Abstract
Background
Anhedonia—a core symptom of major depressive disorder (MDD)—is closely related to diminished reward sensitivity. Nonetheless, the psychopathological and computational mechanism underlying anhedonia in young patients with MDD remains unclear. Therefore, this study aims to investigate reward sensitivity in adolescents and young adults with MDD using computational modelling.
Methods
Overall, 70 patients with MDD and 54 age- and sex-matched healthy controls (HC) completed a probabilistic reward task (PRT) to assess their general behavioral inclination towards more frequently reinforced stimuli (i.e., “response bias”). Bayesian hierarchical drift diffusion modeling (HDDM) was employed to determine changes in reward sensitivity and computational process during decision-making.
Results
Adolescents with depression showed a trend toward reduced response bias compared to those in HC. HDDM analysis revealed wider decision thresholds in both adolescents and young adults with MDD group. Adolescents with MDD exhibited significantly lower drift rates and reduced starting point bias compared to those in HC. Higher anhedonia levels were linked to lower drift rates and wider decision thresholds. Additionally, increased discriminability correlated with higher drift rates, while higher response bias was linked to larger starting points.
Conclusions
Our findings suggest that reduced reward sensitivity and slower evidence accumulation during reward learning may serve as potential indicators of anhedonia in adolescents with MDD. These findings provided crucial insights into the dysregulated positive affect model, underscoring a dysfunctional reward system as a key factor in anhedonia developmental psychopathology in depression.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06353-3.
Keywords: Major depressive disorder, Anhedonia, Reward sensitivity, Adolescent
Introduction
Major depressive disorder (MDD) is a prevalent mental health condition characterized by severe affective symptoms and functional impairments [1]. Its incidence rises sharply during adolescence and young adulthood, marked by rapid psychological and neurological development [2, 3]. Adolescent-onset depression significantly affects mental and social functioning, increasing mortality, disability, and recurrence rates in adulthood [4–6]. Anhedonia, defined as a reduced interest or pleasure, is a core symptom of MDD and a hallmark of adolescent depression [7]. The Research Domain Criteria (RDoC) framework integrates reward-related components into the Positive Valence Systems (PVS), recognizing reward learning as a key component linked to anhedonia [8]. Accumulating studies have shown that diminished reward sensitivity may be an anhedonia marker [9, 10]. Therefore, understanding the age-specific mechanisms of reward sensitivity and anhedonia in MDD could develop targeted interventions that improve treatment outcomes in adolescents and young adults.
Emerging research has suggested that anhedonia may result from reduced sensitivity to rewarding information during the reinforcement probabilistic learning phase [11]. Reward sensitivity— the degree of responsiveness to rewards [12]—is objectively measured using a probabilistic reward task (PRT) based on signal detection theory. In the PRT, participants are presented with two types of stimuli, one rewarded more frequently than the other. Response bias, an indicator of reward sensitivity, is used to assess the preference of an individual for identifying the stimulus with a more frequent reward [13]. Consistent findings from PRT studies in adult depression reveal a significant reduction in reward sensitivity, indicated by a diminished response bias compared to healthy individuals [13–16]. This reduction is particularly pronounced among patients with MDD with heightened anhedonia symptoms, with more severe decreases observed as the task progresses [17].
Research on reduced reward sensitivity in adult MDD has provided valuable insights into the psychopathology of anhedonia. However, evidence has demonstrated that reward dysfunction in depression may be more pronounced during adolescence than in later stages of life [18]. Adolescence is marked by heightened reward-seeking behaviors and impulsivity during the critical developmental period [10]. Unlike the slower responses and more generalized blunted reward sensitivity observed in adults, studies on adolescent depression show faster decision-making responses but reduced response bias under high-probability reward conditions compared to that of healthy controls (HC) [19, 20]. This period is also characterized by rapid neurological and psychological changes, alongside neural circuit imbalances that increase the risk of affective disorders [21]. The dysregulated positive affect theory suggests that adolescents with depression experience reduced positive affect and abnormal reward processing related to these developmental changes, increasing susceptibility to anhedonia and depression [22, 23]. Reward function deficits (e.g., reduced striatal activation to reward) may appear before depressive episodes, indicating that atypical maturation of reward circuits contributes to the vulnerability in reward processing [24]. To date, there is limited research on reward sensitivity in adolescents with MDD, and the mechanisms underlying this sensitivity remain unclear.
The Drift Diffusion Model (DDM) provides a robust framework for analyzing reward-based decision-making processes in the PRT. Compared to traditional analysis on mean behavioral metrics, the DDM models suggest that decision-making involves a constant internal process where evidence accumulates towards one of two responses. It deconstructs response time distributions and choice accuracy across trials into key cognitive components: drift rate (v), representing evidence accumulation rate towards a decision; decision threshold (α), the amount of evidence required to make a decision; non-decision time (t), accounting for unrelated processes such as visual and motor responses; and starting point bias (z), indicating initial bias in evidence accumulation [25]. The DDM quantifies how changes in reward processing influence decision-making behavior, enabling the exploration of age-related variations in reward processing and the identification of potential decision-making deficits across age groups. For instance, the drift rate indicates the speed of evidence accumulation and reflects the rate and quality of information processing, with a lower drift rate indicating less efficiency of evidence accumulation; while the threshold represents the distance between the two boundaries, with a higher threshold indicating greater caution or uncertainty, requiring more evidence to make a decision [25–27]. To our knowledge, few studies have employed the HDDM approach to reward processing, primarily focusing on adult depression, revealing that adults with depression exhibit a slower and less efficient evidence accumulation process, reflected by reduced drift rates [28, 29]. Recent research investigating girls during late childhood and early adolescence found that higher levels of depression were associated with reduced drift rates and lower starting points for frequently received rewards, indicating a need for more evidence to make decisions [30, 31]. These findings provided insights and evidence to explore the mechanisms of reward sensitivity in adolescent with MDD and suggested that slowed evidence accumulation might underlie the failure to develop response bias. However, no studies have directly compared reward sensitivity between adults and adolescents with MDD alongside age-matched control groups. Therefore, this study aimed to investigate these age-specific mechanisms by focusing on the relationship between reward sensitivity and anhedonia in a population of young individuals with depression.
