Abstract
Purpose:
To examine clinical factors associated with long-term optical coherence tomography (OCT)-measured retinal nerve fiber layer thickness (RNFLT) variability in glaucoma.
Design:
Retrospective cohort study.
Methods:
Glaucoma eyes from DIGS/ADAGES with ≥2-years and 4-visits follow-up were included. RNFLT variability was calculated per-visit as the absolute error of optic nerve head RNFLT residuals across longitudinal follow-up. Clinical factors examined included general demographics, baseline ocular measurements, prior and intervening cataract extraction (CE) or glaucoma surgery, scan quality, baseline RNFLT and RNFLT thinning rate, follow-up duration, visit/testing frequency, etc. Three multivariable linear mixed models (full model, baseline model, and parsimonious model) were fit to evaluate the effects of clinical factors on RNFLT variability, with ten-fold cross-validation to estimate real-world model performance.
Results:
A total of 1140 eyes (634 patients) were included. The overall mean (95% CI) RNFLT variability was 1.51(1.45, 1.58) μm. Across different models, African-American race (β[standard error, SE] = 0.18[0.06]), intervening CE (β[SE] = 0.52[0.07]), intervening glaucoma surgeries (β[SE] = 0.15[0.03]), and more positive RNFLT thinning rate (β[SE] = 0.06[0.02] per 1um/year more positive) showed consistent association with greater RNFLT variability, whereas more frequent visits/testing (β[SE] = −0.11[0.05] per 1 visit/year higher) was associated with smaller RNFLT variability (P<0.05 for all).
Conclusions:
Relevant clinical factors affecting long-term RNFLT variability in glaucoma were identified. These data enhance the evaluation of longitudinal structural change. Increasing the testing frequency, especially in eyes at risk of higher measurement variability, and resetting of baseline imaging after intervening procedures may help to more reliably detect OCT progression.
Keywords: glaucoma, OCT, RNFL thickness, variability, reproducibility
INTRODUCTION
With a chronic, progressive and irreversible course,1 clinical monitoring of glaucoma relies heavily on periodic assessment of both functional and structural changes. For assessing the former, the visual field (VF) test has been performed traditionally and routinely. While for the latter, the optical coherence tomography (OCT) has become the most commonly used clinical instrument for structural evaluation.
The measurement variability of VF and OCT both play important roles in the long-term follow-up of glaucoma. Thus, the impact of this variability on the detection of progressive glaucoma is of critical interest. The variability of VF parameters in glaucoma has been well studied.2–6 Several factors have been identified that contribute to the longitudinal fluctuation of VF,6 which can be used to inform clinicians to identify patients that may require more frequent testing and additional evaluation of VF loss.
While VF variability has be extensively evaluated, only limited studies have investigated the variability of OCT-based metrics, particularly long-term variability, despite its key role in glaucoma progression assessment. Although considered less variable than VF,7–10 prior studies still reported a wide range of test-retest variability for OCT on retinal nerve fiber layer thickness (RNFLT) measurement.11–15 This indicates that, similar to VF, there may be individual and inter-instrument differences in the magnitude of OCT measurements fluctuation, and that presence of some characteristics might predict a worse OCT reproducibility in a patient. The factors contributing to a greater RNFLT variability have been largely unexplored. Identification of these factors may reduce false positive results and delayed detection of structural progression. Moreover, this may help to identify patients that require more careful OCT measurements.
This study examined the clinical factors associated with long-term OCT-measured RNFLT variability in order to improve the detection of structural changes and to identify patients requiring additional attention when assessing structural progression based on OCT.
METHODS
This study adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act, and was approved by the University of California San Diego (UCSD) Human Research Protection Program (NCT00221897).
Participants
This is a retrospective cohort study. Primary open angle glaucoma (POAG) and glaucoma suspect patients from the Diagnostic Innovations in Glaucoma Study (DIGS)/ The African Descent and Glaucoma Evaluation Study (ADAGES)16, 17 were included. Inclusion criteria for DIGS/ADAGES were: (1) age >= 18 years; (2) BCVA of 20/40 or better at study entry; (3) open angles on gonioscopy. Routine examinations of DIGS included: (1) gonioscopy and ultrasound pachymetry at the first visit; (2) semi-annual examination of Goldmann applanation tonometry (for intraocular pressure [IOP] measurement), VF, and OCT/OCTA imaging; (3) annual comprehensive ophthalmic examination with dilated fundus examination, slit-lamp biomicroscopy, best-corrected visual acuity (BCVA), and stereoscopic optic disc photography. Relevant clinical information, such as demographics and systemic medical history, was also collected. Written informed consent was obtained from all DIGS participants.
A minimum of 2 years and 4 visits of OCT visits were required to be included in this study. Participants with the following conditions were excluded: (1) axial length ≥ 27mm; (2) uveitis; (3) history of trauma; (4) non-glaucomatous optic neuropathy; (5) coexisting retinal disease including diabetic retinopathy; (6) history of Parkinson’s disease, clinical dementia, or stroke. Glaucoma suspect was defined as having a suspicious-appearing optic disc or an elevated IOP (≥ 22 mm Hg) without repeatable glaucomatous VF damage. POAG was defined as having repeatable (≥ 2 consecutive tests) and reliable (fixation losses and false negatives ≤ 33% and false positives ≤ 15%) abnormal VF results using the 24-2 Swedish Interactive Thresholding Algorithm (SITA) with a PSD outside the 95% confidence limits or a GHT result outside the 99% confidence limit.
