Abstract
Background
An enriched understanding is necessary concerning the association between hypertension and cognitive impairment in older adults, particularly regarding the potential underlying mechanisms at a biological level. This study aimed to explore the mediating role of methylmalonic acid (MMA) in the hypertension-cognition link in the older population.
Methods
A total of 2762 adults (age > = 60 years) from the National Health and Nutrition Examination Survey (NHANES) 2011–2014 participated. Cognitive function was assessed using a combination of the Animal Fluency Test (AFT), the Digit Symbol Substitution Test (DSST), and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Learning Test. Self-reported hypertension diagnosis, antihypertensive medications use, and blood pressure examinations were used to identify hypertension. Serum MMA (sMMA) levels were collected. Weighted multiple linear regressions and mediation analysis were applied. A subgroup analysis by sex and age was performed.
Results
After adjusting for potential confounding factors, we observed a significant mediating effect of the sMMA level in the hypertension-cognition link, accounting for 11.14% (95% CI 4.09%-14.00%, p < 0.001) of the relationship in older adults. The proportion mediated by the sMMA level in the relationship between hypertension and cognitive function was higher in males (15.23%, 95%CI 1.32%-27.00%, p < 0.001) than in females (6.61%, 95%CI 2.12%-10.00%, p < 0.001). This mediating effect of sMMA was observed only in individuals aged 68 years and older (11.31%, 95%CI 3.80%-16.00%, p < 0.001), with no significant mediation detected in those younger than 68 years.
Conclusion
Hypertension may lead to cognitive dysfunction in older adults through MMA. Apart from its role as a biomarker reflecting vitamin B12, MMA may act as an independent neurotoxin capable of inducing brain injury and cognitive impairment. Addressing MMA accumulation, such as through Vitamin B12 supplementation, may have a potential to mitigate hypertension-induced cognitive decline in older adults. Special attention could be paid to hypertensive males with an advanced age (> = 68) to address MMA-related cognitive decline.
Keywords: Hypertension, Cognitive impairment, Methylmalonic acid, Older adults, Mediation analysis
Background
Hypertension, usually defined as persistent systolic blood pressure (SBP) at least 140 mm Hg or diastolic blood pressure (DBP) at least 90 mm Hg [1], poses a significant global health challenge, affecting over 1.3 billion individuals worldwide [2]. It is most prevalent among older adults [3, 4], with nearly two-thirds of this population experiencing high blood pressure (BP) [5], and with the aging population, the number of older adults with hypertension is expected to increase substantially [6–8]. Hypertension is not only a major risk factor for cardiovascular diseases but is also increasingly linked to cognitive decline, a critical public health concern as the population ages [9–14].
Cognitive decline encompasses a range of mental abilities, such as memory, attention, and executive function, which tend to deteriorate with age [15, 16]. Research has shown that age-associated degeneration in cognitive function is strongly linked to the risk of developing dementia [17], resulting in diminished functional independence among the older adults reduced quality of life, and heightened economic and societal burdens [18]. According to a study by Röhr et al. (2020), which analyzed 16 cohorts from 15 countries, the prevalence of subjective cognitive decline—defined as a self-experienced decline in cognitive ability compared to a previously normal status and often considered an early sign preceding more noticeable conditions such as mild cognitive impairment (MCI) and dementia —was approximately 25% in adults aged 60 and older [19]. The overall prevalence of MCI in community-dwelling adults aged 50 and older was estimated to be 15.56% [20]. Currently, more than 55 million people worldwide have dementia, with nearly 10 million new cases each year [21]. Thus, it is imperative to understand the changes in cognitive function in older population and explore all the possibilities to delay the onset of or slow down cognitive decline.
There is growing evidence that hypertension is one of the modifiable risk factors for cerebral vessel dysfunction, which contributes to cognitive decline and dementia [22–26]. Based on a low-income population aged over 60 years in northern China, Bao et al. (2021) had found that the prevalence of cognitive impairment in hypertensive patients was significantly higher than that in non-hypertensive individuals [27]. Similarly, Jia et al. (2020) found that individuals with high blood pressure had an 86% higher risk of developing dementia and a 62% higher risk of MCI compared to those with normal blood pressure [28]. According to Walker et al. (2019), individuals with consistently high blood pressure in both middle and old age faced a 49% higher risk of developing dementia compared to those with normal blood pressure [29].
However, inconsistencies remain, with some studies finding no significant association between late-life hypertension and cognitive decline [30–33]. For example, with a longitudinal study, Posner et al. (2002) reported that hypertension after age 65 years was not associated with mental diseases and did not adversely affect memory, language, or general cognitive function [34]. Although meta-analyses of BP lowering trials indicated a notable decrease in the risk of dementia among individuals receiving antihypertensive treatments, the relative and absolute risk reductions were modest [14]. Furthermore, the mechanisms linking hypertension to cognitive dysfunction remain unclear, though most studies focus on structural changes in the brain and microvascular damage [14, 35, 36]. Questions remain about the development and progression of hypertension-induced cognitive impairment and the targeted treatment. Hence, there is a need for a more comprehensive understanding of the link between hypertension and cognitive impairment, especially the potential underlying mechanisms.
Methylmalonic acid (MMA), a by-product of propionate metabolism in human body, is considered a surrogate biomarker of mitochondrial dysfunction and oxidative stress, and can predict all-cause and cardiovascular mortality in the general population [37, 38]. Impaired mitochondrial function can hinder the production of adenosine triphosphate (ATP) and disrupt energy balance, potentially affecting MMA metabolism and leading to the accumulation of MMA in tissues [39]. Most studies, although not all, have found that elevated MMA levels are associated with cognitive impairment in older adults [40, 41]. According to Doets et al. (2013), individuals with higher MMA concentrations experienced an accelerated decline in overall cognition, with a doubling of MMA concentration being linked to a roughly 60% increase in the rate of cognitive decline [42]. Meanwhile, although the cause-and-effect relation between high BP and mitochondrial dysfunction or oxidative stress remains elusive, some studies have observed elevated MMA levels in patients suffering with hypertension [37, 43–45]. Further, a high MMA level is often viewed as a marker of Vitamin B12 deficiency [46, 47], which suggests that supplementing vitamin B12 may have a potential to reduce the risk of MMA accumulation and that it could be possible to use vitamin B12 to prevent hypertension-induced cognitive decline for older adults. Thus, a study on the role of MMA between hypertension and cognitive decline is promising, having a potential to provide new therapeutic options.
Given that no particular study has explored the association between hypertension, MMA, cognitive decline in the older population, this study aims to elucidate the association between hypertension and cognitive function in this population by assessing the potential mediating role of MMA. It may add new knowledge on the pathway in the hypertension-cognition link and provide valuable insights into the development of appropriate measures for managing hypertension in older adults, especially preventing or delaying hypertension-induced cognitive decline.
Methods
Study design and participants
We conducted a cross-sectional study, utilizing the National Health and Nutrition Examination Survey (NHANES) database 2011–2014. NHANES is a national cross-sectional survey program conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) in two-year cycles, with a goal of providing insights into various health conditions, risk factors, and nutritional patterns of families and populations in the United States (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES 2011–2012 and 2013–2014 cycles, which are the only two cycles providing data on both serum MMA (sMMA) levels and cognitive function assessments with multiple tests, present a unique opportunity for conducting the proposed study. The survey's data collection procedures were approved by the research ethics review board of the National Center for Health Statistics. Written informed consent was obtained from all participants in the NHANES [48]. For protecting the identities of the individuals involved, the NHANES database anonymizes participants’ data and employs unique identifiers called “Respondent sequence number” (SEQN).
Our researchers found relevant datasets, i.e., Demographics Data, Dietary Data, Examination Data, Laboratory Data, and Questionnaire Data, for the years 2011–2012 and 2013–2014 at the section of “Questionnaires, Datasets, and Related Documentation" in NHANES homepage. We downloaded all the datasets and combined them by SEQN for each cycle. We harmonized all the data for making variable names and coding consistent between the two cycles.
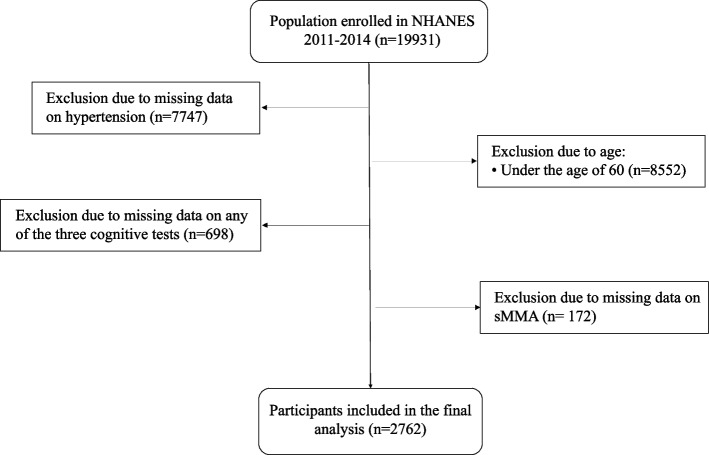
Figure 1 outlines the process of identifying the study population. The inclusion criteria for participant selection were: 1) adults aged 60 years or older, and 2) individuals with complete data on hypertension status, sMMA levels, and cognitive function assessments. Participants were excluded if they were under 60 years of age or had missing or incomplete data on hypertension, sMMA, or cognitive tests. As this study involved individuals aged 60 and above, a total of 19931 participants were identified. After excluding participants with missing information on hypertension, sMMA, and any test of cognitive function, 2762 participants were included in the analysis.
