Abstract
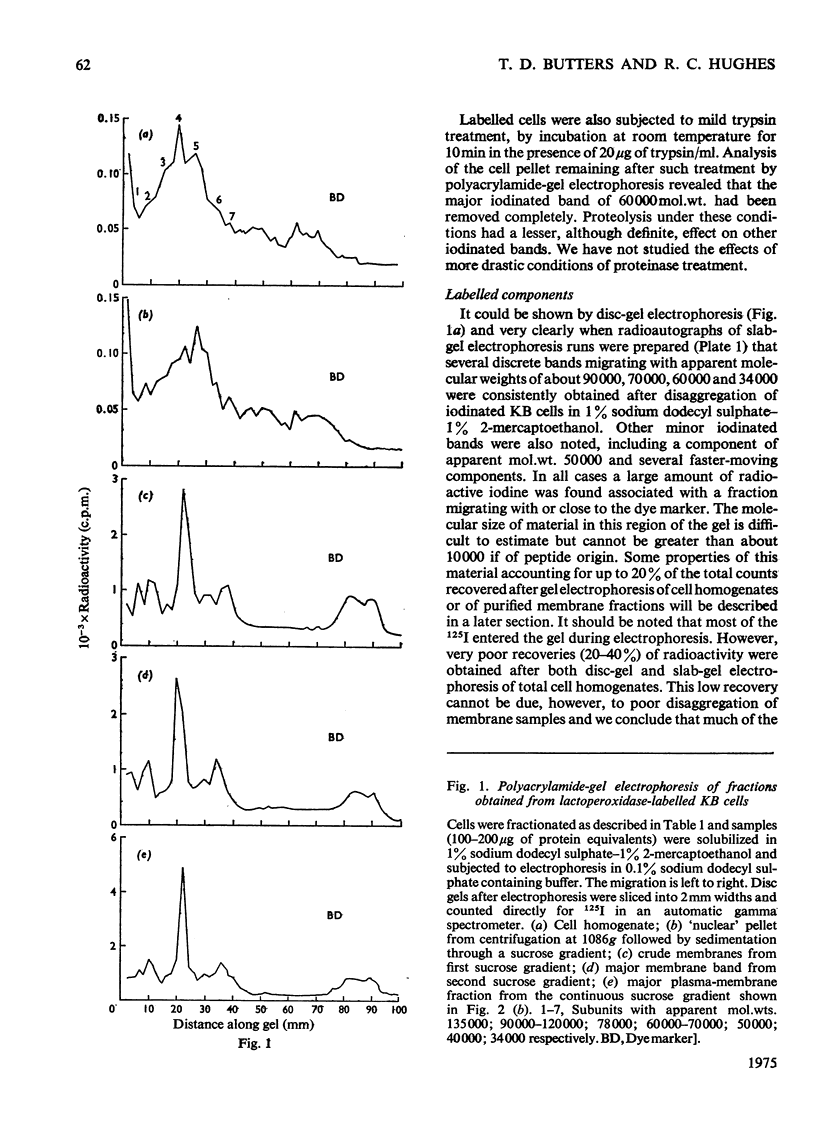
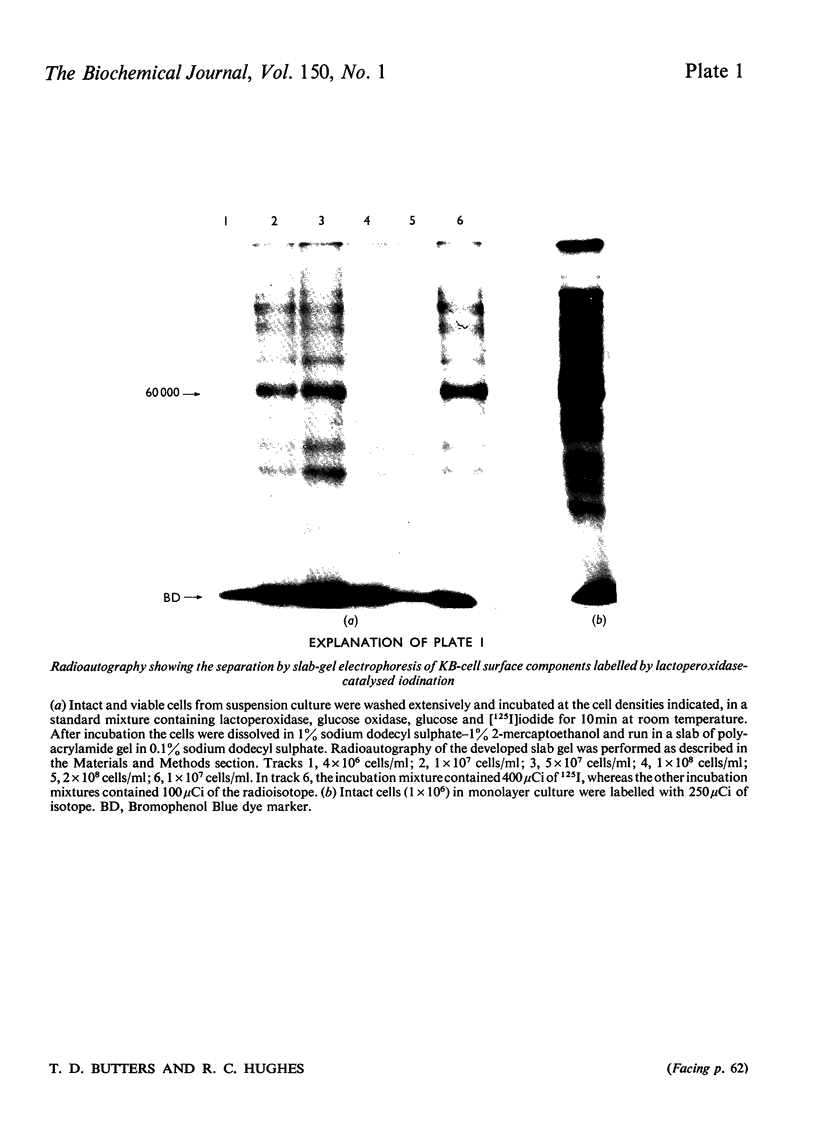
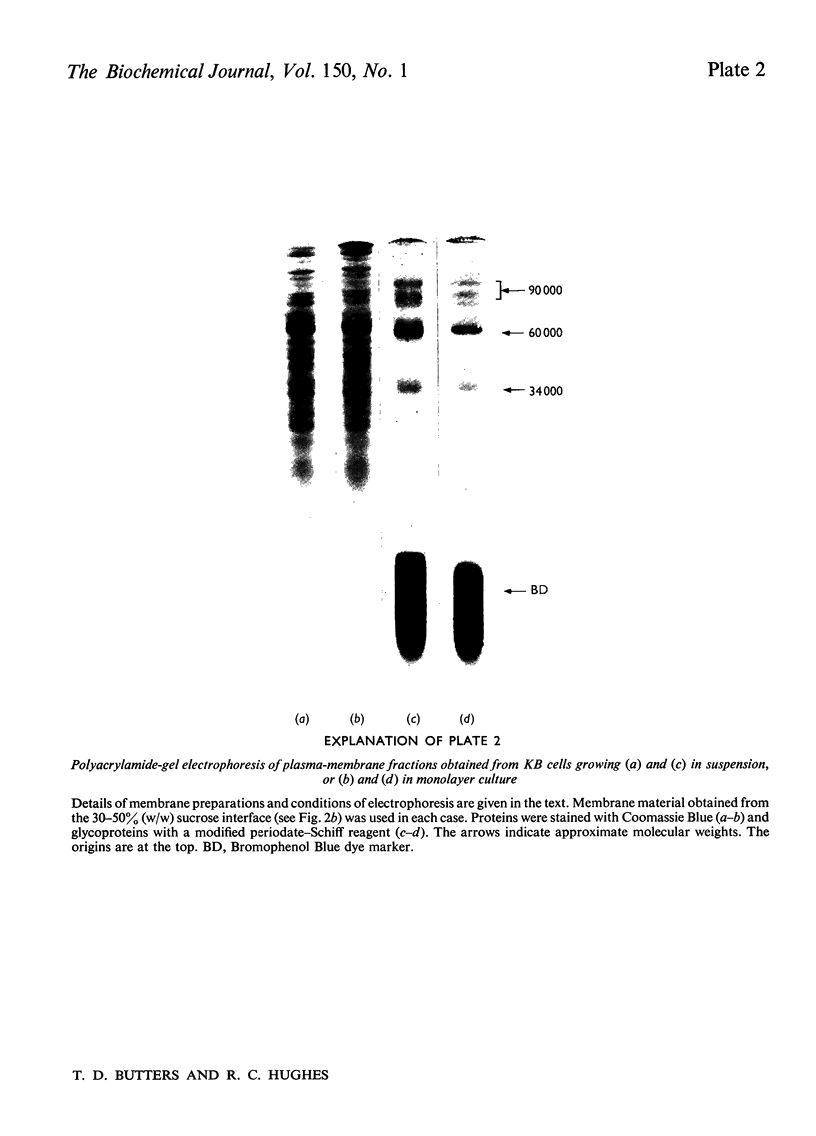
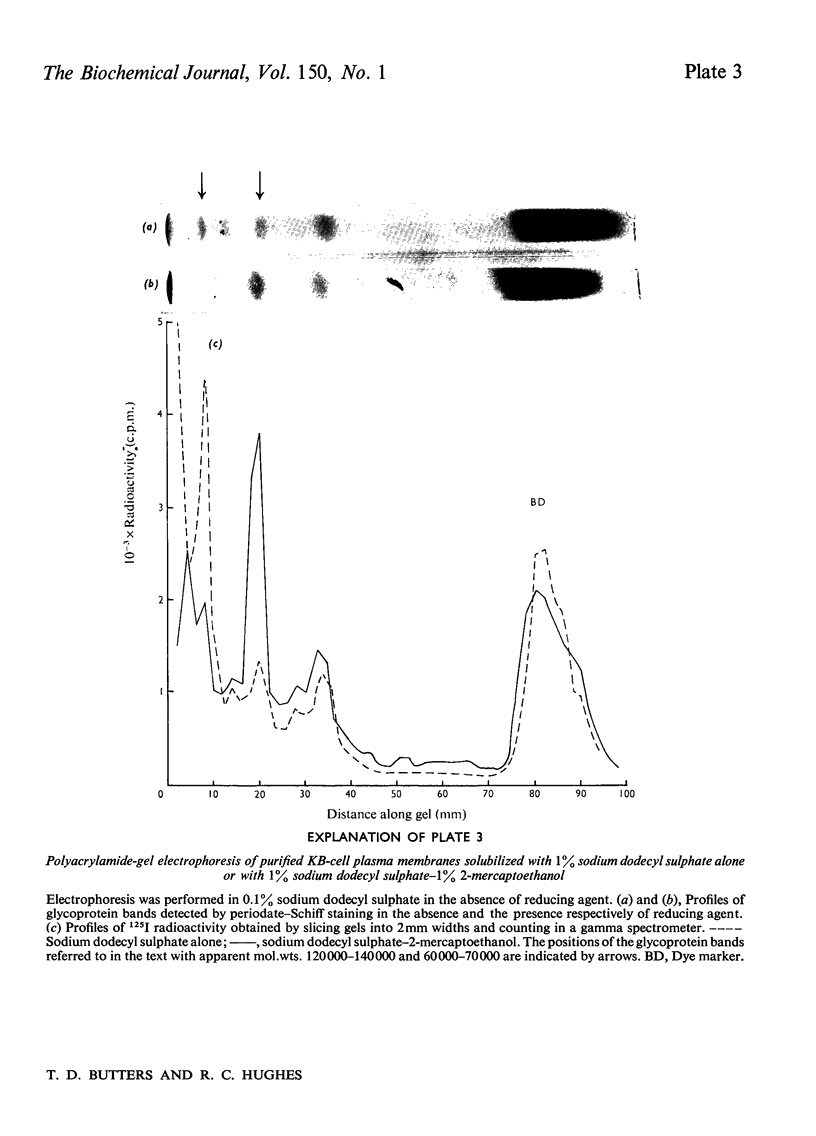
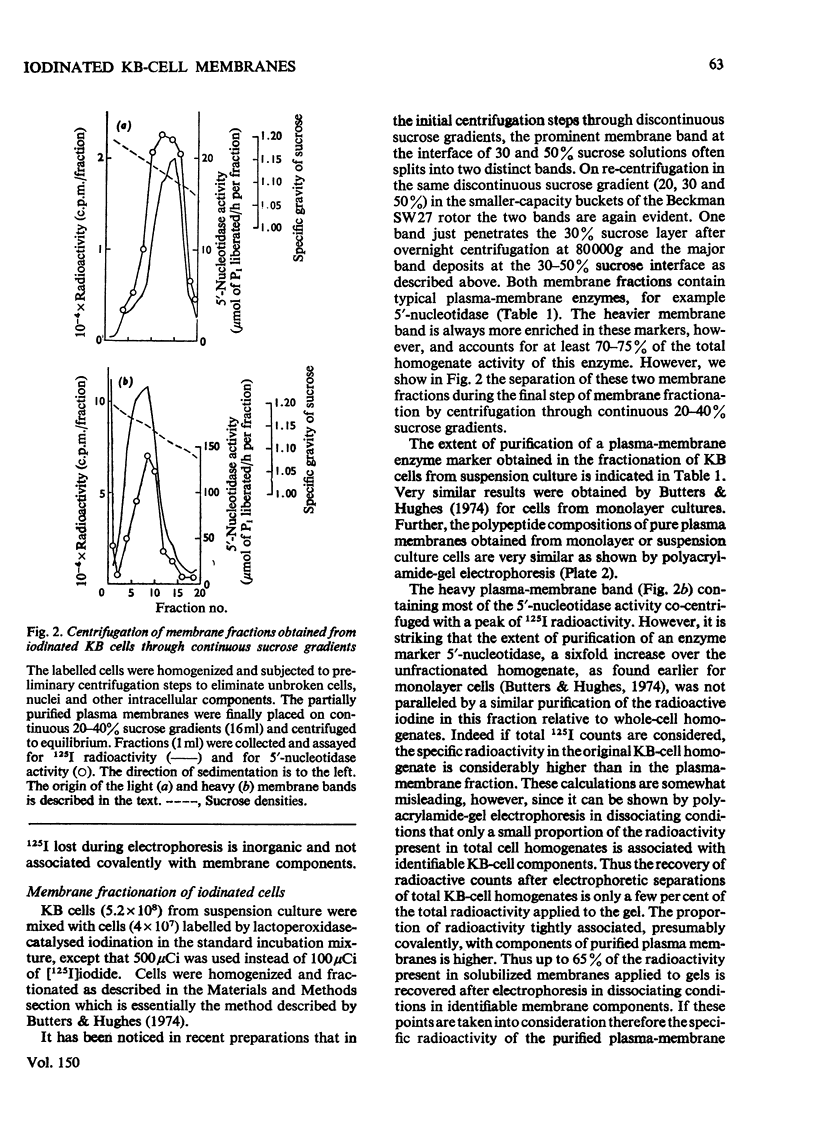
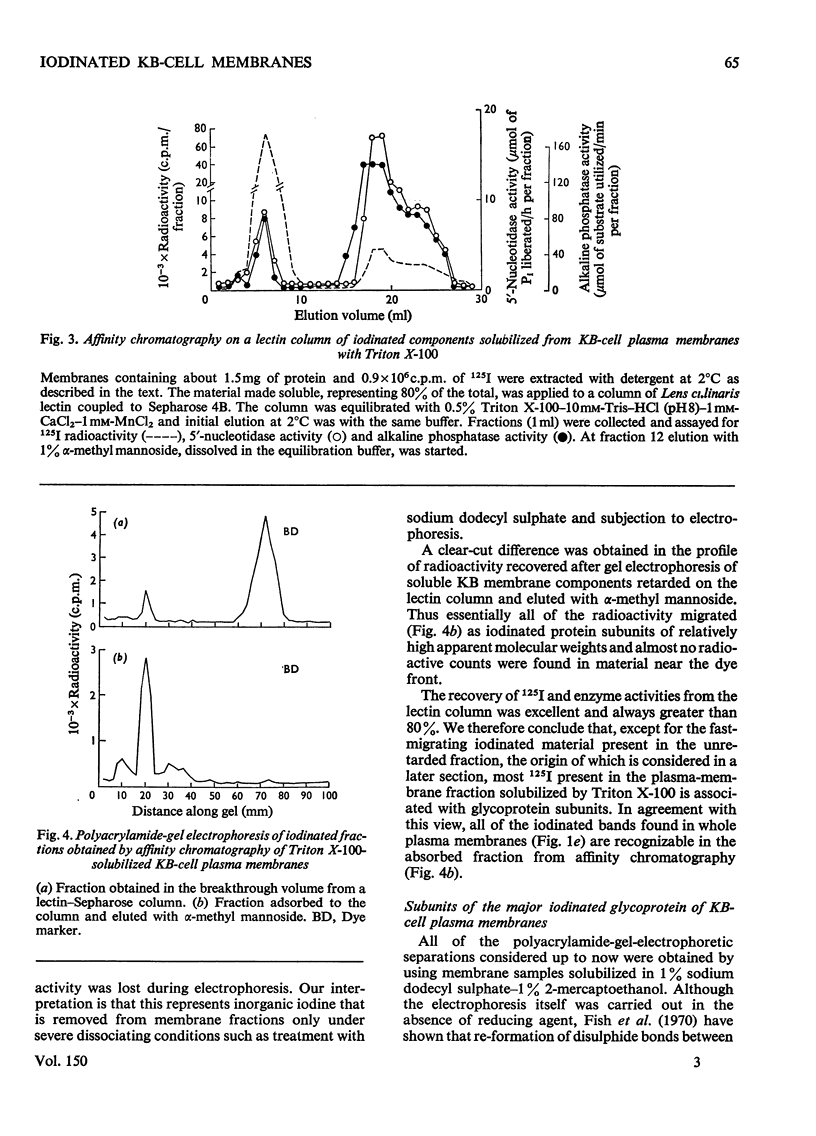
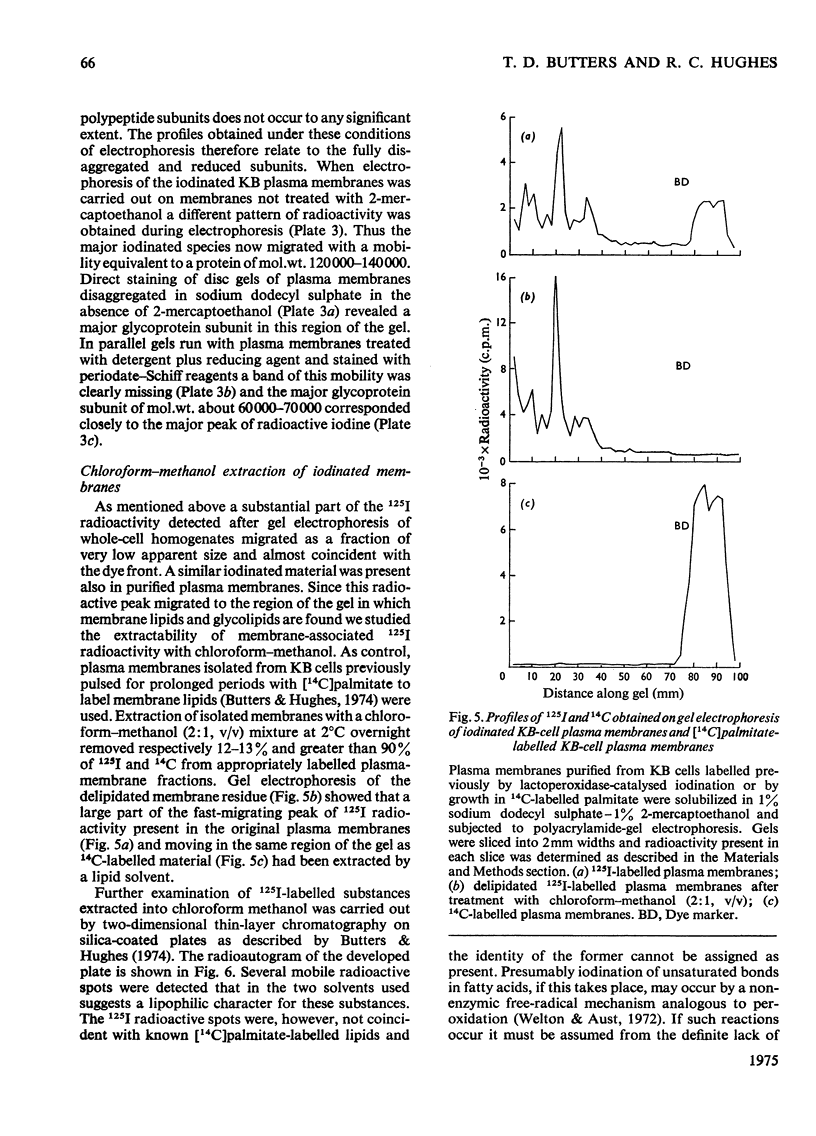