Analysis of OCT measurements variability
The Spectralis spectral domain-OCT (Heidelberg Engineering, GmbH, Heidelberg, Germany) optic nerve head (ONH)-centered retinal nerve fiber layer (RNFL) circle scan (diameter ~ 3.4mm) was used to obtain the mean global RNFLT measurement. In this study, the OCT variability was calculated as the longitudinal absolute error of RNFLT on a per-exam/visit basis, with residuals (the difference between predicted and observed values of RNFLT) being time-varying outcomes derived from a linear mixed-effects model. Our mixed-effects model fit mean global RNFLT across follow-up time (years), with a random slope for longitudinal follow-up time and random intercepts to account for nested subject eye variability and variability derived from Spectralis OCT software version.
Statistical analysis
Demographic and clinical characteristic data were presented as count (%) for categorical variables and mean (95% confidence interval [CI]) for continuous variables. Linear mixed-effects models adjusted for inter-eye correlation as well as Spectralis OCT software version were fit with RNFLT residuals errors (variability of RNFLT) as the dependent variable. Statistical analyses were performed using the R programming language version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) with the packages “lme4”, “nlme”, “lmerTest”, and “performance”. A P-value ≤ 0.05 was considered statistically significant.
Clinical factors selection
Potential clinical factors associated with RNFLT variability initially considered included: (1) baseline variables: age, gender, eye laterality, race, diagnosis (healthy, glaucoma suspect, POAG), systemic hypertension, diabetes, IOP, central corneal thickness (CCT), axial length (AL), spherical equivalent (SE), glaucoma medication use and the number of medications, history of cataract extraction (CE) at baseline, history of glaucoma surgeries at baseline, 24-2 VF MD and PSD, glaucoma severity, RNFLT; (2) longitudinal variables: mean IOP during the follow-up, IOP fluctuation (standard deviation [SD] of the IOP), slope of RNFL thinning (calculated using linear regression), CE during follow-up, glaucoma surgeries during follow-up, OCT scan quality and Automated Real Time (ART)-function, follow-up duration, total number of visits/testing, frequency of visit/testing (per year).
To decide the final set of clinical factors for model fitting, a hierarchical cluster analysis based on squared Spearman correlations was performed. The clustering method clusters all variables based on their correlation patterns, in order to reveal multicollinearity. The goal was to ensure no considerable collinearity existed among the finally included factors (rho2 ≤ 0.30).18 After examining for collinearity, the final set of clinical factors included were: baseline age, gender, race, systemic hypertension, diabetes, eye laterality, baseline AL, baseline CCT, baseline SE, baseline glaucoma medication use, baseline IOP, baseline VF MD, baseline RNFLT, history of CE at baseline, history of glaucoma surgeries (e.g. filtration, laser, and other procedures for glaucoma) at baseline, IOP fluctuation (SD), RNFL thinning rate, CE during follow-up, glaucoma surgeries during follow-up, scan quality, ART, frequency of visit/testing, and follow-up duration. Baseline variables were defined as measurements within 6 months of the first RNFLT included in the analysis. Correlation among the final set of clinical factors is shown in Supplemental Figure S1. All factors exhibited only null-to-fair degrees of correlation (rho2 < 0.27 for all).
The proportion of missing data was calculated for each clinical factor. As shown in Supplemental Figure S2, the percentage of missing data was below 2% for all variables in this study. With the very low data missingness, we did not perform data imputation and presented only the results based on the original data.
Model fitting
Linear mixed models were fit with RNFLT variability (absolute error of time-varying residuals) as the dependent variable. Inter-eye correlation was adjusted by including fellow eye as the random effect. Three separate multivariable models were fit with different set of clinical factors:6 (1) Full model: This model included all eligible factors included after clustering analysis; (2) Baseline model: This model included only eligible factors that were baseline variables; (3) Parsimonious model: This model included factors additionally selected by least absolute shrinkage and selection operator (LASSO) regression.19 R2 was calculated to estimate the total variance explained by each model.20 To estimate the potential model performance for future clinical application,10-fold cross-validation was performed. In the 10-fold cross-validation, the dataset was first randomly divided into 10 equally sized subsets. For each fold, 9 subsets were used to train the model, with 1 subset left out being used as the test sample. This process was repeated 10 times (10-fold) so that all subsets were used as the test sample once, and the mean squared errors derived from all 10 folds were averaged to demonstrate model performance. Results of the univariable model are presented in Supplemental Table S1. A positive co-efficient indicates increased RNFLT variability, while a negative co-efficient indicates decreased RNFLT variability.
RESULTS
Demographic and clinical characteristics of the study population are summarized in Table 1. A total of 1140 eyes (POAG: 632 eyes; glaucoma suspect: 508 eyes) from 634 patients were included. The mean (95% CI) age and 24-2 VF MD at baseline was 64.9 (64.0, 65.7) years and −3.4 (−3.8, −3.1) dB, respectively. Mean (95% CI) baseline RNFLT was 80.5 (79.5, 81.5) μm, and mean (95% CI) rate of RNFL thinning was −0.6 (−0.7, −0.6) μm/year. The mean (95% CI) follow-up duration was 10.3 (10.0, 10.6) years, with a mean (95% CI) of 6.6 (6.5, 6.8) visits throughout the follow-up period. The overall mean (95% CI) RNFLT variability of this cohort was 1.51 (1.45, 1.58) μm. The mean (95% CI) RNFLT variability tended to be higher in African Americans (1.67 [1.47, 1.87] μm) compared to non-African Americans (1.43 [1.35, 1.50] μm), eyes having intervening CE (2.01 [1.81, 2.21] μm) compared to those who did not (1.38 [1.31, 1.44] μm), and eyes undergoing intervening glaucoma surgeries (1.76 [1.55, 1.97] μm) compared to those who did not (1.45 [1.39, 1.52] μm) (P < 0.001 for all). Detailed RNFLT variability stratified by diagnosis and rate of RNFLT thinning is shown in Supplemental Table S2.
