Abstract
Background
Endoscopic bariatric interventions are viable alternatives to traditional weight loss surgeries for patients with a body mass index between 30 and 40 kg/m2. While studies have explored the impact of intragastric balloons on obesity and weight reduction, comprehensive data on real-world device-related complications and failures are limited. This study aimed to investigate mechanical failures leading to patient adverse events reported to the US Food and Drug Administration.
Methods
A retrospective analysis using the Manufacturer and User Facility Device Experience (MAUDE) database examined device-related events and patient outcomes associated with various intragastric balloons from July 2017 to October 2023. Data analysis was performed using SPSS software to assess the reported events and their associations with specific types of intragastric balloons.
Results
Our review included 1393 cases, revealing 1758 device malfunctions and 1760 patient complications. Deflation problems (25.31%) and balloon leakage or rupture (21.90%) were the most common device-related complications. Patient complications were primarily linked to the Orbera balloon, with implant failure (24.30%), abdominal pain (21.92%), and vomiting (21.26%) as leading adverse events.
Conclusion
Our findings highlight the need for continuous surveillance, rigorous monitoring, and enhanced safety protocols for intragastric balloons.
Keywords: Bariatric endoscopy, endoscopy, gastric balloon, nonsurgical weight loss
Endoscopic bariatric therapy has gained traction with the introduction of intragastric balloons (IGBs) in the 1980s as an alternative to bariatric surgery in high-risk individuals or as a bridge to surgery in patients not meeting the criteria of a body mass index ≥40 kg/m2. Endoscopic bariatric therapy comprises IGB placement, endoscopic sleeve gastroplasty, and aspiration therapy. IGBs are either introduced into the stomach endoscopically or swallowed by the patient, can be filled with gas or liquid, and are generally removed within 6 months. Table 1 provides a brief overview on the various types of US Food and Drug Administration (FDA)-approved IGBs, such as Orbera balloon, ReShape Duo, Obalon Capsule, and Spatz Adjustable, that are available for these interventions.1–4
Table 1.
General characteristics of the included intragastric balloons*
| Variable | Orbera | ReShape duo | Obalon capsule | Spatz adjustable |
|---|---|---|---|---|
| Volume (mL) | 400–700 | 900 | 250 | Up to 700 |
| Contents | Methylene blue, saline | Methylene blue, saline | Air mixture | Methylene blue, saline |
| Placement | Endoscopy | Endoscopy | Swallow | Endoscopy |
| Duration (months) | 6 | 6 | 6 | 12 |
Several studies have discussed the efficacy of IGBs for weight loss. The most recent meta-analysis by Weitzner et al5 included 13 studies (8 randomized controlled trials, 3 observational studies) and was one of the most comprehensive and up-to-date studies. The majority of current data on adverse events related to IGBs is limited to clinical trials, case reports, and case series. There is a lack of comprehensive data on real-world device-related complications and failures. Ramai et al6 reported the decline of total adverse events related to IGBs over several years, with 33.1% in 2017 compared to 30.9% in 2018, 23.2% in 2019, and 12.8% in 2020. Another notable prospective study under a single provider in the United Kingdom (2017–2021) with Orbera 365™ IGB revealed a total complication rate of 5.22% (n = 60/1149) but did not discuss the trend.7 Nausea, vomiting, and abdomen pain are the common adverse events associated with IGBs, with other serious adverse events including perforation, ulcers, obstruction, and pancreatitis, to name a few. An extended longitudinal dive into adverse events and device-related failures is warranted because this entire process involves cost and risk to the patient and poses endoscopic challenges to the physician. Premature removal due to adverse events, with the tide of weight gain and loss, demoralizes the individual and increases apprehension toward such procedures. More insight into adverse events can assist us in formulating plans to tackle and improve patient satisfaction and compliance. Our study aimed to examine mechanical failures leading to patient adverse events reported to the FDA in the United States.