Fig. 1.
Flowchart of the study population. Figure 1 presents the flow chart of identifying the study population
NHANES created weights applied to the data to account for oversampling, nonresponse, and noncoverage, thereby forming representative samples of the U.S. civilian noninstitutionalized resident population. In this study involving Mobile Examination Center (MEC) data, the 2-year sample weight (wtmec2yr) accounted for 2 cycles (1/2*wtmec2yr) was used for all analyses, which allowed for the generation of nationally representative estimates with a weighted population estimated to be around 101 million. More information about the weights and their associated process could be found on the NHANES website via the following link: https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx.
Hypertension
In this study, hypertension (no/yes) was defined as systolic blood pressure > = 140 mmHg, diastolic blood pressure > = 90 mmHg [49], taking antihypertensive medications, or self-reported diagnosis of hypertension by the participants. These criteria have been widely used in NHANES-based studies to identify hypertension [50]. Participants who reported not having hypertension but taking antihypertensive medications were considered non-hypertensive, as the medications might be prescribed for reasons other than a high blood pressure.
In the NHANES program, BP is measured by trained clinicians at the MEC using a mercury sphygmomanometer and an appropriately sized blood pressure cuff. Blood pressure is measured after 5 min of sitting still, and three blood pressure readings is taken at 30-s intervals. If one of the three blood pressure measurements is invalid, a fourth reading will be taken. The participants had 1 to 4 blood pressure readings in the study. Some participants had only one reading, which was considered the final record. In cases where participants had more than one reading, the first reading was excluded in blood pressure calculation, which is a common practice in research and clinical practices to mitigate the potential impact of transient factors such as stress or physical activities that may influence the initial reading [51, 52]. In this study, the blood pressure was determined by averaging the readings after excluding the first reading.
Cognitive function
Participants' cognitive function was assessed using a combination of tests, including the Animal Fluency Test (AFT), the Digit Symbol Substitution Test (DSST), and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Learning Test [53]. We employed Z-transformation to standardize the AFT, CERAD wordlist learning test, and DSST scores individually and combined these standardized scores (AFT_Z, CERAD_Z, and DSST_Z) into a composite score called " Total_Score_Z," which served as a representation of the participants' overall cognitive function in this study [54].
The AFT is a verbal fluency test that assesses semantic memory and executive function by requiring participants to name as many animals as possible within 1 min, resulting in a final score representing the total number of correct answers. The CERAD wordlist learning test, commonly utilized in clinical and research settings to assess cognitive function in relation to Alzheimer's disease and other forms of dementia, assess verbal episodic memory and include three consecutive learning trials (CERAD Trial 1 Recall, CERAD Trial 2 Recall, and CERAD Trial 3 Recall) followed by a delayed recall (CERAD Delayed Recall), with the total score (CERAD wordlist learning test total) representing the sum of the four trials. As participants match symbols to 133 adjacent number boxes within 2 min, the DSST, a cognitive performance test from the Wechsler Adult Intelligence Scale-III, measures processing speed, sustained attention, and working memory, with higher scores reflecting better cognitive function.
MMA
For measuring sMMA, blood samples were collected from participants by trained phlebotomists at the MEC, and the amount of blood drawn varied depending on the participant’s age, with 75 μL of serum used for the analysis of sMMA. Serum samples were sent to the Division of Laboratory Sciences at the National Center for Environmental Health, CDC for analysis. For more information about the laboratory procedures of blood withdrawals, please consult the NHANES manual, which can be accessed on NHANES website [55]. An internal standard solution (d3-MMA) was added to 75 μl of serum, followed by MMA extraction through liquid–liquid extraction with tert-butylmethylether/H + . The extracted acid was then derivatized with butanol to form a dibutylester. Subsequently, the derivatized sample was reconstituted in acetonitrile–water, and MMA was separated chromatographically and quantified using liquid chromatography-mass spectrometry (LC–MS/MS) with multiple reaction monitoring [56].
Covariates
Variables, with available data from NHANES 2011–2014 (missing data < 60%), representing baseline characteristics of the participants, and identified as potential confounding factors that could exert an influence on the outcome based on previous studies [57, 58] or discussions conducted in the research team, were considered in this study and grouped into three categories as follows: 1) demographic characteristics including age, sex (male, female), ethnicity/race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race), marital status (married or living with partner, widowed/divorced/separated/never married), education level (below high school, high school or equivalent, college or above); 2) other health conditions, mainly body mass index (BMI) and Vitamin B12 levels (divided into three groups: < 148 pmol/L defined as severe deficiency, 148–220 pmol/L as mild deficiency, and > = 220 pmol/L as normal) [59–64]; 3) health-related lifestyle, including alcohol intake (a. short-term alcohol intake levels: within the recommended daily limits / exceeding the recommended limits [assessed by averaging alcohol consumption per day in grams of two 24 h dietary recall interviews; according to updated national guidelines and recommendations regarding daily alcohol consumption [65–67], the recommended daily alcohol intake is up to 14 g/day for women and up to 28 g/day for men]; b. at least 12 drinks in the past 12 months (no/yes) [defined by participants’ self-report of having at least 12 drinks of any type of alcoholic beverage in the past 12 months]), smoking (based on self-reported information, categorized as “never” if smoked < 100 cigarettes, “former” if not currently smoking but smoked ≥ 100 cigarettes in the past, or “current” if smoked ≥ 100 cigarettes and currently smokes every day or on some days), and physical activity (inactive/moderate/vigorous /both moderate and vigorous).
Statistical analysis
Descriptive statistics included mean with standard deviation (SD) and median with interquartile range (IQR) for continuous data, and frequencies and proportions for categorical data. The normality of the distribution of continuous variables in the dataset was assessed by the Kolmogorov–Smirnov as well as its histogram. As sMMA, a continuous variable, did not satisfy the assumption of normality, log-transformation was performed for its positively skewed distribution (Log_sMMA).
Prior to conducting the regression, we assessed multicollinearity among variables by calculating Variance Inflation Factors (VIFs) and found no evidence of multicollinearity among the variables. Linear regression models were employed to investigate the relationship between hypertension and cognitive function, between hypertension and the sMMA level, as well as between the sMMA level and cognitive function. Both unadjusted and multivariate-adjusted modeling were performed. The standardized regression coefficients (β) and their 95% confidence intervals (CIs) were calculated.
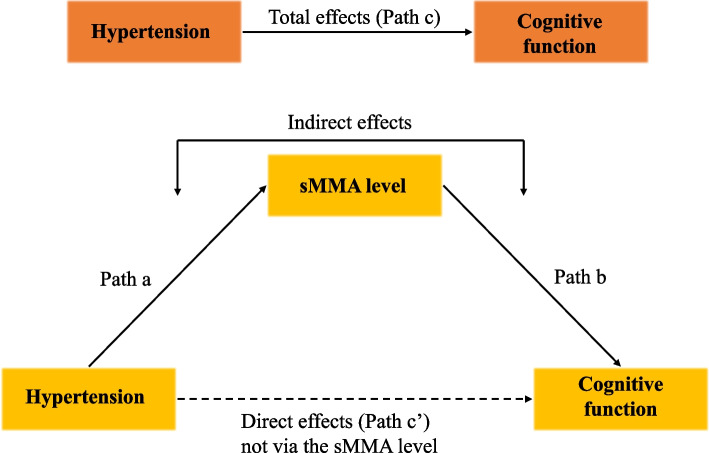
In detecting the potential mediating role of sMMA in the association between hypertension and cognitive function, we applied the strategy of distribution–of–the–product (Fig. 2), by checking 1) the total effect of hypertension on cognitive function while adjusting for all covariates (effect of path c), 2) the direct effect of hypertension on cognitive function after all covariates and sMMA were controlled (effect of path c’), 3) the indirect effect of hypertension on cognitive function through sMMA after all covariates were controlled (effect of path a * effect of path b), 4) the proportion mediated by sMMA (i.e., indirect effect/total effect).
Fig. 2.
Theoretical diagram of the mediation analysis. Figure 2 shows the theoretical model of the mediation analysis for the association between hypertension and cognitive function with the serum methylmalonic acid (sMMA) level as a potential mediator
Previous studies have demonstrated that sex and sex hormones are associated with both hypertension and cognitive function [68–70]. Additionally, research has indicated that sex influences the relationship between hypertension and cognitive function [70]. Taking into account the potential impact of sex on the results, we performed a subgroup analysis to investigate the variations in the mediating effect of sMMA on the relationship between hypertension and cognitive function in males and females.
A two-tailed p-value less than 0.05 was considered statistically significant. All statistical analyses were conducted using R version 4.3.1. The R mediation package was employed to conduct mediation analysis, with nonparametric bootstrapping in 1,000 resamplings [71]. Our analysis incorporated the complex sampling design and considered sampling weight (wtmec2yr/2) to enhance representativeness and accuracy in estimation. The R survey package was used to address the complex sampling design in practice [72].
Results
Participant characteristics
The characteristics, hypertension status, cognitive function scores, and the sMMA level of 2762 older adults (aged 60 years and above) are detailed in Table 1. The median age of the participants was 68 years (IQR: 12). The study population was evenly split between men and women. A significant majority of participants held a college degree or higher (75.00%). More than half were married or in a relationship (57.93%). Nearly half of the population identified as Mexican American (48.90%). Most participants were with abnormal BMI (74.50%). Approximately 70% of participants reported a low level of physical activity, nearly 90% indicated that their short-term alcohol consumption was within recommended limits, about 70% reported consuming more than 12 alcoholic drinks in the past 12 months and nearly 50% stated that they had never smoked. Around 70% of the participants had hypertension.