Table 1:
Demographic and clinical characteristics of included eyes
| N = 1140 eyes (634 patients) | ||
|---|---|---|
|
| ||
| Mean (95% CI) | Median (Range) | |
|
| ||
| Age at baseline (years) | 64.9 (64.0, 65.7) | 65.5 (23.2, 95.9) |
|
| ||
| Sex (Female, n) | 355 (56.0%) | 355 (56.0%) |
|
| ||
| Diagnosis (n) | ||
| Glaucoma suspect | 508 (44.6%) | - |
| POAG | 632 (55.4%) | |
|
| ||
| Race (African American, n) | 239 (37.7%) | - |
|
| ||
| Hypertension (Hypertensive, n) | 311 (49.1%) | - |
|
| ||
| Diabetes (Diabetic, n) | 82 (12.9%) | - |
|
| ||
| Eye laterality (n) | - | |
| Right eye | 563 (49%) | |
| Left eye | 577 (51%) | |
|
| ||
| Baseline IOP (mmHg) | 16.2 (16.1, 16.3) | 16.0 (1.0, 44.0) |
|
| ||
| Mean IOP during follow up (mmHg) | 15.5 (15.3, 15.8) | 15.2 (2.4, 30.0) |
|
| ||
| IOP fluctuation (mmHg) | 2.6 (2.5, 2.6) | 2.3 (0.0, 13.9) |
|
| ||
| Baseline CCT (μm) | 543.9 (541.4, 546.4) | 541.7 (398.5, 694.0) |
|
| ||
| Baseline axial length (mm) | 24.0 (24.0, 24.1) | 24.0 (17.9, 26.9) |
|
| ||
| Baseline spherical equivalent (D) | −0.5 (−0.6, −0.4) | 0.0 (−10.0, 4.25) |
|
| ||
| Baseline 24-2 VF MD (dB) | −3.4 (−3.8, −3.1) | −1.6 (−33.7, 3.0) |
|
| ||
| Baseline 24-2 VF PSD (dB) | 3.9 (3.7, 4.1) | 2.1 (0.9, 16.7) |
|
| ||
| Baseline glaucoma severity (n) | ||
| Mild (MD >= −6 dB) | 931 (81.7%) | - |
| Moderate-advanced (MD < −6 dB) | 209 (18.3%) | |
|
| ||
| Baseline RNFLT (μm) | 80.5 (79.5, 81.5) | 79.0 (33.0, 133.0) |
|
| ||
| Rate of RNFLT thinning (μm/year) | −0.6 (−0.7, −0.6) | −0.6 (−16.0, 13.0) |
|
| ||
| History of cataract extraction at baseline (n) | 203 (17.8%) | - |
|
| ||
| Cataract extraction during follow-up (n) | 242 (21.2%) | - |
|
| ||
| History of glaucoma surgery at baseline (n) | 290 (25.4%) | - |
|
| ||
| Glaucoma surgery during follow-up (n) | 233 (20.4%) | - |
|
| ||
| Glaucoma medication use at baseline | 960 (84.2%) | - |
|
| ||
| Number of glaucoma medication use at baseline | 1.5 (1.4, 1.6) | 1 (0, 4) |
|
| ||
| Scan quality | 28.0 (27.8, 28.1) | 28 (14, 41) |
|
| ||
| Automatic Real Time (ART)-function | 58.2 (57.1, 59.3) | 62.0 (2.0, 100.0) |
|
| ||
| Maximum follow-up visits | 10.3 (10.0, 10.6) | 9 (4, 30) |
|
| ||
| Follow-up time (years) | 6.6 (6.5, 6.8) | 6.2 (2.0, 12.3) |
|
| ||
| Frequency of visits (times/year) | 1.6 (1.6, 1.7) | 1.5 (0.4, 4.4) |
Footnote: Values are shown in mean (95% CI) and median (range), unless otherwise indicated.
Abbreviations: CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; RNFLT= RNFL thickness; POAG = primary open angle glaucoma; PSD = pattern standard deviation, VF = visual field
Figure 1A and Table 2 present the results of the full model (including all factors selected from the clustering analysis). The total R2 was 0.76. African American race (β [standard error, SE] = 0.180[0.068]), higher spherical equivalence (β[SE] = 0.038[0.019], per 1 diopter higher), greater IOP variability (β[SE] = 0.133[0.024], per 1 mmHg higher), CE performed during follow-up (β[SE] = 0.511[0.077]), thicker baseline RNFLT (β[SE] = 0.008[0.002], per 1 μm thicker), more positive RNFL thinning rate (β[SE] = 0.058[0.023], per 1 μm/year more positive), and worse baseline VF MD (β[SE] = −0.017[0.007], per 1 dB higher) were significant predictors of greater RNFLT variability (P < 0.05 for all). While higher baseline IOP (β[SE] = −0.022[0.007], per 1 mmHg higher) and higher visit/testing frequency (β[SE] = −0.115[0.057], per 1 time/year higher) showed association with less RNFLT variability (P < 0.05 for all).
Figure 1.
