We read with interest the article by Jensen and colleagues who proposed a condensed version of the Progressive Supranuclear Palsy Quality of Life scale (PSP‐ShoQoL) as a reliable and practical tool to evaluate quality of life in PSP patients. 1
The proposed PSP‐ShoQoL included 12 items divided into two subscales representing physical (seven items) and mental symptoms (five items) and was administered to 245 patients from the German PSP network. The internal consistency of both total and subscores was high within 0.83 and 0.90. The PSP‐ShoQoL significantly correlated with the Progressive Supranuclear Scale‐Rating Scale (PSP‐RS) and the Geriatric Depression Scale (GDS) but not with the Montreal Cognitive Assessment scale (MoCA). With 12‐month follow‐up data on a subgroup of 94 patients, the authors showed that the PSP‐ShoQoL presented fair sensitivity to change and test–retest reliability.
Herein, we present data on the PSP‐ShoQoL on an independent PSP cohort, the Italian PSP‐NET supported by Fondazione LIMPE.2, 3 413 PSP patients performed the same evaluations used by Jensen et al. except for the GDS that was substituted by the Hospital Anxiety and Depression Scale (HADS). Compared with the German cohort, the PSP‐NET included older (age: mean ± standard deviation [SD] 71.2 ± 8.1 vs. 69.2 ± 7.4) and more severe patients (PSP‐RS: 40.56 ± 16.85 vs. 33.8 ± 13.8) while disease duration was similar (years: 4.44 ± 2.70 vs. 4.1 ± 2.6). Accordingly, PSP‐ShoQoL total and subscores were higher within the PSP‐NET (PSP‐ShoQoL total: 25.33 ± 11.3 vs. 19.27 ± 11.10; PSP‐ShoQoL Physical: 18.6 ± 8.2 vs. 13.74 ± 8.25; PSP‐ShoQoL Mental: 6.7 ± 5.1 vs. 5.53 ± 4.67). We confirm a fair internal consistency for both the total score (Cronbach's alpha: 0.87) and subscores (Physical: 0.89; Mental: 0.80). The PSP‐ShoQoL correlated significantly with the original PSP‐QoL (r = 0.945, P < 0.001), the PSP‐RS (r = 0.646, P < 0.001), the MoCA (−0.340, P < 0.001), and the HADS (r = 0.602, P < 0.001). With 6‐month follow‐up data available for 80 patients, we revealed a significant increase in both PSP‐ShoQoL total score (t = 5.24, P < 0.001) and Physical (t = 5.45, P < 0.001) and Mental (−2.78, P < 0.05) subscores. Test–retest reliability was good both for PSP‐ShoQoL total score (intraclass correlation coefficient [ICC] = 0.78, P < 0.001), as well as for its subscales (Physical ICC = 0.80, P < 0.00; Mental ICC = 0.68, P < 0.001). Finally, by analyzing the area under the curve (AUC) we identified a value of 34.5 as a discriminating cutoff for a significant impairment of quality of patients' life measured by the PSP‐ShoQoL within the PSP‐NET (sensibility: 0.97; specificity: 0.15; AUC: 0.93) (Fig. 1).
FIG. 1.
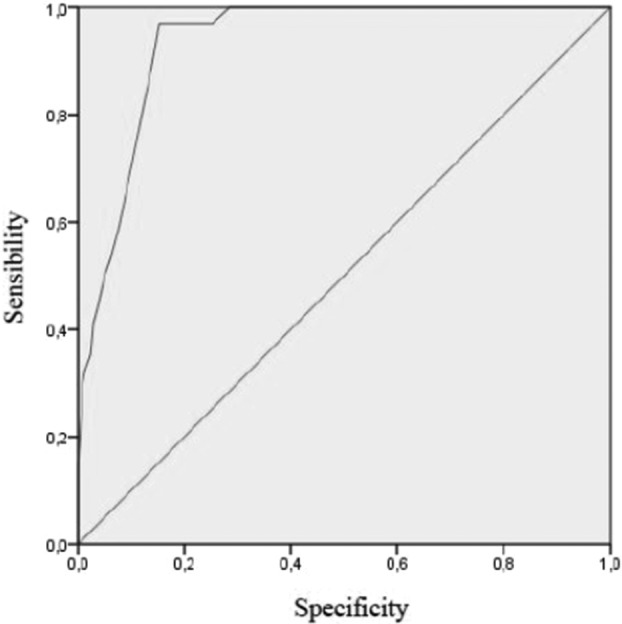
Receiver operating characteristic (ROC) curve of the Short Progressive Supranuclear Palsy Quality of Life scale (PSP‐ShoQoL).
