Abstract
Heilongjiang Province has the third largest bee population in China, producing over 2,000 tons of beeswax waste (BW) each year. Most of this BW is discarded or burned. Therefore, we urgently need to find sustainable applications of BW. Pleurotus ostreatus mushrooms, commonly referred to as oyster mushrooms, are cultivated for both food and medicine. The substrate used to grow P. ostreatus mushrooms often contains wheat bran as a nitrogen source. The goal of this study was to explore the feasibility of substituting this wheat bran with BW to cultivate P. ostreatus mushrooms. Five treatments were established, with BW making up 0%, 3%, 5%, 7%, and 9% of the total substrate, and the effects on the mycelial growth and development, biological efficiency (BE), and yield were evaluated along with changes in the chemical biomass composition of the fruiting bodies. Adding BW increased the number of days needed for primordia initiation and the number of days between flushes of P. ostreatus mushrooms. With increasing BW, the total fresh weight of P. ostreatus mushrooms first increased and then decreased. The 5% BW treatment resulted in the highest yield and biological efficiency (BE) of 1,478.96 ± 9.61 g bag−1 and 92.43 ± 0.60%, respectively, which exceeded the values of the control by 4.14% (control: 1,420.15 ± 9.53 g bag−1 and 88.76 ± 0.60%, respectively). The 5% BW treatment also resulted in the highest mushroom crude protein content (23.47 ± 0.18 g 100 g−1), which was 28.18% higher compared with the control (18.31 ± 0.05 g 100 g−1). The 9% BW treatment resulted in the highest crude polysaccharide content (10.33 ± 0.76 g 100 g−1), which was 2.42-fold that of the control (4.26 ± 0.30 g 100 g−1). This study suggests that BW could serve as an effective source of nitrogen to cultivate P. ostreatus. BW is a promising, cost-effective, and efficient additive to mushroom substrate, improving the yield and quality of P. ostreatus mushrooms while providing a sustainable use for an otherwise difficult to dispose of waste product.
Keywords: Beeswax waste (BW), Pleurotus ostreatus, Yield, Nutritional quality
Introduction
China is one of the primary producers of honey worldwide. The number of bee-keepers and bee colonies and the amount of honey produced in China rank first in the world (Chen et al., 2012). The amount of honey produced in China was estimated to be 461,900 tons in 2022 and 463,500 tons in 2023 (National Bureau of Statistics, 2024; https://www.stats.gov.cn/) with the output increasing each year. Heilongjiang Province has the third largest bee population in the country (Wang, 2023). There were estimated to be more than 1.34 million bee colonies in Heilongjiang Province in 2023 (Li, 2023).
With the rapid development of the bee industry, more waste is being produced. This waste is difficult to dispose of and can negatively impact resources and the environment. In particular, beeswax waste (BW) is the residue left over after extracting beeswax from old honeycombs. The primary components of BW are bee carcasses, feces of bee larvae, residual honey, pollen and cocoons after insect rearing, deteriorated royal jelly, gum, and unpressed beeswax. One hundred pieces of old honeycomb can result in 20–30 kg of BW (Zhao & Zhao, 1991). Therefore, it is estimated that more than 2,000–3,000 tons of BW are produced each year in Heilongjiang Province.
BW is a solid material and is reported to be approximately 30% protein and lipid (Chen, 1979). It has a high potential for use in agricultural sectors to increase soil structure and fertility (Pourghasemian, Moradi & Iriti, 2023) as it is an organic amendment that is inexpensive, natural, eco-friendly, and easily available. However, there is little research on the use of BW in agriculture (Moradi, Pourghasemian & Naghizadeh, 2019). In China, BW is usually piled up on apiaries or burned as waste, which seriously pollutes the environment and wastes resources. This highlights the critical need for scientific analyses on sustainable uses of BW.
Pleurotus ostreatus is one of the most widely cultivated edible fungi in the world (Nam et al., 2018). Commonly known as oyster mushrooms, the fruiting bodies are favored by consumers for their high levels of nutrients, such as fiber, carbohydrates, fat-soluble vitamins and essential minerals, and low fat content (Kong et al., 2020; Abou Fayssal et al., 2021). In addition to their culinary appeal, P. ostreatus mushrooms comprise a group of edible fungi with important medicinal biotechnological and environmentally friendly applications (Elisashvili et al., 2008). P. ostreatus is easy to cultivate and can grow on various agricultural wastes; its short cultivation period and low production cost (Mahari et al., 2020) have led to a substantial demand in recent years and an increasingly broad market. As a result, P. ostreatus is the second most commonly produced variety of mushroom in the world (Grimm & Wösten, 2018; Kuforiji & Fasidi, 2009).