METHODS
A preliminary investigation encompassed both academic literature and online sources to identify the most commonly used IGBs in the US market. This examination led to the identification of several IGBs, namely Orbera balloon, ReShape Duo, Obalon Capsule, and Spatz. A subsequent retrospective investigation was completed via the FDA Manufacturer and User Facility Device Experience (MAUDE)8 database from July 2017 to October 2023. The findings were compiled into an Excel spreadsheet, delineating critical details such as report number, event date, manufacturer, balloon type, device and patient problems, and event specifics. To analyze this information comprehensively, IBM SPSS9 Statistics for Windows, Version 27.0, was employed, enabling a thorough examination of patterns, trends, and noteworthy incidents associated with these specific IGBs during the specified timeframe.
RESULTS
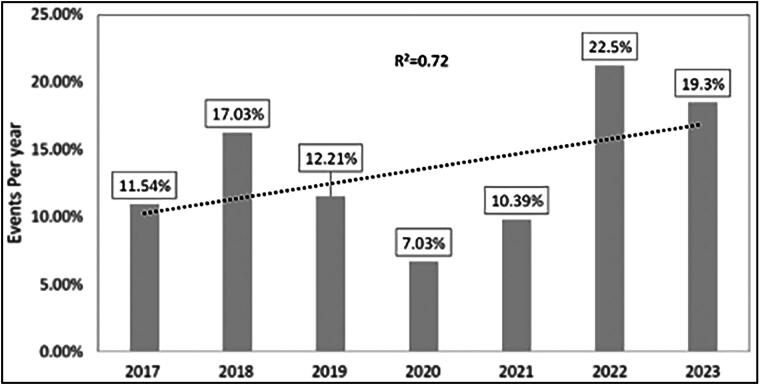
A total of 1393 cases were found, involving 1755 device malfunctions and 1760 patient complications. The most widely used IGB in the US, the Orbera balloon, had 1217 device issues reported, and 396 issues were reported with the Spatz device. Throughout the study, there was an increase in the number of events (R2 = 0.72): 19.3% of events occurred in 2023, compared to 22.5% in 2022, 10.39% in 2021, 7.03% in 2020, 12.21% in 2019, 17.03% in 2018, and 11.54% in 2017 (Figure 1). Forty-seven ReShape Duo device issues and 68 Obalon IGB device issues were reported. Deflation problems accounted for the majority of balloon complications (25.4%), with leakage or balloon rupture (22%) and adverse events without an identified device or use problems (14.1%) following closely behind (Table 2). Table 3 reports other less common device issues. Most patient adverse events were reported for the Orbera balloon (n = 1476), followed by the Spatz (n = 124), Obalon (n = 97), and ReShape Duo balloon (n = 63). Failure of implant (23.2%), abdominal pain (20.9%), vomiting (20.3%), nausea (11.4%), obstruction/occlusion (5.1%), dehydration (2.7%), ulcer formation (2.2%), perforation (1.3%), abdominal distention (1.2%), and death (1.4%) were the most common adverse events reported. Additionally, a subset of 36 patients (2.58%) required care in the intensive care unit. Table 2 shows additional less common patient adverse events.
Figure 1.
Intragastric balloon events per year.
Table 2.