Jensen and coworkers proposed a brief instrument with fair psychometric properties for assessing quality of life in PSP patients. Herein, we have demonstrated the application of the PSP‐ShoQol in an independent, large PSP cohort. Our results largely replicate those of Jensen et al. except for the relationship between the PSP‐ShoQoL and the MoCA. Furthermore, we propose a cutoff of 34.5 as a discriminating value for a significant impairment of quality of patients' life measured by the PSP‐ShoQoL.
Acknowledgments
AC wrote the first draft and conducted the statistical analysis. MP designed the study and revised the manuscript. PB revised the manuscript. All authors in the PSP‐NET Study Group collected data and revised the manuscript. AC has no relevant financial disclosures. MP is supported by the Italian Ministry of Health, the Italian Ministry of University, and Fondazione della Società Italiana di Neurologia. PB received consultancies as a member of the advisory board for Zambon, Lundbeck, UCB, Chiesi, Abbvie, and Acorda. Open access publishing facilitated by Universita degli Studi di Salerno, as part of the Wiley ‐ CRUI‐CARE agreement.
Appendix A. A – Members of the PSP‐NET Study Group
Maria Concetta Altavista1, Vincenzo Moschella1, Maurizio Zibetti2, Leonardo Lopiano2, Laura Bonanni3, Alessandro Tessitore4, Rosa De Micco4, Maria Francesca De Pandis5,6, Maria Gaglione5,6, Matteo Costanzo7,8, Giovanni Fabbrini8,9, Claudio Pacchetti10, Alessio Di Fonzio11, Giulia Lazzeri11, Alessandro Stefani12, Tommaso Schirinzi12, Fabrizio Stocchi13, Laura Vacca13, Nicola Modugno9, Enrica Oliva9, Raffaella Di Giacopo14, Francesca Di Biasio15, Roberta Marchese15, Massimo Cincotta16, Maristella Piccininni16, Maria Gabriella Ceravolo17, Marianna Capecci17, Giovanna Calandra‐Buonaura18, Ilaria Cani18, Luisa Sambati18, Roberto Ceravolo19, Daniela Frosini19, Eleonora Del Prete19, Nicoletti Alessandra20, Calogero Edoardo Cicero20, Andrea Ciammola21, Barbara Poletti21, Francesca Spagnolo22, Colosimo Carlo23, Marta Filidei23, Luca Magistrelli24, Laura De Togni25
1Unità di Neurologia ‐ Ospedale San Filippo Neri Hospital, ASL Roma 1, Roma.
2Dipartimento di Neuroscienze ‐ Università degli Studi di Torino AOU Città della Salute e della Scienza di Torino, Torino.
3Centro Demenze e Disordini del Movimento‐Clinica Neurologica, Neuroscienze, Imaging e Scienze Cliniche ‐ Università G. D'Annunzio di Chieti‐Pescara, Chieti.
4Università della Campania Luigi Vanvitelli, Dipartimento di Scienze Mediche e Chirurgiche Avanzate, Napoli.
5Clinical Trial Center, San Raffaele Cassino (FR).
6Dipartimento di Scienze Umane e Promozione della Qualità della Vita, Università San Raffaele, Roma.
7Dipartimento di Neuroscienze, Istituto Superiore di Sanità, Roma.
8Department of Neuroscienze Umane, Università di Roma Sapienza, Roma.
9IRCCS Neuromed, Pozzilli (IS).
10Centro Parkinson e Disordini del Movimento Istituto Neurologico Nazionale “Fondazione Mondino”, IRCCS, Pavia.
11Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico di Milano, UOC Neurologia, Milano.
