Abstract
Background
We investigated fetal and total DNA levels in maternal plasma in patients bearing fetuses affected with Down syndrome in comparison to controls carrying fetuses with normal karyotype.
Methods
DNA levels in maternal plasma were measured using real-time quantitative PCR using SRY and β-globin genes as markers. Twenty-one pregnant women with a singleton fetus at a gestational age ranging from 15 to 19 weeks recruited before amniocentesis (carried out for reasons including material serum screening and advanced material age), and 16 pregnant women bearing fetuses affected with Down syndrome between 17 to 22 weeks of gestation were involved in the study.
Results
The specificity of the system reaches 100% (no Y signal was detected in 14 women pregnant with female fetuses) and the sensitivity 91.7% (SRY amplification in 22 of 24 examined samples). The median fetal DNA levels in women carrying Down syndrome (n=11) and the controls (n=13) were 23.3 (range 0–58.5) genome-equivalents/ml and 24.5 (range 0–47.5) genome-equivalents/ml of maternal plasma, respectively (P = 0.62). The total median DNA levels in pregnancies with Down syndrome and the controls were 10165 (range 615–65000) genome-equivalents/ml and 7330 (range 1300–36750) genome-equivalents/ml, respectively (P = 0.32). The fetal DNA proportion in maternal plasma was 0%-6 % (mean 0.8%) in women carrying Down syndrome and 0%-2.6 % (mean 0.7 %) in the controls, respectively (P=0.86).
Conclusions
Our study revealed no difference in fetal DNA levels and fetal DNA: maternal DNA ratio between the patients carrying Down syndrome fetuses and the controls.
Background
Current experimental non-invasive methods for the prenatal diagnosis of fetal genetic characteristics use free extracellular fetal DNA and fetal cells isolated from maternal peripheral blood. Free extracellular fetal DNA which can be detected by polymerase chain reaction (PCR) in the serum or plasma of pregnant women [1] has been previously successfully used for the prenatal diagnosis of fetal sex and rhesus D status [2-5]. A novel approach is to monitor elevations in the fetal DNA levels in pregnancies with preeclampsia [7,8], aneuploid fetuses [9,10] and preterm labor [11] using the real-time quantitative PCR technology (RQ-PCR) [6]. The study of Zhong et al. also showed that in preeclampsia, the amounts of both circulatory fetal and maternal DNA were increased [8].
In this study, we investigated the fetal and total DNA levels in maternal plasma in patients bearing fetuses affected with Down syndrome in comparison to the controls carrying fetuses with normal karyotype. The detection of Y DNA was used in male fetuses because a generic fetal DNA marker is not yet available.
Methods
Twenty-one pregnant women with a singleton fetus at a gestational age ranging from 15 - 19 (Φ 17.) weeks with an increased risk for an aneuploid fetus who were undergoing an invasive prenatal diagnostic procedure (amniocentesis), and 16 pregnant women bearing fetuses affected with Down syndrome between 17 - 22 (Φ 20.) weeks of gestation were recruited for this study. Local Ethics Committee approval and informed consent was obtained for all patients in the study.
DNA extraction from plasma samples
Five ml of maternal peripheral blood from Down syndrome and euploid cases was collected into EDTA containing tubes and processed within a few hours (maximally 24 h). In details, blood samples were centrifuged firstly at 1200 g for 10 min., than plasma samples were recentrifuged again and the supernatants were collected and stored at -80 ° C until futher processing. Down syndrome and euploid case samples were matched for time in storage to take under consideration an eventual loss of fetal DNA in maternal plasma as a function of time in freezer storage. The Down syndrome plasma samples were stored in a freezer for 4,6 (range 1–8) months and control samples for 5,1 (range 1–8) months before DNA extraction and RQ-PCR analyses.
DNA was extracted from 400 μl plasma using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. To minimize the risk of contamination, DNA was isolated in laminar air-flow and aerosol resistant tips were used. DNA was eluted in 50 μl Buffer AE and 2,5 μl were used as a template for the RQ-PCR reaction.