Device issues reported according to balloon type
| Device problem | Event | Orbera | Obalon | ReShape | Spatz |
|---|---|---|---|---|---|
| Adverse event without identified device or use problem | 248 (14.11%) | 182 (14.93%) | 14 (20.29%) | 48 (64.86%) | 4 (1.01%) |
| Air/gas in device | 38 (2.16%) | 3 (0.25%) | 1 (1.45%) | 0 (0%) | 34 (8.59%) |
| Appropriate term/code not available | 5 (0.28%) | 5 (0.41%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Compatibility problem | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Connection problem | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Defective component | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Deflation problem | 445 (25.31%) | 171 (14.03%) | 1 (1.45%) | 1 (1.35%) | 272 (68.69%) |
| Detachment of device or device component | 4 (0.23) | 4 (0.33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Device dislodged or dislocated | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Device operates differently than expected | 2 (0.11%) | 2 (0.16%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Difficult to remove | 4 (0.23) | 4 (0.33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Entrapment of device | 1 (0.06%) | 0 (0%) | 1 (1.45%) | 0 (0%) | 0 (0%) |
| Free or unrestricted flow | 63 (3.58%) | 62 (5.09%) | 0 (0%) | 0 (0%) | 1 (0.25%) |
| Fungus in device environment | 21 (1.19%) | 21 (1.72%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Improper or incorrect procedure or method | 2 (0.11%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.51%) |
| Inadequacy of device shape and/or size | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Inflation problem | 73 (4.15%) | 71 (5.82%) | 0 (0%) | 0 (0%) | 2 (0.51%) |
| Insufficient information | 50 (2.84%) | 42 (3.45%) | 0 (0%) | 0 (0%) | 8 (2.02%) |
| Leak/splash | 385 (21.90%) | 330 (27.07%) | 33 (47.83%) | 15 (20.27%) | 7 (1.77%) |
| Material integrity problem | 2 (0.11%) | 2 (0.16%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Material perforation | 5 (0.28%) | 1 (0.08%) | 4 (5.80%) | 0 (0%) | 0 (0%) |
| Material puncture/rupture | 6 (0.34%) | 0 (0%) | 4 (5.80%) | 1 (1.35%) | 1 (0.25%) |
| Material split, cut, or torn | 2 (0.11%) | 1 (0.08%) | 0 (0%) | 1 (1.35%) | 0 (0%) |
| Mechanical problem | 6 (0.34%) | 6 (0.49%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Migration or expulsion of device | 62 (3.53%) | 26 (2.13%) | 3 (4.35%) | 7 (9.46%) | 26 (6.57%) |
| Off-label use | 2 (0.11%) | 1 (0.08%) | 1 (1.45%) | 0 (0%) | 0 (0%) |
| Patient-device incompatibility | 143 (8.13%) | 112 (9.19%) | 2 (2.90%) | 0 (0%) | 29 (7.32%) |
| Position problem | 2 (0.11%) | 0 (0%) | 1 (1.45%) | 1 (1.35%) | 0 (0%) |
| Retraction problem | 1 (0.06%) | 0 (0%) | 1 (1.45%) | 0 (0%) | 0 (0%) |
| Short fill | 3 (0.17%) | 1 (0.08%) | 2 (2.90%) | 0 (0%) | 0 (0%) |
| Unexpected therapeutic results | 1 (0.06%) | 1 (0.08%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unintended deflation | 175 (9.95%) | 165 (13.54%) | 0 (0%) | 0 (0%) | 10 (2.53%) |
| Use of device problem | 2 (0.11%) | 1 (0.08%) | 1 (1.45%) | 0 (0%) | 0 (0%) |
| Total events | 1758 (100%) | 1219 (69.2%) | 69 (3.9%) | 74 (4.2%) | 396 (22.5%) |
Table 3.
