Abstract
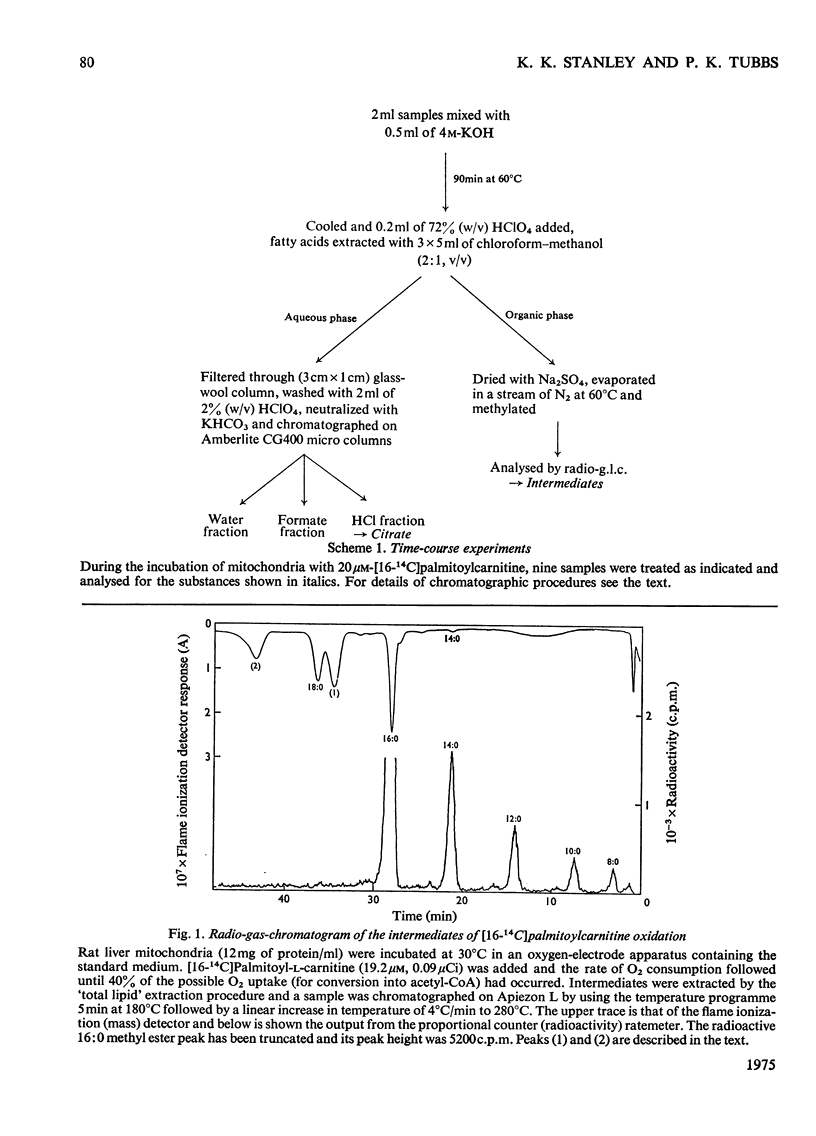
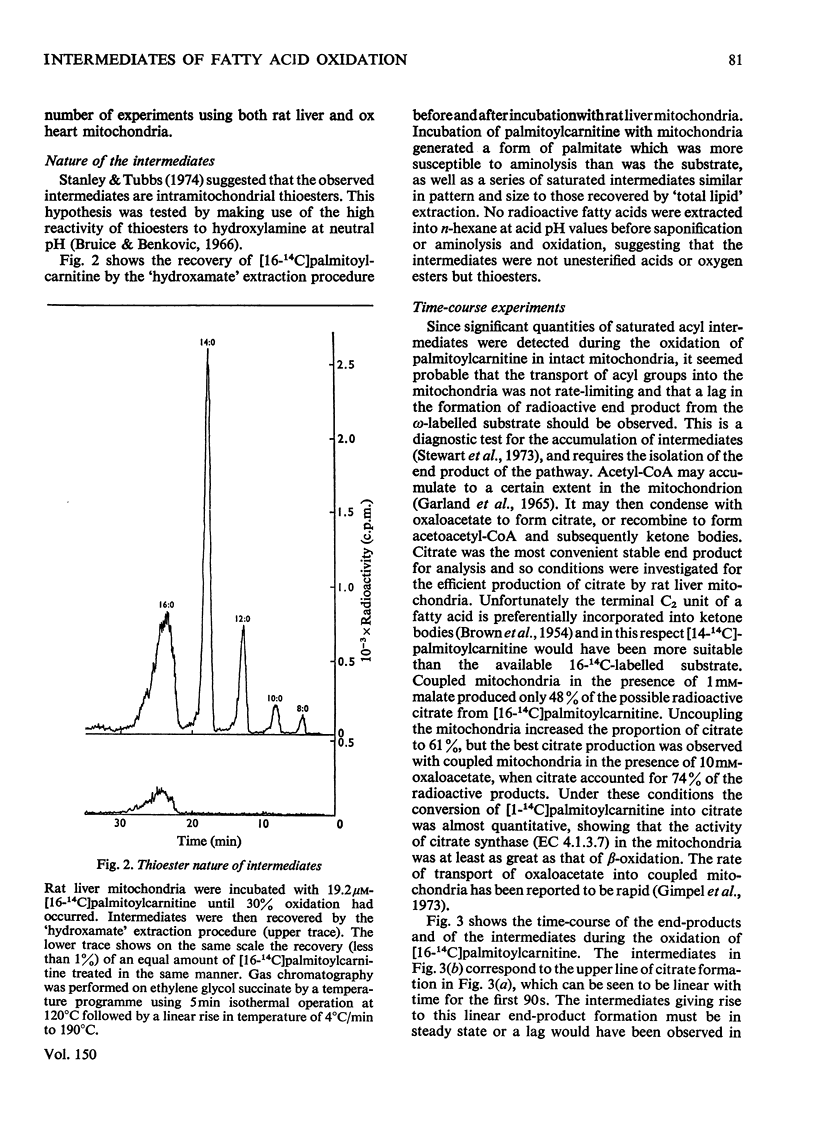
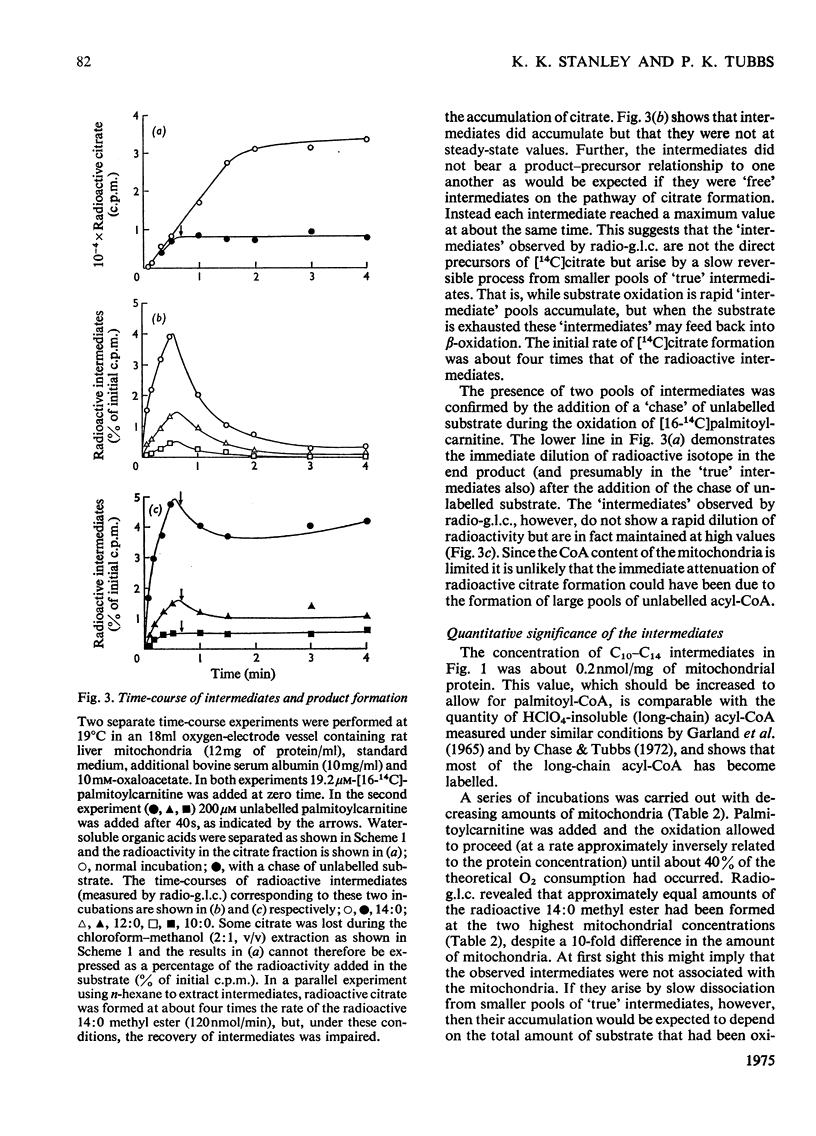
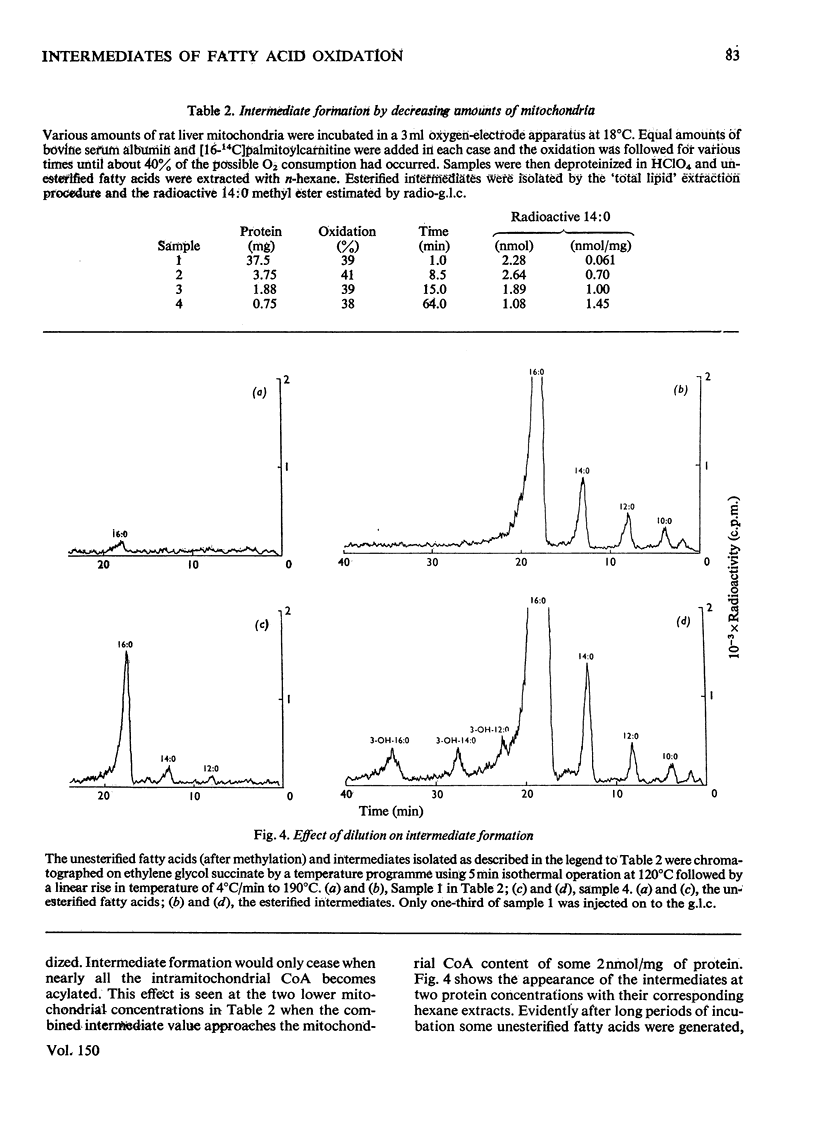
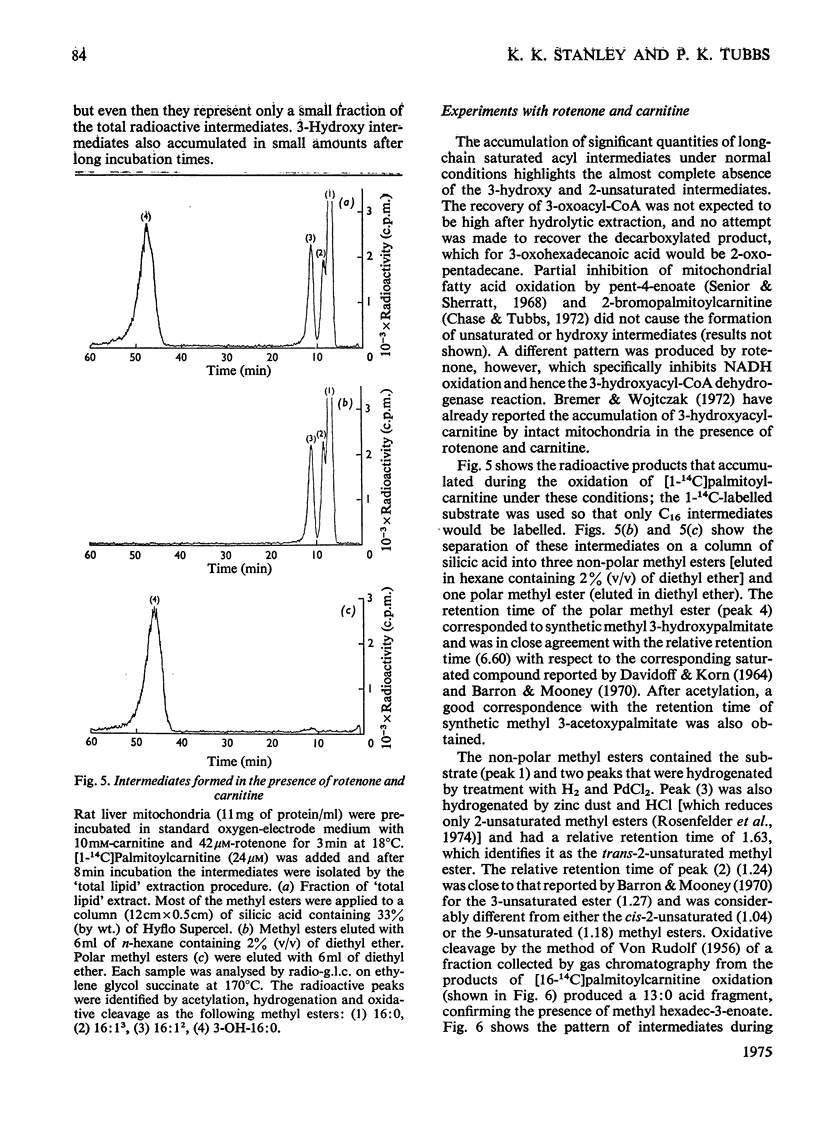
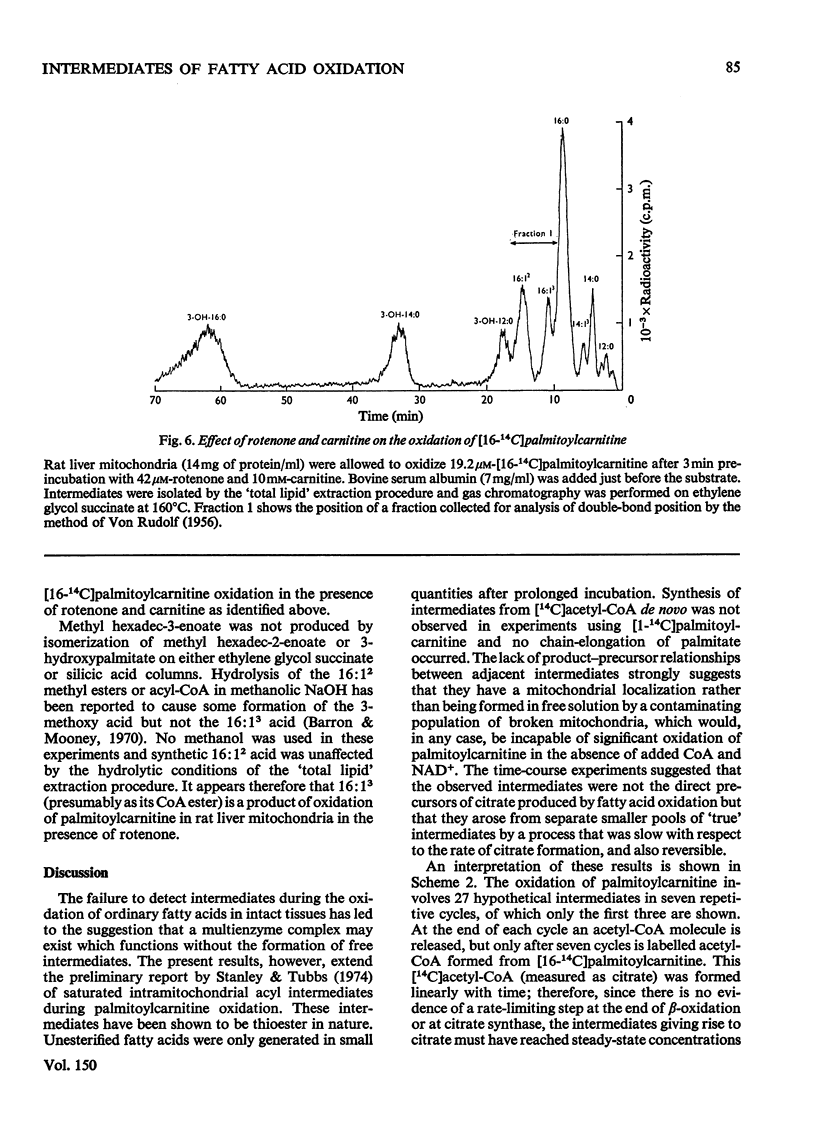
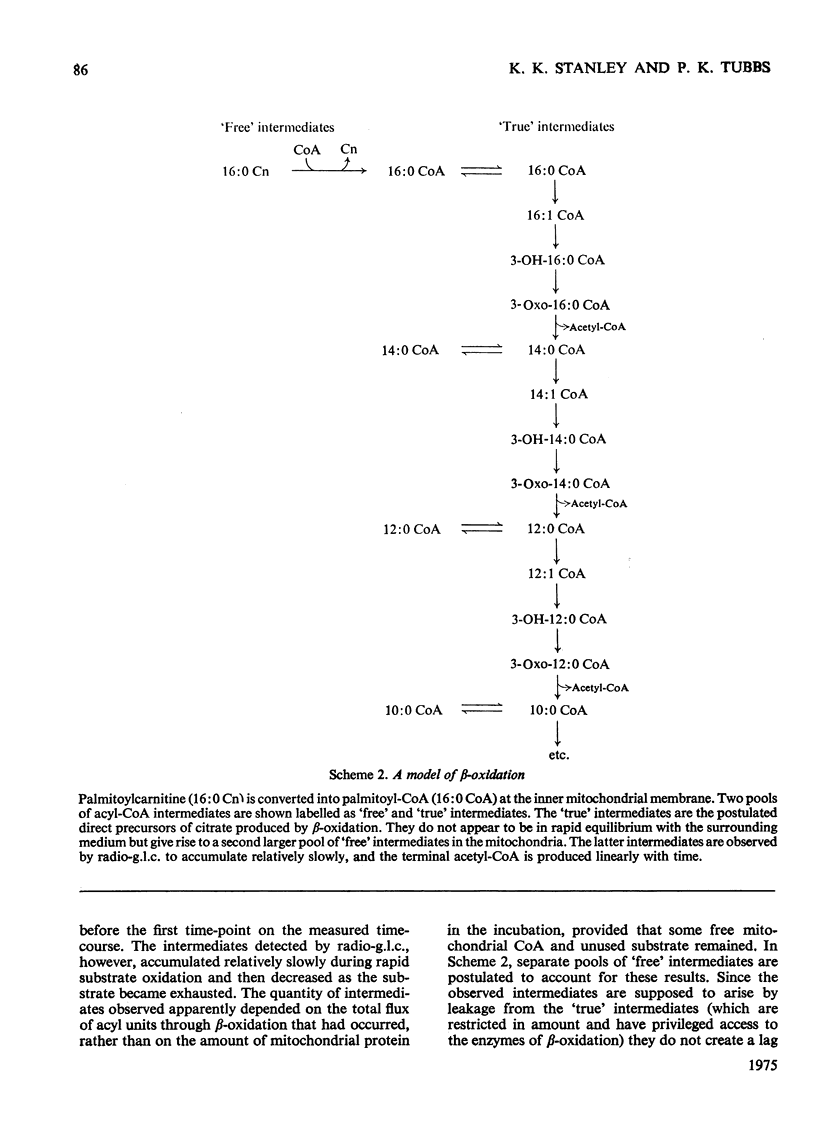
1. Rat liver mitochondria oxidizing [16-14C]palmitoylcarnitine accumulate saturated long-chain thiester intermediates which may be detected by radio-g.1.c.2. Time-courses of intermediate accumulation display no product-precursor relationships and the end product, measured as [14C]citrate, is produced without a detectable initial lag. 3. A short pulse of [16-14C]palmitoylcarnitine followed by unlabelled palmitoylcarnitine showed that the observed intermediates(at least in the greater part)were not the direct precursors of [14C]citrate. 