In recent years, cultivating Pleurotus spp. has become a promising method for converting lignocellulosic residues (plant-based waste materials) into protein-rich food. This approach utilizes renewable resources and contributes to food security by providing a sustainable source of nutrition (Naraian, Narayan & Srivastava, 2014).
In China, many regions cultivate P. ostreatus mushrooms as part of projects to eliminate poverty and promote rural revitalization, with the mushrooms playing an important role in ecological agriculture construction and rural economic development (Yang, 2019).
In the cultivation of P. ostreatus mushrooms, substances with high nitrogen contents, such as rice (Oryza sativa) bran and wheat (Triticum aestivum) bran, are often added to the primary growth substrate to promote the yield and quality of mushrooms (Moonmoon et al., 2011; Elisa et al., 2017). However, this also increases the production costs. Therefore, we need to find low-cost and efficient nitrogen sources to improve the quality and efficiency of P. ostreatus mushroom cultivation.
This study evaluated the effects of adding BW to the growth substrate on various parameters of P. ostreatus, including its mycelial growth and development, enzyme activities, yield of fruiting bodies, and composition of nutrients. The results indicate that BW, if applied at an appropriate percentage (5%), is a novel and sustainable nitrogen source for efficient cultivation of high-quality mushrooms. Moreover, the 9% BW treatment resulted in the highest crude polysaccharide content. To our knowledge, this is the first scientific study on the application of BW in the cultivation of edible fungi in China.
Materials and Methods
Fungal material
P. ostreatus samples were obtained from the Mudanjiang Branch of the Heilongjiang Academy of Agricultural Sciences (Mudanjiang, China). The strain was grown on potato dextrose agar (PDA) (200 g peeled, sliced and boiled potatoes, 20 g dextrose, and 20 g agar L−1) at 25 °C.
Substrate preparation
BW was obtained from the Mudanjiang Branch of the Heilongjiang Academy of Agricultural Sciences and the Raohe Northeast Black Bee Industry (Group) Co., Ltd. (Raohe, China). The other materials used to prepare the substrate were purchased locally.
Beeswax waste preparation
All the materials were dried in the sun. The dried BW was crushed with a Xin-zhuohui-FSJ grinder (Zhuohui Machinery Co., Ltd., Zhengzhou, China) and passed through an inner screen with 2-cm holes.
Analysis of the components of beeswax waste
The dry matter (DM) of the samples was determined after they had been dried to a constant weight at 65 °C. The contents of total carbon (TC) and total nitrogen (TN) were estimated as described by Dundar, Acay & Yildiz (2009).
Beeswax waste fermentation
BW was fermented as follows: The BW was placed in a pile, and the temperature of the pile was recorded. When the temperature of the pile exceeded 60 °C, it was held at this temperature for 24 h. Then, the pile was mixed and re-piled. In general, the pile required mixing 3–4 times during the fermentation period. The water content of the material was maintained at 55–65% (w/w). After the primary fermentation, Aspergillus oryzae was mixed with the BW at 0.15% (w/w) and the mixture was piled for a second round of fermentation. When the pile temperature was 32–40 °C, this temperature was maintained for 48–60 h. The pile was then re-mixed and re-piled. When the pile temperature increased to 55–65 °C, the temperature was maintained for 4–6 d. The pile was then cooled to room temperature (25–26 °C), completing the fermentation process.
Final substrate preparation
Corn cobs were used as the primary material in the growth substrate. First, corn cobs were chopped into small pieces (1–2 cm) with pruning shears, crushed, and passed through a 2-cm screen. Then, corn cobs were pre-wetted, and, after 12 h, wheat bran and lime were evenly spread on the surface of the pre-wetted material. The material was evenly mixed, piled, and then rows of vertical air holes were established in the pile. When the temperature at 20 cm below the maximum height exceeded 60 °C, the temperature was maintained for 24 h. The pile was then re-mixed and re-piled until it reached a temperature of 65 °C for approximately 2 h and then re-piled again. This continued until the pile had been re-mixed and re-piled 3–5 times. After fermentation was complete, the substrate was loose and elastic, not lumpy or sticky. At this stage, BW was added to the corn cob substrate so that the final ratios of each material in the growth substrate for each treatment followed those shown in Table 1. The moisture content in the final substrate was adjusted to 55–65% (w/w).