Real-time quantitative PCR Analysis
The real time quantitative PCR analysis was performed using ABI PRISM 7700 Sequence Detection System (Applied Biosystem, Branchburg, New Jersey, USA). The β-globin TaqMan system consisted of two primers β-globin-354 (forward), 5'-GTG CAC CTG ACT CCT GAG GAG A-3'; β-globin-455 (reverse), 5'-CCT TGA TAC CAA CCT GCC CAG-3' and a dual-labelled fluorescent TaqMan probe β-globin-402T, 5'(FAM) AAG GTG AAC GTG GAT GAA GTT GGT GG (TAMRA)-3' [6]. The SRY TaqMan system consisted of SRY-109 (forward) primer, 5'-TGG CGA TTA AGT CAA ATT CGC-3'; SRY-245 (reverse) primer, 5'-CCC CCT AGT ACC CTG ACA ATG TAT T-3' and a probe SRY-142T, 5'(FAM) AGC AGT AGA GCA GTC AGG GAG GCA GA (TAMRA)-3' [6]. Sequence data were obtained from the GeneBank Sequence database U01317 (β-globin gene) and L08063 (SRY gene) as was previously described by Lo et al. [6].
TaqMan amplification reactions were set up in a reaction volume of 25 μl using the TaqMan Universal PCR Master Mix (Applied Biosystems, Branchburg, New Jersey, USA). DNA amplifications were carried out in 8-well reaction optical tubes/stripes (Applied Biosystem, Branchburg, New Jersey, USA). The TaqMan PCR conditions were used as described in TaqMan guidelines using 40 cycles of 95°C for 15 s and 60°C for 1 min. with 2-min preincubation at 50°C required for optimal AmpErase UNG activity and 10-min preincubation at 95°C required for activation of AmpliTaq Gold DNA polymerase. Each sample was analysed in triplicate.
The calibration curve was run in parallel with each analysis. The concentration of the of SRY and β-globin were expressed in genome-equivalents per millilitre of maternal plasma.