In this study, HDDM was employed to investigate alterations in reward sensitivity and its underlying computational mechanisms in adolescents and young adults with MDD. Based on prior research, we hypothesized that adolescents and young adults with MDD would demonstrate reduced response bias and slower evidence accumulation during reward learning compared to those in HC, with more pronounced impairments in adolescents with depression.
Material and methods
Participants
Seventy-seven patients, comprising 41 adolescents (aged 12–18) and 36 young adults (aged 19–25) with MDD, from Shanghai Mental Health Center and Shanghai Pudong Mental Health Center were enrolled in this study. All participants met the criteria for MDD as defined by the Diagnostic and Statistical Manual of the American Psychiatric Association, Fifth Edition (DSM-5). Additionally, 69 healthy individuals were recruited from the community, comprising 42 adolescents and 27 young adult controls, via advertising handouts and social media platforms. Patients with MDD were excluded from the study based on the following criteria: (a) the presence of additional psychiatric or personality disorders diagnosed following DSM-5 criteria; (b) a history of neurological illness; (c) current or past drug/substance abuse; or (d) having undergone modified electroconvulsive therapy within six weeks before the study. Within the MDD group, 21 patients were undergoing antidepressant treatment, while 49 patients were not administered any medication. HC was included based on the following criteria: (a) a Beck Depression Inventory (BDI) score of ≤ 16 [32]; (b) no current or past DSM-5 disorder; (c) no neurological disorder or history of brain injury; and (d) no current or past drug/substance dependence. The study was approved by the Ethics Committee of East China Normal University (HR 472–2019), and all participants provided written informed consent.
Probabilistic reward task (PRT)
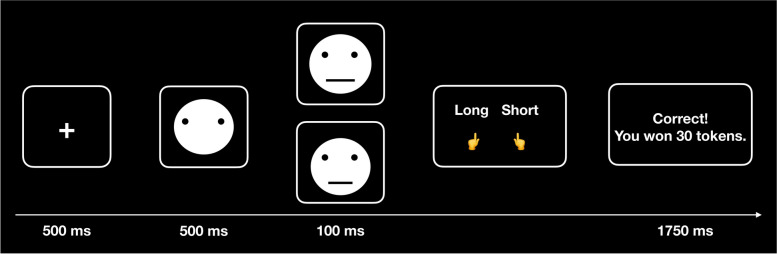
The PRT is a reward-based paradigm designed to measure response bias towards a frequently rewarded stimulus (rich stimulus) over a less frequently rewarded one (lean stimulus) [16]. Participants identified two ambiguous stimuli (a short or long mouth) in 240 trials, divided into three blocks of 80 trials each. The task involved fixation on the screen center (500 ms) followed by a mouthless cartoon face (500 ms), after which the target stimulus (a short (8.7 mm) or long (9.8 mm) mouth) appeared for 100 ms. Participants pressed a button to indicate which target they saw (Fig. 1). Correct responses were associated with positive feedback (“Correct! You won 30 tokens.”) for 1750 ms in 40% of the trials. Specifically, 30% (72 trials) of the rich stimulus and 10% (24 trials) of the lean stimulus) were followed by positive feedback. Therefore, the rich stimulus was rewarded thrice as often as the lean stimulus. The PRT was conducted under two counterbalanced conditions: one where the long mouth was rewarded more across all blocks and another where the short mouth was rewarded more across all blocks.
Fig. 1.
Schematic illustration of the signal detection task (PRT). First, all participants were required to focus on the fixation in the center of the screen (500 ms) followed by a mouthless cartoon face (500 ms). Target stimulus (a short or long mouth) appeared in 100 ms and participants were asked to press the button using their left or right fingers to indicate a short or long mouth. Feedback was provided to the participants for 1750 ms to inform whether they correctly responded to the target and how many tokens were awarded
Clinical assessments and self-reported scales
Depressive symptom severity was clinically assessed using the Hamilton Depression Scale (HAMD) [33]. The Chinese version of the HAMD [34] was demonstrated to be reliable and valid for detecting depression.
The BDI [35], a widely used 21-item self-rating scale, measures depression severity on a 4-point scale (0 to 3). A modified Chinese version of the BDI [36] with high reliability (Cronbach’s α = 0.97) was used in this study.
The Snaith–Hamilton Pleasure Scale (SHAPS) was used to evaluate anhedonia severity [37]. The Chinese version of SHAPS, consisting of 14 items on a 4-point self-report scale, was employed in this study (Cronbach’s α = 0.92). Higher SHAPS scores indicate more severe anhedonia.
Anticipatory and consummatory pleasure experiences were measured using the Temporal Experience of Pleasure Scale (TEPS) [38]. The Chinese version of TEPS [39], consisting of 20 items, each rated on a 6-point scale, measures the ability of an individual to experience pleasure with good reliability and validity (Cronbach’s α = 0.89). Higher TEPS scores indicate greater pleasure capacity.