Table 1.
Basic characteristics of the study sample (NHANES 2011-2014)
| Characteristics | Overall (N=2762) |
|---|---|
| Age, N (%) | |
| <68 | 1266(45.80) |
| >=68 | 1496(54.20) |
| Sex, N (%) | |
| Male | 1355(49.10) |
| Female | 1407(50.90) |
| Ethnicity/Race, N (%) | |
| Non-Hispanic White | 244(8.80) |
| Non-Hispanic Black | 625(22.60) |
| Mexican American | 1351(48.90) |
| Other Hispanic | 278(10.10) |
| Other Race | 264(9.60) |
| Education Level, N (%) | (n=2760) |
| Below high school | 310(11.23) |
| High school or equivalent | 379(13.73) |
| College or above | 2071(75.04) |
| Marital status, N (%) | (n=2760) |
| Married/Living with partner | 1599(57.93) |
| Widowed/Divorced/Separated/ Never married | 1161(42.07) |
| BMI, kg/m2, N (%) | (n=2722) |
| 18.5-24.9 | 694(25.50) |
| <18.5 | 39(1.43) |
| 25-29.9 | 966(35.49) |
| >=30 | 1023(37.58) |
| Vitamin B12, pmol/L, N (%) | (n=2757) |
| <148 | 74(2.68) |
| 148-220 | 257(9.32) |
| >=220 | 2426(88.00) |
| Physical Activity, N (%) | |
| Inactive | 1919(69.48) |
| Moderate | 543(19.66) |
| Vigorous | 80(2.90) |
| Both moderate and vigorous | 220(7.96) |
| Short-term Alcohol Intake, gm, N (%) | (n=2393) |
| <= 28 (Male) / <=14 (Female) | 2140(89.43) |
| >28 (Male) / >14 (Female) | 253(10.57) |
| Alcohol Drinks >=12 in the Past 12 Months, N (%) | (n=2715) |
| No | 850(31.31) |
| Yes | 1865(68.69) |
| Smoking, N (%) | (n=2760) |
| Never | 1362(49.30) |
| Former | 1040(37.70) |
| Current | 358(13.00) |
| Hypertension, N (%) | |
| No | 821(29.03) |
| Yes | 1941(70.97) |
| sMMA, nmol/L, median (IQR) | 210.96(101.50) |
| Log_sMMA, mean (SD) | 5.18(0.52) |
| Total_Score, mean (SD) | 87.47(24.71) |
| Total_Score_Z, mean (SD) | 0.44(2.41) |
Categorical variables are number of subjects(percentage),continuous variables are mean±standard deviation. BMI Body mass index, sMMA Serum methylmalonic acid, Log_sMMA The log transformed sMMA; Total_Score: sum of CERAD wordlist learning test, AFT and DSST scores; Total_Score_Z: the sum of standardized scores of CERAD wordlist learning test, AFT and DSST
Association between hypertension and cognitive function
Table 2 demonstrates that hypertension was significantly associated with cognitive function (β [95% CI]: −0.982[−1.283, −0.680], p < 0.001). After sequentially introducing demographic characteristics, other health conditions, health-related lifestyle factors, and Log_MMA into the model, there was still a significant association between hypertension and cognitive function (β [95% CI]: −0.640[−0.935, −0.346], p < 0.001). Specifically, the individuals with hypertension had lower cognitive abilities than the ones without hypertension. From the crude model to Model 4, by gradually adding controlled variables, the R-square increased significantly, with F change values being statistically significant, except for the transition from model 1 to model 2 with the addition of BMI and Vitamin B12. This indicated that most additional variables had a notable effect on the dependent variable, namely the cognitive function score, and the model's explanatory power increased significantly with the addition of controlled variables.
Table 2.
Regressions exploring the association between hypertension and cognitive function
| Crude model | Model 1 | Model2 | Model 3 | Model 4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | P | β | 95%CI | P | β | 95%CI | P | β | 95%CI | P | β | 95%CI | P | |
| Hypertension | |||||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Yes | −0.982 | −1.283, −0.680 | < 0.001 | −0.640 | −0.931, −0.358 | < 0.001 | −0.666 | −0.956, −0.375 | < 0.001 | −0.699 | −0.983, −0.416 | < 0.001 | −0.640 | −0.935, −0.346 | < 0.001 |
| Age | |||||||||||||||
| < 68 | Ref | Ref | Ref | Ref | |||||||||||
| > = 68 | −1.582 | −1.803, −1.361 | < 0.001 | −1.536 | −1.771, −1.300 | < 0.001 | −1.439 | −1.666, −1.213 | < 0.001 | −1.376 | −1.601, −1.151 | < 0.001 | |||
| Sex | |||||||||||||||
| Male | Ref | Ref | Ref | Ref | |||||||||||
| Female | 0.590 | 0.387, 0.793 | < 0.001 | 0.608 | 0.385, 0.831 | < 0.001 | 0.699 | 0.429, 0.970 | < 0.001 | 0.681 | 0.405, 0.956 | < 0.001 | |||
| Ethnicity/Race | |||||||||||||||
| Non-Hispanic White | Ref | Ref | Ref | Ref | |||||||||||
| Non-Hispanic Black | −1.397 | −1.711, −1.082 | < 0.001 | −1.416 | −1.736, −1.095 | < 0.001 | −1.289 | −1.628, −0.941 | < 0.001 | −1.385 | −1.754, −1.016 | < 0.001 | |||
| Mexican American | −0.766 | −1.165, −0.368 | < 0.001 | −0.799 | −1.199, −0.398 | < 0.001 | −0.829 | −1.243, −0.416 | 0.001 | −0.896 | −1.327, −0.466 | 0.001 | |||
| Other Hispanic | −1.538 | −1.874, −1.203 | < 0.001 | −1.535 | −1.864, −1.206 | < 0.001 | −1.408 | −1.744, −1.071 | < 0.001 | −1.468 | −1.842, −1.094 | < 0.001 | |||
| Other Race | −0.755 | −1.115, −0.394 | < 0.001 | −0.701 | −1.077, −0.325 | 0.001 | −0.468 | −0.884, −0.053 | 0.031 | −0.558 | −0.975, −0.140 | 0.015 | |||
| Education Level | |||||||||||||||
| Below high school | Ref | Ref | Ref | Ref | |||||||||||
| High school or equivalent | 1.022 | 0.585,1.459 | < 0.001 | 0.998 | 0.559, 1.437 | < 0.001 | 0.837 | 0.345, 1.329 | 0.004 | 0.796 | 0.311, 1.282 | 0.005 | |||
| College or above | 2.436 | 2.046,2.827 | < 0.001 | 2.420 | 2.024, 2.816 | < 0.001 | 2.125 | 1.647, 2.603 | < 0.001 | 2.072 | 1.562, 2.581 | < 0.001 | |||
| Marital Status | |||||||||||||||
| Married/Living with partner | Ref | Ref | Ref | Ref | |||||||||||
| Widowed/Divorced/Separated/Never married | −0.382 | −0.689, −0.075 | 0.017 | −0.382 | −0.694, −0.069 | 0.020 | −0.330 | −0.651, −0.010 | 0.045 | −0.280 | −0.609, 0.050 | 0.087 | |||
| BMI | |||||||||||||||
| 18.5–24.9 | Ref | Ref | Ref | ||||||||||||
| < 18.5 | −0.482 | −1.337, 0.372 | 0.250 | −0.510 | −1.546, 0.526 | 0.299 | −0.458 | −1.498, 0.583 | 0.346 | ||||||
| 25–29.9 | 0.085 | −0.245, 0.415 | 0.593 | 0.073 | −0.277, 0.422 | 0.653 | 0.056 | −0.289, 0.401 | 0.722 | ||||||
| > = 30 | 0.205 | −0.151, 0.560 | 0.242 | 0.206 | −0.143, 0.555 | 0.217 | 0.194 | −0.159, 0.547 | 0.246 | ||||||
| Vitamin B12 | |||||||||||||||
| < 148 | Ref | Ref | Ref | ||||||||||||
| 148–220 | 0.563 | −0.050, 1.176 | 0.070 | 0.554 | −0.142, 1.251 | 0.107 | 0.343 | −0.417, 1.103 | 0.334 | ||||||
| > = 220 | 0.504 | −0.040, 1.047 | 0.067 | 0.503 | −0.063, 1.068 | 0.076 | 0.129 | −0.429, 0.688 | 0.613 | ||||||
| Physical Activity | |||||||||||||||
| Inactive | Ref | Ref | |||||||||||||
| Moderate | 0.275 | −0.021, 0.571 | 0.065 | 0.267 | −0.038, 0.572 | 0.079 | |||||||||
| Vigorous | 0.144 | −0.396, 0.684 | 0.566 | 0.211 | −0.313, 0.735 | 0.386 | |||||||||
| Both moderate and vigorous | 0.436 | 0.008, 0.864 | 0.047 | 0.395 | −0.048, 0.839 | 0.075 | |||||||||
| Short-term Alcohol Intake, gm, N (%) | |||||||||||||||
| < = 28 (Male) / < = 14 (Female) | Ref | Ref | |||||||||||||
| > 28 (Male) / > 14 (Female) | 0.192 | −0.269, 0.654 | 0.374 | 0.184 | −0.286, 0.654 | 0.399 | |||||||||
| Alcohol Drinks > = 12 in the Past 12 Months, N (%) | |||||||||||||||
| No | Ref | Ref | |||||||||||||
| Yes | 0.590 | 0.315, 0.864 | < 0.001 | 0.578 | 0.305, 0.852 | < 0.001 | |||||||||
| Smoking | |||||||||||||||
| Never | Ref | Ref | |||||||||||||
| Former | −0.285 | −0.628, 0.057 | 0.093 | −0.304 | −0.646, 0.038 | 0.076 | |||||||||
| Now | −0.596 | −1.015, −0.177 | 0.010 | −0.590 | −1.019, −0.160 | 0.013 | |||||||||
| Log_sMMA | −0.476 | −0.725, −0.227 | 0.002 | ||||||||||||
| R-squared | 0.0370 | 0.3120 | 0.3130 | 0.3290 | 0.3360 | ||||||||||
| Adjusted R-squared | 0.0367 | 0.311 | 0.3100 | 0.3261 | 0.3329 | ||||||||||
| F-change | 825.225, p < 0.001 | 5.886, p = 0.344 | 34.814, p = 0.017 | 18.703, p = 0.002 | |||||||||||
Crude model: Non-adjusted
Model 1: Adjusted for age, sex, ethnicity, education level, marital status
Model 2: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12
Model 3: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, smoking
Model 4: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, smoking, Log_sMMA
BMI Body mass index, Log_sMMA The logarithm of sMMA
Association between hypertension and the sMMA level
Following adjustments for age, sex, ethnicity/race, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, and smoking, it was observed that there was a significant association between hypertension and the sMMA level (β [95% CI]:0.123[0.078, 0.169], p < 0.001). Specifically, the individuals with hypertension had a higher sMMA level than the ones without hypertension. Table 3 shows the results of regressions regarding the association between the hypertension and the sMMA level.
