. 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- 31.Zintl A, Finnerty EJ, Murphy TM, de Waal T, Gray JS. Babesias of red deer (Cervus elaphus) in Ireland. Vet Res. 2011;42:1–6. 10.1186/1297-9716-42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015;8:1–7. 10.1186/s13071-015-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgroi G, Iatta R, Veneziano V, Bezerra-Santos MA, Lesiczka P, Hrazdilová K, et al. Molecular survey on tick-borne pathogens and Leishmaniainfantum in red foxes (Vulpes vulpes) from southern Italy. Ticks Tick Borne Dis. 2021;12:101669. 10.1016/j.ttbdis.2021.101669. [DOI] [PubMed] [Google Scholar]
- 34.Panait LC, Hrazdilová K, Ionică AM, Deak G, Chişamera GB, Adam C, et al. Babesiapisicii n. sp. and Babesiacanis infect European wild cats, Felissilvestris, in Romania. Microorganisms. 2021;9:1474. 10.3390/microorganisms9071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hrazdilová K, Červená B, Blanvillain C, Foronda P, Modrý D. Quest for the type species of the genus Hepatozoon–phylogenetic position of hemogregarines of rats and consequences for taxonomy. Syst Biodivers. 2021;9:622–31. 10.1080/14772000.2021.1903616. [Google Scholar]
- 36.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thrusfield M. Veterinary epidemiology. 4th ed. John Wiley & Sons; 2018. p. 896 (ISBN: 978-1-118-28028-7). [Google Scholar]
- 38.Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL. Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol. 2014;3:171–7. 10.1016/j.ijppaw.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett BD. Dogs of the Galapagos Islands: evolution, ecology, impact and control (feral, Galapagos, dogs). PhD Thesis. University of California, Davis; 1985.
- 40.Diaz NM, Mende GS, Grijalva CJ, Walden HS, Cruz M, Aragon E, et al. Dog overpopulation and burden of exposure to canine distemper virus and other pathogens on Santa Cruz Island, Galapagos. Prev Vet Med. 2016;123:128–37. 10.1016/j.prevetmed.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Adams DJ, Rosenberg DE, Yirui H. Prevalence of vector-borne diseases in a sample of client-owned dogs on Santa Cruz in the Galápagos Islands: a pilot study. Vet Parasitol Reg Stud Reports. 2016;6:28–30. 10.1016/j.vprsr.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Barnett BD, Rudd RL. Feral dogs of the Galapagos Islands: impact and control. Int J Stud Anim Prob 1983;4:44–58. [Google Scholar]
- 43.Levy JK, Crawford PC, Lappin MR, Dubovi EJ, Levy MG, Alleman R, et al. Infectious diseases of dogs and cats on Isabela Island, Galapagos. J Vet Intern Med. 2008;22:60–5. 10.1111/j.1939-1676.2007.0034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285. 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 45.Otranto D, Capelli G, Genchi C. Changing distribution patterns of canine vector borne diseases in Italy: leishmaniosis vs. dirofilariosis. Parasit Vectors. 2009;26:S1–2. 10.1186/1756-3305-2-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esteban-Mendoza MV, Arcila-Quiceno V, Albarracín-Navas J, Hernández I, Flechas-Alarcón MC, Morchón R. Current situation of the presence of Dirofilaria immitis in dogs and humans in Bucaramanga, Colombia. Front Vet Sci. 2020;7:488. 10.3389/fvets.2020.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez-Escolar I, Hernández-Lambraño RE, Sánchez-Agudo JÁ, Collado M, Pérez-Pérez P, Morchón R. Current risk of Dirofilariosis transmission in the Iberian Peninsula (Spain and Portugal) and the Balearic Islands (Spain) and its future projection under climate change scenarios. Animals. 2023;13:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chocobar MLE, Schmidt EMDS, Weir W, Panarese R. The distribution, diversity, and control of dirofilariosis in Brazil: a comprehensive review. Animals. 2024;14:2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuchprayoon S. DNA-based diagnosis of lymphatic filariasis. Southeast Asian J Trop Med Public Health. 2009;40:904. [PubMed] [Google Scholar]
- 50.Ramos RAN, do Rêgo AGD, de Farias Firmino ED, do Nascimento Ramos CA, de Carvalho GA, Dantas-Torres F, et al. Filarioids infecting dogs in northeastern Brazil. Vet Parasitol. 2016;226:26–9. 10.1016/j.vetpar.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Kamyingkird K, Junsiri W, Chimnoi W, Kengradomkij C, Saengow S, Sangchuto K, et al. Prevalence and risk factors associated with Dirofilaria immitis infection in dogs and cats in Songkhla and Satun provinces, Thailand. Agric Nat Resour. 2017;51:299–302. 10.1016/j.anres.2017.05.003. [Google Scholar]
- 52.Moreira HR, Madeira EA, Cunha DNL, Scofield A, Góes-Cavalcante G, Abel I, et al. Dirofilaria immitis infection in dogs in Algodoal Island, Brazilian Amazon. Pesquisa Veterinária Brasileira. 2019;39:510–5. [Google Scholar]
- 53.Soares LA, Matias IC, Silva SS, Ramos MEO, Silva AP, Barretto ML, et al. Parasitological, serological and molecular diagnosis of Dirofilaria immitis in dogs in Northeastern Brazil. Exp Parasitol. 2022;236:108233. 10.1016/j.exppara.2022.108233. [DOI] [PubMed] [Google Scholar]
- 54.Roblejo-Arias L, Díaz-Corona C, Piloto-Sardiñas E, Díaz-Sánchez AA, Zając Z, Kulisz J, et al. First molecular characterization of Dirofilaria immitis in Cuba. BMC Vet Res. 2023;19:239. 10.1186/s12917-023-03803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferasin L, Knight D. Filarial infections. In: Shaw SE, Day MJ (eds) Arthropod-borne Infectious Diseases of the Dog and Cat. Lippincott, Williams and Wilkins, Baltimore. 2005. p. 51–61.
