Abstract
Background
Unilateral subthalamic nucleus (STN) ablation using magnetic resonance–guided focused ultrasound (MRgFUS) is being explored as a new treatment for asymmetric Parkinson's disease (PD).
Objectives
The aims were to study the efficacy and safety of this treatment in asymmetric PD patients and to characterize the lesions.
Methods
This prospective, single‐center, open‐label study evaluated asymmetric PD patients at 6 (n = 20) and 12 months (n = 12) after MRgFUS lesion of the STN. The primary outcome was the change in the Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III (MDS‐UPDRS III), score in off medication on the treated side and the adverse events (AEs) at 6‐month follow‐up. We also evaluated cognitive–neuropsychological changes, self‐assessment of clinical improvement, and the correlation of the lesion volume with the motor outcomes.
Results
On the treated side, the MDS‐UPDRS III score (mean difference = 13.8) and the scores in rigidity, bradykinesia, and tremor improved (P < 0.001) throughout the follow‐up compared to baseline (at 6 months: rigidity mean difference = 2.8, improvement: 83.5%; bradykinesia mean difference = 6.0, improvement: 69.4%; tremor mean difference = 4.7, improvement: 91.5%). One patient had severe weakness in the treated hemibody, 1 had moderate dyskinesia, and 1 was in moderate confusional state that became mild (weakness) or completely resolved (dyskinesia and confusional state) at 6 months. The rest of the AEs were mild. We observed no clinically relevant changes in cognitive–neuropsychological tests. The percentage of ablation of the STN correlated with the improvement in the total MDS‐UPDRS III and contralateral tremor scores (P < 0.05).
Conclusion
Unilateral MRgFUS lesion of the STN resulted in a significant motor improvement. We observed no persistent severe AEs, although mild, mostly transient AEs were frequent. © 2024 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease, subthalamic nucleus (STN), magnetic resonance–guided focused ultrasound
Hyperactivity of the subthalamic nucleus (STN) is a key pathophysiological feature of Parkinson's disease (PD). 1 , 2 Unilateral lesions of the STN in a primate model of parkinsonism 3 , 4 exhibited a marked improvement in the treated hemibody. Subsequent unilateral 5 , 6 , 7 and bilateral 8 STN lesions using radiofrequency in PD patients resulted in a significant reduction in parkinsonian features without relevant adverse events (AEs). When STN deep brain stimulation (DBS) was developed and showed similar results, 9 , 10 , 11 , 12 ablative procedures were relegated to specific patients without access to stimulation treatments.
Recent developments in ultrasound technology and neuroimaging facilitated improvements in the security and precision of ablative procedures. 13 Magnetic resonance–guided focused ultrasound (MRgFUS) ablation in the ventral intermediate nucleus (Vim) of the thalamus is a well‐known treatment for essential tremor and PD patients. 14 Since then, investigations have focused on other nuclei to be targeted using MRgFUS in PD such as the STN, which has the European Union's approval (Conformité Européene, also known as “CE mark”). Martínez‐Fernández et al 15 , 16 recently demonstrated the safety and efficacy of unilateral lesion of the STN using MRgFUS. These results have, however, not been replicated, and there is controversy regarding the benefit/risk of this therapy compared to the other targets used in PD, namely Vim 14 and the internal segment of the globus pallidus (GPi). 17
Furthermore, the relationship between the volume and location of the lesion and the clinical outcome is important. Previous data suggest that radiofrequency lesions located within the dorsolateral region of the STN induced greater motor improvement. 18 Similarly, in patients treated with MRgFUS, reports have suggested an association between the improvement in parkinsonian features and the location of the lesion in the STN. 19 However, confirmation of this finding is necessary to define the hot spot for this treatment. In the latter study, 19 MR data were obtained 24 hours after the treatment, whereas clinical data were obtained 4 months after the procedure. Thus, the volume of the lesions analyzed included a small area of necrotic tissue surrounded by cytotoxic edema, which may not completely correspond to the final lesion volume and location.
In this study we aimed to evaluate the safety and efficacy of the unilateral lesion of the STN using MRgFUS on parkinsonian motor, cognitive, and neuropsychiatric features, and the characteristics of the lesions 3 months after treatment, and determine their correlation with the clinical outcome of patients.
Patients and Methods
Study Design and Patients
In this prospective, open‐label study, we included 20 patients with PD 20 treated with unilateral MRgFUS lesion of the STN between January 2020 and November 2021 at Clínica Universidad de Navarra (Spain). The Ethics Committee approved the protocol, and patients provided written informed consent.
Inclusion criteria were patients aged >30 years (with no upper age limit), improvement of at least 33% in the Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III (MDS‐UPDRS III), score after a levodopa challenge, asymmetric parkinsonism (based on the different motor scores of the most and least affected hemibody, and on the clinical judgment of the neurologist [M.C.R.‐O.] and neurosurgeon [J.G.] experts in movement disorders), and a Hoehn and Yahr scale score of ≤2.5. We excluded patients with severe dyskinesias (score of 3 or 4 in items 4.1 and 4.2 of the MDS‐UPDRS IV), clinically relevant cognitive impairment (Montreal Cognitive Assessment ≤24), 21 serious neuropsychiatric conditions not adequately controlled (depression, impulse control disorders [ICD], addictions), or a skull density ratio <0.40 calculated from a head computed tomography (CT). Other exclusion criteria are included in the Supplementary Material.
Evaluations
We performed motor evaluations in both off and on medication states before treatment, and in off medication state at 1, 3, 6, and 12 months after treatment, using the MDS‐UPDRS III by the same neurologists (M.C.R.‐O. and L.A.‐G.). We evaluated the total score; the scores of the treated and nontreated sides; and the subscores for tremor (items 3.15–17), rigidity (item 3.3), and bradykinesia (items 3.4–3.8) on the treated side. At each visit, we completed the Patient Global Impression of Change (PGI‐C) scale 22 and calculated the levodopa equivalent daily dose (LEDD). 23
We performed the safety assessment by evaluating AEs defined as any new clinical feature or a decline of previous conditions after the lesion of the STN. We recorded and categorized them at every visit—according to the definition from the Food and Drug Administration 24 and based on the interference with the patient's daily activities—as mild, moderate, or severe.
We performed patients' neuropsychological assessments while on medication at baseline and 6 months after the lesion of the STN, using a comprehensive neuropsychological battery evaluating global cognition, different cognitive domains, and other neuropsychiatric symptoms (Supplementary Material).
Because of the COVID‐19 lockdown and the fact that some patients lived far away, we were forced to do online follow‐up for 6 patients for whom, in consequence, it was not possible to evaluate rigidity, although we assessed the remaining items included in the physical exam. For 4 of them we also performed a modified online neuropsychological evaluation (Supplementary Material).