Table 3.
Regressions exploring the association between hypertension and sMMA
| β | 95%CI | P-value | |
|---|---|---|---|
| Crude Model | 0.127 | 0.080,0.173 | < 0.001 |
| Model 1 | 0.111 | 0.063,0.160 | < 0.001 |
| Model 2 | 0.121 | 0.077, 0.165 | < 0.001 |
| Model 3 | 0.123 | 0.078, 0.169 | < 0.001 |
Crude model: Non-adjusted
Model 1: Adjusted for age, sex, ethnicity, education level, marital status
Model 2: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12
Model 3: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, smoking
The association between the sMMA level and cognitive function
There was a significant relationship between the sMMA level and cognitive function score (p < 0.001). Following adjustments for age, sex, ethnicity/race, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, and smoking, it was observed that Total_Score_Z decreased by around 0.567 SDs (approximately 1.366 points in the original scale) for each SD increase in the Log_sMMA (approximately 0.52 points in the natural scale). Table 4 shows the results of regressions regarding the association between the sMMA level and cognitive function.
Table 4.
Regressions exploring the association between sMMA and cognitive function
| β | 95%CI | P-value | |
|---|---|---|---|
| Crude Model | −0.887 | −1.144, −0.631 | < 0.001 |
| Model 1 | −0.557 | −0.774, −0.340 | < 0.001 |
| Model 2 | −0.588 | −0.789, −0.388 | < 0.001 |
| Model 3 | −0.567 | −0.801, −0.334 | < 0.001 |
Crude model: Non-adjusted
Model 1: Adjusted for age, sex, ethnicity, education level, marital status
Model 2: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12,
Model 3: Adjusted for age, sex, ethnicity, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, smoking
The mediating role of sMMA in the association between hypertension and cognitive function
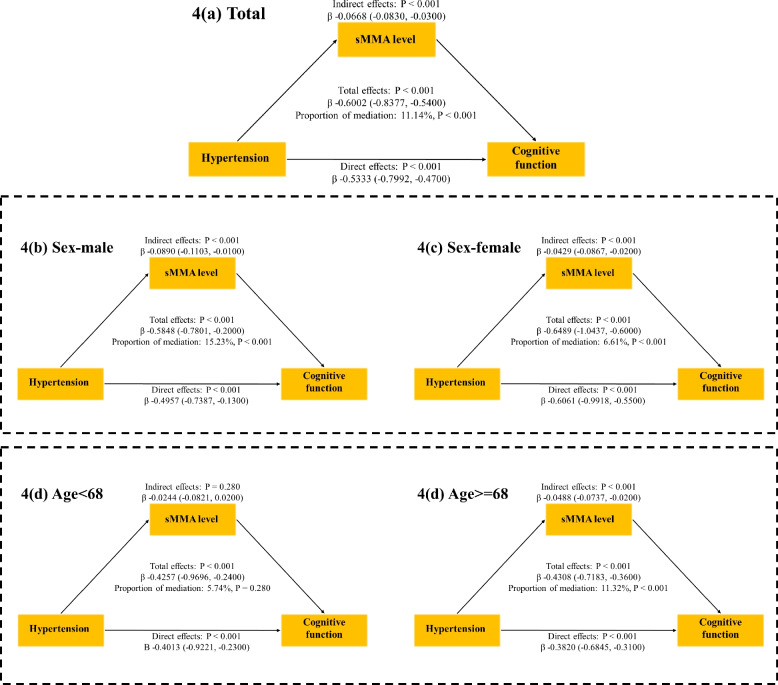
We further explored the potential mediating effect of sMMA on the relationship between hypertension and cognitive function reflected by Total_Score_Z. Table 5 and Fig. 3 show that after adjusting for controlled variables, the sMMA significantly mediated the link between hypertension and cognitive function (proportion mediated: 11.14%; Indirect effect: −0.0668[−0.0830, −0.0300], p < 0.001). In the subgroup analysis based on sex (male and female), after adjusting for all other covariates, the proportion mediated by the sMMA level in the relationship between hypertension and cognitive function was higher in males (15.23%, 95%CI 1.32%−27.00%, p < 0.001) than in females (6.61%, 95%CI 2.12%−10.00%, p < 0.001). Furthermore, this mediating effect was observed in individuals aged 68 years and older (11.32%, 95% CI: 3.80%—16.00%, p < 0.001), with no significant mediation detected in those younger than 68 years.
Table 5.
Direct and indirect effects of hypertension on cognitive function with sMMA as a mediator
| Adjusted Models | ||||
|---|---|---|---|---|
| Estimate | 95%CI lower | 95%CI upper | P-value | |
| Indirect effect | ||||
| Total | −0.0668 | −0.0830 | −0.0300 | < 0.001 |
| Sex | ||||
| Male | −0.0890 | −0.1103 | −0.0100 | < 0.001 |
| Female | −0.0429 | −0.0867 | −0.0200 | < 0.001 |
| Age | ||||
| < 68 | −0.0244 | −0.0821 | 0.0200 | 0.280 |
| > = 68 | −0.0488 | −0.0737 | −0.0200 | < 0.001 |
| Direct effect | ||||
| Total | −0.5333 | −0.7992 | −0.4700 | < 0.001 |
| Sex | ||||
| Male | −0.4957 | −0.7387 | −0.1300 | < 0.001 |
| Female | −0.6061 | −0.9918 | −0.5500 | < 0.001 |
| Age | ||||
| < 68 | −0.4013 | −0.9221 | −0.2300 | < 0.001 |
| > = 68 | −0.3820 | −0.6845 | −0.3100 | < 0.001 |
| Total effect | ||||
| Total | −0.6002 | −0.8377 | −0.5400 | < 0.001 |
| Sex | ||||
| Male | −0.5848 | −0.7801 | −0.2000 | < 0.001 |
| Female | −0.6489- | −1.0437 | −0.6000 | < 0.001 |
| Age | ||||
| < 68 | −0.4257 | −0.9696 | −0.2400 | < 0.001 |
| > = 68 | −0.4308 | −0.7183 | −0.3600 | < 0.001 |
| Proportion mediated | ||||
| Total | 0.1114 | 0.0409 | 0.1400 | < 0.001 |
| Sex | ||||
| Male | 0.1523 | 0.0132 | 0.2700 | < 0.001 |
| Female | 0.0661 | 0.0212 | 0.1000 | < 0.001 |
| Age | ||||
| < 68 | 0.0574 | −0.0313 | 0.1700 | 0.280 |
| > = 68 | 0.1132 | 0.0380 | 0.1600 | < 0.001 |
Adjusted Analyses: Adjusted for age, ethnicity, education level, marital status, BMI, Vitamin B12, physical activity, short-term alcohol intake, alcohol drinks > = 12 in the past 12 months, smoking. Sex was adjusted in the analyses for the total population but not in subgroup analyses of gender (female and male). Age was adjusted in the analyses for the total population (age was introduced into the models as a continuous variable) but not in subgroup analyses of age (< 68 and > = 68)
Fig. 3.
Mediating role of methylmalonic acid between hypertension and cognitive function. Figure 3 shows the results of the mediation analyses for the association between hypertension and cognitive function with the serum methylmalonic acid (sMMA) level as a potential mediator, with subgroup analyses for age and sex
Discussion
Our results revealed a significant association between hypertension and lower cognitive function, with sMMA mediating a significant portion of this relationship. The mediating effect of sMMA was more pronounced in males and individuals aged 68 years and older. This study highlights the potential importance of addressing both hypertension and MMA levels to mitigate cognitive decline in older adults.