12Università Roma Tor Vergata ‐ Policlinico Tor Vergata, Dipartimento Medicina dei Sistemi – Neurologia, Roma.
13IRCCS San Raffaele, Roma.
14Unità di Neurologia, Ospedale Santa Maria del Carmine, Rovereto (TN).
15IRCCS Ospedale Policlinico San Martino, Genova.
16Unità di Neurologia di Firenze, Asl Toscana Centrale, Firenze.
17Dipartimento di Medicina Sperimentale e Clinica ‐ Università Politecnica delle Marche, Ancona.
18UO NeuroMet, IRCCS Istituto delle Scienze Neurologiche, Azienda USL di Bologna, Bologna.
19SCDU Neurologia, Dipartimento di Medicina Clinica e Sperimentale, UO Neurologia, Azienda Ospedaliera Universitaria Pisana, Pisa.
20Ambulatorio Malattia di Parkinson e Disordini del Movimento, Dipartimento di Scienze Mediche, Chirurgiche e Tecnologie Avanzate “G.F. Ingrassia”, Sezione di Neuroscienze, Clinica Neurologica, A.O.U. Policlinico‐San Marco, Presidio “G. Rodolico”, Catania.
21Dipartimento di Neurologia e Laboratorio di Neuroscienze, IRCCS Istituto Auxologico Italiano, Milano.
22UOC Neurologia, Ambulatorio Disturbi del Movimento, Ospedale Antonio Perrino, Brindisi.
23Centro per i Disturbi Cognitivi e le Demenze (CDCD), Azienda Ospedaliera Santa Maria, Terni.
24Istituto Parkinson di Milano, ASST G. Pini‐CTO, Milano.
25Unità di Neurologia Ospedale Magalini Villafranca, Verona.
Members of the PSP‐NET Study Group are listed in Appendix A.
Relevant conflicts of interest/financial disclosures: All authors have no relevant conflicts of interest with the present work.
Funding agency: PSP‐NET is supported by Fondazione LIMPE per il Parkinson onlus.
Contributor Information
Marina Picillo, Email: mpicillo@unisa.it.
The PSP‐NET Study Group.:
Maria Concetta Altavista, Vincenzo Moschella, Maurizio Zibetti, Leonardo Lopiano, Laura Bonanni, Alessandro Tessitore, Rosa De Micco, Maria Francesca De Pandis, Maria Gaglione, Matteo Costanzo, Giovanni Fabbrini, Claudio Pacchetti, Alessio Di Fonzio, Giulia Lazzeri, Alessandro Stefani, Tommaso Schirinzi, Fabrizio Stocchi, Laura Vacca, Nicola Modugno, Enrica Oliva, Raffaella Di Giacopo, Francesca Di Biasio, Roberta Marchese, Massimo Cincotta, Maristella Piccininni, Maria Gabriella Ceravolo, Marianna Capecci, Giovanna Calandra‐Buonaura, Ilaria Cani, Luisa Sambati, Roberto Ceravolo, Daniela Frosini, Eleonora Del Prete, Nicoletti Alessandra, Calogero Edoardo Cicero, Andrea Ciammola, Barbara Poletti, Francesca Spagnolo, Colosimo Carlo, Marta Filidei, Luca Magistrelli, and Laura De Togni
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Jensen I, Stiel S, Bebermeier S, et al. A short progressive supranuclear palsy quality of life scale. Mov Disord 2024;39(9):1602–1609. 10.1002/mds.29936. [DOI] [PubMed] [Google Scholar]
- 2. Picillo M, Cuoco S, Amboni M, et al. Validation of the Italian version of the PSP quality of life questionnaire. Neurol Sci 2019;40(12):2587–2594. 10.1007/s10072-019-04010-2 [DOI] [PubMed] [Google Scholar]
- 3. Fabbri M, Ledda C, Schirinzi T, et al. Multidisciplinary care use in neurodegenerative complex diseases: the example of progressive supranuclear palsy and advanced Parkinson's disease in real‐life. Parkinsonism Relat Disord 2024;125:107047. 10.1016/j.parkreldis.2024.107047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