Procedure
Lesions were performed using Insightec's Exablate hardware with software version 7.33 (Insightec, Tirat at Carmel, Israel). The target was calculated with stereotactic coordinates using brain images from a CT scan and a 3‐T MR (Siemens Skyra, Erlangen, Germany) before the procedure. The initial coordinates were 12 mm lateral, 3 mm posterior, and 4 mm inferior to the mid‐commissural point. We applied any required adjustments by visualizing the STN using T2 and SWI sequences. After having confirmed the correct alignment with three sublesional sonications, we performed a so‐called “verifying” sonication to check for the clinical benefit and AEs at a temperature ranging between 48°C and 52°C. If there were no AEs and a clinical response was observed, we performed therapeutic sonications at higher temperatures by gradually increasing the power and/or the duration of the sonication. We performed additional changes in the target coordinates during the procedure, generally with a second lesion in the sensorimotor part of the nucleus (lateral and posterior to the first one) and a third lesion in a more dorsal position overlapping with the fasciculus lenticularis to minimize the risk of dyskinesia with a pallidotomy‐like effect. 18 We aimed to perform two sonications above 55°C in each of the three locations and made further adjustments depending on the intraoperative clinical feedback (Supplementary Material and Table S1).
Posttreatment MR Acquisition and Analysis
We performed a 3‐T MR at the 3‐month visit in 8 patients. We calculated lesion volume based on focal hypointense areas shown by T1‐weighted images (Fig. 2A), based on a recent study showing that T1‐weighted images are more accurate than T2‐weighted images in detecting the effective extent of MRgFUS lesions. 25
FIG. 2.
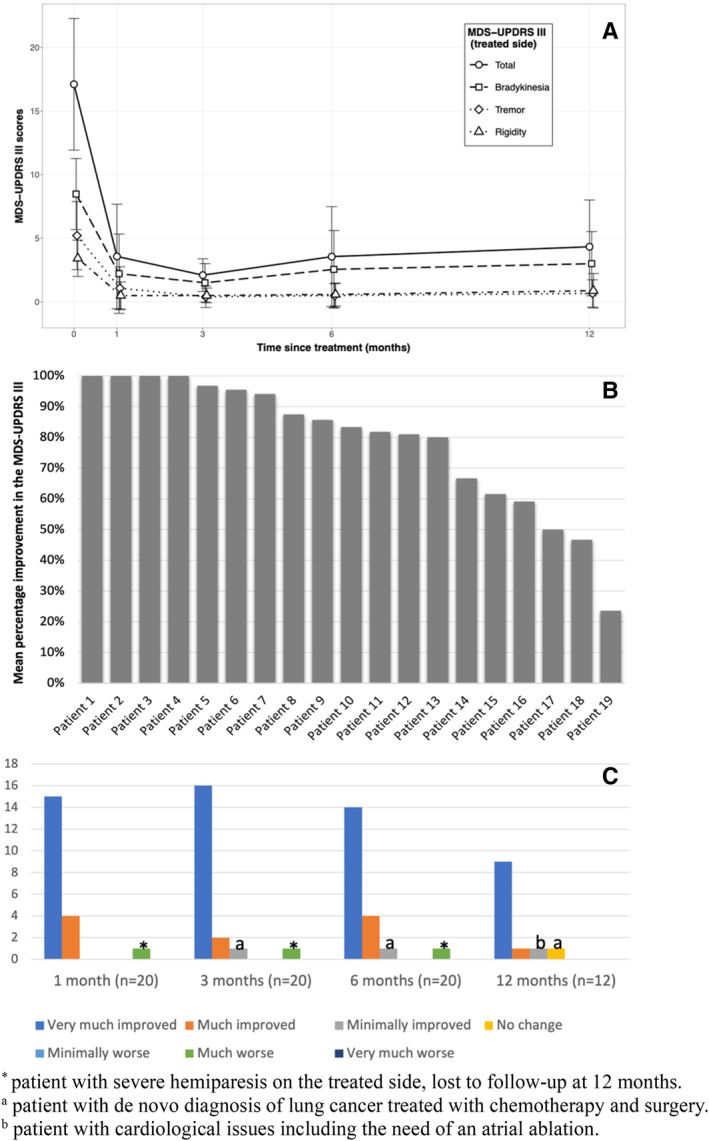
(A) Change in the MDS‐UPDRS III (Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III) scores on the treated side during follow‐up. (B) Mean percentage of improvement in the MDS‐UPDRS III score on the treated side of each patient 6 months after treatment. (C) Patient Global Impression of Change (PGI‐C) during follow‐up. [Color figure can be viewed at wileyonlinelibrary.com]
Morphometric transformations derived from the baseline (prior to treatment) T1‐weighted images were applied to the distal STN atlas 26 to obtain representations of the STN in the images of each patient. MR acquisition and morphometric processing are shown in the Supplementary Material. Lesion volumes corresponding to the STN were calculated by computing the voxels common to the lesion and the STN. The percentage of the STN that overlapped with the lesion was also calculated.
Outcomes
Our primary efficacy endpoint was the change in the MDS‐UPDRS III score in off medication in the treated hemibody (range: 0–44) from that at baseline to that at 6 months. The primary safety outcome was the frequency and severity of AEs at 6 months.
As secondary outcomes, we evaluated the change at 6 months in the subitems of rigidity (range: 0–8), bradykinesia (range: 0–20), total tremor (range: 0–16), rest (range: 0–8), postural tremor (range: 0–4), and kinetic tremor (range: 0–4) on the treated side in off medication and the change in the LEDD, in the total score of the MDS‐UPDRS III (range: 0–132) and in the score of the ipsilateral side (range: 0–44) in off medication. Other secondary outcomes were the change in the MDS‐UPDRS III scores in off medication from baseline to 1, 3, and 12‐month visits, and the frequency and severity of AEs at each follow‐up. Our nonmotor secondary outcomes were the change in cognitive and neuropsychiatric tests at 6‐month follow‐up, and the PGI‐C at 6‐ and 12‐month follow‐up. As exploratory outcomes, we studied the relationship between the total lesion volume and the change in the total score and subscores of the MDS‐UPDRS III on the treated side in off medication at 6‐month follow‐up. The same analysis was performed to determine the percentage of the volume of the STN overlapping with the lesion.
Statistical Analysis
We calculated the sample size of 20 using STATA (version 14), bearing in mind findings from a previous study, 15 potential patient attrition, and interstudy variability, while applying a type I error of 0.05 and a type II error of 0.02 (power of 98%).
We estimated the change in MDS‐UPDRS III score and dopaminergic treatment from baseline to other time points utilizing the estimated marginal means derived from a series of linear mixed models, with time point, age, sex, and duration of disease included as independent variables. We assessed the statistical significance of these changes using the t test corrected using Satterthwaite's method. For our secondary analyses we employed linear regression models to evaluate the association between the improvement in MDS‐UPDRS III and baseline clinical variables. We evaluated assumptions underlying the statistical tests primarily using graphical methods. We also calculated the mean percentage changes between baseline and other time points. We performed these statistical analyses using the R software, version 4.2.0, 27 and the lmerTest 3.1‐3 package. 28
In the Supplementary Material we describe the statistical analyses for other secondary outcomes.