The association between hypertension and cognitive function found in the study was consistent with numerous large-scale cross-sectional or longitudinal studies across different regions and age groups [29, 73–75]. For example, Wei et al. (2018), using longitudinal data from the China Health and Retirement Longitudinal Study (CHARLS), found that uncontrolled hypertension had a negative impact on cognitive function, particularly in the aspects of episodic memory and executive function [75]. However, inconsistent results have been reported in other studies, with some indicating no significant impact of hypertension on cognitive decline in older adults [76–78]. This variation could be partially attributed to differences in the characteristics of the studied populations, the approaches to defining hypertension, as well as the methods used to assess cognitive function or other confounding factors. Our results emphasize the importance of addressing hypertension in older individuals to prevent adverse cognitive decline and offering hypertensive older adults with proactive screening and tailored interventions.
Moreover, given the potential impact of age, sex, ethnicity/race, education level, marital status, and smoking on cognitive function, it may be crucial to offer additional support to older men from non-white ethnic groups, with lower levels of education, without a partner, or who are smoking. Interestingly, alcohol intake > = 12 drinks in the past 12 months was positively associated with cognitive function, while short-term alcohol intake showed no significant association with cognitive performance. This may be due to the fact that only a small proportion of the participants (n = 253, 9.2%) exceeded the recommended daily alcohol consumption limits, while the majority engaged in light to moderate alcohol consumption, which has been shown to have protective effects on cognitive function [79–81]. The protective effects of moderate alcohol intake on cognition are likely due to mechanisms such as improved cardiovascular health, which is closely linked to brain health [82, 83]. These findings highlight the importance of considering both the quantity and frequency of alcohol consumption, as well as capturing habitual drinking patterns alongside recent consumption behaviors, when assessing the effects of alcohol intake on cognition.
An important observation from this study is that the sMMA level was associated with hypertension. Recently, similar to our study, Wang et al. (2022) using data from NHANES 2013–2014, and Dhar et al. (2023) using data from two large clinical cohorts in Norway, found that a higher level of sMMA was associated with hypertension [43, 84]. According to Forte et al. (2020), hypertension accelerates vascular aging, primarily through oxidative stress and mitochondrial dysfunction [85]. These processes are reflected in elevated MMA levels, which are recognized as key mechanisms contributing to the functional and structural changes in aging blood vessels. Other researchers have suggested that the signaling involved in regulating blood pressure may contribute to vascular mitochondrial dysfunction and increase MMA [86, 87].
Previous studies noted that elevated MMA levels were associated with cognitive decline [88] and the decline in overall cognitive function was accelerated in individuals with higher MMA concentrations [42]. By controlling for B12 levels in our regressions, our study further corroborated this by observing that MMA may have an independent effect on cognition, distinct from the effect of B12. Apart from its role as a biomarker reflecting the status of other substances such as B12, MMA, a pathogenic substance, can also be considered an independent neurotoxin capable of causing brain injury and cognitive impairment [89], which deserves special attention. Some studies revealed MMA could lead to neuronal damage by inhibiting the mitochondrial respiratory chain [90, 91], the transmitochondrial malate shuttle [92], pyruvate carboxylase activity [93] and β-hydroxybutyrate [94], resulting in neuron apoptosis through mechanisms involving oxidative stress injury [44, 95], neuroinflammation [96, 97] and DNA damage [98].
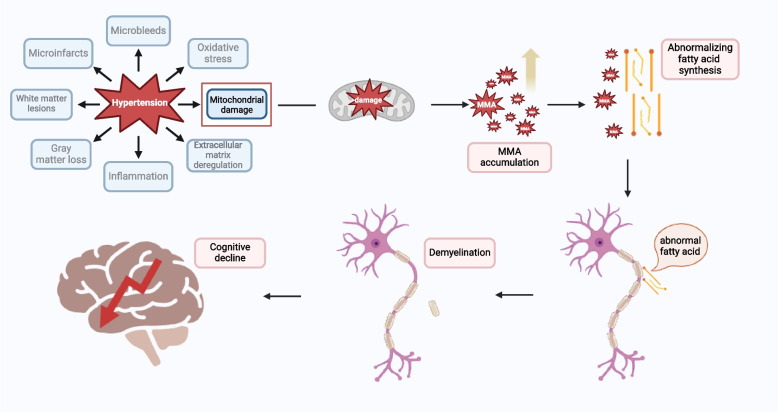
The key finding in this study was that sMMA exhibited a significant mediating effect (11.14%) between hypertension and cognitive function in older adults (Fig. 3). This suggests that hypertension may impact cognitive decline through the accumulation of MMA, highlighting the potential importance of MMA in hypertension-related cognitive decline in older adults. Previous understanding on the pathway through which hypertension leads to cognitive decline was mainly related to vascular pathology [99]. Long-term exposure to hypertension contributes to microvascular damage in the brain arteries, resulting in dysfunction of the blood–brain barrier (BBB), neuroinflammation, and the accumulation of neurotoxic molecules, which initiates and promotes neurodegeneration and cognitive impairment [100, 101]. Our results provide additional perspectives and insights into the potential mechanisms involving MMA in the pathway from hypertension to cognitive decline in older adults. One possible explanation could be that high blood pressure may cause mitochondrial damage [85], promoting the accumulation of MMA, a type of neurotoxic molecules [102], and then the accumulation of MMA can disrupt normal fatty acid synthesis by interfering with metabolic pathways, potentially substituting for malonic acid (propanedioic acid) in the fatty acid synthesis process [103]. This disruption can impair the production of essential fatty acids needed for cellular functions. Those abnormal or defected acids may penetrate the myelin, causing it to become fragile and resulting in demyelination [104, 105], which makes neuronal function and survival compromised, leading to axonal degeneration and progressive deterioration in neurological function [106]. Figure 4 illustrates a proposed mechanistic pathway linking hypertension to cognitive decline, highlighting the potential mediating role of MMA in older adults.
Fig. 4.
A hypothesized mechanistic pathway between hypertension and cognitive decline via methylmalonic acid. Figure 4 illustrates a proposed mechanistic pathway linking hypertension to cognitive decline, highlighting the potential mediating role of MMA. (This illustration was created with BioRender.com)
It has been suggested that B12-deficiency may facilitate the accumulation of MMA [107, 108], and that vitamin B12 could increase methionine for promoting the synthesis of myelin and neurotransmitters, and promote remyelination [108], meanwhile decrease the accumulation of MMA for preventing demyelination [107–112]. Evidence has shown that vitamin B12 supplementation could decrease serum MMA levels in older adults [113]. Therefore, supplementing with vitamin B12 may help in reducing hypertension-induced MMA accumulation and preventing cognitive decline for older adults with hypertension.
This study also suggests that the mediating effect of MMA may be more pronounced in males than in females. Gender differences in hypertension have been observed, likely due to variations in hormones and gene dosage on sex chromosomes [114]. Gender has also been associated with health behaviours, such as higher smoking rates in males, which increase the risk of developing chronic diseases, including hypertension [115, 116]. Sex hormones may influence cognitive function, with estradiol potentially being associated with better performance in certain cognitive domains in females [117]. It was noted by large-scale studies that there was a significant association between sex hormones and cognitive function [117]. It may be the sex hormones and lifestyles of older males that contribute to their susceptibility to hypertension-induced and MMA-related cognitive decline, and more efforts may need to be made for monitoring and addressing males’ MMA among hypertensive older adults.
Additionally, the observation of age-related differences in the mediating effects of MMA suggests that MMA’s role as a mediator may be more pertinent for older adults with a more advanced age. Studies have shown that the prevalence of hypertension, which is notably higher in older elderly compared to younger elderly [7, 118, 119], and cognitive decline is also notably pronounced after age 65 and accelerates significantly in the final years of life [120–122]. Gomes et al. (2020) found that sMMA accumulated with age, potentially fostering disease progression [123]. As, older elderly may have a longer duration of exposure to both hypertension and related metabolic changes, they could be more vulnerable to hypertension-induced and MMA-related cognitive decline.
Strength and limitations
The key strength of this study with a large population from NHANES 2011–2014 lies in its pioneering revelation of the potential mediation of the association between hypertension and cognitive function by the sMMA level. This contribution sheds new light on the understanding of the relationship between hypertension and cognitive decline, opening up avenues for further research and potential interventions. Furthermore, in contrast to previous studies using only one indicator [124, 125] to measure cognitive function, our study incorporated three reliable cognitive assessments (AFT, CERAD wordlist learning test, and DSST), which could provide a more robust and multifaceted evaluation of cognitive function. Meanwhile, aligning our approaches to identifying diseases and clinical conditions with that of other studies enhances the comparability of our findings with the other relevant research.
Another key strength of this study lies in the comprehensive measurement of alcohol intake, which was assessed using both short-term and long-term consumption metrics. By incorporating two methods—short-term alcohol intake based on two separate 24-h dietary recall interviews and long-term consumption defined as consuming at least 12 drinks in the past 12 months—the study captured both recent drinking behaviors and habitual patterns. This dual approach provides a more nuanced understanding of alcohol consumption and its potential impact on cognitive function and strengthens the study's findings.
However, it is important to acknowledge the limitations of our study. First, in this cross-sectional study, we were unable to establish a definitive causal relationship between hypertension, the sMMA level, and cognitive function in older adults. To address this, future research could replicate this investigation using longitudinal data to validate the associations.