Table 1. Substrate composition for Pleurotus ostreatus cultivation.
| Material | Percentage (DM) in each treatment | ||||
|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | |
| Corncob | 85 | 85 | 85 | 85 | 85 |
| Wheat bran | 12 | 9 | 7 | 5 | 3 |
| BW | 0 | 3 | 5 | 7 | 9 |
| Lime | 3 | 3 | 3 | 3 | 3 |
Note:
DM, dry matter; BW, beeswax waste; T, treatment; CK, control.
Mushroom cultivation
The prepared substrates were placed in 25 cm × 42 cm polyethylene bags that were 0.0015 cm thick with a packing density of 1,600 g of substrate (dry weight) per bag. The spawn was spread on the substrates to inoculate the cultivation bags using 20% (w/w) of substrate dry weight. In total there were 30 bags per replicate and three replicates per treatment. The inoculated bags were kept in the dark in the spawn running room at 25 °C and 60–65% relative humidity (RH). Then, bags were moved to a greenhouse at 17–22 °C and 80–90% RH and 100–200 Lux for primordia initiation and the fruiting body growth. The mycelial growth rate was determined as the height of the mycelia in the colonized culture bags (mm) divided by the incubation time (d). The time that it took the primordia to initiate (days from inoculation to the development of pinhead fruiting bodies) was noted. The time interval between flushes was considered to be the number of days between the first flush of mushrooms and the second flush.
Harvest and determination of the biological efficiency
Mushrooms were harvested when the mushroom caps were slightly rolled up at the margins as described by Yang, Guo & Wan (2013). The fruiting bodies were weighed after harvest, and the number of harvested bags was counted. These data were used to calculate the mushroom weights and biological efficiency (BE) at the end of the harvesting period. The yield was calculated as shown in Eq. (1):
| (1) |
The BE was calculated as shown in Eq. (2):
| (2) |
Determination of enzyme activity
The substrate was sampled at 10, 20, and 30 d after inoculation, as well as during the formation of primordia and the maturation of fruiting bodies. Each sample size was 2 g. The sampling point was approximately 2 cm below the material surface. Three samples were randomly selected and quickly frozen in liquid nitrogen. Frozen samples were stored at −80 °C until further use. The substrates were thawed at 4 °C in an ice water bath. The substrates were then centrifuged at 8,000 × g or 10,000 × g for 10 min at 4 °C, and the supernatants that contained the enzymes were assayed.
Commercial test kits (Suzhou Comin Biotechnology Company, Ltd., Suzhou, China) were used to assay enzymes according to the manufacturer’s instructions. Briefly, laccase was assayed using the 2,2-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) method (Han et al., 2021). The amount of enzyme required to convert 1.0 μmol min−1 of ABTS was defined as one unit of activity. Carboxymethyl cellulase (CMCase) was assayed as described by Santa-Rose et al. (2018) using an enzyme-labeled instrument (RT-6100; Nidek, San Jose, CA, USA).
Fruiting body nutrient analysis
Fruiting bodies were randomly collected from each treatment group in the first flush, and the nutritional content was analyzed by Beijing Jinyan Innovation Technology Co., Ltd. (Beijing, China). The contents of crude polysaccharide (China Agricultural Press, 2023), crude fat (GB 5009.6, 2016), crude fiber (GB/T 5009.10, 2003), crude protein (GB 5009.5, 2016), and ash (GB 5009.4, 2016) were determined as described in the National Food Safety Standards.
Statistical analysis
One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple range tests were used to determine differences in variables among the five treatments at a 95% confidence level (P < 0.05). All statistics were performed using SPSS 26.0 for Windows (IBM, Inc., Armonk, NY, USA). The normality of the data was tested using the Shapiro-Wilk method. Correlations were determined using the Pearson coefficient. Figures were initially plotted using GraphPad Prism 8 (San Diego, CA, USA) and then modified manually to improve presentation.
Results
Components of beeswax waste
The carbon-nitrogen ratio (C/N) of substrate is one of the key factors affecting the cultivation of edible fungi. The contents of total carbon (TC) and total nitrogen (TN) of BW were determined in this study. The TC content of the BW was 49.56%, while that of the TN was 1.86%. The C/N ratio of the BW was 26.65:1.