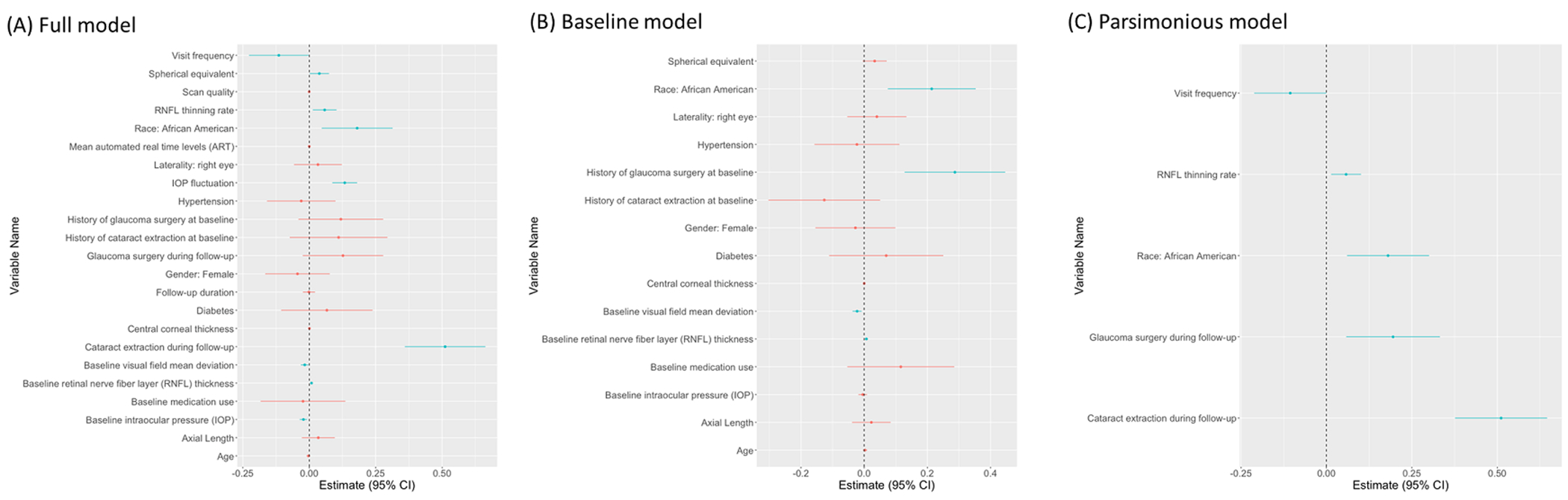
Forest plot showing the (A) full model (R2=0.76) (B) baseline model (R2=0.68) (C) parsimonious model (R2=0.77) for clinical factors predicting retinal nerve fiber layer thickness variability. Dots and bars indicate the coefficient estimates and the 95% confidence intervals (CI), respectively (blue = significant effects; red = insignificant effects). Estimates for continuous variables are intended for a 1-unit increase as indicated in the tables, unless specified otherwise.
Table 2.
Effects of clinical factors on OCT measurement variability - Full model (Multivariable analysis)
| Variable | β Coefficient | SE | P-value |
|---|---|---|---|
| (Intercept) | −0.041 | 1.602 | 0.980 |
| Age, per 1 year older | −0.003 | 0.003 | 0.349 |
| Gender: Female (vs. male) | −0.044 | 0.062 | 0.475 |
| Race: African American (vs. others) | 0.180 | 0.068 | 0.009 |
| Diabetes (yes/no) | 0.066 | 0.088 | 0.451 |
| Hypertension (yes/no) | −0.030 | 0.066 | 0.646 |
| Laterality: Right eye | 0.033 | 0.046 | 0.479 |
| CCT, per 1 μm thicker | 0.001 | 0.001 | 0.486 |
| AL, per 1 mm longer | 0.034 | 0.032 | 0.289 |
| Spherical equivalent, per 1 D higher | 0.038 | 0.019 | 0.042 |
| Baseline VF MD, per 1 dB higher | −0.017 | 0.007 | 0.019 |
| Baseline IOP, per 1 mmHg higher | −0.022 | 0.007 | 0.002 |
| IOP fluctuation, per 1 mmHg higher | 0.133 | 0.024 | < 0.001 |
| Baseline medication use (yes/no) | −0.024 | 0.081 | 0.773 |
| History of CE at baseline (yes/no) | 0.110 | 0.094 | 0.240 |
| CE during follow-up (yes/no) | 0.511 | 0.077 | < 0.001 |
| History of glaucoma surgery at baseline (yes/no) | 0.119 | 0.081 | 0.144 |
| Glaucoma surgery during follow-up (yes/no) | 0.127 | 0.077 | 0.101 |
| Baseline RNFLT, per 1 μm thicker | 0.008 | 0.002 | 0.001 |
| RNFL thinning rate, per 1 μm/year more positive | 0.058 | 0.023 | 0.012 |
| Scan quality, per 1 unit higher | 0.001 | 0.001 | 0.681 |
| Mean ART, per 1 unit higher | 0.001 | 0.001 | 0.987 |
| Follow-up duration, per 1 year longer | −0.001 | 0.012 | 0.913 |
| Visit/testing frequency, per 1 time/year higher | −0.115 | 0.057 | 0.047 |
| Total R2: 0.76 | |||
A positive co-efficient indicates increased RNFLT variability, while a negative co-efficient indicates decreased RNFLT variability.
Abbreviation: AL = axial length, ART = automated real time value, CCT = central corneal thickness, CE = cataract extraction, IOP = intraocular pressure, OCT = optical coherence tomography, RNFL = retinal nerve fiber layer, RNFLT= RNFL thickness, SE = standard error, VF MD = visual field mean deviation
Figure 1B and Table 3 show the results of the baseline model (including only baseline clinical factors), with a total R2 of 0.68. In this model, African American race (β[SE] = 0.214[0.071]), history of glaucoma surgery at baseline (β[SE] = 0.287[0.081]), thicker baseline RNFLT (β[SE] = 0.007[0.003], per 1 μm thicker), and worse baseline VF MD (β[SE] = −0.022[0.007], per 1 dB higher) predicted greater RNFLT variability (P < 0.05 for all).