Data collection and reduction
Based on prior research [13, 16], PRT data was filtered by excluding trials (above 15% of the total trials) with reaction time (RT) < 150 ms or > 2500 ms and RT (naturally log-transformed) falling beyond the mean ± 3 standard deviations (SD). This led to the exclusion of 14 participants—7 with MDD and 7 HC—along with 8 HC with a BDI score above 16. Overall, 70 patients with MDD and 54 HC were included in the final analysis, yielding 124 participants out of the original 146 (84.93%).
Task performance behavioral metrics evaluation encompassed hit rates, RTs, discriminability, and response bias. Discriminability measures the ability of an individual to differentiate between two stimuli types, reflecting the difficulty level of the task. It was calculated as follows:
Response bias indicates the degree to which participants tend to prefer higher-rewarded stimuli. A higher response bias implies a stronger tendency toward the rewarding stimulus during implicit reinforcement learning, indicating heightened reward sensitivity. The algorithm utilized for calculation was as follows:
Statistical analyses
Group differences in demographic characteristics, clinical symptoms, and performances of PRT
Independent sample t-tests were performed with a significance level of p < 0.05 to statistically compare demographic characteristics and clinical variables between the MDD and HC groups. Sex distribution differences were assessed using chi-square analysis. To analyze the differences in depression (i.e., HAMD and BDI) and anhedonia levels (i.e., SHAPS and TEPS) between adolescents and adults with MDD, independent sample t-tests were performed. Detailed findings can be found in the supplementary materials.
To assess the effects of age on RTs and hit rates in MDD and HC groups across blocks, linear mixed-effects models (LMMs) were used, with fixed effects for age, group, stimulus type, block, and their interactions, controlling for the educational level (see Fig. S1 for results). To assess the interaction between age and group on discriminability and response bias, models were established incorporating fixed effects for age, group, and block, with the education level included as the covariate. Random effect predictors were incorporated to accommodate varying intercepts and slopes across participants. Bonferroni correction was employed for multiple comparisons, with degrees of freedom estimated using the Satterthwaite method.
Hierarchical drift diffusion modelling
We employed a Bayesian Hierarchical Drift Diffusion Model (HDDM) to analyze response and RTs in the PRT. Stimuli and responses were categorized as "rich" or "lean." The four model parameters including drift rate (v), decision threshold (α), non-decision time (t), and starting point bias (z) varied by group and age [26]. We defined the model based on prior research on PRT [28, 29, 40] using the StimulusCoding tool in HDDM. We applied a mixture model in HDDM, which assumes that 5% of the RT trials are outliers to improve the model fit by reducing the impact of outliers on parameter estimates [26]. The analysis comprised 10,000 samples with an initial burn-in period of 5,000 samples, retaining every fifth sample for evaluation. Chain convergence was assessed using the Gelman–Rubinstein convergence diagnostic [41], yielding maximum R-hat values of 1.016 across runs, indicating convergence below the acceptable threshold of 1.1. To evaluate the ability of the model to replicate key data patterns, we conducted posterior predictive checks (PPC), simulating data for each parameter value by sampling 500 values from the posterior distribution. Summary statistics validated that the observed results remained within the 95% credible interval of the simulated data. Using a Bayesian framework can provide flexible and robust estimates with credible intervals for the derived parameters, enabling direct hypothesis testing on the posterior distributions [42]. Groupwise parameter comparisons were conducted using Bayesian inference, reporting results through posterior probabilities (PP|D) for hypotheses of interest, with a posterior probability of ≥ 0.95 deemed statistically significant.
Relationships between response bias, behavioral parameters, and other variables
We conducted Pearson correlations to examine the relationships between response bias, model parameters, and other variables in patients with MDD. These variables included hedonic capacity, measured by the SHAPS and TEPS scores, as well as clinical characteristics such as the BDI scores, HAMD scores, duration of illness, medication course, and antidepressant dosage (also see Table S3). To further understand the relationship between HDDM parameters and behavioral metrics, we also performed Pearson correlations among model parameters, response bias, and discriminability in MDD and HC (Table S4).
Statistical analyses and modeling were performed using R (http://www.r-project.org/; v.4.2.0) and the HDDM toolbox (http://ski.clps.brown.edu/hddm_docs/; version 0.9.8; Python 3). Mixed-effects models were fitted using the lmer function from the “lme4” package (https://cran.r-project.org/web/packages/lme4/index.html; v.1.1.29).
Results
Demographic characteristics and clinical symptoms
Table 1 presented no significant difference in age (t (122) = −0.45, p = 0.652) or sex (χ2 (1) = 1.13, p = 0.289) between groups. However, patients with MDD had lower education levels than those of HC (t (122) = −2.03, p = 0.044). Among adolescent participants, significant group differences were observed in age (t (61) = −3.68, p = 0.001) and education levels (t (61) = −4.24, p = 0.001) compared to those of HC (Table S1). For young adults, patients with MDD were older than those of HC (t (59) = 2.92, p = 0.01) (Table S2). As expected, patients with MDD exhibited higher scores on the BDI, HAMD, SHAPS, and all subscales of TEPS than those of HC (all ps < 0.001). The educational level as the covariate was controlled in subsequent analyses.
Table 1.