Mycelial growth and development
To assess the effect of BW on P. ostreatus mycelial growth and development, five levels of BW application—0% (CK), 3% (T1), 5% (T2), 7% (T3), and 9% (T4)—were investigated and the growth rate of mycelia as well as the primordial initiation time and the time interval between flushes were determined. The results showed that the addition of BW to P. ostreatus growth substrate affected the rate of mycelial growth, the time of primordial initiation, and the time interval between flushes (Table 2, Tables S1 and S2). The rate of mycelial growth in the different treatments followed the trend: T2 > T3 > T1 > CK > T4. The mycelia in T2 (5% BW) grew significantly faster than T4 mycelia (P < 0.05). However, there were no significant differences between the BW treatments and the control treatment. BW addition delayed primordial initiation. In T4 (9% BW), the primordial initiation time was 50.33 ± 1.53 d, which was significantly longer than that of other treatments (P < 0.05), including the control. BW also increased the time interval between flushes of P. ostreatus. T3 (7% BW) resulted in the longest time interval (13.67 ± 0.58 d), significantly longer than that of the control (11.67 ± 1.53 d) (P < 0.05).
Table 2. Growth and development of Pleurotus ostreatus mushrooms on different substrates.
| Treatment | Mycelial growth rate (mm d−1) |
Primordial initiation time (days) | Time interval between flushes (days) |
|---|---|---|---|
| CK | 6.42 ± 0.45ab | 43.33 ± 1.15c | 11.67 ± 1.53b |
| T1 | 6.43 ± 0.56ab | 46.33 ± 1.15b | 12.33 ± 0.58ab |
| T2 | 6.89 ± 0.56a | 47.33 ± 1.53b | 12.67 ± 1.53ab |
| T3 | 6.69 ± 0.58ab | 47.00 ± 1.73b | 13.67 ± 0.58a |
| T4 | 6.34 ± 0.52b | 50.33 ± 1.53a | 13.33 ± 0.68ab |
Notes:
Means ± SD are shown. SD, standard deviation.
Different lowercase letters (a, b, c) indicate significant differences (α = 0.05, ANOVA, LSD test).
T, treatment; BW, beeswax waste; CK, control.
CK: 85% corncob, 12% wheat bran, 3% lime; T1: 85% corncob, 9% wheat bran, 3% BW, 3% lime; T2: 85% corncob, 7% wheat bran, 5% BW, 3% lime; T3: 85% corncob, 5% wheat bran, 7% BW, 3% lime; T4: 85% corncob, 3% wheat bran, 9% BW, 3% lime.
Yield and biological efficiency
The fresh weight of each flush, BE, and total yield of P. ostreatus are shown in Table 3 and Table S4. The mushrooms in the first flush of T1 and T2 weighed 674.19 ± 14.30 g bag−1 and 678.16 ± 4.03 g bag−1, respectively, and these values did not differ significantly from those of the control (681.42 ± 5.08 g bag−1). However, T3 and T4 resulted in significantly lower mushroom weights (651.97 ± 12.91 g bag–1 and 649.07 ± 9.97 g bag−1, respectively) than the other BW treatments and the control treatment (P < 0.05).
Table 3. Fresh weight and biological efficiency of the Pleurotus ostreatus mushrooms grown on different substrates (mean ± SD).
| Treatment | Fresh weight of the mushrooms (g bag−1) | BE (%) | |||
|---|---|---|---|---|---|
| First flush | Second flush | Third flush | Total | ||
| CK | 681.42 ± 5.08a | 473.53 ± 9.77ab | 265.21 ± 7.11b | 1420.15 ± 9.53b | 88.76 ± 0.60b |
| T1 | 674.19 ± 14.30a | 475.17 ± 9.51ab | 277.50 ± 7.38b | 1426.85 ± 10.19b | 89.18 ± 0.64b |
| T2 | 678.16 ± 4.03a | 482.05 ± 4.58a | 318.75 ± 9.42a | 1478.96 ± 9.61a | 92.43 ± 0.60a |
| T3 | 651.97 ± 12.91b | 465.47 ± 6.21bc | 281.09 ± 10.91b | 1398.53 ± 7.50c | 87.41 ± 0.47c |
| T4 | 649.07 ± 9.97b | 457.26 ± 11.25c | 240.48 ± 8.55c | 1346.81 ± 5.36d | 84.18 ± 0.34d |
Notes:
Different lowercase letters (a, b, c) indicate significant differences (α = 0.05, ANOVA, LSD test).