Table 3.
Effects of clinical factors on OCT measurement variability - Baseline model (Multivariable analysis)
| Variable | β Coefficient | SE | P-value |
|---|---|---|---|
| (Intercept) | −0.131 | 1.388 | 0.925 |
| Age, per 1 year older | 0.003 | 0.003 | 0.436 |
| Gender: Female (vs. male) | −0.028 | 0.065 | 0.669 |
| Race: African American (vs. others) | 0.214 | 0.071 | 0.003 |
| Diabetes (yes/no) | 0.070 | 0.092 | 0.449 |
| Hypertension (yes/no) | −0.023 | 0.069 | 0.739 |
| Laterality: Right eye | 0.040 | 0.048 | 0.401 |
| CCT, per 1 μm thicker | 0.001 | 0.001 | 0.743 |
| AL, per 1 mm longer | 0.023 | 0.031 | 0.470 |
| Spherical equivalent, per 1 D higher | 0.033 | 0.019 | 0.088 |
| Baseline VF MD, per 1 dB higher | −0.022 | 0.007 | 0.003 |
| Baseline IOP, per 1 mmHg higher | −0.005 | 0.007 | 0.511 |
| Baseline medication use (yes/no) | 0.116 | 0.086 | 0.181 |
| History of CE at baseline (yes/no) | −0.126 | 0.090 | 0.163 |
| History of glaucoma surgery at baseline (yes/no) | 0.287 | 0.081 | < 0.001 |
| Baseline RNFLT, per 1 μm thicker | 0.007 | 0.003 | 0.006 |
| Total R2: 0.68 | |||
A positive co-efficient indicates increased RNFLT variability, while a negative co-efficient indicates decreased RNFLT variability.
Abbreviation: AL = axial length, CCT = central corneal thickness, CE = cataract extraction, IOP = intraocular pressure, OCT = optical coherence tomography, RNFLT= RNFL thickness, SE = standard error, VF MD = visual field mean deviation
Results of the parsimonious model (including only factors selected by LASSO regression) are presented in Figure 1C and Table 4. The total R2 was 0.77. African-American race (β[SE] = 0.180[0.061]), intervening CE (β[SE] = 0.511[0.069]), intervening glaucoma surgeries (β[SE] = 0.195[0.070]), and more positive RNFLT thinning rate (β[SE] = 0.057[0.022], per 1 μm/year more positive) were associated with greater RNFLT variability, while a higher visit/testing frequency (β[SE] = −0.106[0.054] per 1 time/year higher) was associated with smaller RNFLT variability (P < 0.05 for all).
Table 4.
Effects of clinical factors on OCT measurement variability – Parsimonious model (Multivariable analysis)
| Variable | β Coefficient | SE | P-value |
|---|---|---|---|
| (Intercept) | 1.492 | 0.757 | 0.049 |
| Race: African American (vs. others) | 0.180 | 0.061 | 0.003 |
| CE during follow-up (yes/no) | 0.511 | 0.069 | < 0.001 |
| Glaucoma surgery during follow-up (yes/no) | 0.195 | 0.070 | 0.006 |
| RNFL thinning rate, per 1 μm/year more positive | 0.057 | 0.022 | 0.010 |
| Visit/testing frequency, per 1 times/year higher | −0.106 | 0.054 | 0.050 * |
| Total R2: 0.77 | |||
A positive co-efficient indicates increased RNFLT variability, while a negative co-efficient indicates decreased RNFLT variability.
Abbreviation: CE = cataract extraction, OCT = optical coherence tomography, RNFL= retinal never fiber layer, SE = standard error
P-value = 0.0496
DISCUSSION
This study examined the clinical factors associated with long-term OCT-measured RNFLT variability in glaucoma. A major strength of the study is the examination of RNFLT variability with the residuals as time-varying outcomes over longitudinal follow-up; this provides a more clinically meaningful quantification of measurement fluctuation and predictor effects over time. Overall, the RNFLT variability was small and only a limited number of factors showed an effect of > 0.1 μm. The main factors that affected the RNFLT variability were African American race, intervening CE, intervening glaucoma surgeries, and more frequent visit/testing. All models based on the original data achieved a satisfactory R2, demonstrating robust performances.
After VF testing, OCT imaging is an invaluable diagnostic test for assessing glaucoma progression. While OCT is less variable than VF,21–23 measurement fluctuation is an intrinsic issue that is particularly relevant to diseases with a chronic and progressive course such as glaucoma. Although RNFL progression has generally shown a strong association with VF loss,24, 25 some patients might be more prone to RNFLT measurement fluctuation as the factors related to testing variability differ across diagnostic approaches. This could not only lead to false positive results, but also missed or delayed detection of true glaucoma progression.