Regarding MR analysis, descriptive statistics were used to summarize volume lesion and overlap with the STN. Kendall's tau (KT) rank correlations were performed between the total lesion volume and the improvement in MDS‐UPDRS III total score in off medication and subscores of the treated side at 6‐month follow‐up (MDS‐UPDRS III, rigidity, tremor, and bradykinesia). This analysis was also performed for the percentage of the volume of the STN overlapping with the lesion. STATA (version 14) was used for statistical analysis (see Supplementary Material for further information).
Results
We treated 20 PD patients using MRgFUS lesion of the STN (Table 1).
TABLE 1.
Clinical and demographic characteristics at baseline and change in MDS‐UPDRS III score in the treated side and frequency of AEs at 6 months in all patients
| Total, n = 20 | Not followed at 12 months, n = 8 | Followed at 12 months, n = 12 | P | |
|---|---|---|---|---|
| Age | 62.5 (56.8–66.0) | 64.5 (56.8–69.2) | 61.5 (55.8–64.2) | 0.511 |
| Sex, male | 18 (90.0) | 7 (87.5) | 11 (91.7) | 0.999 |
| Treated side, right | 12 (60.0) | 3 (37.5) | 9 (75.0) | 0.167 |
| Disease duration (y) | 7.5 (3.8–9.2) | 8.0 (6.8–9.5) | 5.5 (3.8–9.2) | 0.534 |
| LEDD (mg) | 585.0 (450.0–812.5) | 660.0 (450.0–900.0) | 578.5 (450.0–775.0) | 0.938 |
| Levodopa (mg) | 400.0 (300.0–712.5) | 450.0 (375.0–712.5) | 362.5 (300.0–525.0) | 0.483 |
| Dopamine agonists (mg) | 100.0 (37.5–302.5) | 85.0 (0.0–175.0) | 100.0 (87.5–302.5) | 0.365 |
| MDS‐UPDRS III (off) | ||||
| Total | 32.0 (27.0–40.2) | 31.5 (29.2–37.8) | 34.0 (25.2–41.5) | 0.877 |
| Treated side | 17.0 (14.8–21.2) | 16.5 (15.0–19.0) | 17.0 (12.8–21.2) | 0.587 |
| Rigidity | 4.0 (2.8–4.0) | 4.0 (2.0–4.0) | 3.5 (3.0–4.0) | 0.968 |
| Bradykinesia | 8.0 (6.8–10.2) | 8.0 (7.5–10.2) | 8.0 (6.8–10.2) | 0.725 |
| Rest tremor | 3.0 (3.0–5.0) | 3.0 (3.0–4.2) | 3.5 (2.8–5.0) | 0.999 |
| Postural tremor | 1.0 (0.0–2.0) | 1.5 (1.0–2.0) | 0.5 (0.0–2.0) | 0.211 |
| Kinetic tremor | 0.0 (0.0–1.0) | 1.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.067 |
| Untreated side | 7.0 (4.5–11.2) | 8.0 (5.2–9.5) | 7.0 (4.5–12.2) | 0.877 |
| Hoehn and Yahr scale |
0.387 |
|||
| 1 | 3 (15.0) | 2 (25.0) | 1 (8.3) | |
| 1.5 | 2 (10.0) | 0 (0.0) | 2 (16.7) | |
| 2 | 14 (70.0) | 5 (62.5) | 9 (75.0) | |
| 2.5 | 1 (5.0) | 1 (12.5) | 0 (0.0) | |
| Reduction in the MDS‐UPDRS III score in the treated side at 6 months | 14.0 (10.0–16.5) | 15.0 (13.5–15.5) | 12.0 (8.8–17.0) | 0.611 |
| Adverse effects at 6 months | 7 (35.0) | 4 (50.0) | 3 (25.0) | 0.356 |
Quantitative variables are summarized as median (interquartile range). Qualitative variables are summarized as frequency (percentage).
P‐values correspond to Wilcoxon's rank‐sum test or Fisher's exact test comparing patients followed up for <12 versus ≥12 months.
Abbreviations: MDS‐UPDRS III, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III; AE, adverse event; LEDD, levodopa equivalent daily dose.
Motor Outcomes
We excluded 1 patient attending the 6‐month follow‐up in on medication from the motor analysis. There was a significant reduction in the MDS‐UPDRS III score of the treated hemibody between baseline and 6 months (primary outcome) (P < 0.001), and between baseline and the other visits (secondary outcomes) (P < 0.001) (Table 2; Fig. 1A). The mean reduction in the MDS‐UPDRS III score on the treated side at 6 months with respect to baseline was 78.6% (13.7 points from 17.5 ± 5.0 to 3.8 ± 4.0, P < 0.001). The percentage reduction for each patient is shown in Fig. 1B. All subscores were significantly reduced in all visits (P < 0.001) (at 6 months: rigidity score reduction from 3.5 ± 1.4 to 0.5 ± 0.9, improvement: 83.5%; bradykinesia score reduction from 8.8 ± 2.9 to 2.9 ± 3.3, improvement: 69.4%; tremor score reduction from 5.2 ± 2.6 to 0.5 ± 0.9, improvement: 91.5%) (Table 2).
TABLE 2.