4. The quantity of saturated intermediates depended on the total accumulated flux of acyl units through the pathway provided that some mitochondrial CoA and unused substrate remained. 5. In the presence of rotenone and carnitine, 2-unsaturated, 3-unsaturated and 3-hydroxy intermediates were formed as well as saturated intermediates...
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESSLER R., WAKIL S. J. Studies on the mechanism of fatty acid synthesis. XI. The product of the reaction and the role of sulfhydryl groups in the synthesis of fatty acids. J Biol Chem. 1962 May;237:1441–1448. [PubMed] [Google Scholar]
- BROWN G. W., Jr, CHAPMAN D. D., MATHESON H. R., CHAIKOFF I. L., DAUBEN W. G. Acetoacetate formation in liver. III. On the mechanism of acetoacetate formation from palmitic acid. J Biol Chem. 1954 Aug;209(2):537–548. [PubMed] [Google Scholar]
- Barron E. J., Mooney L. A. Identification of possible intermediates in the mitochondrial fatty acid chain elongation system. Biochemistry. 1970 May 12;9(10):2143–2152. doi: 10.1021/bi00812a017. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Cress E. A., Stephens N., Snyder F. On the analysis of long-chain alkane diols and glycerol ehters in biochemical studies. J Lipid Res. 1971 Sep;12(5):638–640. [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Holman R. T. Chain shortening of acyl-coenzyme A by rat liver microsomes. Biochim Biophys Acta. 1972 Sep 7;280(1):17–21. doi: 10.1016/0005-2760(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972 Aug;129(1):55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DAVIDOFF F., KORN E. D. THE CONVERSION OF LONG CHAIN SATURATED FATTY ACIDS TO THEIR ALPHA, BETA-UNSATURATED, BETA, GAMMA-UNSATURATED, AND BETA-HYDROXY DERIVATIVES BY ENZYMES FROM THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM. J Biol Chem. 1964 Aug;239:2496–2506. [PubMed] [Google Scholar]