Summary of demographic and clinical characteristics
|
MDD (n = 70) M (SD) |
HC (n = 54) M (SD) |
t / 2-value | p-value | Cohen’s d / φ | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 18.24 (3.75) | 18.50 (2.56) | −0.45 | .652 | 0.08 |
| Sex (%female) | 55 (78.57%) | 37 (68.52%) | 1.13 | .289 | 0.10 |
| Education (years) | 11.72 (3.56) | 12.79 (2.30) | −2.03 | .044 | 0.35 |
| Age of onset (years) | 17.22 (3.43) | ||||
| Clinical characteristics | |||||
| Duration of illness (month) | 15.35 (20.94) | ||||
| Course of medication (month) | 3.98 (6.06) | ||||
| Dosage of drug (Fluoxetine equivalence, mg/day) | 29.20 (16.19) | ||||
| HAMD | 18.69 (5.28) | 2.32 (2.23) | 20.06 | < .001 | 3.78 |
| Self-report scale score | |||||
| BDI | 31.49 (10.10) | 4.15 (3.95) | 18.63 | < .001 | 3.51 |
| SHAPS | 33.31 (6.74) | 19.77 (5.46) | 11.59 | < .001 | 2.19 |
| TEPS_T | 64.05 (15.35) | 87.76 (10.75) | −9.52 | < .001 | 1.77 |
| TEPS-AA | 12.18 (4.91) | 20.22 (2.96) | −10.56 | < .001 | 1.94 |
| TEPS-CA | 13.29 (5.32) | 17.87 (3.53) | −5.43 | < .001 | 1.00 |
| TEPS-AC | 20.05 (7.18) | 27.93 (4.73) | −6.93 | < .001 | 1.27 |
| TEPS-CC | 12.24 (5.06) | 17.17 (3.08) | −6.26 | < .001 | 1.15 |
Abbreviations: MDD major depressive disorder, HC healthy controls, BDI Beck Depression Inventory, HAMD Hamilton Depression Scale, SHAPS Snaith-Hamilton Pleasure Scale, TEPS Temporal Experience of Pleasure Scale, AA Abstract Anticipatory, CA Contextual Anticipatory, AC Abstract Consummatory; CC Contextual Consummatory
Discriminability and response bias in adolescents and young adults with depression
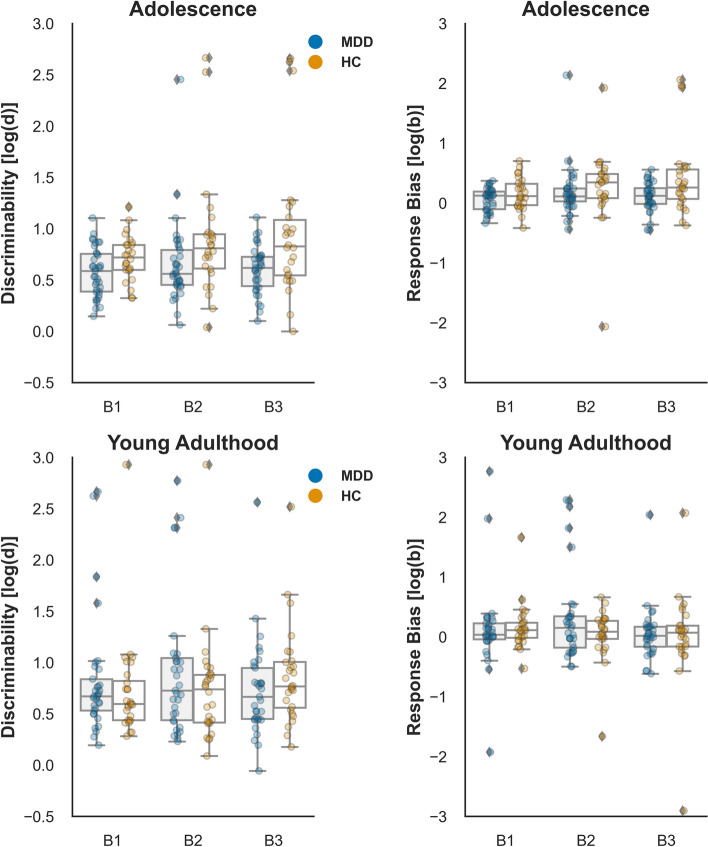
To assess potential group differences in task difficulty, we employed a LMM on discriminability in the adolescents and young adults with MDD and HC. The main effect of the Group (F (1,119) = 1.68, p = 0.197, η2 = 0.01) and Age (F (1,119) = 0.37, p = 0.542, η2 = 0.003) were not significant. However, a significant Group × Age interaction was observed (F (1,119) = 4.66, p = 0.033, η2 = 0.04). Simple effects analysis revealed that adolescents with MDD showed a tendency of lower discriminability compared to adults with MDD (β = −0.21, SE = 0.12, t (119) = −1.70, p = 0.093), while no significant difference was found between adolescents and adults in the HC group (β = 0.09, SE = 0.11, t (119) = 0.87, p = 0.388). Additionally, lower discriminability was observed in the MDD group compared to HC in Block3 (β = −0.48, SE = 0.18, t (288) = −2.73, p = 0.007), as reflected by the significant Group × Block interaction (F (2, 240) = 3.56, p = 0.030, η2 = 0.03) (see Fig. 2).
Fig. 2.