MDS‐UPDRS III scores in off state and LEDD at baseline and throughout follow‐up
| Baseline (n = 20) | 1 Month (n = 14) | 3 Months (n = 11) | 6 Months (n = 19) | 12 Months (n = 12) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | EMM (SE) | Mean (SD) | Change % | Mean difference* (P) | Mean (SD) | Change % | Mean difference* (P) | Mean (SD) | Change % | Mean difference* (P) | Mean (SD) | Change % | Mean difference* (P) | |
| MDS‐UPDRS III | ||||||||||||||
| Total | 35.5 (12.9) | 37.9 (4.4) | 15.6 (12.1) | 57.5 | 19.4 (<0.0001) | 11.6 (8.3) | 64.5 | 22.1 (<0.001) | 15.6 (10.7) | 57.8 | 20.1 (<0.001) | 19.9 (10.6) | 50.0 | 16.1 (<0.001) |
| Treated side | 17.5 (5.0) | 16.9 (1.7) | 3.6 (4.1) | 78.8 | 13.5 (<0.001) | 3.0 (3.2) | 82.4 | 14.1 (<0.001) | 3.8 (4.0) | 78.6 | 13.8 (<0.001) | 5.0 (3.7) | 68.5 | 12.4 (<0.001) |
| Bradykinesia | 8.8 (2.9) | 8.3 (1.4) | 2.2 (3.1) | 74.6 | 6.1 (<0.001) | 2.5 (3.5) | 76.4 | 6.6 (<0.001) | 2.9 (3.3) | 69.4 | 6.0 (<0.001) | 3.7 (3.0) | 55.5 | 5.7 (<0.001) |
| Rigidity | 3.5 (1.4) | 3.9 (0.5) | 0.5 (1.1) | 88.5 | 3.1 (<0.001) | 0.4 (0.5) | 88.3 | 3.0 (<0.001) | 0.5 (0.9) | 83.5 | 2.8 (<0.001) | 0.9 (1.4) | 79.8 | 2.6 (<0.001) |
| ‐ Total tremor | 5.2 (2.6) | 5.0 (0.6) | 1.1 (1.7) | 72.0 | 4.1 (<0.001) | 0.4 (0.8) | 92.4 | 4.7 (<0.001) | 0.5 (0.9) | 91.5 | 4.7 (<0.001) | 0.6 (0.9) | 89.1 | 4.6 (<0.001) |
| Rest tremor | 3.6 (1.4) | 3.5 (0.4) | 0.8 (1.2) | 68.8 | 2.8 (<0.001) | 0.4 (0.8) | 87.9 | 3.2 (<0.001) | 0.4 (0.7) | 87.4 | 3.2 (<0.001) | 0.6 (0.9) | 85.6 | 3.1 (<0.001) |
| Postural tremor | 0.6 (0.9) | 1.0 (0.2) | 0.2 (0.4) | 68.8 | 0.9 (<0.001) | 0.0 (0.0) | 100.0 | 1.1 (<0.001) | 0.1 (0.3) | 93.1 | 1.0 (<0.001) | 0.0 (0.0) | 100.0 | 1.1 (<0.001) |
| Kinetic tremor | 0.4 (0.7) | 0.4 (0.1) | 0.1 (0.3) | 83.3 | 0.4 (0.005) | 0.0 (0.0) | 100.0 | 0.5 (0.002) | 0.0 (0.0) | 100.0 | 0.4 (<0.001) | 0.0 (0.0) | 100.0 | 0.4 (0.002) |
| Untreated side | 8.3 (5.3) | 10.2 (2.2) | 8.3 (6.6) | −29.1 | −0.1 (0.989) | 5.8 (5.5) | 11.2 | 1.1 (0.264) | 7.5 (5.7) | −11.2 | 0.7 (0.373) | 10.5 (6.2) | −86.9 | −1.6 (0.115) |
| LEDD | ||||||||||||||
| Total | 737.5 (513.8) | 648.0 (172.2) | 593.7 (471.8) | 20.2 | 146.1 (<0.001) | 535.5 (433.3) | 27.3 | 198.6 (<0.001) | 544.1 (436.6) | 24.8 | 194.4 (<0.001) | 562.3 (448.8) | 13.1 | 158.9 (0.002) |
| Levodopa | 483.8 (330.5) | 429.5 (126.0) | 400.0 (252.7) | 16.2 | 83.8 (0.004) | 368.8 (288.8) | 23.1 | 115.0 (<0.001) | 368.8 (287.0) | 22.3 | 115.0 (<0.001) | 356.2 (215.9) | 5.8 | 81.6 (0.017) |
| Dopamine Agonists | 253.8 (367.8) | 216.5 (120.0) | 191.6 (320.1) | 13.9 | 62.3 (0.021) | 170.5 (277.6) | 22.7 | 83.3 (0.002) | 173.1 (279.2) | 21.5 | 80.7 (0.003) | 206.1 (334.4) | 10.0 | 77.9 (0.016) |
Mean differences are differences in estimated marginal means between baseline and other time points; therefore, positive values indicate clinical benefit. P‐values correspond to t tests of these differences corrected using Satterthwaite's method.
Abbreviations: MDS‐UPDRS III, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III; LEDD, levodopa equivalent daily dose (in mg); SD, standard deviation; EMM, estimated marginal mean; SE, standard error; change %, median percentage of improvement.
FIG. 1.
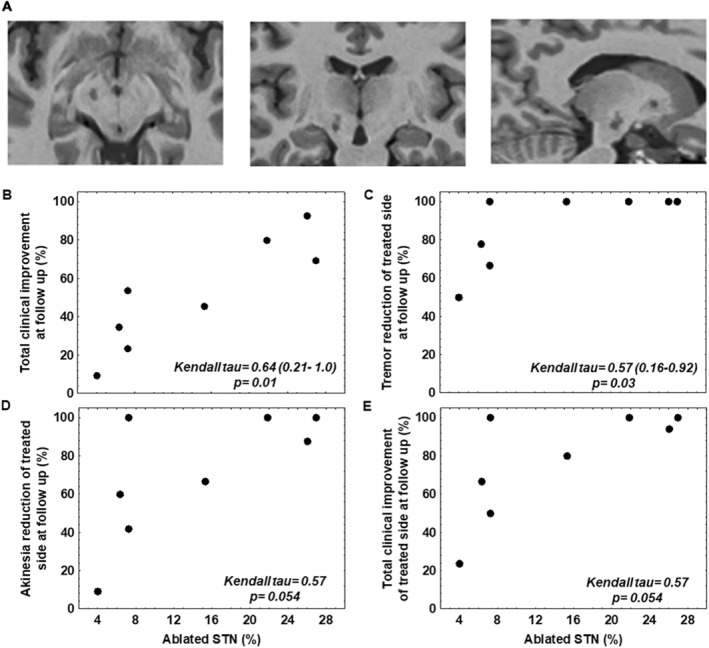
(A) T1‐weighted images showing the STN (subthalamic nucleus) lesion. Correlation between the percentage of ablated STN and the reduction (B) in the MDS‐UPDRS III (Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Part III) total score, (C) in the tremor score on the treated side, (D) in the akinesia score on the treated side, and (E) in the total score of MDS‐UPDRS III on the treated side.
Despite the significant reduction in the LEDD (P < 0.001), we observed no significant change in the MDS‐UPDRS III score in off medication throughout the follow‐up on the untreated side (Table 2).
No significant results were observed in the linear regression models we performed to study the association between the improvement in the MDS‐UPDRS III score at 6 months on the treated side and age (P = 0.8), sex (P = 0.2), duration of PD (P = 0.7), and the MDS‐UPDRS III score at baseline (P = 0.8).
We also evaluated a subgroup of 12 patients at 12 months (see Supplementary Material for dropouts). We detected no significant difference between patients followed up for 6 months and those followed up for 12 months (Table 1). A significant improvement was observed in their MDS‐UPDRS III score on the treated side (mean difference: 12.5 from 17.5 ± 5.0 to 5.0 ± 3.7, P < 0.001). In the subscales, a significant change was observed (P < 0.001), with a rigidity score reduction from 3.5 ± 1.4 to 0.9 ± 1.4, indicating a 79.8% improvement; a bradykinesia score reduction from 8.8 ± 2.9 to 3.7 ± 3.0, indicating a 55.5% improvement; and a tremor score reduction from 5.2 ± 2.6 to 0.6 ± 0.9, indicating an 89.1% improvement.