Moreover, it is important to acknowledge that the cognitive function indexes (AFT, CERAD wordlist learning test, and DSST scores) utilized in this study as well as the data processing methods employed to create the composite score for assessing overall cognitive performance, may introduce potential biases in the results. While similar strategies have been utilized to assess participants' cognition in previous studies based on the NHANES dataset, the reliability and validity of this overall assessment still require further investigation, and other validated methods to assess cognitive function or decline should be considered in future studies.
Additionally, the scope of our study, the availability of data in NHANES, and limitations in sample size for successful modeling may have constrained the selection and categorization of covariates, potentially leading to the omission of certain important covariates that should be considered. For example, psychiatric disorders such as schizophrenia and depression [126, 127], which are often associated with cognitive decline, were not included in our models due to the fact that adding these variables caused model overloading and led to failures in model fitting. Thus, more potential confounders, particularly those reflecting mental health, should be included and addressed in future studies. While our study investigated the partially mediating role of sMMA in the association between hypertension and cognitive function, it is important to acknowledge that there may be other mechanisms or mediating factors at play. Further research is needed to explore these additional mechanisms in order to gain a deeper understanding of this complex relationship.
We also have to acknowledge that a large number of participants were excluded from this study due to being under the age of 60, as shown in Fig. 1. While our focus was on older adults due to the higher prevalence of both hypertension and cognitive decline in this population, future studies could explore the relationship between hypertension, MMA, and cognitive function in younger adults. Examining these factors in a younger cohort may provide insights into the early effects of hypertension and metabolic changes on vascular and cognitive health, potentially identifying earlier opportunities for intervention. In addition, as the majority of our participants probably engaged in little to moderate alcohol drinking, the overrepresentation of light to moderate drinkers may introduce bias, potentially underestimating or overlooking the negative cognitive impacts of heavier drinking. Further research could include a more diverse population with varying levels of alcohol consumption to provide more rigorous insights.
Conclusion
This study with a large population suggests that the sMMA level may mediate the link between hypertension and cognitive decline in older adults. Thus, reducing sMMA levels in older adults with hypertension could be critical for preventing cognitive decline and maintaining mental health. Monitoring B12 levels and Vitamin B12 supplementation could be considered in providing care to hypertensive older adults, particularly for males with an advanced age.
Acknowledgements
We express our gratitude to all the staff at NHANES for their valuable contribution in data acquisition.
Clinical trial number
The authors of this study utilized data from NHANES (The National Health and Nutrition Examination Survey of the U.S., which provides open access to datasets). No clinical trial was conducted by the authors, and therefore, a clinical trial number was not available.
Abbreviations
- AFT
Animal fluency test
- ATP
Adenosine triphosphate
- BBB
Blood-brain barrier
- BP
Blood pressure
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- CDC
Centers for disease control and prevention
- CHARLS
China health and retirement longitudinal study
- CI
Confidence interval
- DBP
Diastolic blood pressure
- DSST
Digit Symbol Substitution Test
- IQR
Interquartile range
- MCI
Mild cognitive impairment
- MEC
Mobile examination center
- MMA
Methylmalonic acid
- NCHS
National center for health statistics
- NHANES
National health and nutrition examination survey
- SBP
Persistent systolic blood pressure
- SD
Standard deviation
- SEQN
Respondent sequence number
- sMMA
Serum methylmalonic acid
- VIFs
Variance Inflation Factors
Authors’ contributions
(CRediT author statement). Ying Xu: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization. Rucheng Chen: Methodology, Validation, Writing - Review & Editing, Paulus Torkki: Conceptualization, Methodology, Validation, Writing - Review & Editing, Weijun Zheng: Conceptualization, Methodology, Validation, Investigation, Writing - Review & Editing, Supervision. An Chen: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Funding
This study was supported by Funds for Cultivating Young Talents of Zhejiang Chinese Medical University (701100E005, 701100E029).
Data availability
The National Health and Nutrition Examination Survey (NHANES) data are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
The NHANES protocol was approved by the Institutional Review Board of the CDC's National Center for Health Statistics. Written informed consent was obtained from each participant prior to their participation in NHANES programme.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Xu and An Chen are co-first authors and contributed equally to this work.
Contributor Information
Weijun Zheng, Email: zcmu_zwj@163.com.
An Chen, Email: chenan198710@outlook.com.
References
- 1.Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension.
- 2.Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. 2024;21(6):396–416. 10.1038/s41569-023-00964-1. [DOI] [PubMed]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Mattix-Kramer HJ, Basile JN, Sutherland SE. Managing hypertension in older adults. Curr Hypertens Rep. 2024;26(4):157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA. 2022;328(18):1849–61. [DOI] [PubMed] [Google Scholar]
- 10.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH Jr. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76(22):1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH Jr, Coresh J, Szklo M, Carvalho MS, Selvin E. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78(2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–74. [DOI] [PubMed] [Google Scholar]
- 14.Canavan M, O’Donnell MJ. Hypertension and cognitive impairment: a review of mechanisms and key concepts. Front Neurol. 2022;13:821135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor L, Watkins SL, Marshall H, Dascombe BJ, Foster J. The impact of different environmental conditions on cognitive function: a focused review. Front Physiol. 2015;6:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Wang D, Hou W, Li H. Cognitive decline associated with aging. Adv Exp Med Biol. 2023;1419:25–46. [DOI] [PubMed] [Google Scholar]
- 17.Collyer TA, Murray AM, Woods RL, Storey E, Chong TT, Ryan J, Orchard SG, Brodtmann A, Srikanth VK, Shah RC, et al. Association of dual decline in cognition and gait speed with risk of dementia in older adults. JAMA Netw Open. 2022;5(5):e2214647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastva AM, Hugenschmidt CE, Kitzman DW, Nelson MB, Brenes GA, Reeves GR, Mentz RJ, Whellan DJ, Chen H, Duncan PW. Cognition, physical function, and quality of life in older patients with acute decompensated heart failure. J Card Fail. 2021;27(3):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Röhr S, Pabst A, Riedel-Heller SG, Jessen F, Turana Y, Handajani YS, Brayne C, Matthews FE, Stephan BCM, Lipton RB, et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res Ther. 2020;12(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai W, Chen P, Cai H, Zhang Q, Su Z, Cheung T, Jackson T, Sha S, Xiang YT. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a metaanalysisand systematic review of epidemiology studies. Age Ageing. 2022;51(8):afac173. 10.1093/ageing/afac173. [DOI] [PubMed]
- 21.Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia.
- 22.Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension. 2023;80(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer’s disease. Lancet. 2021;397(10284):1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, Tapia AL, DavisThomas S, Windham BG, Coker L, Schneider ALC, Alonso A, Coresh J, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(11):1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71(10):1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao J, Liu J, Li Z, Zhang Z, Su X, Sun J, Tu J, Wang J, Li J, Song Y, et al. Relationship between hypertension and cognitive function in an elderly population: a population-based study in Rural Northern China. Front Neurol. 2022;13:885598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, Li Y, Li Y, Zhu M, Jiao H, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661–71. [DOI] [PubMed] [Google Scholar]
- 29.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, et al. Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. JAMA. 2019;322(6):535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce OC, McHugh C, Mockler D, Wilson F, Kelly ÁM. Midlife hypertension is a risk factor for some, but not all, domains of cognitive decline in later life: a systematic review and meta-analysis. J Hypertens. 2024;42(2):205–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malone JE, Elkasaby MI, Lerner AJ. Effects of Hypertension on Alzheimer’s Disease and Related Disorders. Curr Hypertens Rep. 2022;24(12):615–25. [DOI] [PubMed] [Google Scholar]
- 32.Zúñiga Salazar G, Zúñiga D, Balasubramanian S, Mehmood KT, Al-Baldawi S. The relation between arterial hypertension and cognitive impairment: a literature review. Cureus. 2024;16(1):e52782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension. 2016;67(1):171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58(8):1175–81. [DOI] [PubMed] [Google Scholar]
- 35.Baggeroer CE, Cambronero FE, Savan NA, Jefferson AL, Santisteban MM. Basic mechanisms of brain injury and cognitive decline in hypertension. Hypertension. 2024;81(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheon EJ. Hypertension and cognitive dysfunction: a narrative review. J Yeungnam Med Sci. 2023;40(3):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Liu Y, Liu J, Tian W, Zhang X, Cai H, Fang S, Yu B. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 2020;37:101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao B, Xue Y, Liu D. The association between methylmalonic acid, a biomarker of mitochondria dysfunction, and phenotypic age acceleration: a population-based study. Arch Gerontol Geriatr. 2024;117:105176. [DOI] [PubMed] [Google Scholar]
- 39.Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D, Hargreaves IP. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J Clin Med. 2017;6(7):71. 10.3390/jcm6070071. [DOI] [PMC free article] [PubMed]
- 40.Pascoe MC, Linden T. Folate and MMA predict cognitive impairment in elderly stroke survivors: a cross sectional study. Psychiatry Res. 2016;243:49–52. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Wu L, Wang S, Pan Y, Zhang A. Increased serum methylmalonic acid levels were associated with the presence of cognitive dysfunction in older chronic kidney disease patients with albuminuria. BMC Geriatr. 2024;24(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doets EL, van Wijngaarden JP, Szczecińska A, Dullemeijer C, Souverein OW, Dhonukshe-Rutten RA, Cavelaars AE, van ’t Veer P, Brzozowska A, de Groot LC. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol Rev. 2013;35:2–21. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Li W, Xiang M. Increased serum methylmalonic acid levels were associated with the presence of cardiovascular diseases. Front Cardiovasc Med. 2022;9:966543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Wang S, Zhang X, Cai H, Liu J, Fang S, Yu B. The regulation and characterization of mitochondrial-derived methylmalonic acid in mitochondrial dysfunction and oxidative stress: from basic research to clinical practice. Oxid Med Cell Longev. 2022;2022:7043883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griendling KK, Camargo LL, Rios FJ, Alves-Lopes R, Montezano AC, Touyz RM. Oxidative stress and hypertension. Circ Res. 2021;128(7):993–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li A, Du M, Chen Y, Marks LAM, Visser A, Xu S, Tjakkes GE. Periodontitis and cognitive impairment in older adults: The mediating role of mitochondrial dysfunction. J Periodontol. 2022;93(9):1302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riphagen IJ, Minović I, Groothof D, Post A, Eggersdorfer ML, Kootstra-Ros JE, de Borst MH, Navis G, Muskiet FAJ, Kema IP, et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: results from the prospective Lifelines-MINUTHE study. BMC Med. 2020;18(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat 2. 2013;160:1–23. [PubMed] [Google Scholar]
- 49.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):1269–324. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Wang Z. Association between joint physical activity and healthy dietary patterns and hypertension in US adults: cross-sectional NHANES study. BMC Public Health. 2024;24(1):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Z. Association between blood lead level with high blood pressure in US (NHANES 1999–2018). Front Public Health. 2022;10:836357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miao H, Liu Y, Tsai TC, Schwartz J, Ji JS. Association between blood lead level and uncontrolled hypertension in the US population (NHANES 1999–2016). J Am Heart Assoc. 2020;9(13):e015533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou L. Association of vitamin B2 intake with cognitive performance in older adults: a cross-sectional study. J Transl Med. 2023;21(1):870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smagula SF, Zhang G, Gujral S, Covassin N, Li J, Taylor WD, Reynolds CF 3rd, Krafty RT. Association of 24-Hour Activity Pattern Phenotypes With Depression Symptoms and Cognitive Performance in Aging. JAMA Psychiat. 2022;79(10):1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.NHANES 2013–2014 Laboratory Data Overview. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2013.