In this study, African American race was associated with higher RNFLT variability as compared to non-African Americans. Interestingly, a prior study has also shown an association between African American race and a higher long-term VF variability in glaucoma.26 The presence of racial/ethnic differences in ocular biometry, such as retinal thickness, have been reported in many studies and may account for some of the racial difference in measurement variablity.27–30 It has been reported that African Americans have thinner global and temporal RNFLT,28, 31, 32 and a higher risk of developing functional damage.33–35 Although no study has directly examined the relationship between RNFLT variability and glaucoma severity, based on available evidences for VF,36–38 it is possible the greater OCT variability of African Americans may be partially related to the worse glaucoma severity and the faster deterioration (African Americans vs. non-African Americans: baseline VF MD = −4.1 vs. −3.1 dB; RNFLT thinning rate = −0.77 vs. −0.58 μm/year; P<0.05 for both). This hypothesis remains to be examined in future studies. Of note, a potentially delayed detection of glaucoma progression in African Americans due to the greater VF variability has been suggested.39 With a similarly greater variability for RNFLT, such differences should be considered and, perhaps, managed with more frequent testing when evaluating glaucoma in this population.40
In the baseline and full models, a worse baseline VF MD showed a small but significant association with greater RNFLT variability. Similar correlation has also been reported for macular OCT measurements.9 This finding is consistent with prior literatures suggesting an increased RNFLT variability in more severe glaucoma,11, 41, 42 which might pose challenge to clinicians when assessing structural change in patients with worse baseline disease. Interestingly, we found an association between a more positive RNFLT thinning rate and greater RNFLT variability, which has not been reported previously. Although this seems to suggest RNFLT may be reliable when evaluating rapidly progressing eyes (with more negative RNFLT change rate) before reaching the OCT floor,43 considering the small effect size and the insignificant result in the univariable model, whether the association was clinically significant remains to be examined. The non-linearity of data might also partially explain this finding. Nonetheless, clinically, it might indeed be more difficult to detect progression when the slope of RNFLT change is small, which can make differentiation between noises and true changes more challenging, In general, these suggest the incorporation of other clinical tests on top of OCT when assessing glaucomatous progression. Additionally, clinicians should be aware of possible false-negative results when a disproportionately small/slow change in RNFLT was found despite other symptoms and signs suggesting true progression.
A greater RNFLT variability was found associated with intervening CE and glaucoma surgeries. It is known the presence of significant cataract often causes falsely decreased RNFLT due to attenuated OCT signal strength, and that the scan quality and thickness measurement usually increase once the lens opacity is removed.44–46 Therefore, the higher RNFLT variability associated with intervening CE might be related to the rise in RNFLT measurement after CE or the presence of a significant cataract during the follow-up period. On the other hand, the post-operative change in RNFLT after glaucoma surgeries is usually not as significant as that observed after CE.47 However, short-term fluctuation in RNFLT might still be observed, and can be a source of the greater variability.48 Considering the more prominent effects of intervening surgeries on RNFLT variability, the establishment of a new imaging baseline after the procedures might be beneficial for subsequent progression evaluation for patients receiving intervening CE and/or glaucoma surgeries. Based on this, a supplemental subgroup analysis was additionally performed on patients without history of intervening surgeries (Supplemental Table S3), which may help to understand the possible effects of other clinical factors when accounting for these predictors.
Finally, the association between a lower RNFLT variability and a higher visit/testing frequency has clinical importance. Information on factors associated with a lower measurement variability, especially modifiable factors, can be incorporated clinically to improve measurement interpretation. Our findings suggest that, by performing more frequent monitoring, clinicians will likely be able to detect structural progression by OCT more accurately, which can be applied to cases at higher risk of measurement fluctuation. Consistent findings showing increased VF visit/test frequency leading to earlier detection of VF progression has been previously reported,6, 49 suggesting this strategy may be applicable to various clinical examinations.
This study has several limitations. First, the RNFLT variability are likely worse in clinical setting than in this academic research setting, since our technicians are very experienced, and the scans have gone through quality review. However, this suggests that clinical factors associated with measurement fluctuation should have even more effects in real-world clinics. Second, whether the study population is representative of the clinical population is unclear, and it is possible that other OCT instruments may have different characteristics and reproducibility. To confirm the generalizability of our results, more studies with diverse enrollment and examination settings are required. Third, there might be residual clinical factors not considered in the analysis, including variation in dynamic factors such as ocular pulse, which may also affect structural measurements. Last, despite providing insight into some potential relationships, the effects of most clinical factors are small, and some are not be well explained. Future studies are needed to clarify the clinical implication of these findings.
In conclusion, this study identified relevant clinical factors associated with the long-term OCT-measured RNFLT variability in glaucoma, which will enhance the evaluation of longitudinal structural change. The overall small RNFLT variability suggests this parameter is suitable for longitudinal glaucoma assessment, and only a limited number of factors showed clinically significant effects on the variability. Furthermore, increasing the testing frequency, especially in eyes at risk of higher measurement variability, and resetting of baseline imaging after intervening procedures may more reliably assist to detect glaucoma progression by OCT.
Supplementary Material
a. Funding:
This work is supported by National Institutes of Health/National Eye Institute Grants (R01EY034148, R01EY029058, R01EY011008, R01EY019869, R01EY027510, R01EY026574, R01EY018926, P30EY022589); University of California Tobacco Related Disease Research Program (T31IP1511), and an unrestricted grant from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
b. Financial Disclosure:
Sasan Moghimi reported grants from the National Eye Institute. Takashi Nishida is a consultant for Topcon. Jeffrey M Liebmann is a consultant to Alcon, Allergan, Thea, Genentech. AdvanceSight, and Carl Zeiss Meditech. Massimo A. Fazio reported grants from the National Eye Institute; grants from Heidelberg Engineering and Topcon; nonfinancial support from Wolfram Research. Christopher A. Girkin reported research support from Heidelberg Engineering and Topcon. Linda Zangwill reported grants from the National Eye Institute; grants from Heidelberg Engineering and nonfinancial support from Carl Zeiss Meditec, Optovue, Heidelberg Engineering, and Topcon; and patents from Carl Zeiss Meditec and AiSight Health. Linda Zangwill is a consultant of Abbvie and Topcon. Robert N. Weinreb is a consultant of Abbvie, Alcon, Allergan, Amydis, Equinox, Eyenovia, Iantrek, IOPtic, Implandata, Nicox, Santen and Topcon. Robert N. Weinreb reported nonfinancial support from Heidelberg Engineering, Carl Zeiss Meditec, Optovue, Centervue, and Topcon; grants from the National Eye Institute, National Institute of Minority Health Disparities, and Research to Prevent Blindness, patents from Toromedes, Carl Zeiss Meditec to UCSD; all outside the submitted work. No other disclosures were reported.