Discriminability and response bias in HC and patients with MDD during the adolescence and young adulthood
Regarding response bias (Fig. 2), a marginally significant Age x Group interaction effect was observed (F (1, 119) = 3.66, p = 0.058, η2 = 0.03). Simple effects analysis revealed that adolescents with MDD tended to exhibit lower response bias than that of the HC group (β = −0.32, SE = 0.19, t (119) = −1.66, p = 0.099), while no differences were observed between MDD and HC in young adults (β = 0.19, SE = 0.18, t (119) = 1.06, p = 0.290). Furthermore, adolescents showed a trend towards a higher response bias than that of young adults in Block 3 (β = 0.43, SE = 0.22, t (244) = 1.98, p = 0.049), as indicated by a marginally significant Age x Block interaction (F (2, 240) = 2.81, p = 0.062, η2 = 0.02).
Drift diffusion modeling
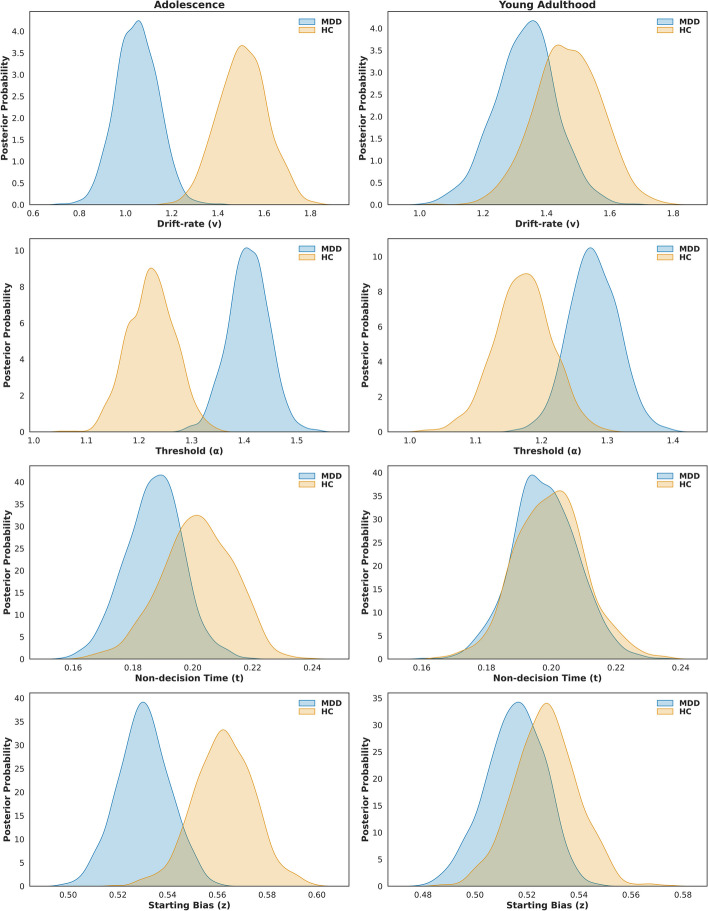
Overall, patients with MDD showed lower drift rates (v) (P P|D = 1.0) and starting point bias (z) (P P|D = 0.976) than those of the HC group. Conversely, patients with MDD had significantly larger decision thresholds (α) than those of HC (P P|D = 1.0). No difference was observed in non-decision time (t) between patients with MDD and HC (P P|D = 0.788).
Both adolescents (P P|D = 1.0) and young adults (P P|D = 0.977) with depression exhibited larger thresholds (α) than those of the HC group. Adolescents with depression showed lower drift rates (v) (P P|D = 1.0) and starting point bias (z) (P P|D = 0.979) compared to adolescent HC, while young adults did not exhibit significant group differences in drift rates (v: P P|D = 0.833; z: P P|D = 0.772). No differences were observed in non-decision time (t) between adolescent and young adult patients with MDD and HC (adolescent: P P|D = 0.834; adult: P P|D = 0.545). Moreover, adolescents with depression showed significantly lower drift rates (v) (P P|D = 0.984) and larger thresholds (α) (P P|D = 0.991) than those of young adults. However, no significant group differences were found in non-decision time (t) (P P|D = 0.790) or starting point bias (z) (P P|D = 0.839). (Fig. 3).
Fig. 3.