- 56.Mineva EM, Zhang M, Rabinowitz DJ, Phinney KW, Pfeiffer CM. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal Bioanal Chem. 2015;407(11):2955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin J, Gong R, Zhang M, Ding L, Shen T, Cai Y, He S, Peng D. Associations between sleep disturbance, inflammatory markers and depressive symptoms: Mediation analyses in a large NHANES community sample. Prog Neuropsychopharmacol Biol Psychiatry. 2023;126:110786. [DOI] [PubMed] [Google Scholar]
- 58.Liu D, Zhou L, Yang M, McIntyre RS, Cao B. Oxidative stress mediates the association between dietary fat intake and cognition in US older adults. Am J Geriatr Psychiatry. 2022;30(7):761–73. [DOI] [PubMed] [Google Scholar]
- 59.Warendorf JK, van Doormaal PTC, Vrancken A, Verhoeven-Duif NM, van Eijk RPA, van den Berg LH, Notermans NC. Clinical relevance of testing for metabolic vitamin B12 deficiency in patients with polyneuropathy. Nutr Neurosci. 2022;25(12):2536–46. [DOI] [PubMed] [Google Scholar]
- 60.Green R, Miller JW. Vitamin B12 deficiency. Vitam Horm. 2022;119:405–39. [DOI] [PubMed] [Google Scholar]
- 61.Wolffenbuttel BH, Owen PJ, Ward M, Green R. Vitamin B(12). BMJ. 2023;383:e071725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology. 2011;77(22):1947–50. [DOI] [PubMed] [Google Scholar]
- 63.Siswanto O, Smeall K, Watson T, Donnelly-Vanderloo M, O’Connor C, Foley N, Madill J. Examining the Association between Vitamin B12 Deficiency and Dementia in High-Risk Hospitalized Patients. J Nutr Health Aging. 2015;19(10):1003–8. [DOI] [PubMed] [Google Scholar]
- 64.Tu MC, Lo CP, Chen CY, Huang CF. Correlation of Tc-99 m ethyl cysteinate dimer single-photon emission computed tomography and clinical presentations in patients with low cobalamin status. BMC Neurol. 2015;15:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dietary Guidelines for Americans, 2020–2025. https://health.gov/healthypeople/tools-action/browse-evidence-based-resources/dietary-guidelines-americans-2020-2025.
- 66.Alcohol use and your health. 2022. https://www.cdc.gov/alcohol/about-alcohol-use/index.html.
- 67.Rethinking Drinking. https://rethinkingdrinking.niaaa.nih.gov/.
- 68.Hogervorst E, Matthews FE, Brayne C. Are optimal levels of testosterone associated with better cognitive function in healthy older women and men? Biochim Biophys Acta. 2010;1800(10):1145–52. [DOI] [PubMed] [Google Scholar]
- 69.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. [DOI] [PubMed] [Google Scholar]
- 70.Kritz-Silverstein D, Laughlin GA, McEvoy LK, Barrett-Connor E. Sex and Age Differences in the Association of Blood Pressure and Hypertension with Cognitive Function in the Elderly: The Rancho Bernardo Study. J Prev Alzheimers Dis. 2017;4(3):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59(5):1–38.26917999 [Google Scholar]
- 72.Meneses-León J, León-Maldonado L, Macías N, Torres-Ibarra L, Hernández-López R, Rivera-Paredez B, Flores M, Flores YN, Barrientos-Gutiérrez T, Quezada-Sánchez AD, et al. Sugar-sweetened beverage consumption and risk of hyperuricemia: a longitudinal analysis of the Health Workers Cohort Study participants in Mexico. Am J Clin Nutr. 2020;112(3):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X, Dong C, Levin BE, Caunca M, Zeki Al Hazzourie A, DeRosa JT, Stern Y, Cheung YK, Elkind MSV, Rundek T, et al. Systolic blood pressure and cognition in the elderly: the Northern Manhattan study. J Alzheimers Dis. 2021;82(2):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Menezes ST, Giatti L, Brant LCC, Griep RH, Schmidt MI, Duncan BB, Suemoto CK, Ribeiro ALP, Barreto SM. Hypertension, Prehypertension, and Hypertension Control: Association With Decline in Cognitive Performance in the ELSA-Brasil Cohort. Hypertension. 2021;77(2):672–81. [DOI] [PubMed] [Google Scholar]
- 75.Wei J, Yin X, Liu Q, Tan L, Jia C. Association between hypertension and cognitive function: A cross-sectional study in people over 45 years old in China. J Clin Hypertens (Greenwich). 2018;20(11):1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuyumcu ME, Yesil Y, Yavuz BB, Halil M, Cankurtaran M, Arıoğul S. Relationship between blood pressure and physical and cognitive function in the oldest old. J Am Geriatr Soc. 2013;61(5):828–9. [DOI] [PubMed] [Google Scholar]
- 77.Farmer ME, Kittner SJ, Abbott RD, Wolz MM, Wolf PA, White LR. Longitudinally measured blood pressure, antihypertensive medication use, and cognitive performance: the Framingham Study. J Clin Epidemiol. 1990;43(5):475–80. [DOI] [PubMed] [Google Scholar]
- 78.Zhu L, Viitanen M, Guo Z, Winblad B, Fratiglioni L. Blood pressure reduction, cardiovascular diseases, and cognitive decline in the mini-mental state examination in a community population of normal very old people: a three-year follow-up. J Clin Epidemiol. 1998;51(5):385–91. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Li Y, Zheng X, Zhu L, Xu B. Association between alcohol consumption in midlife and cognitive function in old age: Findings from the China health and Nutrition Survey. Nutr Metab Cardiovasc Dis. 2021;31(11):3044–53. [DOI] [PubMed] [Google Scholar]
- 80.Zhang R, Shen L, Miles T, Shen Y, Cordero J, Qi Y, Liang L, Li C. Association of low to moderate alcohol drinking with cognitive functions from middle to older age among US adults. JAMA Netw Open. 2020;3(6):e207922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reas ET, Laughlin GA, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK. Moderate, regular alcohol consumption is associated with higher cognitive function in older community-dwelling adults. J Prev Alzheimers Dis. 2016;3(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Gaetano G, Costanzo S, Di Castelnuovo A, Badimon L, Bejko D, Alkerwi A, Chiva-Blanch G, Estruch R, La Vecchia C, Panico S, et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr Metab Cardiovasc Dis. 2016;26(6):443–67. [DOI] [PubMed] [Google Scholar]
- 83.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dhar I, Lysne V, Ulvik A, Svingen GFT, Pedersen ER, Bjørnestad E, Olsen T, Borsholm R, Laupsa-Borge J, Ueland PM, et al. Plasma methylmalonic acid predicts risk of acute myocardial infarction and mortality in patients with coronary heart disease: A prospective 2-cohort study. J Intern Med. 2023;293(4):508–19. [DOI] [PubMed] [Google Scholar]
- 85.Forte M, Stanzione R, Cotugno M, Bianchi F, Marchitti S, Rubattu S. Vascular ageing in hypertension: focus on mitochondria. Mech Ageing Dev. 2020;189:111267. [DOI] [PubMed] [Google Scholar]
- 86.Vereczki V, Mansour J, Pour-Ghaz I, Bodnar I, Pinter O, Zelena D, Oszwald E, Adam-Vizi V, Chinopoulos C. Cyclophilin D regulates lifespan and protein expression of aging markers in the brain of mice. Mitochondrion. 2017;34:115–26. [DOI] [PubMed] [Google Scholar]
- 87.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial Cyclophilin D in Vascular Oxidative Stress and Hypertension. Hypertension. 2016;67(6):1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kvestad I, Hysing M, Shrestha M, Ulak M, Thorne-Lyman AL, Henjum S, Ueland PM, Midttun Ø, Fawzi W, Chandyo RK, et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am J Clin Nutr. 2017;105(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 89.Kölker S, Okun JG. Methylmalonic acid–an endogenous toxin? Cell Mol Life Sci. 2005;62(6):621–4. [DOI] [PubMed] [Google Scholar]
- 90.Brusque AM, Borba Rosa R, Schuck PF, Dalcin KB, Ribeiro CA, Silva CG, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, et al. Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int. 2002;40(7):593–601. [DOI] [PubMed] [Google Scholar]
- 91.Proctor EC, Turton N, Boan EJ, Bennett E, Philips S, Heaton RA, Hargreaves IP. The effect of methylmalonic acid treatment on human neuronal cell coenzyme Q10 status and mitochondrial function. Int J Mol Sci. 2020;21(23):9137. 10.3390/ijms21239137. [DOI] [PMC free article] [PubMed]