Footnotes
Supplemental Material available at AJO.com
REFERENCES
- 1.Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults-screening, diagnosis, and management: A Review. Jama. Jan 12 2021;325(2):164–174. doi: 10.1001/jama.2020.21899 [DOI] [PubMed] [Google Scholar]
- 2.Tattersall CL, Vernon SA, Menon GJ. Mean deviation fluctuation in eyes with stable Humphrey 24–2 visual fields. Eye. 2007/March/01 2007;21(3):362–366. doi: 10.1038/sj.eye.6702206 [DOI] [PubMed] [Google Scholar]
- 3.Al-Sheikh M, Ghasemi Falavarjani K, Akil H, et al. Impact of image quality on OCT angiography based quantitative measurements. International Journal of Retina and Vitreous. 2017/May/15 2017;3(1):13. doi: 10.1186/s40942-017-0068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. Aug 15 1989;108(2):130–5. doi: 10.1016/0002-9394(89)90006-8 [DOI] [PubMed] [Google Scholar]
- 5.Urata CN, Mariottoni EB, Jammal AA, et al. Comparison of short- and long-term variability in standard perimetry and spectral domain optical coherence tomography in glaucoma. American Journal of Ophthalmology. 2020/February/01/ 2020;210:19–25. doi: 10.1016/j.ajo.2019.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabiolo A, Morales E, Kim JH, et al. Predictors of long-term visual field fluctuation in glaucoma patients. Ophthalmology. 2020/June/01/ 2020;127(6):739–747. doi: 10.1016/j.ophtha.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 7.Miraftabi A, Amini N, Gornbein J, et al. Local variability of macular thickness measurements with sd-oct and influencing factors. Translational Vision Science & Technology. 2016;5(4):5–5. doi: 10.1167/tvst.5.4.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KE, Yoo BW, Jeoung JW, et al. Long-Term reproducibility of macular ganglion cell analysis in clinically stable glaucoma patients. Investigative Ophthalmology & Visual Science. 2015;56(8):4857–4864. doi: 10.1167/iovs.14-16350 [DOI] [PubMed] [Google Scholar]
- 9.Nishida T, Moghimi S, Hou H, et al. Long-term reproducibility of optical coherence tomography angiography in healthy and stable glaucomatous eyes. British Journal of Ophthalmology. 2021:bjophthalmol-2021–320034. doi: 10.1136/bjophthalmol-2021-320034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JH, Moghimi S, Nishida T, et al. Evaluation of the long-term variability of macular OCT/OCTA and visual field parameters. Br J Ophthalmol. Dec 30 2022;doi: 10.1136/bjo-2022-322470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazirani J, Kaushik S, Pandav SS, et al. Reproducibility of retinal nerve fiber layer measurements across the glaucoma spectrum using optical coherence tomography. Indian J Ophthalmol. Apr 2015;63(4):300–5. doi: 10.4103/0301-4738.158064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis ASC, Jammal AA, Zangalli C, et al. Repeatability of macular and optic nerve head oct parameters in advanced glaucoma. Investigative Ophthalmology & Visual Science. 2019;60(9):1423–1423. [Google Scholar]
- 13.Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. Oct 2011;20(8):470–6. doi: 10.1097/IJG.0b013e3181f3eb64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan BB, Natividad M, Chua KC, et al. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. Apr-May 2012;21(4):266–73. doi: 10.1097/IJG.0b013e3182071cdd [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Sung KR, Shin JW. Progression detection capabilities of circumpapillary and macular vessel density in advanced glaucomatous eyes. Scientific Reports. 2022/July/15 2022;12(1):12109. doi: 10.1038/s41598-022-16083-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. Sep 2009;127(9):1136–45. doi: 10.1001/archophthalmol.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. May 2010;128(5):541–50. doi: 10.1001/archophthalmol.2010.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akoglu H User's guide to correlation coefficients. Turk J Emerg Med. Sep 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibshirani R Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B (Methodological). 1996;58(1):267–288. [Google Scholar]
- 20.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4(2):133–142. doi: 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- 21.Pearce JG, Maddess T. Inter-visit test-retest variability of OCT in glaucoma. Optom Vis Sci. Mar 2017;94(3):404–410. doi: 10.1097/opx.0000000000001022 [DOI] [PubMed] [Google Scholar]
- 22.Budenz DL, Chang RT, Huang X, et al. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. Jul 2005;46(7):2440–3. doi: 10.1167/iovs.04-1174 [DOI] [PubMed] [Google Scholar]
- 23.Langenegger SJ, Funk J, Töteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of spectralis SD-OCT in glaucomatous and healthy control eyes. Investigative Ophthalmology & Visual Science. 2011;52(6):3338–3344. doi: 10.1167/iovs.10-6611 [DOI] [PubMed] [Google Scholar]