Adverse events reported according to balloon type
| Adverse event | Events | Orbera | Obalon | ReShape | Spatz |
|---|---|---|---|---|---|
| Abdominal distention | 22 (1.31%) | 17 (1.20%) | 1 (1.10%) | 0 (0%) | 4 (3.36%) |
| Abdominal pain | 368 (21.92%) | 260 (18.36%) | 30 (32.97%) | 9 (16.98%) | 69 (57.98%) |
| Aspiration | 15 (0.89%) | 10 (0.71%) | 0 (0%) | 3 (5.66%) | 2 (1.68%) |
| Dehydration | 47 (2.80%) | 45 (3.18%) | 0 (0%) | 2 (3.77%) | 0 (0%) |
| Dyspnea | 2 (0.12%) | 0 (0%) | 2 (2.20%) | 0 (0%) | 0 (0%) |
| Failure of implant | 408 (24.30%) | 396 (27.97%) | 0 (0%) | 1 (1.89%) | 11 (9.24%) |
| Gastritis | 18 (1.07%) | 16 (1.13%) | 0 (0%) | 2 (3.77%) | 0 (0%) |
| Hiccups | 1 (0.06%) | 0 (0%) | 1 (1.10%) | 0 (0%) | 0 (0%) |
| Gastroparesis | 5 (0.30%) | 5 (0.35%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypersensitivity/allergic reaction | 2 (0.12%) | 2 (0.14%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Malaise | 22 (1.31%) | 22 (1.55%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 201 (11.97%) | 161 (11.37%) | 22 (24.18%) | 7 (13.21%) | 11 (9.24%) |
| Obstruction/occlusion | 90 (5.36%) | 82 (5.79%) | 3 (3.30%) | 4 (7.55%) | 1 (0.84%) |
| Pancreatitis | 25 (1.49%) | 23 (1.62%) | 0 (0%) | 0 (0%) | 2 (1.68%) |
| Pulmonary embolism | 8 (0.48%) | 4 (0.28%) | 0 (0%) | 4 (7.55%) | 0 (0%) |
| Pyrosis/heartburn | 13 (0.77%) | 13 (0.92%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Regurgitation | 21 (1.25%) | 21 (1.48%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Renal failure | 7 (0.42%) | 7 (0.49%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ulcer formation | 38 (2.26%) | 15 (1.06%) | 11(12.09%) | 8 (15.09%) | 4 (3.36%) |
| Unspecified infection | 9 (0.54%) | 9 (0.64%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 357 (21.26%) | 308 (21.75%) | 21(23.08%) | 13 (24.53%) | 15 (12.61%) |
| Subtotal | 1679 (95.40%) | 1416 (84.34%) | 91 (93.81%) | 53 (84.13%) | 119 (95.97%) |
| Serious adverse events | |||||
| Cardiac arrest | 10 (12.35%) | 10 (16.67%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Death | 24 (29.63%) | 21 (35.00%) | 0 (0%) | 2 (20%) | 1 (20%) |
| Hemorrhage/bleeding | 12 (14.81%) | 11 (18.33%) | 0 (0%) | 1 (10%) | 0 (0%) |
| Laceration(s) of esophagus | 4 (4.94%) | 2 (3.33%) | 1 (16.67%) | 1 (10%) | 0 (0%) |
| Perforation | 23 (28.40%) | 10 (16.67%) | 5 (83.33%) | 5 (50%) | 3 (60%) |
| Sepsis | 8 (9.88%) | 6 (10%) | 0 (0%) | 1 (10%) | 1 (20%) |
| Subtotal | 81 (4.60%) | 60 (16.67%) | 6 (6.19%) | 10 (15.87%) | 5 (4.03%) |
| Total events | 1760 (100%) | 1476 (83.86%) | 97 (5.51%) | 63 (3.58%) | 124 (7.05%) |
DISCUSSION
Our understanding of IGB device-related problems and reported adverse events is limited to information gathered from pivotal clinical trials, oligocenter studies, and sporadic case reports and case series. Information on the safety, efficacy, and incidence of adverse events from pivotal clinical trials is valuable for obtaining institutional approval, such as from the FDA, followed by monitoring of data from postmarketing studies. In our study with the MAUDE database, we aimed to discuss ground-level numbers in clinical practice and trends over the years.
In our study, Orbera accounted for 69.3% (n = 1217) of total device-related problems and 83.8% (n = 1476) of total patient adverse events. The unequal distribution of reported device problems and adverse events among devices is related to the fact that Orbera received FDA approval in 2015 and has been on the market for several years. In a Brazilian consensus study involving data on 40,000 IGBs, Orbera constituted more than two-thirds of the devices (78.2%).10 Obalon, although approved by the FDA in 2016, has noticeably lower reported device problems. This can be attributed to the widespread acceptance and higher sales of Orbera compared to Obalon. Reshape Company was acquired by Apollo Endosurgery, and then Reshape Balloon was gradually phased out in 2020, focusing on its flagship Orbera. Spatz was FDA approved in 2021, which explains the small share in the current market and thus the lower reported number of adverse events and device problems.