- DAVIDOFF F., KORN E. D. THE REACTIONS OF TRANS-ALPHA, BETA-HEXADECENOYL COENZYME A AND CIS- AND TRANS-BETA, GAMMA-HEXADECENOYL COENZYME A CATALYZED BY ENZYMES FROM GUINEA PIGS LIVER MITOCHONDRIA. J Biol Chem. 1965 Apr;240:1549–1558. [PubMed] [Google Scholar]
- ELOVSON J. CHAIN-SHORTENING OF STEARIC ACID IN THE INTACT RAT. Biochim Biophys Acta. 1965 Feb 1;98:36–40. doi: 10.1016/0005-2760(65)90006-8. [DOI] [PubMed] [Google Scholar]
- EVANS J. R., OPIE L. H., SHIPP J. C. METABOLISM OF PALMITIC ACID IN PERFUSED RAT HEART. Am J Physiol. 1963 Oct;205:766–770. doi: 10.1152/ajplegacy.1963.205.4.766. [DOI] [PubMed] [Google Scholar]
- Fiecchi A., Galli-Kienle M., Scala A., Galli G., Paoletti R. The beta-oxidative cleavage of long-chain fatty acids in rat-liver cytoplasm. Eur J Biochem. 1973 Oct 18;38(3):516–528. doi: 10.1111/j.1432-1033.1973.tb03087.x. [DOI] [PubMed] [Google Scholar]
