Abstract
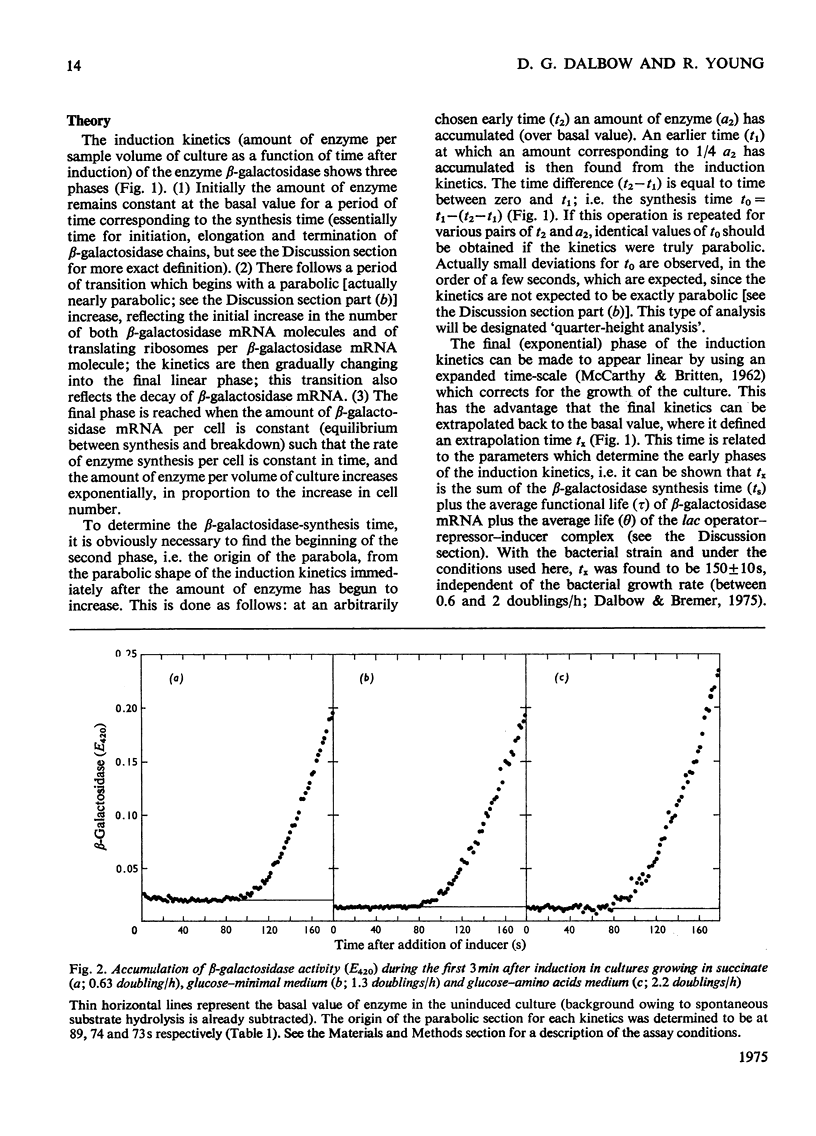
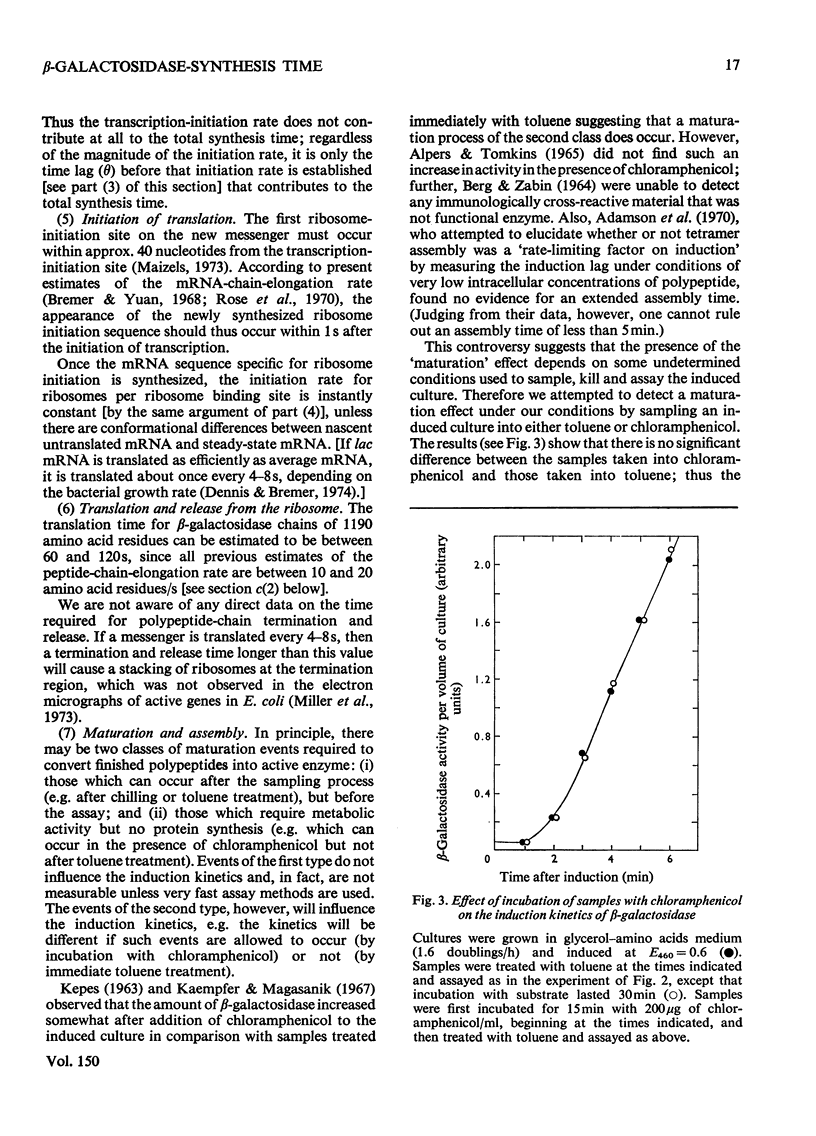
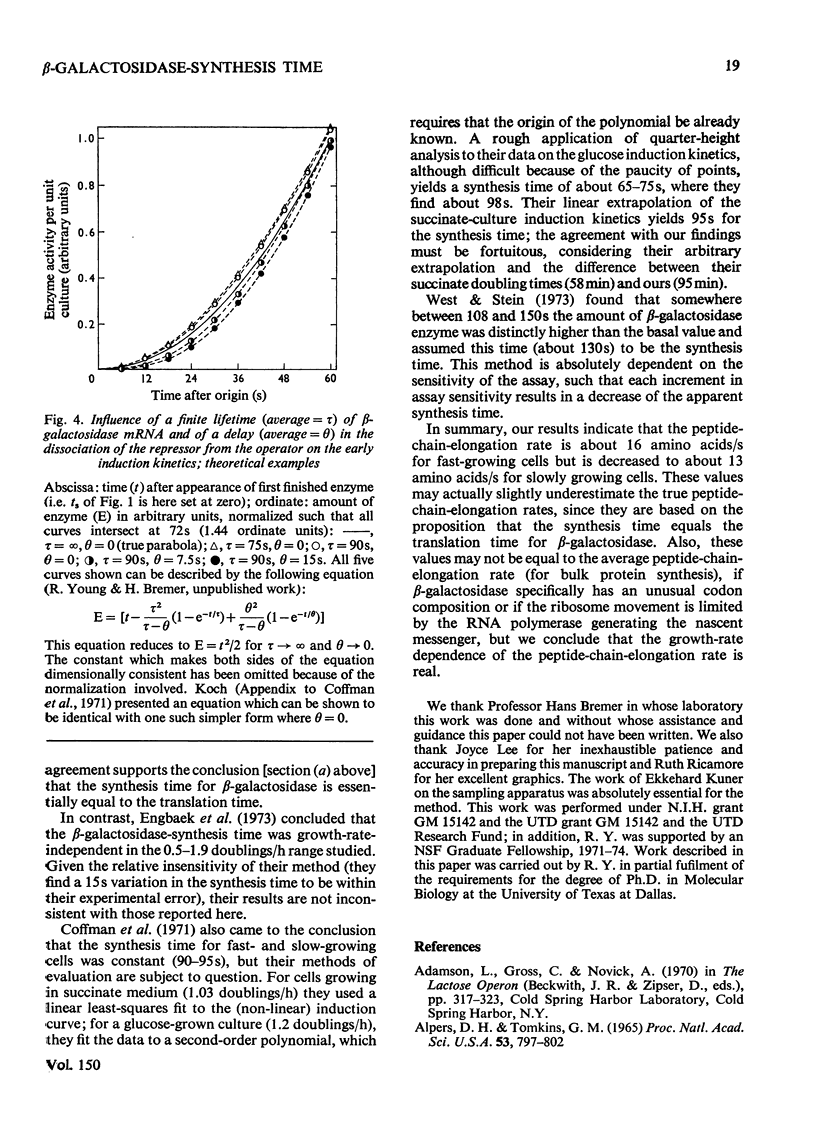
By analysing the kinetics of beta-galactosidase accumulation after induction, the synthesis time of beta-galactosidase in Escherichia coli B/r was found to be 75s in rapidly growing cells (1.36 and 2.1 doublings/h), and 90s in slowly growing cells (0.63 doubling/h). These values correspond to peptide-chain-elongation rates of 16 and 13 amino acids/s respectively, in agreement with previous findings, indicating that the peptide-chain growth rate is constant (presumably maximal) in fast-growing bacteria, but decreased in slowly growing bacteria [Forchhammer & Lindahl (1971) J. Mol. Biol. 55, 563-568].