Posterior Distributions of HDDM Parameters. The posterior distributions of key parameters from the HDDM analysis, including drift rate, decision threshold, non-decision time and starting point bias. Starting point bias refers to the initial bias at the start of evidence accumulation in the decision process. A starting bias of 0.5 indicates no initial preference for either of the two decision boundaries
Correlations between dynamics of reward sensitivity, depression severity, and anhedonia level
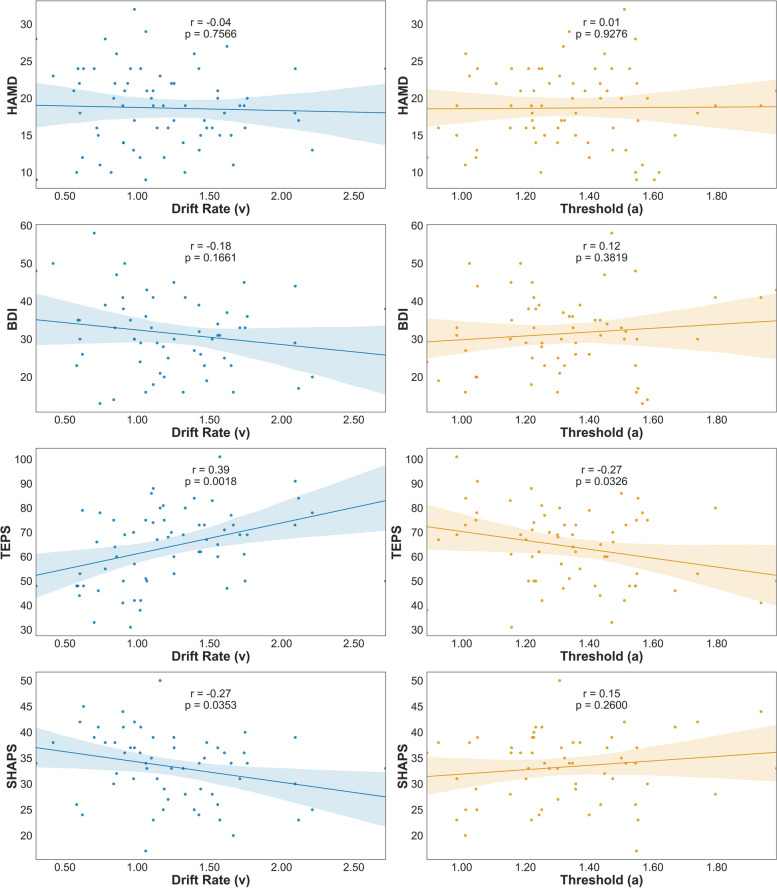
For patients with MDD, drift rates showed a significant inverse correlation with SHAPS scores (r = −0.27, p = 0.035) (Fig. 4). A significant positive association was also observed between drift rates and total TEPS scores (r = 0.39, p = 0.002). A negative association was observed between the thresholds and TEPS total scores (r = −0.27, p = 0.033), while no significant correlation was found between the thresholds and the SHAPS scores (r = 0.15, p = 0.260). However, no significant correlation was observed between depression levels and HDDM parameters (ps > 0.05). Moreover, the discriminability showed a significant positive correlation between drift rates (r = 0.56, p < 0.001) and non-decision time (r = 0.27, p = 0.025) in MDD and HC groups. We also found that the starting point bias was positively related to response bias (r = 0.66, p < 0.001) (Table S4).
Fig. 4.
Relationship of behavioral parameters, depression and hedonic capacity among adolescent and young adult patients with MDD
Discussion
This study investigated reward sensitivity and its underlying computational mechanisms in adolescents and young adults with MDD, emphasizing the common and unique patterns of response bias and evidence accumulation across these age groups. Our findings revealed a tendency for reduced response bias among adolescent patients with MDD. Adolescents with MDD exhibited lower drift rates and starting point bias, with these lower drift rates closely associated with elevated levels of anhedonia. Furthermore, both adolescent and young adult patients with MDD exhibited broader decision thresholds than those of their healthy counterparts, indicating a need for more evidence to make the decision.
Adolescence is a critical phase of neurodevelopment characterized by heightened activity in the mesolimbic dopamine reward system. This enhanced reward responses and reward-seeking behavior [43, 44]. Consistent with prior research findings, our analysis of age-related effects revealed that healthy adolescents exhibited enhanced reward sensitivity, evidenced by a significant increase in response bias. However, on the other hand, the development of reward-related regions during adolescence supports the hypothesis of an imbalance in maturation, with the mesolimbic system maturing earlier than that of the prefrontal cortex, which governs higher-order cognitive functions [45]. In contrast, adolescents with MDD in our study showed reduced response bias and lower drift rates, consistent with prior findings from PRT studies on adults with MDD [13, 16, 17]. Compared to the HC group, adolescents with MDD exhibited pronounced abnormalities in reward learning. They failed to develop a general response bias and struggled to modulate their behaviors effectively in response to reinforcing rewards. The dysregulated positive affect model [22, 23] suggests that impaired reward sensitivity may indicate vulnerability to anhedonia in adolescent depression. This discrepancy likely relates to the developmental characteristics and deviations from typical neurodevelopmental trajectories affecting reward function during adolescence. Prior evidence has demonstrated alterations, such as reduced striatal responses, even before the onset of adolescent MDD [24]. These findings suggested that early developmental imbalances in reward processing might contribute to difficulties in maintaining reward sensitivity.
Despite previous research consistently indicating blunted reward sensitivity in adults with depression, our study found no significant group effects on response bias in the young adult population. The observed reduction in response bias may reflect the severity of anhedonia symptoms [9]. In our analysis, we found that young adults with MDD exhibited lower levels of anhedonia compared to adolescents with MDD. This difference in anhedonia severity might be one of the potential reasons contributing to the slightly higher tendency for response bias in young adults with MDD. Alternatively, individuals with higher discriminability (i.e., an ability to differentiate between stimuli) may not exhibit a preference for the more frequently rewarded stimuli [28]. Our data showed that young adults with MDD exhibited a tendency of higher accuracy for the rich and lean stimuli and greater discriminability than that of adolescents, which may partially explain the absence of reduced response bias in young adults with MDD.