SD, standard deviation; T, treatment; BE, biological efficiency; BW, beeswax waste.
CK: 85% corncob, 12% wheat bran, 3% lime; T1: 85% corncob, 9% wheat bran, 3% BW, 3% lime; T2: 85% corncob, 7% wheat bran, 5% BW, 3% lime; T3: 85% corncob, 5% wheat bran, 7% BW, 3% lime; T4: 85% corncob, 3% wheat bran, 9% BW, 3% lime.
The fresh weight of the second flush ranged from 457.26 ± 11.25 to 482.05 ± 4.58 g bag−1 and followed the trend: T2 > T1 > CK > T3 > T4. There were no significant differences in yield between T1, T2, or T3 and the control. However, T4 (457.26 ± 11.25 g bag−1) resulted in a significantly lower yield compared with the control treatment (473.53 ± 9.77 g bag−1).
For the third flush, the mushroom fresh weights for T1, T2, T3 and T4 were 277.50 ± 7.38, 318.75 ± 9.42, 281.09 ± 10.91, and 240.48 ± 8.55 g bag−1, respectively. However, the fresh weights of T1 and T3 did not differ significantly nor were they significantly different from the fresh weight of control (265.21 ± 7.11 g bag−1). The mushroom yield of T2 was significantly higher than yields of the other treatments, and T4 had a significantly lower yield than yields of the other treatments (P < 0.05).
BW substantially affected total P. ostreatus mushroom yield. The total yields of CK and T1 were similar (1,420.15 ± 9.53 and 1,426.85 ± 10.19 g bag−1, respectively). The total yield and BE of T2 were 1,478.96 ± 9.61 g bag−1 and 92.43 ± 0.60%, respectively, which was significantly higher than those of the other treatments (P < 0.05). The total yields of T3 and T4 were 1,398.53 ± 7.50 and 1,346.81 ± 5.36 g bag−1, respectively. These two treatments had significantly different total yields from each other and significantly lower yields than those of the other treatments (P < 0.05).
Enzyme activities
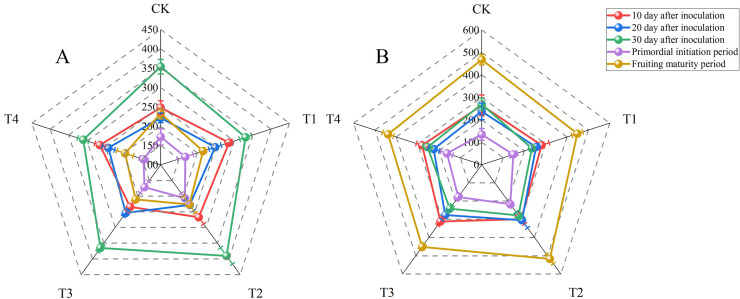
Laccase and carboxymethyl cellulase (CMCase) are the main enzymes secreted by P. ostreatus mycelia. These enzymes degrade the lignocellulose in the growth substrate. The nutritional content of the growth substrate can affect mycelial cells and therefore extracellular enzyme activity. Laccase activity fluctuated considerably with sampling time in all treatments (Fig. 1, Tables S3 and S6). The laccase activities for all treatments were determined at 10 d after inoculation and ranged from 234.32 ± 15.06 to 285.92 ± 9.88 U L−1. The laccase activity did not differ significantly between T1 and T2, but these treatments had significantly higher laccase activities than other treatments (P < 0.05). There were no significant differences in laccase activity at 20 d after inoculation between T1, T2, or T3 and T4. However, T3 resulted in significantly higher laccase activity compared with the control treatment (P < 0.05). The laccase activity at fruiting maturity in T4 was significantly lower than that in the control group (P < 0.05). However, there were no significant differences between the other BW treatments and the control treatment. The highest laccase activities for all treatments were observed at 30 d after inoculation and ranged from 310.32 ± 14.90 to 389.85 ± 26.21 U L−1. The laccase activity then decreased sharply, and the lowest values for all treatments were observed during the primordial initiation period (146.38 ± 11.23 to 206.80 ± 16.22 U L−1). Both at 30 d after inoculation and during the primordial initiation period, T2 had the highest laccase activities, with activities significantly higher than those of the control group (P < 0.05).