1. Human tumour KB cells growing in suspension culture were labelled by lactoperoxidase-catalysed iodination. Several major radioactively labelled proteins were detected by poly-acrylamide-gel electrophoresis in sodium dodecyl sulphate. 2. After reduction with 2-mercaptoethanol the major radioactive electrophoretic bands migrated as substances with apparent molecular weights of about 90,000, 70,000, 60,000, 50,000 and 34,000 and corresponded closely to the positions at which the major glycosylated polypeptide subunits of KB-cell homogenates migrated during electrophoresis under the same conditions. 3. All the iodinated protein bands except one were present in purified preparations of KB plasma membranes. 4. Most of the 50,000-molecular-weight species, supposedly a surface protein component labelled during iodination of intact and viable KB cells by a non-penetrating enzyme reagent, appeared in a crude nuclear pellet during fractionation. 5. The glyco-protein nature of the major external iodinated species of KB cells was confirmed by adsorption chromatography of these substances, dissolved in low concentrations of Triton X-100, on a lectin-Sepharose column. Two major enzyme markers of the KB plasma membrane, 5'-nucleotidase and alkaline phosphatase were also found to be glycoproteins. 6. Enzyme-catalysed incorporation of radioactive iodine into a fraction of low molecular weight and soluble in chloroform-methanol mixtures also occurred during lactoperoxidase treatment of intact KB cells. The partial characterization of this fraction is briefly described.