Safety
During the procedure of lesion creation, the most frequently encountered AEs were dizziness (n = 11, 55%), headache (n = 6, 30%), and nausea (n = 2, 10%). All three were bearable, well controlled with medication, 29 and transient as they disappeared in minutes or hours (Table 3).
TABLE 3.
Adverse events during the procedure to induce the lesion and follow‐up
| During procedure (n = 20) | 24 h posttreatment (n = 20) | 1 Month (n = 20) | 3 Months (n = 20) | 6 Months (n = 20) | 12 Months (n = 12) | |
|---|---|---|---|---|---|---|
| Facial asymmetry | 2 (10%) | 4 (20%) | 0 | 0 | 0 | 0 |
| Weakness | 0 | 3 (15%) | 3 (15%) | 3 (15%) | 2 (10%) | 1 (8%) |
| Dysarthria | 4 (20%) | 2 (10%) | 0 | 0 | 0 | 0 |
| Paresthesia | 0 | 0 | 2 (10%) | 1 (5%) | 0 | 0 |
| Gait disturbance (dystonic pseudo foot drop) a | – | 3 (15%) | 3 (15%) | 3 (15%) | 1 (5%) | 1 (8%) |
| Behavioral changes (hypomania) | 0 | 0 | 6 (30%) | 3 (15%) | 0 | 0 |
| Weight gain | 0 | 0 | 7 (35%) | 2 (10%) | 1 (5%) | 1 (8%) |
| Confusional state | 0 | 1 (5%) | 1 (5%) | 1 (5%) | 0 | 0 |
| De novo dyskinesia (off) | 4 (20%) | 0 | 2 (10%) | 2 (10%) | 1 (5%) | 0 |
| De novo dyskinesia (on) | – | 0 | 3 (15%) | 3 (15%) | 1 (5%) | 0 |
Two patients had foot drop and 1 patient had a turned inward position without weakness or pyramidal signs. They did not improve with dopaminergic medication.
At 6‐month follow‐up, there were no moderate or severe AEs; however, there were six mild AEs (30%). Throughout the follow‐up, all AEs were of mild intensity, except for those in 3 patients (Table 3). One patient suffered severe weakness of the treated hemibody 1 week after treatment, which improved with physical therapy and was mild at the 6‐month visit; however, the patient was lost to follow‐up at 12 months. Another patient exhibited moderate dyskinesia in off medication in the treated lower limb, which required no treatment and was completely resolved at 6 months. The third patient had a moderate confusional state that was completely resolved by the 6‐month visit.
Three patients (15%) presented weakness 24 hours after treatment. One had severe weakness (the patient described earlier), whereas the other 2 patients had mild weakness, of whom 1 recovered at 6 months and the other still had mild weakness at 6‐ and 12‐month follow‐up. Mild dysarthria was observed in 2 patients (10%) after 24 hours: one experienced severe weakness, and the other had mild weakness. Both patients recovered by the 1‐month follow‐up. Gait disturbance was observed in 3 patients (15%), 2 of whom had dystonic pseudo foot drop, and 1 had a foot inversion without weakness or pyramidal signs that did not improve with dopaminergic medication. Two patients recovered at 6 months, whereas 1 patient still exhibited the condition at the 6‐ and 12‐month follow‐up.
In the remaining patients with mild AEs, the most frequent were a slight weight gain (n = 7, 35%) and hypomania, with cheerful behavior reported as verborrhea, inappropriate comments, and excitement (n = 6, 30%), that were completely resolved by the 6‐month visit (Table 3), except for 1 patient who retained weight gain at 6 and 12 months. Mild paresthesia in the treated hand (n = 2, 10%) was less frequent and observed only at 1 and 3 months.
Dyskinesia
Of the 8 patients who had mild dyskinesia in on medication before treatment, 5 did not have dyskinesias after treatment in either off‐ or on medication. In the remaining 3 patients, the severity was unchanged throughout the follow‐up period. Four other patients (20%) exhibited mild dyskinesia lasting only a few minutes during the procedure. During off medication, 2 patients (10%) had de novo dyskinesia; one had moderate intensity, as previously reported, and the other had mild intensity. The former case was completely resolved at the 6‐month visit, and the latter remained mild at 6 months and resolved at the 12‐month visit. Three other patients (15%) presented with on medication de novo mild dyskinesia at 1‐ and 3‐month visits, which persisted in only 1 patient (5%) at 6 months and was resolved at the 12‐month visit (Table 3).
Nonmotor Secondary Outcomes
Regarding cognition and neuropsychiatric assessments, we observed significant improvement in the scores of tests evaluating visual memory (Rey–Osterrieth complex figure‐delayed recall: 2.09, P = 0.04), depression (Geriatric Depression Scale: –2.13, P = 0.02), and impulsivity (Barratt Impulsiveness Scale II: –7.99, P = 0.03), as well as worsening in tests evaluating semantic fluency (−2.31, P = 0.03) and processing speed (Stroop word: –4.98, P = 0.03) (Table S2).
Subjective Impression of Improvement
With one exception, all patients reported a subjective impression of improvement in the PGI‐C scale at 6 month follow‐up. At 6 months, 90% (n = 18) of the patients scored their situation as “very much improved” or “much improved” (Fig. 1C).
Image Analysis
The mean volume of the lesion was 70.45 ± 27 mm3, and the volume of the ablated STN was 25.9 ± 18.54 mm3. The percentage of the ablated STN was significantly positively correlated with 6‐month improvements in the MDS‐UPDRS III total score (ΚΤ = 0.64 [0.21, 1.0], P = 0.01) and tremor score on the treated side (ΚΤ = 0.57 [0.16, 0.9], P = 0.03). The correlation with the MDS‐UPDRS III score (KT = 0.57, P = 0.056), bradykinesia score (KT = 0.57, P = 0.056), and rigidity score (KT = −0.07, P = 0.45) on the treated side was not statistically significant (Fig. 2). The total lesion volume showed no significant correlation with any clinical score (Table S3).
Discussion
Our study demonstrates that MRgFUS unilateral lesion of the STN can improve all PD motor manifestations on the treated side without permanent moderate or severe AEs.