We focus on the most common adverse events, the current process measures to mitigate them, device-related inherent problems, existing evidence of worldwide consensus, reasons for the concerning upward trend in reported adverse events over the years, and the future course and implications. The most common adverse events of nausea/vomiting and abdominal pain are related to the gastric accommodation of a foreign body. It is not uncommon for these events to lead to premature device removal. A meta-analysis of randomized controlled trials from 2018 comparing Orbera, Obalon, Reshape, and Ellipse showed that Orbera had the highest meta-analytic rate of nausea (81.97%) and vomiting (72.16%) when compared to former IGB subtypes.11 Medications can successfully manage most of these symptoms during the perioperative period. American Gastroenterological Association clinical practice guidelines suggest using intraoperative anesthetics with the lowest incidence of nausea and scheduled antiemetics for 2 weeks after IGB placement based on the limited available evidence.12 This Brazilian study recommends prescription medications such as ondansetron and hyoscine/scopolamine for 3 to 5 days to avoid reactive symptomatology after implantation.10 Scheduled close outpatient follow-ups, symptomatic treatment during the immediate postoperative period, and formulation of a plan together with the patient can reduce the rates of premature device removal.
IGBs are foreign bodies that are constantly in touch with the gastric mucosa and can induce morphological changes such as mucosal erosion, ulcer formation (n = 38), luminal perforation (n = 23), and bleeding (n = 12). In addition to these adverse events, we also noticed gastritis (n = 18), heartburn/pyrosis (n = 13), and regurgitation (n = 21). No clinical trials have assessed and compared the role of proton pump inhibitor (PPI) dose and duration in patients with IGBs. Usually, IGB insertion involves a diagnostic esophagogastroduodenoscopy to evaluate for concomitant pathologies that contraindicate placement, such as peptic ulcer disease, severe grade esophagitis, large hiatal hernias, strictures, and varices. In patients with relative contraindications such as grade A or B esophagitis, nonbleeding angioectasias, and gastritis, PPI therapy can be considered. Patients with indications for PPI prior to IGB placement can discuss with their endoscopist adjustments in dosage and frequency for the in situ duration for better symptom control. Regarding the risk of bleeding, the Brazilian consensus statement largely concurs that IGBs are contraindicated in patients receiving anticoagulants.10
A significant number of balloon obstruction/occlusion events (n = 90) were reported in our study, and these can be linked to several device-related problems such as issues with deflation (n = 620), leak/splash (n = 385), and migration or expulsion of the device (n = 62). IGBs are often filled with methylene blue (with the exception of Obalon, which is air-filled), which, in the event of a leak, is absorbed systemically and gives urine a bluish-green color that alerts patients to seek immediate medical assistance. Though leaked IGBs within the gastric cavity can be safely removed under endoscopy, the risk of intestinal migration requiring surgical extraction persists. The procedureless ellipse gastric balloon device, not included in our study, deserves special mention as it is a swallowable capsule that automatically deflates after 4 months and passes through the digestive system. Under investigation are next-generation swallowable, self-inflating capsules made of biodegradable material.13 Perhaps these adverse events should decrease with the newer generation of IGBs.
Other serious adverse events include pancreatitis (n = 25), sepsis (n = 6), cardiac arrest (n = 10), and death (n = 24). Although the exact cause of IGB-associated pancreatitis is unknown and limited to case reports in the literature, hypothesized mechanisms include device migration into the duodenum and pancreatic duct compression.5 Thirty-six subjects needed intensive care unit–level of care for serious adverse events in our study, but it is not feasible to determine the cause of clinical deterioration or patient-specific comorbidities due to the reporting system used in the MAUDE database.