Full text
PDF



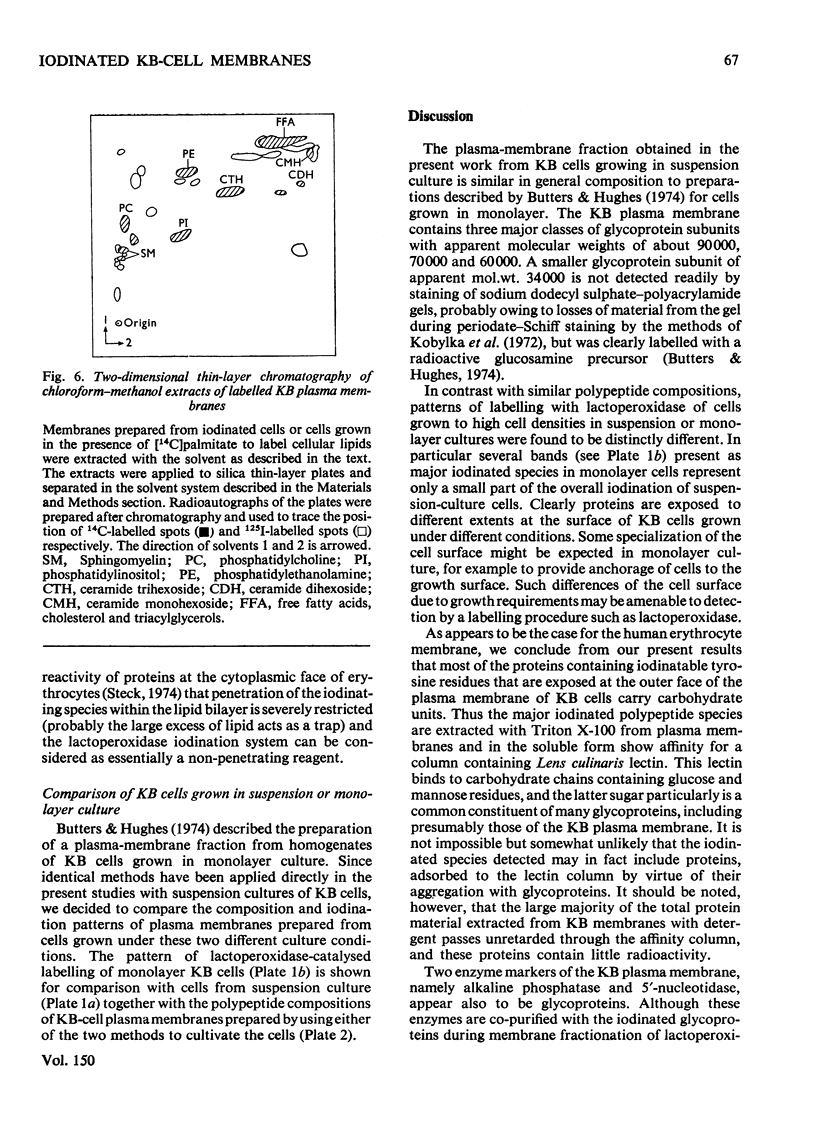


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butters T. D., Hughes R. C. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. Biochem J. 1974 Jun;140(3):469–478. doi: 10.1042/bj1400469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. Glycoproteins as components of cellular membranes. Prog Biophys Mol Biol. 1973;26:189–268. doi: 10.1016/0079-6107(73)90020-5. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. The proteins of the erythrocyte membrane. Biochim Biophys Acta. 1973 Dec 28;300(4):341–378. doi: 10.1016/0304-4157(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Kobylka D., Khettry A., Shin B. C., Carraway K. L. Proteins and glycoproteins of the erythrocyte membrane. Arch Biochem Biophys. 1972 Feb;148(2):475–487. doi: 10.1016/0003-9861(72)90166-x. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Hubbard A. L., Sabatini D. D. On the spatial arrangememt of proteins in microsomal membranes from rat liver. J Cell Biol. 1974 Mar;60(3):616–627. doi: 10.1083/jcb.60.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pekarthy J. M., Short J., Lansing A. I., Lieberman I. Function and control of liver alkaline phosphatase. J Biol Chem. 1972 Mar 25;247(6):1767–1774. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Silva P. P., Nicolson G. L. Freeze-etch localization of concanavalin A receptors to the membrane intercalated particles of human erythrocyte ghost membranes. Biochim Biophys Acta. 1974 Sep 23;363(3):311–319. doi: 10.1016/0005-2736(74)90071-6. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F. The dispositions of proteins in the plasma membranes of animal cells: analytical approaches using controlled peptidolysis and protein labels. Biochim Biophys Acta. 1972 Feb 14;265(1):61–83. doi: 10.1016/0304-4157(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Lipid peroxidation during enzymatic iodination of rat liver endoplasmic reticulum. Biochem Biophys Res Commun. 1972 Nov 1;49(3):661–666. doi: 10.1016/0006-291x(72)90462-7. [DOI] [PubMed] [Google Scholar]