The computational model results indicated that adolescent and young adult populations with MDD exhibited wider decision thresholds, suggesting a greater need for evidence before making decisions. This finding not only corroborated previous research findings on adults [29] but also underscored that adolescents showed similar patterns. The wider decision thresholds reflect a more cautious decision-making strategy aimed at achieving greater accuracy, which may explain the absence of significant abnormalities in discriminability among patients with MDD. Adolescents with MDD further showed lower drift rates than those of their HC counterparts and young adults with MDD. This observation aligned with previous research findings, highlighting that slow evidence accumulation was a marker of deficits in reward processing among individuals with depression [28–31]. Further correlational analyses confirmed previous findings [28, 40] that slower evidence accumulation was closely associated with lower discriminability. The relatively low discriminability observed in our study of adolescents with MDD likely contributed to their reduced rate of evidence accumulation. This relationship helped explain why adolescents with MDD showed significantly slower evidence accumulation. Additionally, recent computational evidence from healthy populations has revealed that as age increases, cognitive control abilities and the speed of evidence accumulation during reward anticipation tasks improve [46]. From the perspective of relative maturation in the prefrontal cortex and cognitive function enhancements in adults, these factors may contribute to maintaining evidence accumulation efficiency. Regarding the starting point bias, adolescents and young adults with MDD and HC groups exhibited the starting point bias above the midpoint, indicating a greater tendency to choose more frequently rewarded stimuli. However, the starting point bias of adolescents with MDD was closer to the midpoint than that of their healthy counterparts, suggesting they require relatively more evidence to reach the rich boundary. Our research findings also highlighted a strong relationship between response bias and starting point bias, aligning with recent findings that starting point bias was a reliable predictor of response bias [40]. This observation provided evidence for a more pronounced reduction in reward sensitivity among adolescents with MDD in our study. Therefore, linking the computational results with discriminability and response bias when assessing features of reward sensitivity can help explain age-related reward learning processes and their collective influence on individual task performance.
Derived from subjective and objective measures, our study revealed that higher levels of anhedonia were strongly associated with reduced drift rates and wider decision thresholds. These findings suggested that slower evidence accumulation and a greater demand for evidence during reward learning might be contributing factors to anhedonia. Consistent with the hypothesis that impaired behavioral modulation in response to rewards underlies diminished hedonic capacity in MDD [16, 17, 47], the current findings elucidated the psychopathology of anhedonia during adolescence as linked to aberrant reward processing in depression.
The present study has some limitations that should be acknowledged. First, as a cross-sectional study, we could not investigate the long-term effect of reduced reward sensitivity on the developmental psychopathology of depression. Second, while the behavioral aspects of reward sensitivity in young people with depression were examined in this study, further research is needed to explore the underlying neural mechanisms of abnormal reward sensitivity during adolescence using neuroimaging techniques. Third, we utilized only monetary reinforcement as a reward stimulus, which may not fully capture differences in reward processing among patients with MDD, as these differences might vary based on the type of reward used [48]. Additionally, we did not measure anxiety levels in patients with MDD, despite excluding other mental disorders, and we did not account for medication status or differences in age and education levels between the HC and MDD groups. Consequently, future studies should consider matching these variables to better control for their potential effect on reward processing.
Conclusion
Our findings revealed that altered reward biases were present in both adolescents and young adults with MDD, reflected by higher decision thresholds. Adolescents with MDD exhibited significantly reduced reward sensitivity, which was also closely associated with slower evidence accumulation and lower starting point bias in decision-making. This finding aligned with the dysregulated positive effect model, which connected developmental changes in the reward system to increased vulnerability to depression, offering valuable insights into the developmental psychopathology of anhedonia.
Supplementary Information
Acknowledgements
None.
Authors’ contributions
CY: Conceptualization; supervision; writing – review and editing. LS: Formal analysis; writing – original draft. YXH: Data curation; formal analysis. QYL: Data curation. ZHY: Data curation. JBG: writing – review and editing.
Funding
This work was funded by the National Natural Science Foundation of China [32171084], the East China Normal University and Health Joint Fund [2022JKXYD09003], and Open Research Fund of Shanghai Key Laboratory of Brain Functional Genomics (East China Normal University) [23SKBFGKF2, 24SKBFGZR1]. These funding agents had no role in the study design; collection, analysis, and interpretation of the data; or writing of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
We have complied with American Psychological Association (APA) ethical standards and the current work was approved by the Ethics Committee of East China Normal University (HR 472–2019).
Consent for publication
All authors gave their consent for the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Shen and Ya-xin Hu contributed equally to this work.
References
- 1.Association APsychiatric. Diagnostic and statistical manual of mental disorders. 2013. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=1811753.