Statistics
Two-tailed t-test was performed using the SPSS software package for Windows. A P value of < 0.05 was taken as statistically significant.
Results
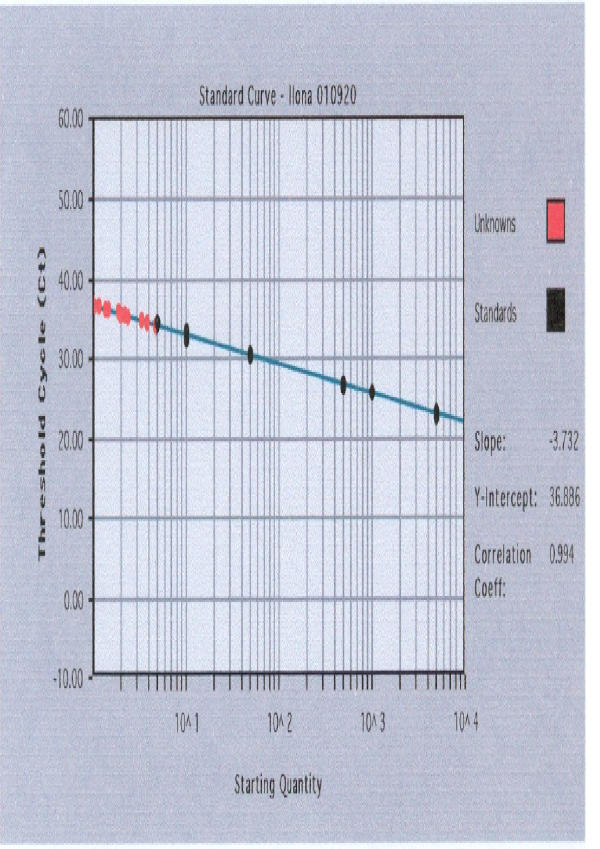
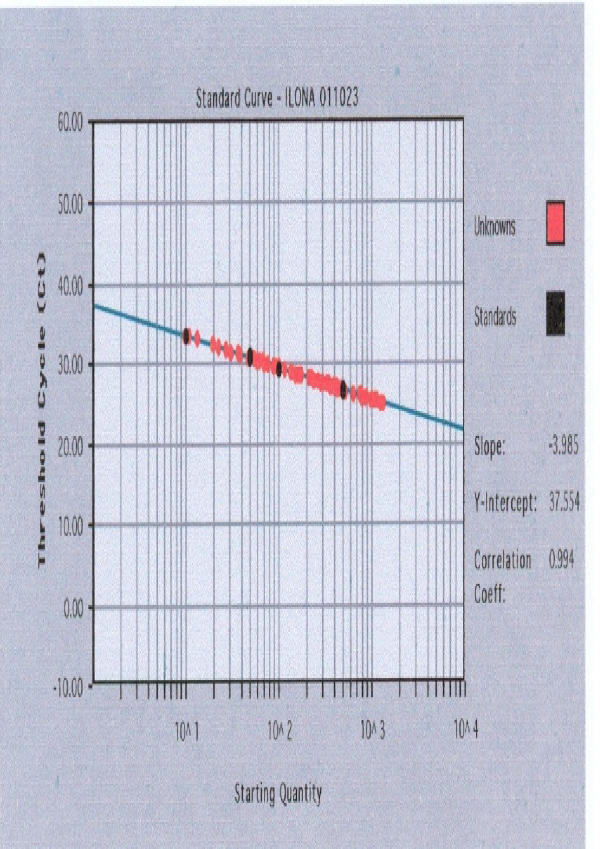
Figure 1 and Figure 2 demonstrate standard curves for SRY and β-globin genes plotting the threshold cycle (CT) against known concentrations of serially diluted mixed DNA. Target quantity of unknowns was interpolated from given the CT of any unknown.
Figure 1.
Standard curve for SRY gene (in logarithmic scale) plotting the threshold cycle (CT) against known concentrations of serially diluted mixed DNA.
Figure 2.
Standard curve for β-globin gene (in logarithmic scale) plotting the threshold cycle (CT) against known concentrations of serially diluted mixed DNA.
In our setting the sensitivity of the RQ-PCR system was 91,7 % as SRY specific PCR amplicons were detected in 22 out of 24 studied samples and the specificity 100 % since no Y chromosome positive signals were detected in 14 women currently pregnant with a female fetus.
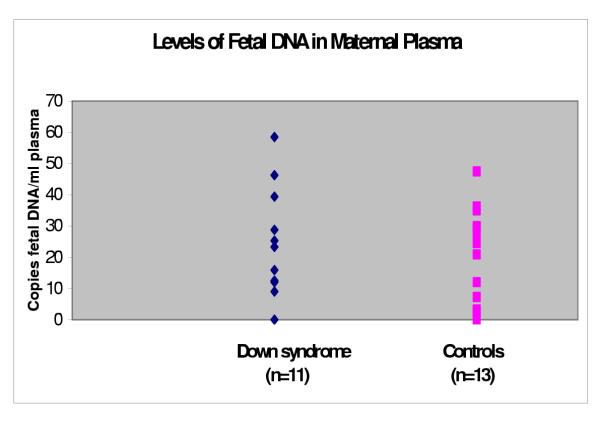
The median fetal DNA levels in maternal plasma obtained from women carrying Down syndrome (n = 11) and the controls carrying fetuses with normal karyotype (n = 13) were 23,3 (range 0–58.5) genome-equivalents/ml and 24,5 (range 0–47.5) genome-equivalents/ml. The difference between the two groups did not reach statistical significance (P= 0.62), (Figure 3).
Figure 3.
Levels of fetal DNA in maternal plasma.