Figure 1. Radar maps of enzyme activities (units: U L−1) at 10, 20, and 30 d after inoculation, the primordial initiation period, and the fruiting maturity period for each treatment.
(A) Laccase activity; (B) carboxymethyl cellulase activity. BW, beeswax waste; CK, control; T, treatment. CK: 85% corncob, 12% wheat bran, 3% lime; T1: 85% corncob, 9% wheat bran, 3% BW, 3% lime; T2: 85% corncob, 7% wheat bran, 5% BW, 3% lime; T3: 85% corncob, 5% wheat bran, 7% BW, 3% lime; T4: 85% corncob, 3% wheat bran, 9% BW, 3% lime.
Similar to laccase, the lowest carboxymethyl cellulase (CMCase) activities were observed during the primordial initiation period (135.05 ± 25.98 to 216.77 ± 16.18 U L−1). Conversely, the highest CMCase activities were observed during the fruiting maturity period (438.30 ± 26.16 to 516.79 ± 26.48 U L−1). While there were no significant differences in CMCase activity among the treatments on day 10 after inoculation, T2 had significantly higher CMCase activity than that of the control on day 20 after inoculation as well as in the primordial initiation and fruiting maturity periods (P < 0.05). However, the CMCase activity of T2 did not differ significantly from than that of the control on day 30 after inoculation.
Nutritional value
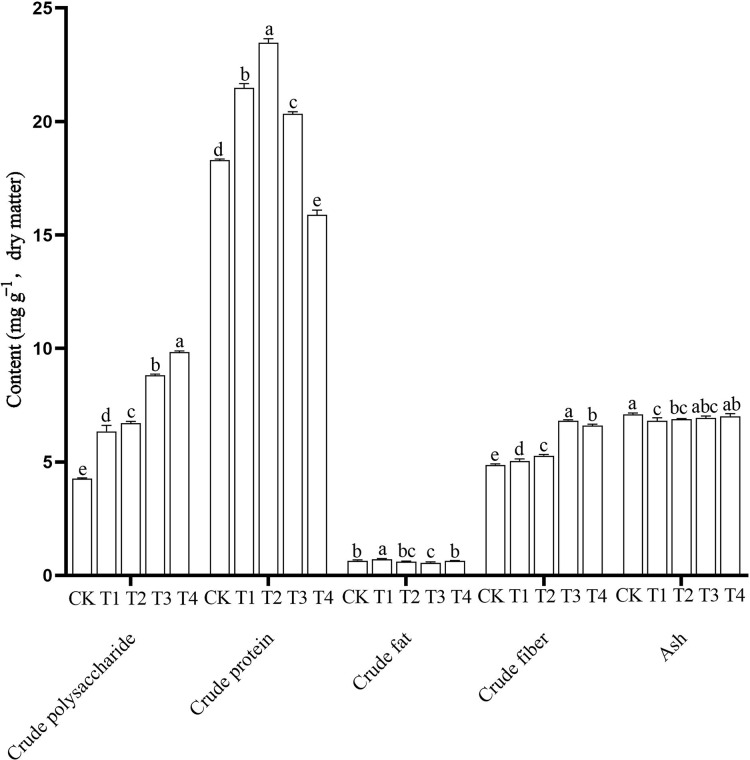
The nutrient content of edible fungi, including the higher crude polysaccharide, higher crude protein, lower crude fat, proper crude fiber and ash content, are important for its value as a food. In this study, nutrient contents in the P. ostreatus fruiting bodies differed significantly among the different treatments (Fig. 2 and Table S5). The CK mushrooms had the highest ash content (7.10 ± 0.06 g 100 g−1) and the lowest crude polysaccharide (4.26 ± 0.30 g 100 g−1) and crude fiber contents (4.86 ± 0.05 g 100 g−1). The T1 mushrooms had the highest crude fat content (0.72 ± 0.03 g 100 g−1) and the lowest ash content (6.81 ± 0.13 g 100 g−1). The T2 mushrooms had the highest crude protein content (23.47 ± 0.18 g 100 g−1). The T3 mushrooms had the highest crude fiber content (6.82 ± 0.04 g 100 g−1) and the lowest crude fat content (0.56 ± 0.04 g 100 g−1). The T4 mushrooms had the highest crude polysaccharide content (10.33 ± 0.76 g 100 g−1) and the lowest crude protein content (15.89 ± 0.20 g 100 g−1).