The improvement was highly significant and sustained throughout the follow‐up period in all cardinal features, whereas it was higher in rigidity and tremor (>80%) than in bradykinesia (about 70%). Importantly, at the 6‐month follow‐up visit compared to baseline, almost 70% of patients presented at least an 80% reduction in the MDS‐UPDRS III scores in off medication on the treated side. Notably, of the 2 patients with less than 50% motor improvement, the overall condition of 1 declined due to de novo lung cancer diagnosed between the 3‐ and 6‐month visits post‐lesion of the STN. He underwent surgery and received chemotherapy during the follow‐up period. Despite the 24.8% reduction in dopaminergic treatment at the 6‐month visit, the total MDS‐UPDRS III score was much improved without a significant worsening of the untreated side. These findings support the few reports of unilateral STN lesions, using either radiofrequency 7 , 30 or MRgFUS, 15 , 16 and are similar to those shown after unilateral STN‐DBS. 31 , 32
Importantly, we observed no permanent moderate or severe AEs. Although the number of mild AEs after the procedure was high, most of them were transient, improving progressively until the 6‐month visit, with a few cases persisting at this visit. This may be attributed to the edema surrounding lesions made by MRgFUS that is typically observed in the first months after the procedure. One patient had a severe AE (weakness in the treated hemibody), and 2 patients experienced moderate AEs (off‐medication dyskinesias and confusional state) that were mild (weakness) or had completely resolved at the 6‐month visit. Notably, the patient with a moderate transient confusional state had scores in the low normal range in attention and executive functions at baseline. The most frequent mild AEs were transient hypomania and weight gain, both of which completely resolved in all cases, except for 1 patient who retained a slight weight gain 6 months after treatment. Permanent weight gain after STN‐DBS is a common AE whose mechanism is unknown. 33 Behavioral changes such as logorrhea and disinhibition have been described not only after STN lesions 34 but also with STN‐DBS. 35 , 36 , 37 They are conceived as reduced control of appropriate behavior and are somewhat comparable to the lack of motor inhibition associated with dyskinesia. 38 Other mild, mostly transient AEs—such as weakness and gait disturbance due to dystonic pseudo foot drop 39 in the treated hemibody and dysarthria—occurred in a few cases, with no limitations in daily functioning.
In our study, we observed no worsening of preexisting dyskinesias, and in most cases they were not present after the treatment. De novo dyskinesias—during either off‐ or on medication—were infrequent, and transient and mild when they occurred (except in one case in which they were moderate, as indicated earlier). The low incidence of dyskinesias might be due to the third lesion overlapping with the fasciculus lenticularis with a pallidotomy‐like effect. 18 The reduction in dopaminergic treatment may also play a role. The dyskinesias and behavioral changes are mostly transient and may be related to the lesion‐like effect of perilesional edema. They may also reflect a transitory reorganization of the neuronal discharges in the area surrounding the lesion, implying both the limbic‐associative circuit and the motor circuit. Local field potentials recorded in the STN in patients treated with STN‐DBS have indeed shown increased θ‐α activity associated with ICD or dyskinesias 40 in the limbic‐associative or motor area, respectively. Even though our patients did not have ICD, it is known that PD patients with ICD often have a euphoric/hypomaniac state similar to the one described in our patients, both being in the spectrum of emotional disorders related to abnormal activity in the limbic‐associative circuit. 38 Moreover, stimulation of the STN through contacts of the electrodes placed in the limbic area of the STN can induce mania in the absence of the typical ICD behaviors in PD patients. 41 Despite the limitation of the small number of patients, our study indicates that the risk of persistent dyskinesia is not as frequent as previously reported. 34
Most patients manifested a high level of satisfaction. The vast majority subjectively reported their clinical situation at 6 months as “very much improved” or “much improved,” indicating that the AEs were tolerable. Indeed, except for the patient who suffered moderate hemiparesis, those reporting lower satisfaction had been affected by comorbidities (cancer and cardiopathies), greatly impacting their quality of life.
Interestingly, a subset of 12 patients evaluated at 12 months showed similar results to those at 6 months. We acknowledge the attrition rate at this evaluation; therefore, these data should be interpreted with caution. However, we observed no significant differences between both features at baseline and motor benefit or AEs at 6 months when comparing patients followed up for 12 months and those who dropped out.
Regarding neuropsychiatric assessment, we observed both improvement in the scores of tests evaluating visual memory, depression, and impulsivity and decline in those assessing procedural speed and semantic fluency. Nevertheless, neither the patients nor their relatives noticed any clinical impact, as the scores, both at baseline and at 6‐month follow‐up, fell within the normal range. Similar findings have been reported in the few studies performed with STN unilateral lesions using radiofrequency, 7 , 34 , 42 , 43 , 44 and in the only study performed using MRgFUS, 15 , 16 indicating that a controlled lesion of the STN does not convey any deleterious cognitive effect. 34
MRgFUS and DBS may not be affordable in unwealthy countries, where lesions can be induced by radiofrequency. With regard to the available data, unilateral STN lesions induced using both MRgFUS and radiofrequency provide similar antiparkinsonian benefits. 7 , 15 , 16 , 31 , 32 However, a distinction between side effects related to the lesion itself and those related to the technique used for inducing the lesion must be considered. Side effects related to the lesion itself are similar with both techniques, whereas radiofrequency treatments have been associated with scalp infections and the risk of intracerebral hemorrhage, although the risk of such complications is low. 34 Thus, we believe that unilateral radiofrequency STN lesion induced by experts is also a useful treatment for PD patients under specific circumstances.
Regarding the lesion features, we found correlation between the volume of the lesions in the STN and the improvement in tremor on the treated side and in the total motor score. The results of the correlation analyses for the bradykinesia score (P = 0.056) and the MDS‐UPDRS III score on the treated side (P = 0.056), although not statistically significant, are quite interesting in the context of our research and are in keeping with previous studies using radiofrequency 7 , 18 or MRgFUS 19 and with the “sweet spot” for electrode placement in STN‐DBS. 45 , 46 In contrast, we found no correlation between the total volume of the lesion and the clinical outcome. These results have to be interpreted with caution as this is an exploratory analysis in a few patients. Nonetheless, we must acknowledge that a part of the lesion is placed in the subthalamic area surrounding the STN, which includes areas such as the pallidothalamic tract, fields of Forel, prelemniscal radiation, zona incerta or posterior subthalamic area. Given that lesions in these areas have shown antiparkinsonian benefit, 42 , 47 , 48 we cannot exclude the fact that part of the benefits could be associated with lesions in these areas.
We conducted this study in selected patients with asymmetric parkinsonism, relatively short duration of disease, and nontroublesome dyskinesia and remarkable bradykinesia, rigidity, and tremor. Despite the open‐label nature of our study and its small sample size, our results and those of previous studies 15 , 16 suggest that the benefit–risk ratio of unilateral treatments using MRgFUS in the STN in such PD patients might be better than that in other targets used for PD. Even though a direct comparison is not possible due to the different designs (randomized placebo‐controlled, blind evaluation trials), sample sizes, and outcomes of the studies evaluating the results, lesions in the Vim are less effective in alleviating parkinsonian tremor than those in the STN, reducing it to about 60% from baseline. 14 A recent study demonstrated that unilateral lesions of the GPi can induce an improvement in the MDS‐UPDRS III score on the treated side. The motor improvement is, however, lower than that observed in STN lesions, with a similar AE profile. 17
This study has several limitations. First, it was an open‐label study, the sample size was small, and only 2 women were included. Considering the COVID‐19 pandemic and large distances at which some patients lived, some neurological assessments were performed through video calls. Therefore, we could not obtain data on the rigidity and complete cognitive, neuropsychological, and MR evaluation of the whole sample. Furthermore, the subgroup followed up for 12 months was limited, emphasizing the need for additional studies with larger samples and longer follow‐up to expand our knowledge of this treatment.
