Abstract
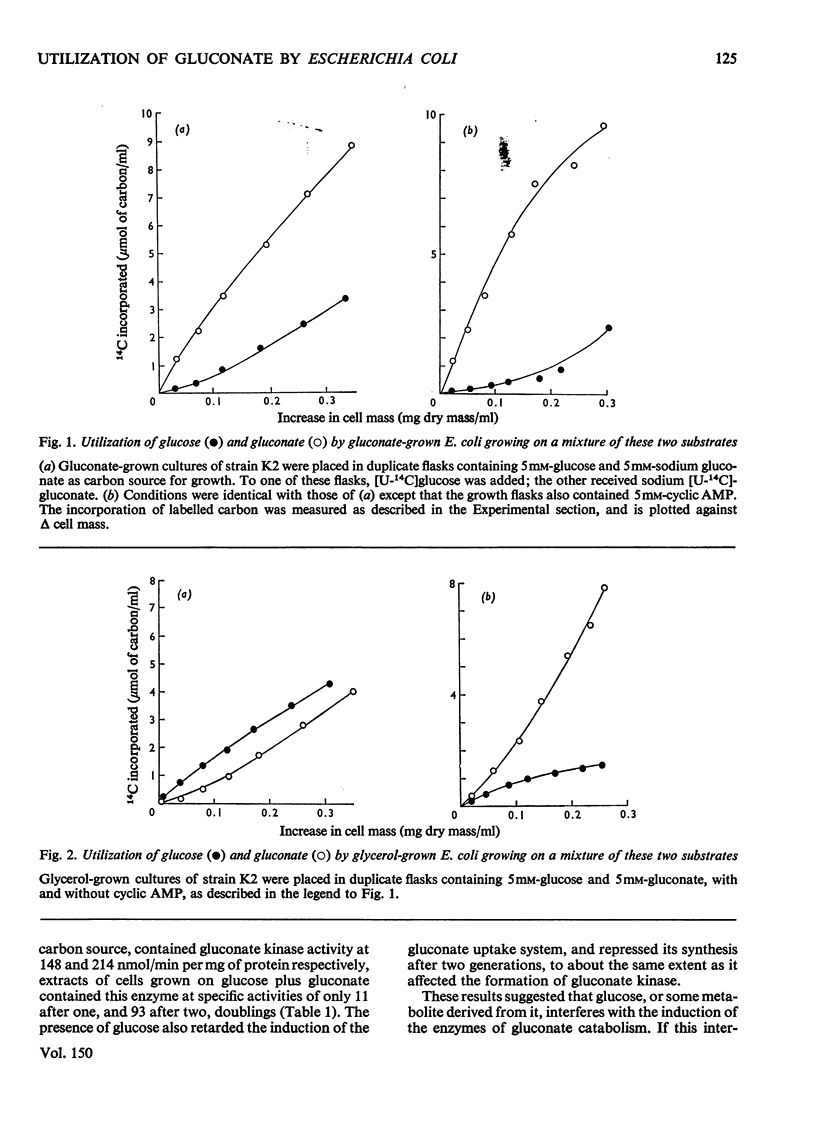
1. Cultures of Escherichia coli growing on gluconate use both gluconate and glucose when glucose is added. 2. Glycerol-grown cells adapt to gluconate utilization even in media containing glucose as well as gluconate. 3. The rates of gluconate utilization by cells growing on a mixture of glucose and gluconate, and the specific activities of the gluconate uptake system and of gluconate kinase, are greater if adenosine 3':5'-cyclic monophosphate (cyclic AMP) is present in the medium than in its absence. 4. Growth on media containing gluconate and cyclic AMP is accompanied by the formation of methyl glyoxal and pyruvate, and progressive inhibition of growth. 5. A mutant devoid of adenylate cyclase activity (cya) grew well on glucose in the absence of exogenous cyclic AMP but grew only poorly on gluconate; neither the gluconate uptake system nor gluconate kinase was adequately induced. The addition of cyclic AMP promoted growth on gluconate and facilitated the induction of proteins required for gluconate catabolism. 6. Phage Pl-mediated transduction of cya+ into the cya-mutant also restored the wild-type phenotype in its ability to adapt to gluconate utilization.