Figure 2. Nutritional components of Pleurotus ostreatus mushrooms grown on different substrates.
Different lowercase letters (a, b, c) above bars indicate significant differences (α = 0.05, ANOVA, LSD test). BW, beeswax waste; CK, control; T, treatment. CK: 85% corncob, 12% wheat bran, 3% lime; T1: 85% corncob, 9% wheat bran, 3% BW, 3% lime; T2: 85% corncob, 7% wheat bran, 5% BW, 3% lime; T3: 85% corncob, 5% wheat bran, 7% BW, 3% lime; T4: 85% corncob, 3% wheat bran, 9% BW, 3% lime.
With increasing BW, the crude polysaccharide content increased and the crude protein content first increased and then decreased. There were no clear patterns in crude fat content. The addition of BW significantly increased the crude fiber content of the P. ostreatus mushrooms. Adding BW decreased ash content compared with the control, but the ash content increased with increasing BW addition.
Correlation analysis
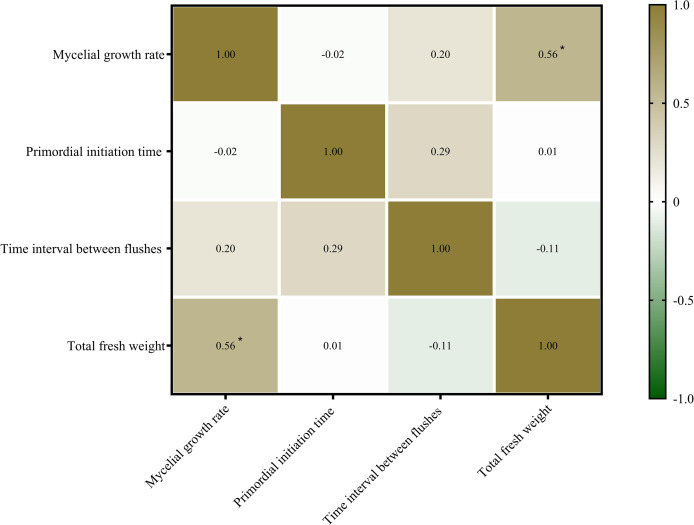
Mycelial growth and fruiting body formation are the two primary stages of edible fungus cultivation. The mycelial growth rate, primordium initiation time, and time interval between flushes are three important indicators of mycelial growth and development. In this study, the relationship between mycelial growth and fruiting body yield (fresh weight) of P. ostreatus was determined (Fig. 3). The mycelial growth rate was significantly positively correlated with the total fresh weight of P. ostreatus fruiting body (P < 0.05), while neither the primordium initiation time nor time interval between flushes were significantly correlated with the total yield of P. ostreatus fruiting body.
Figure 3. Correlations among agronomic characteristics and total fresh weight of Pleurotus ostreatus mushrooms.
Values are Pearson’s correlation coefficients. Green and brown indicate the degree of association. Brown, positive correlation. Green, negative correlation. *p < 0.05.
Discussion
This study aimed to explore the feasibility of substituting wheat bran with BW as a nitrogen source in P. ostreatus cultivation. The key findings indicate that BW addition at different weight ratios had different effects on the growth and development of P. ostreatus mycelia, enzyme activity, the total fresh weight and BE, and the nutrient content of P. ostreatus mushroom.
The cultivation substrate can influence P. ostreatus mushroom growth (Das et al., 2014; Sher, Al-Yemeni & Khan, 2011). In the current study, the number of days required for primordial initiation varied from 43.33 ± 1.15 d to 50.33 ± 1.53 d, which is within the range reported by Zakil et al. (2021). In their article, the lowest growth period (29 ± 3.00 d) for pin head formation of P. ostreatus was obtained in 100% rubber tree sawdust and a long duration (131 ± 20.07 d) for primordial development was recorded in 100% oil palm empty fruit bunch. Baysal et al. (2003) reported that P. ostreatus grown on waste paper took 2–3 weeks for fruit body formation after spawn running. Our results agree with their findings. The number of days between the first flush and the second flush in our study ranged from 11.67 ± 1.53 d to 13.33 ± 0.68 d, which was longer than the 6–10 d reported by Mandeel, Al-Laith & Mohamed (2005).