- 2.Schubert KO, Clark SR, Van LK, Collinson JL, Baune BT. Depressive symptom trajectories in late adolescence and early adulthood: A systematic review. Aust N Z J Psychiatry. 2017. 10.1177/0004867417700274. [DOI] [PubMed] [Google Scholar]
- 3.Thapar A, Eyre O, Patel V, Brent D. Depression in young people. The Lancet. 2022;400:617–31. [DOI] [PubMed] [Google Scholar]
- 4.Bentley SM, Pagalilauan GL, Simpson SA. Major Depression. Med Clin. 2014;98:981–1005. [DOI] [PubMed] [Google Scholar]
- 5.Chang K, Kuhlman KR. Adolescent-onset depression is associated with altered social functioning into middle adulthood. Sci Rep. 2022;12:17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory EJ, Gerin MI, Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry – the contribution of functional brain imaging. J Child Psychol Psychiatry. 2017;58:338–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. Journal of child and adolescent psychopharmacology. 2015Apr 1;25(3):194–200. 10.1089/cap.2014.0105https://home.liebertpub.com/cap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 2010;167:748–51. [DOI] [PubMed] [Google Scholar]
- 9.Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Depression. 2015;4:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely BA, Nguyen TNB, Tobe RH, Walker AM, Gabbay V. Multimodal Investigations of Reward Circuitry and Anhedonia in Adolescent Depression. Front Psychiatry. 2021;12. [DOI] [PMC free article] [PubMed]
- 11.Ng TH, Alloy LB, Smith DV. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. 2019;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alloy LB, Olino T, Freed RD, Nusslock R. Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behav Ther. 2016;47:600–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol Psychiatry. 2005;57:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halahakoon DC, Kieslich K, O’Driscoll C, Nair A, Lewis G, Roiser JP. Reward-Processing Behavior in Depressed Participants Relative to Healthy Volunteers: A Systematic Review and Meta-analysis. JAMA Psychiat. 2020;77:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emot. 2000;14:711–24. [Google Scholar]
- 16.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced Reward Learning Predicts Outcome in Major Depressive Disorder. New Insights Treat Mood Disord. 2013;73:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, Dahl RE. Research Review: Altered reward function in adolescent depression: what, when and how? J Child Psychol Psychiatry. 2012;53:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes EE, Shaw DS, Dahl RE. Alterations in Reward-Related Decision Making in Boys with Recent and Future Depression. Biol Psychiatry. 2007;61:633–9. [DOI] [PubMed] [Google Scholar]
- 20.Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. J Child Psychol Psychiatry. 2005;46:995–1005. [DOI] [PubMed] [Google Scholar]
- 21.Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–58. [DOI] [PubMed] [Google Scholar]
- 22.Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–36. [DOI] [PubMed] [Google Scholar]
- 23.Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstrand KL, Lenniger CJ, Forbes EE. Development of Reward Circuitry During Adolescence: Depression, Social Context, and Considerations for Future Research on Disparities in Sexual and Gender Minority Youth. Annu Rev Dev Psychol. 2022;4:231–52. [Google Scholar]
- 25.Ratcliff R, Smith PL, Brown SD, McKoon G. Diffusion Decision Model: Current Issues and History. Trends Cogn Sci. 2016;20:260–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiecki TV, Sofer I, Frank MJ. HDDM: Hierarchical Bayesian estimation of the Drift-Diffusion Model in Python. Front Neuroinformatics. 2013;7. [DOI] [PMC free article] [PubMed]
- 27.Ratcliff R, McKoon G. The Diffusion Decision Model: Theory and Data for Two-Choice Decision Tasks. Neural Comput. 2008;20:873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillon DG, Belleau EL, Origlio J, McKee M, Jahan A, Meyer A, et al. Using Drift Diffusion and RL Models to Disentangle Effects of Depression On Decision-Making vs. Learning in the Probabilistic Reward Task. Comput Psychiatry. 2024;8(1):46–69. [DOI] [PMC free article] [PubMed]
- 29.Lawlor VM, Webb CA, Wiecki TV, Frank MJ, Trivedi M, Pizzagalli DA, et al. Dissecting the impact of depression on decision-making. Psychol Med. 2020;50:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitliya RJ, Nelson BD, Hajcak G, Jin J. Drift-Diffusion Model Reveals Impaired Reward-Based Perceptual Decision-Making Processes Associated with Depression in Late Childhood and Early Adolescent Girls. Res Child Adolesc Psychopathol. 2022;50:1515–28. [DOI] [PubMed] [Google Scholar]
- 31.Pitliya RJ, Burani K, Nelson BD, Hajcak G, Jin J. Reward-Related Brain Activity Mediates the Relationship Between Decision-Making Deficits and Pediatric Depression Symptom Severity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2024. 10.1016/j.bpsc.2024.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, Park J. Diagnostic Test Accuracy of the Beck Depression Inventory for Detecting Major Depression in Adolescents: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022;31:1481–90. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Zhao J, Phillips M, Liu J, Cai M, Sun S, et al. Validity and Reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry. 1988;152:660–4. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Beamesderfer A. Assessment of depression: The depression inventory. In: Pichot P, Olivier-Martin R, (Eds.). Psychological measurements in psychopharmacology. S. Karger; 1974. 10.1159/000395074.
- 36.Zhang YX, Wang Y, Qian MY. Reliability and validity of Back Depression Inventory (BDI) examined in Chinese samples. Chin Ment Health J. 1990;4:22–6. [Google Scholar]
- 37.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A Scale for the Assessment of Hedonic Tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. [DOI] [PubMed] [Google Scholar]
- 38.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan RCK, Wang Y, Yan C, Zhao Q, McGrath J, Hsi X, et al. A Study of Trait Anhedonia in Non-Clinical Chinese Samples: Evidence from the Chapman Scales for Physical and Social Anhedonia. PLoS ONE. 2012;7: e34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillon DG, Lazarov A, Dolan S, Bar-Haim Y, Pizzagalli DA, Schneier FR. Fast evidence accumulation in social anxiety disorder enhances decision making in a probabilistic reward task. Emotion. 2022;22:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Stat Sci. 1992;7:457–72. [Google Scholar]
- 42.Kruschke JK. Bayesian Analysis Reporting Guidelines. Nat Hum Behav. 2021;5:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galván A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4. [DOI] [PMC free article] [PubMed]
- 44.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. The dual systems model: Review, reappraisal, and reaffirmation. Spec Sect Dev Neurosci Adolesc Revisiting Refin Extending Semin Models. 2016;17:103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casey BJ, Jones RM, Somerville LH. Braking and Accelerating of the Adolescent Brain. J Res Adolesc. 2011;21:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Wang D, Wang C, Xiao T, Shi J. The influence of reward anticipation on conflict control in children and adolescents: Evidences from hierarchical drift-diffusion model and event-related potentials. Dev Cogn Neurosci. 2022;55: 101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frey A-L, McCabe C. Impaired social learning predicts reduced real-life motivation in individuals with depression: A computational fMRI study. J Affect Disord. 2020;263:698–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.