- 56.Furtado AP, Melo FT, Giese EG, dos Santos JN. Morphological redescription of Dirofilaria immitis. J Parasitol. 2010;96:499–504. 10.1645/GE-2178.1. [DOI] [PubMed] [Google Scholar]
- 57.Pachnicke S, Stanneck D, Mencke N. Helminths in veterinary practice. Leverkusen: Bayer Animal Health GmbH; 2015. [Google Scholar]
- 58.Hendrix C, Brunner C, Bellamy L. Natural transmission of Dirofilaria immitis by Aedes aegypti. J Am Mosquito Control Assoc. 1986;2:48–51. [PubMed] [Google Scholar]
- 59.Páez-Rosas D, Torres J, Espinoza E, Marchetti A, Seim H, Riofrío-Lazo M. Declines and recovery in endangered Galapagos pinnipeds during the El Niño event. Sci Rep. 2021. 10.1038/s41598-021-88350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarzosa MS, Duignan P, DeRango EJ, Field C, Ríos C, Sanchez S, et al. Occurrence of mycoplasmas in Galapagos sea Lions (Zalophus wollebaeki) and their association with other respiratory pathogens. J Wildl Dis. 2021;57:623–7. 10.7589/JWD-D-20-00081. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Saenz J, Barragan V, Grijalva-Rosero CJ, Diaz EA, Páez-Rosas D. Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) confirms the presence of canine distemper virus in rookeries of San Cristóbal Island. Animals. 2023;13:3657. 10.3390/ani13233657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Páez-Rosas D, Guevara N. Management strategies and conservation status in populations of Galapagos sea lion (Zalophus wollebaeki). In: Tropical pinnipeds, bio-ecology, threats and conservation. CRC Press/Taylor & Francis Group; 2017. p. 159–75. [Google Scholar]
- 63.De la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, et al. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol. 2017;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 65.Charles R, Basu A, Sanford B, King-Cenac A, Melville-Edwin S, Pow-Brown P, et al. Survey of ticks of domestic dogs and cattle in three Caribbean islands. Transbound Emerg Dis. 2020;67:129–34. 10.1111/tbed.13384. [DOI] [PubMed] [Google Scholar]
- 66.Šlapeta J, Halliday B, Chandra S, Alanazi AD, Abdel-Shafy S. Rhipicephalus linnaei (Audouin, 1826) recognised as the “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato: Neotype designation, redescription, and establishment of morphological and molecular reference. Ticks Tick-borne eases. 2022;13:102024. 10.1016/j.ttbdis.2022.102024. [DOI] [PubMed] [Google Scholar]
- 67.Jalovecka M, Sojka D, Ascencio M, Schnittger L. Babesia life cycle–when phylogeny meets biology. Trends Parasitol. 2019;35:356–68. 10.1016/j.pt.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Lack JB, Reichard MV, Van Den Bussche RA. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol. 2012;42:353–63. 10.1016/j.ijpara.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 69.George N, Bhandari V, Reddy DP, Sharma P. Molecular and phylogenetic analysis revealed new genotypes of Theileria annulata parasites from India. Parasit Vectors. 2015;8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lempereur L, Beck R, Fonseca I, Marques C, Duarte A, Santos M, et al. Guidelines for the detection of Babesia and Theileria parasites. Vector-Borne Zoonotic Dis. 2017;17:51–65. 10.1089/vbz.2016.1955. [DOI] [PubMed] [Google Scholar]
- 71.Uilenberg G, Gray J, Kahl O. Research on Piroplasmorida and other tick-borne agents: are we going the right way? Ticks Tick Borne Dis. 2018;9:860–3. 10.1016/j.ttbdis.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Panti-May JA, Rodríguez-Vivas RI. Caninebabesiosis: a literature review of prevalence, distribution, and diagnosis in Latin America and the Caribbean. Vet Parasitol Reg Stud Rep. 2020;21:100417. 10.1016/j.vprsr.2020.100417. [DOI] [PubMed] [Google Scholar]
- 73.Schnittger L, Ganzinelli S, Bhoora R, Omondi D, Nijhof AM, Florin-Christensen M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights. Parasitol Res. 2022;121:1207–45. 10.1007/s00436-022-07424-8. [DOI] [PubMed] [Google Scholar]
- 74.Kumar B, Maharana BR, Thakre B, Brahmbhatt NN, Joseph JP. 18S rRNA gene-based piroplasmid PCR: an assay for rapid and precise molecular screening of Theileria and Babesia species in animals. Acta Parasitol. 2022;67:1697–707. 10.1007/s11686-022-00625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krawczak FDS, Calchi AC, Neves LC, Dias SA, da Silva BBF, Paula WVDF, et al. Phylogenetic inferences based on distinct molecular markers confirm a novel Babesia Species (Babesiagoianiaensis nov. sp.) in Capybaras (Hydrochoerushydrochaeris) and associated ticks. Microorganisms. 2023. 10.3390/microorganisms11082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards MC, Gibbs RA. Multiplex PCR: advantages, development, and applications. PCR Methods Appl. 1994;3:S65-75. 10.1101/gr.3.4.S65. [DOI] [PubMed] [Google Scholar]