In conclusion, our study demonstrates that unilateral MRgFUS lesion of the STN in PD patients with asymmetric presentation and mild dyskinesias resulted in significant motor improvement. Severe and moderate AEs may occur early after the treatment but are not frequent, and they improved in the following months. No severe or moderate permanent AEs were observed at 6 months. Mild and mostly transient AEs were frequent. We achieved these results through the accurate placement of the lesion in the nucleus and subthalamic area. Multicenter control studies with longer follow‐up are crucial to confirm these findings.
Author Roles
Research project: A. Conception and design, B. Organization, C. Execution;
Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
Manuscript Preparation: A. Writing of the first draft, B. Editing, C. Review and Critique;
LAG: 1B, 1C, 2A, 2B, 2C, 3A, 3B, 3C
CASC: 1B, 1C, 2A, 2B, 2C, 3A, 3B, 3C
AJH: 2A, 2B, 2C, 3A, 3B, 3C
IAO: 1C, 2C, 3C
GM: 1C, 2A, 2B, 2C
AG: 3C
AMB: 3C
LHGQ: 1C, 3C
JG: 1C, 2C, 3C
MCRO: 1A, 1B, 1C, 2A, 2C, 3A, 3B, 3C
Full financial disclosures of all authors for the preceding 12 months
M.C.R.‐O. received financial support for attending scientific meetings and lectures from Boston Scientific, AbbVie, Insightec, and Esteve. I.A.‐O has received speaking honoraria from Boston Scientific, Medtronic, and Esteve along with travel and congress meeting support from Bial. A.J.‐H. received financial support from Lilly for attending a scientific meeting. A.M.‐B. received honoraria from Zambon, Bial, and Esteve for travel expenses. L.H.G.‐Q. received honoraria from Insightec, Palex, Medtronic, Siemens, and Boston Scientific for lectures, travel, and accommodation to attend scientific meetings. The other authors report no competing interests.
Supporting information
Data S1. Supporting Information.
Table S1. MRgFUS (magnetic resonance–guided focused ultrasound) lesion of the subthalamic nucleus procedure details.
Table S2. Neuropsychological evaluations at baseline and 6‐month follow‐up.
Table S3. Correlation analyses between lesion volume and clinical changes at 6‐month follow‐up.
Acknowledgment
We acknowledge Insightec for its support in MR acquisitions at 3‐month follow‐up.
Data Availability Statement
Data available on request from the authors.
References
- 1. DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007;64(1):20–24. 10.1001/archneur.64.1.20 [DOI] [PubMed] [Google Scholar]
- 2. Obeso JA, Rodríguez‐Oroz MC, Benitez‐Temino B, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord 2008;23(Suppl 3):S548–S559. 10.1002/mds.22062 [DOI] [PubMed] [Google Scholar]
- 3. Wichmann T, Bergman H, DeLong MR, The primate subthalamic nucleus. III . Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 1994;72(2):521–530. 10.1152/jn.1994.72.2.521 [DOI] [PubMed] [Google Scholar]
- 4. Guridi J, Herrero MT, Luquin MR, et al. Subthalamotomy in parkinsonian monkeys Behavioural and biochemical analysis. Brain 1996;119:1717–1727. [DOI] [PubMed] [Google Scholar]
- 5. Gill SS, Heywood P. Bilateral dorsolateral subthalamotomy for advanced Parkinson's disease. Lancet 1997;350(9086):1224. 10.1016/s0140-6736(05)63455-1 [DOI] [PubMed] [Google Scholar]
- 6. Alvarez L, Macias R, Guridi J, et al. Dorsal subthalamotomy for Parkinson's disease. Mov Disord 2001;16(1):72–78. [DOI] [PubMed] [Google Scholar]
- 7. Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson's disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry 2009;80(9):979–985. 10.1136/jnnp.2008.154948 [DOI] [PubMed] [Google Scholar]
- 8. Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson's disease: initial and long‐term response. Brain 2005;128(3):570–583. 10.1093/brain/awh397 [DOI] [PubMed] [Google Scholar]
- 9. Deep‐Brain Stimulation for Parkinson's Disease Study Group , Obeso JA, Olanow CW, et al. Deep‐brain stimulation of the subthalamic nucleus or the pars Interna of the Globus Pallidus in Parkinson's disease. N Engl J Med 2001;345(13):956–963. 10.1056/NEJMoa000827 [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez‐Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow‐up. Brain 2005;128(10):2240–2249. 10.1093/brain/awh571 [DOI] [PubMed] [Google Scholar]
- 11. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced parkinson disease: a randomized controlled trial. J Am Med Assoc 2009;301(1):63–73. 10.1001/jama.2008.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355(9):896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]
- 13. Wang TR, Bond AE, Dallapiazza RF, et al. Transcranial magnetic resonance imaging‐guided focused ultrasound thalamotomy for tremor: technical note. Neurosurg Focus 2018;44(2):E3. [DOI] [PubMed] [Google Scholar]
- 14. Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication‐refractory, tremor‐dominant Parkinson disease a randomized clinical trial. JAMA Neurol 2017;74(12):1412–1418. 10.1001/jamaneurol.2017.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez‐Fernández R, Rodríguez‐Rojas R, del Álamo M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol 2018;17(1):54–63. 10.1016/S1474-4422(17)30403-9 [DOI] [PubMed] [Google Scholar]
- 16. Martínez‐Fernández R, Máñez‐Miró JU, Rodríguez‐Rojas R, et al. Randomized trial of focused ultrasound Subthalamotomy for Parkinson's disease. N Engl J Med 2020;383(26):2501–2513. 10.1056/nejmoa2016311 [DOI] [PubMed] [Google Scholar]
- 17. Krishna V, Fishman PS, Eisenberg HM, et al. Trial of Globus Pallidus focused ultrasound ablation in Parkinson's disease. N Engl J Med 2023;388(8):683–693. 10.1056/nejmoa2202721 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez‐Rojas R, Carballo‐Barreda M, Alvarez L, et al. Subthalamotomy for Parkinson's disease: clinical outcome and topography of lesions. J Neurol Neurosurg Psychiatry 2018;89(6):572–578. 10.1136/jnnp-2017-316241 [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez‐Rojas R, Pineda‐Pardo JA, Mañez‐Miro J, et al. Functional topography of the human subthalamic nucleus: relevance for Subthalamotomy in Parkinson's disease. Mov Disord 2022;37(2):279–290. 10.1002/MDS.28862 [DOI] [PubMed] [Google Scholar]
- 20. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 22. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 23. Schade S, Mollenhauer B, Trenkwalder C. Levodopa equivalent dose conversion factors: an updated proposal including Opicapone and safinamide. Mov Disord Clin Pract 2020;7(3):343–345. 10.1002/MDC3.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration . What is a Serious Adverse Event? https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event 2023.