- 24.Nouri-Mahdavi K, Mohammadzadeh V, Rabiolo A, et al. Prediction of visual field progression from OCT structural measures in moderate to advanced glaucoma. American Journal of Ophthalmology. 2021/June/01/ 2021;226:172–181. doi: 10.1016/j.ajo.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao HL, Zangwill LM, Weinreb RN, et al. Structure-function relationship in glaucoma using spectral-domain optical coherence tomography. Archives of Ophthalmology. 2011;129(7):864–871. doi: 10.1001/archophthalmol.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gracitelli CP, Zangwill LM, Diniz-Filho A, et al. Detection of glaucoma progression in individuals of African descent compared with those of European descent. JAMA ophthalmology. 2018;136(4):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon LY, Antar H, Tsikata E, et al. Effects of age, race, and ethnicity on the optic nerve and peripapillary region using spectral-domain OCT 3D volume scans. Transl Vis Sci Technol. Nov 2018;7(6):12. doi: 10.1167/tvst.7.6.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashani AH, Zimmer-Galler IE, Shah SM, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. Mar 2010;149(3):496–502.e1. doi: 10.1016/j.ajo.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. Jun 2007;114(6):1046–52. doi: 10.1016/j.ophtha.2006.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowd C, Zangwill LM, Weinreb RN, et al. Racial differences in rate of change of spectral-domain optical coherence tomography–measured minimum rim width and retinal nerve fiber layer thickness. American Journal of Ophthalmology. 2018/December/01/ 2018;196:154–164. doi: 10.1016/j.ajo.2018.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nousome D, McKean-Cowdin R, Richter GM, et al. Retinal nerve fiber layer thickness in healthy eyes of black, chinese, and latino Americans: A population-based multiethnic study. Ophthalmology. Jul 2021;128(7):1005–1015. doi: 10.1016/j.ophtha.2020.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Archives of Ophthalmology. 2010;128(5):541–550. doi: 10.1001/archophthalmol.2010.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khachatryan N, Medeiros FA, Sharpsten L, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): predictors of visual field damage in glaucoma suspects. Am J Ophthalmol. Apr 2015;159(4):777–87. doi: 10.1016/j.ajo.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. Nov 14 1991;325(20):1412–7. doi: 10.1056/nejm199111143252004 [DOI] [PubMed] [Google Scholar]
- 35.Pleet A, Sulewski M, Salowe RJ, et al. Risk factors associated with progression to blindness from primary open-angle glaucoma in an African-American population. Ophthalmic Epidemiol. Aug 2016;23(4):248–56. doi: 10.1080/09286586.2016.1193207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell RA, Crabb DP, Malik R, et al. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Investigative Ophthalmology & Visual Science. 2012;53(10):5985–5990. [DOI] [PubMed] [Google Scholar]
- 37.Artes PH, Iwase A, Ohno Y, et al. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Investigative ophthalmology & visual science. 2002;43(8):2654–2659. [PubMed] [Google Scholar]
- 38.Henson DB, Chaudry S, Artes PH, et al. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Investigative Ophthalmology & Visual Science. 2000;41(2):417–421. [PubMed] [Google Scholar]
- 39.Gracitelli CPB, Zangwill LM, Diniz-Filho A, et al. Detection of glaucoma progression in individuals of African descent compared with those of European descent. JAMA Ophthalmol. Apr 1 2018;136(4):329–335. doi: 10.1001/jamaophthalmol.2017.6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez CI, Chansangpetch S, Mora M, et al. Ethnicity-specific database improves the diagnostic ability of peripapillary retinal nerve fiber layer thickness to detect glaucoma. American Journal of Ophthalmology. 2021;221:311–322. doi: 10.1016/j.ajo.2020.07.043 [DOI] [PubMed] [Google Scholar]
- 41.Blumenthal EZ, Horani A, Sasikumar R, et al. Correlating structure with function in end-stage glaucoma. Ophthalmic Surg Lasers Imaging. May-Jun 2006;37(3):218–23. doi: 10.3928/15428877-20060501-06 [DOI] [PubMed] [Google Scholar]
- 42.Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. Jun 2015;99(6):732–7. doi: 10.1136/bjophthalmol-2014-305745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moghimi S, Bowd C, Zangwill LM, et al. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology. Jul 2019;126(7):980–988. doi: 10.1016/j.ophtha.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mwanza JC, Bhorade AM, Sekhon N, et al. Effect of cataract and its removal on signal strength and peripapillary retinal nerve fiber layer optical coherence tomography measurements. J Glaucoma. Jan 2011;20(1):37–43. doi: 10.1097/IJG.0b013e3181ccb93b [DOI] [PubMed] [Google Scholar]
- 45.El-Ashry M, Appaswamy S, Deokule S, et al. The effect of phacoemulsification cataract surgery on the measurement of retinal nerve fiber layer thickness using optical coherence tomography. Curr Eye Res. May 2006;31(5):409–13. doi: 10.1080/02713680600646882 [DOI] [PubMed] [Google Scholar]
- 46.van Velthoven ME, van der Linden MH, de Smet MD, et al. Influence of cataract on optical coherence tomography image quality and retinal thickness. Br J Ophthalmol. Oct 2006;90(10):1259–62. doi: 10.1136/bjo.2004.097022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez FG, Sanders DS, Moon JJ, et al. Effect of trabeculectomy on OCT measurements of the optic nerve head neuroretinal rim tissue. Ophthalmol Glaucoma. Jan-Feb 2020;3(1):32–39. doi: 10.1016/j.ogla.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghu N, Pandav SS, Kaushik S, et al. Effect of trabeculectomy on RNFL thickness and optic disc parameters using optical coherence tomography. Eye (Lond). Aug 2012;26(8):1131–7. doi: 10.1038/eye.2012.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nouri-Mahdavi K, Zarei R, Caprioli J. Influence of visual field testing frequency on detection of glaucoma progression with trend analyses. Archives of ophthalmology. 2011;129(12):1521–1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