- 77.Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000;13:559–70. 10.1128/cmr.13.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jefferies R, Ryan UM, Irwin PJ. PCR – RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet Parasitol. 2007;144:20–7. 10.1016/j.vetpar.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 79.Inácio EL, Pérez-Macchi S, Alabi A, Bittencourt P, Müller A. Prevalence and molecular characterization of piroplasmids in domestic dogs from Paraguay. Ticks Tick-borne Dis. 2019;10:321–7. 10.1016/j.ttbdis.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Zygner W, Gójska-Zygner O, Bartosik J, Górski P, Karabowicz J, Kotomski G. Canine babesiosis caused by large Babesia species: global prevalence and risk factors—a review. Animals. 2023;13:2612. 10.3390/ani13162612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obeta SS, Ibrahim B, Lawal IA, Natala JA, Ogo NI, Balogun EO. Prevalence of canine babesiosis and their risk factors among asymptomatic dogs in the federal capital territory, Abuja, Nigeria. Parasite Epidemiol Control. 2020;11:e00186. 10.1016/j.parepi.2020.e00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.André MR, Adania CH, Teixeira RHF, Allegretti SM, Machado RZ. Molecular and serological detection of Babesia spp. in neotropical and exotic carnivores in Brazilian zoos. J Zoo Wildl Med. 2011;42:139–43. [DOI] [PubMed] [Google Scholar]
- 83.Simking P, Wongnakphet S, Stich RW, Jittapalapong S. Detection of Babesia vogeli in stray cats of metropolitan Bangkok, Thailand. Vet Parasitol. 2010;173:70–5. 10.1016/j.vetpar.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 84.Kelly P, Marabini L, Dutlow K, Zhang J, Loftis A, Wang C. Molecular detection of tick-borne pathogens in captive wild felids, Zimbabwe. Parasites Vectors. 2014;7:1–6. 10.1186/s13071-014-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krücken J, Czirják GÁ, Ramünke S, Serocki M, Heinrich SK, Melzheimer J, et al. Genetic diversity of vector-borne pathogens in spotted and brown hyenas from Namibia and Tanzania relates to ecological conditions rather than host taxonomy. Parasit Vectors. 2021;14:328. 10.1186/s13071-021-04835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dantas-Torres F. Canine vector-borne diseases in Brazil. Parasit Vectors. 2008;1:25. 10.1186/1756-3305-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Miranda RL, O’Dwyer LH, de Castro JR, Metzger B, Rubini AS, Mundim AV. Prevalence and molecular characterization of Hepatozoon canis in dogs from urban and rural areas in Southeast Brazil. Res Vet Sci. 2014;97:325–8. 10.1016/j.rvsc.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 88.Rojas A, Rojas D, Montenegro V, Gutierrez R, Yasur-Landau D, Baneth G. Vector-borne pathogens in dogs from Costa Rica: first molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet Parasitol. 2014;199:121–8. [DOI] [PubMed] [Google Scholar]
- 89.Wei L, Kelly P, Ackerson K, El-Mahallawy HS, Kaltenboeck B, Wang C. Molecular detection of Dirofilariaimmitis, Hepatozooncanis, Babesia spp., Anaplasmaplatys and Ehrlichiacanis in dogs on Costa Rica. Acta Parasitol. 2015;60:21–5. [DOI] [PubMed] [Google Scholar]
- 90.Maggi RG, Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit Vectors. 2019;12:1–37. 10.1186/s13071-019-3407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Primers sets used for the amplification of DNA.
Supplementary Material 2. BLAST comparisons between the obtained sequences and the GenBank sequences.
Supplementary Material 3. Consensus sequences derived from the obtained sequences.
Supplementary Material 4. GLM analysis results for Dirofilaria immitis and Babesia vogeli in dogs.
Supplementary Material 5. BLAST comparisons between the obtained sequences and the GenBank sequences.
Data Availability Statement
Data are provided within the manuscript and in the Supplementary Information files. Our accesion numbers include: PQ044876-PQ044877 for D. immitis, PQ136446–PQ136456 for B. vogeli, PQ136654–PQ136655 and PQ136656–PQ136657 for H. canis.