Full text
PDF



Selected References
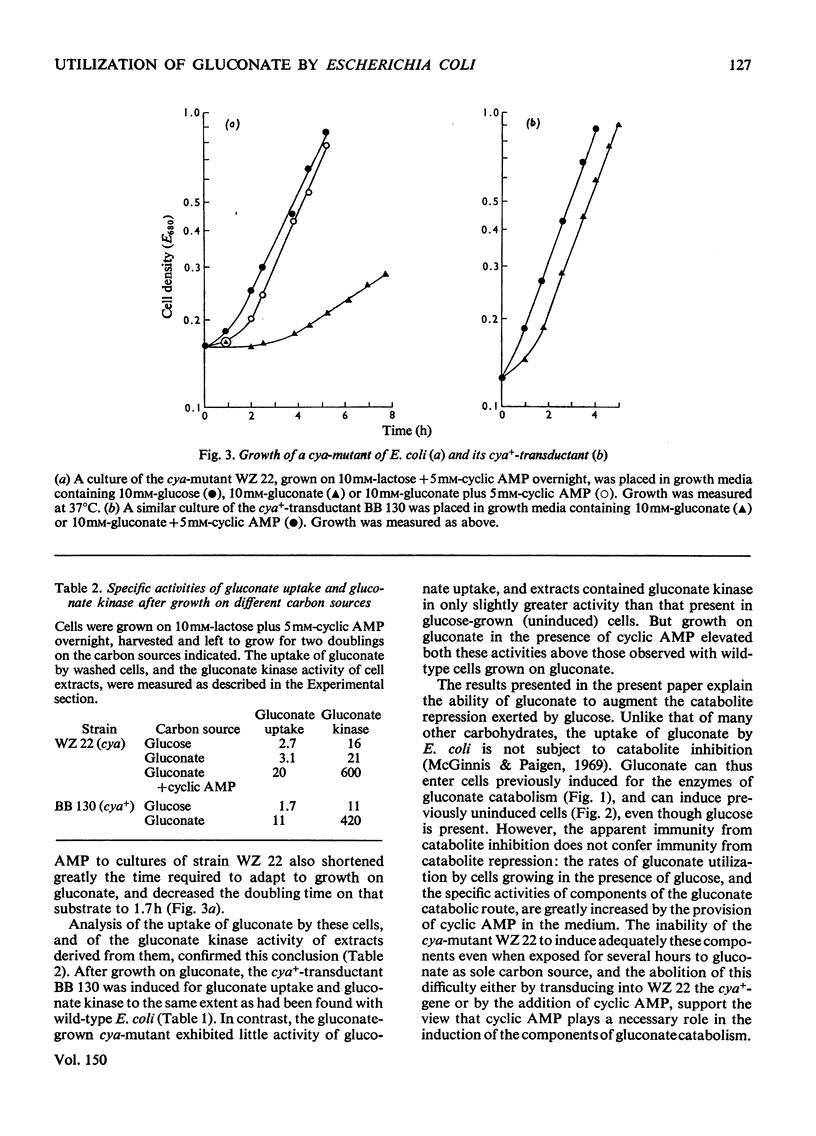
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman R. S., Cozzarelli N. R., Epstein W. Accumulation of toxic concentrations of methylglyoxal by wild-type Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):357–362. doi: 10.1128/jb.119.2.357-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location of a gene specifying phosphopyruvate synthase activity on the genome of Escherichia coli, K12. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):281–292. doi: 10.1098/rspb.1967.0066. [DOI] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970 Dec 11;11(4):273–276. doi: 10.1016/0014-5793(70)80546-4. [DOI] [PubMed] [Google Scholar]
- Goldenbaum P. E., Dobrogosz W. J. The effect of cyclic 3',5'-AMP on catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem Biophys Res Commun. 1968 Dec 9;33(5):828–833. doi: 10.1016/0006-291x(68)90235-0. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Fine control of sugar uptake by Escherichia coli. Symp Soc Exp Biol. 1973;27:175–193. [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Soutar A. K. Utilization of gluconate by Escherichia coli. Induction of gluconate kinase and 6-phosphogluconate dehydratase activities. Biochem J. 1973 Jun;134(2):489–498. doi: 10.1042/bj1340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Untersuchungen zum Glukose-Effekt bei der Synthese der Galaktose-Enzyme von Escherichia coli. Z Vererbungsl. 1966;98(3):203–229. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka R. T., Dobrogosz W. J. Catabolite repression and pyruvate metabolism in Escherichia coli. J Bacteriol. 1967 May;93(5):1644–1650. doi: 10.1128/jb.93.5.1644-1650.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Cyclic AMP in metabolism. Nat New Biol. 1971 Jan 6;229(1):5–7. doi: 10.1038/newbio229005a0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. M., Stoeber F. R. Rameau dégradatif commun des hexuronates chez Escherichia coli K12. Mécanisme d'induction des enzymes assurant le métabolisme du 2-céto-3-désoxy-gluconate. Eur J Biochem. 1972 Nov 7;30(3):479–494. doi: 10.1111/j.1432-1033.1972.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Rekarte U. D., Zwaig N., Istúriz T. Accumulation of methylglyoxal in a mutant of Escherichia coli constitutive for gluconate catabolism. J Bacteriol. 1973 Sep;115(3):727–731. doi: 10.1128/jb.115.3.727-731.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968 Nov;2(1):57–60. doi: 10.1016/0014-5793(68)80100-0. [DOI] [PubMed] [Google Scholar]