There was an upward trend in the number of adverse events from 2017 to 2023. This can be attributed to an increase in the total number of IGB placements over the years and increased patient awareness about available options for weight loss. The drop in adverse events in 2020 (7.03%) and 2021 (10.39%) could be attributed to the COVID-19 pandemic and reduced numbers of elective procedures, as well as patient perceptions regarding safety. In this study, we attempted to provide a broader snapshot of data (2017–2023), underline the concerning upward trend, and emphasize the need for a nationwide clinician consensus and clinical practice guidelines outlining comprehensive care after IGB placement.
The MAUDE database is a collection of medical device reports from health care providers, institutions, device manufacturers, and patients. Reported events are often not comprehensive and have incomplete or insufficient information, making it challenging to ascertain a causal relationship. The database does not include the total number of devices implanted during a specific timeframe, making it difficult to perform additional estimates such as incidence rates. Despite the limitations, the MAUDE database provides us with valuable information regarding nationwide ground-level numbers in clinical practice.
Although IGBs are associated with an increasing trend of reported adverse events, they have been shown to be effective for weight loss. It is essential to educate patients regarding common adverse outcomes so they can recognize and seek medical attention promptly. Appropriate postimplantation care and routine follow-up visits to identify early symptoms and avert adverse events need to be incorporated into multidisciplinary clinical practice.
Disclosure statement/Funding
The authors report no funding or conflicts of interest.
References
- 1.Orbera . Apollo Endosurgery. https://apolloendo.com/patients/orbera/. Accessed March 6, 2024.
- 2.News about ReShape Ready: Orbera® Managed Weight Loss System . https://www.orbera.com/reshape. Accessed March 6, 2024.
- 3.Spatz3: The world’s first & only adjustable gastric balloon . https://www.spatzmedical.com/. Accessed March 6, 2024.
- 4.Obalon® Balloon System Level Information Manual . https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160001c.pdf.
- 5.Weitzner ZN, Phan J, Begashaw MM, et al. Endoscopic therapies for patients with obesity: a systematic review and meta-analysis. Surg Endosc. 2023;37(11):8166–8177. doi: 10.1007/s00464-023-10390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramai D, Bhandari P, Facciorusso A, et al. Real-world experience of intragastric balloons for obesity: insights from the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Obes Surg. 2021;31(7):3360–3364. doi: 10.1007/s11695-021-05324-x. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins T, Sharma O, Sarfaraz Y, et al.. Safety and efficacy of 12-month intra-gastric balloon-series of over 1100 patients. Obes Surg. 2023;34(1):176–182. doi: 10.1007/s11695-023-06953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MAUDE – Manufacturer and User Facility Device Experience . https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed March 6, 2024.
- 9.IBM SPSS Statistics . www.ibm.com. https://www.ibm.com/products/spss-statistics?utm_content=SRCWW&p1 = Search&p4=43700078595924100&p5 = p&gad_source = 1&gclid=CjwKCAiAxaCvBhBaEiwAvsLmWEVrdDUvxKeK33gEOxfW8AzmLTy431nsSva-cpXnuSx4Ls0i4amfBxoC46sQAvD_BwE&gclsrc=aw.ds. Accessed March 6, 2024.
- 10.Neto MG, Silva LB, Grecco E, et al. Brazilian Intragastric Balloon Consensus Statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis. 2018;14(2):151–159. doi: 10.1016/j.soard.2017.09.528. [DOI] [PubMed] [Google Scholar]
- 11.Trang J, Lee SS, Miller A, et al. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients – a systematic review and meta-analysis. Int J Surg. 2018;57:22–29. doi: 10.1016/j.ijsu.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Muniraj T, Day LW, Teigen LM, et al. AGA clinical practice guidelines on intragastric balloons in the management of obesity. Gastroenterology. 2021;160(5):1799–1808. doi: 10.1053/j.gastro.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Shirin H, Neeland IJ, Ryan DH, et al. Effects of an oral biodegradable device used for 12 weeks on weight reduction, cardiovascular risk factors, satiety, snacking, and meal size. Obes Pillars. 2023;8:100094. doi: 10.1016/j.obpill.2023.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]