- 92.Halperin ML, Schiller CM, Fritz IB. The inhibition by methylmalonic acid of malate transport by the dicarboxylate carrier in rat liver mitochondria. A possible explantation for hypoglycemia in methylmalonic aciduria. J Clin Invest. 1971;50(11):2276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberholzer VG, Levin B, Burgess EA, Young WF. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967;42(225):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dutra JC, Dutra-Filho CS, Cardozo SE, Wannmacher CM, Sarkis JJ, Wajner M. Inhibition of succinate dehydrogenase and beta-hydroxybutyrate dehydrogenase activities by methylmalonate in brain and liver of developing rats. J Inherit Metab Dis. 1993;16(1):147–53. [DOI] [PubMed] [Google Scholar]
- 95.Royes LF, Gabbi P, Ribeiro LR, Della-Pace ID, Rodrigues FS, de Oliveira Ferreira AP, da Silveira Junior ME, da Silva LR, Grisólia AB, Braga DV, et al. A neuronal disruption in redox homeostasis elicited by ammonia alters the glycine/glutamate (GABA) cycle and contributes to MMA-induced excitability. Amino Acids. 2016;48(6):1373–89. [DOI] [PubMed] [Google Scholar]
- 96.Gabbi P, Ribeiro LR, Jessié Martins G, Cardoso AS, Haupental F, Rodrigues FS, Machado AK, Sperotto Brum J, Medeiros Frescura Duarte MM, Schetinger MR, et al. Methylmalonate induces inflammatory and apoptotic potential: a link to glial activation and neurological dysfunction. J Neuropathol Exp Neurol. 2017;76(3):160–78. [DOI] [PubMed] [Google Scholar]
- 97.Li Q, Song W, Tian Z, Wang P. Aminoguanidine alleviated MMA-induced impairment of cognitive ability in rats by downregulating oxidative stress and inflammatory reaction. Neurotoxicology. 2017;59:121–30. [DOI] [PubMed] [Google Scholar]
- 98.Andrade VM, Dal Pont HS, Leffa DD, Damiani AP, Scaini G, Hainzenreder G, Streck EL, Ferreira GC, Schuck PF. Methylmalonic acid administration induces DNA damage in rat brain and kidney. Mol Cell Biochem. 2014;391(1–2):137–45. [DOI] [PubMed] [Google Scholar]
- 99.Habes M, Grothe MJ, Tunc B, McMillan C, Wolk DA, Davatzikos C. Disentangling Heterogeneity in Alzheimer’s Disease and Related Dementias Using Data-Driven Methods. Biol Psychiatry. 2020;88(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Anda-Duran I, Woltz SG, Bell CN, Bazzano LA. Hypertension and cognitive function: a review of life-course factors and disparities. Curr Opin Cardiol. 2022;37(4):326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cortes-Canteli M, Iadecola C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(8):942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun S, Jin H, Rong Y, Song W, Li Q. Methylmalonic acid levels in serum, exosomes, and urine and its association with cblC type methylmalonic acidemia-induced cognitive impairment. Front Neurol. 2022;13:1090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tejero J, Lazure F, Gomes AP. Methylmalonic acid in aging and disease. Trends Endocrinol Metab. 2024;35(3):188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duffield MS, Phillips JI, Vieira-Makings E, Van der Westhuyzen J, Metz J. Demyelinisation in the spinal cord of vitamin B12 deficient fruit bats. Comp Biochem Physiol C Comp Pharmacol Toxicol. 1990;96(2):291–7. [DOI] [PubMed] [Google Scholar]
- 105.Poitelon Y, Kopec AM, Belin S. Myelin fat facts: An overview of lipids and fatty acid metabolism. Cells. 2020;9(4):812. 10.3390/cells9040812. [DOI] [PMC free article] [PubMed]
- 106.Crawford AH, Chambers C, Franklin RJ. Remyelination: the true regeneration of the central nervous system. J Comp Pathol. 2013;149(2–3):242–54. [DOI] [PubMed] [Google Scholar]
- 107.Bailey RL, Jun S, Murphy L, Green R, Gahche JJ, Dwyer JT, Potischman N, McCabe GP, Miller JW. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am J Clin Nutr. 2020;112(6):1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Collaboration HS. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–22. [DOI] [PubMed] [Google Scholar]
- 109.Hu J, Melchor GS, Ladakis D, Reger J, Kim HW, Chamberlain KA, Shults NV, Oft HC, Smith VN, Rosko LM, et al. Myeloid cell-associated aromatic amino acid metabolism facilitates CNS myelin regeneration. NPJ Regen Med. 2024;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toyoshima S, Watanabe F, Saido H, Pezacka EH, Jacobsen DW, Miyatake K, Nakano Y. Accumulation of methylmalonic acid caused by vitamin B12-deficiency disrupts normal cellular metabolism in rat liver. Br J Nutr. 1996;75(6):929–38. [DOI] [PubMed] [Google Scholar]
- 111.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A. 2007;104(50):19995–20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toohey JI. Vitamin B12 and methionine synthesis: a critical review. Is nature’s most beautiful cofactor misunderstood? Biofactors. 2006;26(1):45–57. [DOI] [PubMed] [Google Scholar]
- 113.Koehler KM, Romero LJ, Stauber PM, Pareo-Tubbeh SL, Liang HC, Baumgartner RN, Garry PJ, Allen RH, Stabler SP. Vitamin supplementation and other variables affecting serum homocysteine and methylmalonic acid concentrations in elderly men and women. J Am Coll Nutr. 1996;15(4):364–76. [DOI] [PubMed] [Google Scholar]
- 114.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim BJ, Han JM, Kang JG, Kim BS, Kang JH. Association between cotinine-verified smoking status and hypertension in 167,868 Korean adults. Blood Press. 2017;26(5):303–10. [DOI] [PubMed] [Google Scholar]
- 116.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50(10):1385–401. [DOI] [PubMed] [Google Scholar]
- 117.Boss L, Kang DH, Marcus M, Bergstrom N. Endogenous sex hormones and cognitive function in older adults: a systematic review. West J Nurs Res. 2014;36(3):388–426. [DOI] [PubMed] [Google Scholar]
- 118.Tan S, Liu D, Zhang Y, Li S, Zhang K, Zuo H. Trends in blood pressure and hypertension among older adults and oldest-old individuals in China between 2008–2018. Hypertens Res. 2023;46(5):1145–56. [DOI] [PubMed] [Google Scholar]
- 119.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salthouse TA. Trajectories of normal cognitive aging. Psychol Aging. 2019;34(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen-Mansfield J, Skornick-Bouchbinder M, Brill S. Trajectories of End of Life: A Systematic Review. J Gerontol B Psychol Sci Soc Sci. 2018;73(4):564–72. [DOI] [PubMed] [Google Scholar]
- 122.MacDonald SW, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop? J Gerontol B Psychol Sci Soc Sci. 2011;66(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gomes AP, Ilter D, Low V, Endress JE, Fernández-García J, Rosenzweig A, Schild T, Broekaert D, Ahmed A, Planque M, et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature. 2020;585(7824):283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heffernan KS, Stoner L, Meyer ML, Loprinzi PD. Association Between Estimated Pulse Wave Velocity and Cognitive Performance in Older Black and White Adults in NHANES. J Alzheimers Dis. 2022;88(3):985–93. [DOI] [PubMed] [Google Scholar]
- 125.Frith E, Loprinzi PD. Physical activity and cognitive function among older adults with hypertension. J Hypertens. 2017;35(6):1271–5. [DOI] [PubMed] [Google Scholar]
- 126.Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci. 2022;272(7):1139–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang L, Deng YT, Leng Y, Ou YN, Li YZ, Chen SD, He XY, Wu BS, Huang SY, Zhang YR, et al. Depression, Depression Treatments, and Risk of Incident Dementia: A Prospective Cohort Study of 354,313 Participants. Biol Psychiatry. 2023;93(9):802–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The National Health and Nutrition Examination Survey (NHANES) data are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.