- 25. Pineda‐Pardo JA, Urso D, Martínez‐Fernández R, et al. Transcranial magnetic resonance‐guided focused ultrasound Thalamotomy in essential tremor: a comprehensive lesion characterization. Neurosurgery 2020;87(2):256–265. 10.1093/neuros/nyz395 [DOI] [PubMed] [Google Scholar]
- 26. Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2017;2018(170):271–282. 10.1016/j.neuroimage.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 27. R Core Team R . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 28. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017;82(13):1–26. 10.18637/JSS.V082.I13 [DOI] [Google Scholar]
- 29. Cacho‐Asenjo E, Honorato‐Cia C, Nuñez‐Cordoba JM, et al. Factors associated with headache and nausea during magnetic resonance–guided focused ultrasound for tremor. Mov Disord Clin Pract 2021;8(5):701–708. 10.1002/mdc3.13210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su PC, Tseng H, Liu H, Yen R, Liou H. Treatment of advanced Parkinson's disease by subthalamotomy: one‐year results. Mov Disord 2003;18(5):531–538. 10.1002/mds.10393 [DOI] [PubMed] [Google Scholar]
- 31. Ehm G, Kim H‐J, Kim J, et al. Effect of unilateral subthalamic deep brain stimulation in highly asymmetrical Parkinson's disease: 7‐year follow‐up. J Neurosurg 2019;131(5):1508–1513. 10.3171/2018.5.JNS172006 [DOI] [PubMed] [Google Scholar]
- 32. Slowinski JL, Putzke JD, Uitti RJ, et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg 2007;106(4):626–632. 10.3171/JNS.2007.106.4.626 [DOI] [PubMed] [Google Scholar]
- 33. Balestrino R, Baroncini D, Fichera M, et al. Weight gain after subthalamic nucleus deep brain stimulation in Parkinson's disease is influenced by dyskinesias' reduction and electrodes' position. Neurol Sci 2017;38(12):2123–2129. 10.1007/s10072-017-3102-7 [DOI] [PubMed] [Google Scholar]
- 34. Máñez‐Miró JU, Rodríguez‐Rojas R, Del Álamo M, Martínez‐Fernández R, Obeso JA. Present and future of subthalamotomy in the management of Parkinson's disease: a systematic review. Expert Rev Neurother 2021;21(5):533–545. 10.1080/14737175.2021.1911649 [DOI] [PubMed] [Google Scholar]
- 35. Voon V, Kubu C, Krack P, Houeto JL, Tröster AI. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 2006;21(S14): S305–S327. 10.1002/mds.20963 [DOI] [PubMed] [Google Scholar]
- 36. Castrioto A, Lhommée E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson's disease. Lancet Neurol 2014;13(3):287–305. 10.1016/S1474-4422(13)70294-1 [DOI] [PubMed] [Google Scholar]
- 37. Cartmill T, Skvarc D, Bittar R, McGillivray J, Berk M, Byrne LK. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: a meta‐analysis of mood effects. Neuropsychol Rev 2021;31(3):385–401. 10.1007/s11065-020-09467-z [DOI] [PubMed] [Google Scholar]
- 38. Voon V, Napier TC, Frank MJ, et al. Impulse control disorders and levodopa‐induced dyskinesias in Parkinson's disease: an update. Lancet Neurol 2017;16(3):238–250. 10.1016/S1474-4422(17)30004-2 [DOI] [PubMed] [Google Scholar]
- 39. Aquino CC, Slow E, Lang AE. Dystonic pseudo foot drop. Mov Disord Clin Pract 2015;2(3):295–298. 10.1002/mdc3.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodriguez‐Oroz MC, López‐Azcárate J, Garcia‐Garcia D, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson's disease. Brain 2011;134(1):36–49. 10.1093/brain/awq301 [DOI] [PubMed] [Google Scholar]
- 41. Chopra A, Tye SJ, Lee KH, et al. Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson's disease: a review of the literature. J Neuropsychiatry Clin Neurosci 2012;24(1):102–110. 10.1176/appi.neuropsych.10070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCarter RJ, Walton NH, Rowan AF, Gill SS, Palomo M. Cognitive functioning after subthalamic nucleotomy for refractory Parkinson's disease. J Neurol Neurosurg Psychiatry 2000;69(1):60–66. 10.1136/jnnp.69.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Obeso I, Casabona E, Rodríguez‐Rojas R, et al. Unilateral subthalamotomy in Parkinson's disease: cognitive, psychiatric and neuroimaging changes. Cortex 2017;94:39–48. 10.1016/j.cortex.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 44. Patel NK, Heywood P, O'Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson's disease. Brain 2003;126(5):1136–1145. 10.1093/brain/awg111 [DOI] [PubMed] [Google Scholar]
- 45. Garcia‐Garcia D, Guridi J, Toledo JB, Alegre M, Obeso JA, Rodríguez‐Oroz MC. Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson's disease: a new approach for active contact localization. J Neurosurg 2016;125(5):1068–1079. 10.3171/2015.9.JNS15868 [DOI] [PubMed] [Google Scholar]
- 46. Hamel W, Köppen JA, Alesch F, et al. Targeting of the subthalamic nucleus for deep brain stimulation: a survey among Parkinson disease specialists. World Neurosurg 2017;99:41–46. 10.1016/J.WNEU.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 47. Aziz TZ, Peggs D, Agarwal E, Sambrook MA, Crossman AR. Subthalamic nucleotomy alleviates parkinsonism in the 1 ‐methyl‐4‐phenyl‐1,2,3,6‐ tetrahydropyridine (MPTP)‐exposed primate. Br J Neurosurg 1992;6(6):575–582. 10.3109/02688699209002375 [DOI] [PubMed] [Google Scholar]
- 48. Gill SS, Heywood P. Bilateral subthalamic nucleotomy can be accomplished safely. Mov Disord 1998;13(Suppl 2):201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Table S1. MRgFUS (magnetic resonance–guided focused ultrasound) lesion of the subthalamic nucleus procedure details.
Table S2. Neuropsychological evaluations at baseline and 6‐month follow‐up.
Table S3. Correlation analyses between lesion volume and clinical changes at 6‐month follow‐up.
Data Availability Statement
Data available on request from the authors.