- Fleming P. J., Hajra A. K. Biosynthesis and characterization of a phosphatidic acid analog containing beta-hydroxy fatty acid. Biochem Biophys Res Commun. 1973 Dec 10;55(3):743–751. doi: 10.1016/0006-291x(73)91207-2. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpel J. A., de Haan E. J., Tager J. M. Permeability of isolated mitochondria to oxaloacetate. Biochim Biophys Acta. 1973 Apr 5;292(3):582–591. doi: 10.1016/0005-2728(73)90006-6. [DOI] [PubMed] [Google Scholar]
- Goldman R., Katchalski E. P. Kinetic behavior of a two-enzyme membrane carrying out a consecutive set of reactions. J Theor Biol. 1971 Aug;32(2):243–257. doi: 10.1016/0022-5193(71)90163-9. [DOI] [PubMed] [Google Scholar]
- Greville G. D., Tubbs P. K. The catabolism of long chain fatty acids in mammalian tissues. Essays Biochem. 1968;4:155–212. [PubMed] [Google Scholar]
- LYNEN F. Biosynthesis of saturated fatty acids. Fed Proc. 1961 Dec;20:941–951. [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Rabinowitz J. L., Hercker E. S. Incomplete oxidation of palmitate and leakage of intermediary products during anoxia. Arch Biochem Biophys. 1974 Apr 2;161(2):621–627. doi: 10.1016/0003-9861(74)90345-2. [DOI] [PubMed] [Google Scholar]
- Rebeiz C., Castelfranco P. An Extra-Mitochondrial Enzyme System from Peanuts Catalyzing the beta-Oxidation of Fatty Acids. Plant Physiol. 1964 Nov;39(6):932–938. doi: 10.1104/pp.39.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder G., Lüderitz O., Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974 May 15;44(2):411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Carbohydrate metabolism. Biochem J. 1968 Dec;110(3):521–527. doi: 10.1042/bj1100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The occurrence of intermediates in mitochondrial fatty acid oxidation. FEBS Lett. 1974 Mar 1;39(3):325–328. doi: 10.1016/0014-5793(74)80141-9. [DOI] [PubMed] [Google Scholar]
- Stewart H. B., Tubbs P. K., Stanley K. K. Intermediates in fatty acid oxidation. Biochem J. 1973 Jan;132(1):61–76. doi: 10.1042/bj1320061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Eker, Assad H., Sprecher H. Enzymatic studies on the mechanism of the retroconversion of C22-polyenoic fatty acids to their C20-homologues. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1545–1554. doi: 10.1515/bchm2.1970.351.2.1545. [DOI] [PubMed] [Google Scholar]
- WEINMAN E. O., CHAIKOFF I. L., DAUBEN W. G., GEE M., ENTENMAN C. Relative rates of conversion of the various carbon atoms of palmitic acid to carbon dioxide by the intact rat. J Biol Chem. 1950 Jun;184(2):735–744. [PubMed] [Google Scholar]