Full text
PDF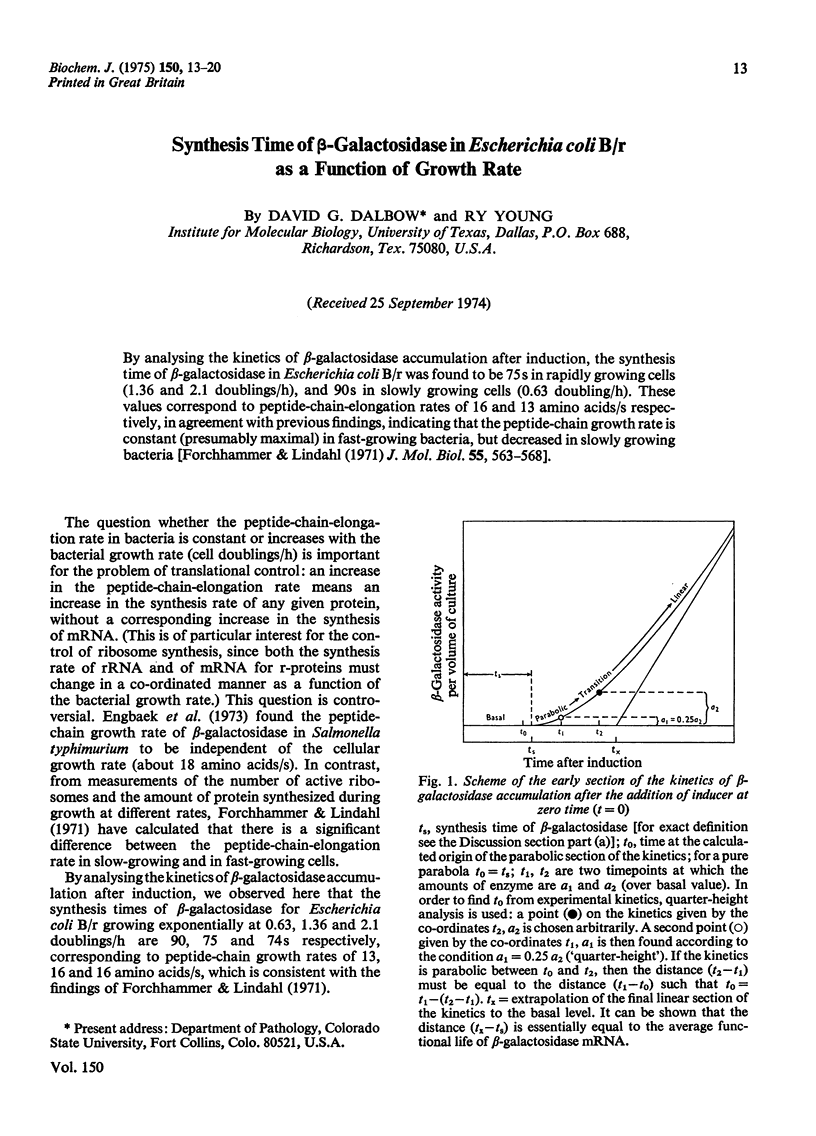




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., TOMKINS G. M. THE ORDER OF INDUCTION AND DEINDUCTION OF THE ENZYMES OF THE LACTOSE OPERON IN E. COLI. Proc Natl Acad Sci U S A. 1965 Apr;53:797–802. doi: 10.1073/pnas.53.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG A., ZABIN I. IMMUNOLOGICAL STUDIES ON BETA-GALACTOSIDASE AND THIOGALACTOSIDE TRANSACETYLASE: PROTEINS OF THE LACTOSE SYSTEM IN ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:289–294. doi: 10.1016/s0022-2836(64)80047-4. [DOI] [PubMed] [Google Scholar]
- Bremer H., Dalbow D. G. Regulatory state of ribosomal genes and physiological changes in the concentration of free ribonucleic acid polymerase in Escherichia coli. Biochem J. 1975 Jul;150(1):9–12. doi: 10.1042/bj1500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dalbow D. G., Bremer H. Metabolic regulation of beta-galactosidase synthesis in Escherichia coli. A test for constitutive ribosome synthesis. Biochem J. 1975 Jul;150(1):1–8. doi: 10.1042/bj1500001b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbaek F., Kjeldgaard N. O., Maaloe O. Chain growth rate of -galactosidase during exponential growth and amino acid starvation. J Mol Biol. 1973 Mar 25;75(1):109–118. doi: 10.1016/0022-2836(73)90532-9. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. In vivo experiments on the mechanism of action of L-arabinose C gene activator and lactose repressor. J Mol Biol. 1973 Nov 5;80(3):433–444. doi: 10.1016/0022-2836(73)90414-2. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Kepes A., Beguin S. Peptide chain initiation and growth in the induced synthesis of beta-galactosidase. Biochim Biophys Acta. 1966 Sep;123(3):546–560. doi: 10.1016/0005-2787(66)90222-x. [DOI] [PubMed] [Google Scholar]
- LONBERG-HOLM K. K. A direct study of intracellular glycolysis in Ehrlich's ascites tumor. Biochim Biophys Acta. 1959 Oct;35:464–472. doi: 10.1016/0006-3002(59)90396-8. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- West I. C., Stein W. D. The kinetics of induction of -galactoside permease. Biochim Biophys Acta. 1973 Apr 21;308(7):161–167. doi: 10.1016/0005-2787(73)90133-0. [DOI] [PubMed] [Google Scholar]