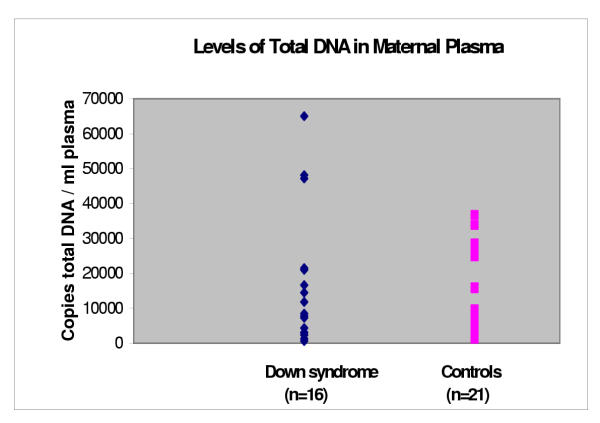
The total median DNA levels in patients bearing fetuses affected with Down syndrome (n = 16) and the controls carrying fetuses with normal karyotype (n = 21) were 10165 (range 615 – 65000) genome-equivalents/ml and 7330 (range 1300 – 36750) genome-equivalents/ml of maternal plasma, respectively (P = 0.32), (Figure 4).
Figure 4.
Levels of total DNA in maternal plasma.
The fetal DNA proportion in maternal plasma was 0 % – 6 % (mean 0,8 %) in women carrying Down syndrome and 0%–2,6 % (mean 0,7 %) in the controls carrying fetuses with normal karyotype, respectively (P = 0.86).
Discussion
Recently, Lo et al. [9] reported findings of very high concentrations of circulating cell-free fetal DNA in maternal plasma in a proportion of pregnancies involving trisomy 21 fetuses. The median cell-free fetal DNA in women carrying trisomy 21 fetuses was found to be 2.96-fold and 1.97-fold higher than that of women carrying euploid fetuses in prospective studies done on samples collected from two centres. Consequently, Zhong et al. [10] confirm these results by showing elevated levels of fetal DNA in pregnancies with trisomy 21 fetuses (mean 185.6 genome-equivalents/ml) when compared to pregnancies with normal male fetuses (mean 83.1 genome-equivalents/ml).
Our results showed that fetal DNA levels in maternal plasma were not significantly elevated in pregnancies with Down syndrome fetuses when comparing to the controls carrying fetuses with normal karyotype. The median fetal DNA levels in maternal plasma obtained from women carrying Down syndrome (n = 11) and the controls carrying fetuses with normal karyotype (n = 13) were 23,3 (range 0–58.5) genome-equivalents/ml and 24,5 (range 0–47.5) genome-equivalents/ml, respectively (P= 0.62). Plasma samples from women carrying euploid fetuses were recruited before amniocentesis for indications including maternal serum screening and advanced maternal age and compared with samples from women carrying Down syndrome fetuses sampling immediately as soon as possible after karyotype determination. Our data might confirm recently published results of Ohashi et al. independent study who observed no significant differencies in the levels of fetal DNA between pregnancies with fetuses of normal karyotype and those with Down and Edwards syndrome [12]. Several studies reported that plasma DNA concentration could be affected by different sample processing protocols [13,14]. The examination of only circulatory fetal DNA, any of initial processing steps concerning a single centrifugation of the blood sample or use of a discontinuous Percoll density gradient, as well as plasma fractions subsequently cleared further by high-speed centrifugation or filtration appear not to make significant difference in the concentration of fetal DNA [13,14]. However in case of maternal plasma, DNA concentrations may vary in various studies due to the level of clearing the plasma of any maternal cellular remnants. By using sample processing protocol concerning the double centrifugation of blood samples at 1200 g we found out comparable fetal DNA levels as those reported by Lo et al. who separated the plasma using double centrifugation at 3000 g [6]. Differences in total DNA levels and fetal DNA: maternal DNA ratio in maternal plasma between our results and those reported by Lo et al. [6] might be caused by the use of lower-speed and lower-time centrifugation protocol we used for plasma preparation in our study.
Conclusions
Our study revealed no difference in fetal DNA levels and fetal DNA: maternal DNA ratio between the patients carrying Down syndrome and the controls carrying fetuses with normal karyotype.
Competing interests
None declared.
Authors' contributions
IH designed the study and drafted the manuscript. BH, DH, SV carried out plasma sampling, DNA extraction and RQ-PCR studies. PC, KN, JK, DS, JD collected biological samples and reported clinical data. OC and JV participated in the design of the study.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This project was supported by the Internal Grant Agency, Ministry of Health, Czech Republic, project no: 4537–3 and by 2nd Medical faculty, Charles University in Prague, no: VZ 111300003.
Contributor Information
Ilona Hromadnikova, Email: ilona.hromadnikova@lfmotol.cuni.cz.
Bela Houbova, Email: ilona.hromadnikova@lfmotol.cuni.cz.
Dana Hridelova, Email: ilona.hromadnikova@lfmotol.cuni.cz.
Sona Voslarova, Email: ilona.hromadnikova@lfmotol.cuni.cz.
Pavel Calda, Email: calda@obgyn.anet.cz.
Katerina Nekolarova, Email: calda@obgyn.anet.cz.
Josef Kofer, Email: josef.kofer@mnul.cz.
David Stejskal, Email: david.stejskal@gennet.cz.
Jindrich Doucha, Email: ilona.hromadnikova@lfmotol.cuni.cz.
Ondrej Cinek, Email: ondrej.cinek@lfmotol.cuni.cz.
Jan Vavrirec, Email: jan.vavrinec@lfmotol.cuni.cz.
References
- Lo YMD, Corbetta N, Chamberlain PF, Sargent JL. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- Faas BH, Beuling EA, Christiaens GC, von dem Borne AE, van der Achoot CE. Detection of fetal RhD – specific sequences in maternal plasma. Lancet. 1998;352:1196. doi: 10.1016/s0140-6736(05)60534-x. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, Poon PM, Redman CW, Wainscoat JS. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N engl J Med. 1998;339:1734–1738. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- Hahn S, Zhong XY, Burk MR, Troeger C, Holzgreve W. Multiplex and realtime quantitative PCR on fetal DNA in maternal plasma: a comparison with fetal cells isolated from maternal blood: In Circulating DNA in Plasma, M Stroun, P Anker (eds). Ann N Y Acad Sci. 2000;906:148–152. doi: 10.1111/j.1749-6632.2000.tb06605.x. [DOI] [PubMed] [Google Scholar]
- Zhong XY, Holzgreve W, Hahn S. Detection of fetal rhesus D and sex using fetal DNA from maternal plasma by multiplex PCR. BJOG. 2000;107:766–769. doi: 10.1111/j.1471-0528.2000.tb13338.x. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Tein MSC, Lau TK, Haines CJ, Leung TN, Poon PMK, Wainscoat JS, Johnson PJ, Chang AMZ, Hjelm NM. Quantitative analysis of fetal DNA in maternal plasma and serum. Implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62:768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YMD, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin Chem. 1999;45:184–188. [PubMed] [Google Scholar]
- Zhong XY, Laivuori H, Livingston JC, Ylikorbala O, Sibai BM, Holzgreve W, Hahn S. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am J Obstet Gynecol. 2001;184:414–419. doi: 10.1067/mob.2001.109594. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Lau TK, Zhang J, Leung TN, Chang AM, Hjelm NM, Elmas RS, Bianchi DW. Increased fetal DNA concentrations in the plasma of pregnant women carrying fetuses with trisomy 21. Clin Chem. 1999;45:1747–1751. [PubMed] [Google Scholar]
- Zhong XY, Burk MR, Troeger C, Jackson LR, Holzgreve W, Hahn S. Fetal DNA in maternal plasma is elevated in pregnancies with aneuploid fetuses. Prenat Diagn. 2000;20:795–798. doi: 10.1002/1097-0223(200010)20:10<795::AID-PD897>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YMD. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352:1904–1905. doi: 10.1016/S0140-6736(05)60395-9. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Miharu N, Honda H, Samura O, Ohama K. Quantitation of fetal DNA in maternal serum in normal and aneuploid prenancies. Hum Genet. 2001;108:123–127. doi: 10.1007/s004390100457. [DOI] [PubMed] [Google Scholar]
- Hahn S, Zhong XY, Holzgreve W. Quantification of Circulating DNA: In the Preparation Lies the Rub. Clin Chem. 2001;47:1577–1578. [PubMed] [Google Scholar]
- Chiu RWK, Poon LLM, Lau TK, Leung TN, Wong EMC, Lo YMD. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin Chem. 2001;47:1607–1613. [PubMed] [Google Scholar]