In our study, the mycelial growth rate was positively correlated with mushroom yield (total fresh weight). This result is similar to that of Pleurotus florida cultivated on corncob substrate (Naraian et al., 2008), but in contrast with P. ostreatus cultivated on different lignocellulosic by-products (Obodai, Cleland-Okine & Vowotor, 2003) and Lepista sordida cultivated on grain crop residues (Sheng et al., 2024), in these studies the mycelial growth rate did not correlate with yield or BE. This may be because the mycelial growth and development of P. ostreatus is not only related to the cultivation substrate but also to the genotype, cultivation conditions, and other factors (Shen et al., 2021; Zhang & Yang, 2017; Ejigu et al., 2022). In this study, laccase activity peaked at day 30 after inoculation, which corresponded with the time that it took the mycelium to fill the cultivation bag. This indicates that the mycelium was active at this time and accumulating energy for the initiation of primordia. Then, during primordial initiation, laccase activity was lower, likely because of the change in the physical structure of the mycelium during this time. For CMCase, the lowest activities were observed during primordial initiation, while activities peaked during the period of fruiting body maturity. These results agree with those of Shen et al. (2010). Interestingly, the activities of both enzymes were significantly higher in T2 than in the control at the points when enzyme activities were highest and lowest. T2 also resulted in a higher yield and BE, indicating that this treatment provided a good nutrient supply to mycelial cells, resulting in rapid growth of hyphae and high nutrient contents in the fruiting bodies.
Nitrogen is an important nutrient for the growth and development of edible mushrooms (Moonmoon et al., 2011). Different fungi strains respond differently to nitrogen sources. Within a certain range, increasing the nitrogen content can increase the yield of fruiting bodies (Wen et al., 2018). Our study found that with increasing BW supplementation, the total yield and BE of P. ostreatus tended to first increase and then decrease. This result is similar to those of Guo et al. (2024) and Hu et al. (2022), who reported an initial increase and then decrease in the yield of mushrooms as more nitrogen was added. A lack of nitrogen in the substrate can lead to an imbalance of nutrients in the fungus and thus is not conducive to high yields. According to nitrogen utilization by fungi, nitrogen sources can be divided into primary and secondary nitrogen sources (Marzluf, 1997). In this study, T2 had a significantly higher total yield than the control. However, the mushroom yields of the first and second flushes from T2 did not differ significantly from those of the control, while the third flush of mushrooms had significantly higher yields than the control. We speculated that wheat bran was used as a primary nitrogen source and BW was a secondary nitrogen source. This indicates that BW is a slow-release and long-acting source of nitrogen. Moreover, there was evidence that BW stimulated the secretion of extracellular laccase and CMCase by P. ostreatus mycelia, which likely accelerated the degradation of nutrients, such as cellulose and lignin in the substrate. This could explain the increased mycelial growth, mushroom yields, and accumulation of nutrients in mushrooms. The potential mechanism by which BW acts as a supplemental nitrogen source merits further study. In our study, the first mushroom flush produced approximately 50% of the total yield, while the second flush produced 30% of the total yield. This was consistent with the cultivation of P. ostreatus on Hypsizigus marmoreus mushroom substrates (Wang et al., 2015). The BE and yields of the first flush were consistently higher than those of the second flush across all treatments. These results are similar to those of a study in which Agrocybe cylindracea and P. ostreatus were cultivated on agricultural and forestry byproducts (Koutrotsios et al., 2014). In contrast, when Auricularia polytricha was cultivated on the sawdust waste of spent mushrooms (Wu, Liang & Liang, 2020), the second flush had higher yields and BE than the first flush.
The nutritional composition of mushrooms varies among different species and strains (Hoa, Wang & Wang, 2015), and is substantially affected by the environmental conditions and cultivation substrate (Xu et al., 2016; Gupta et al., 2013). In the current study, the substrate composition significantly affected the nutritional content of the mushrooms. The average crude protein content of P. ostreatus mushrooms in T2 was 23.47%, which was within the crude protein range reported in other articles (7.02% to 41.6%, Table 4). The average crude fat content in this study was 0.60%, which is low compared with values from other studies (0.50–5.45%). The ash concentration of oyster mushrooms ranges from 5.49% to 9.80%, while the value of P. ostreatus in this study was 6.88%. However, the average crude polysaccharide content of P. ostreatus in this study was 6.77%, which was higher than that of P. ostreatus mushrooms grown on a substrate containing common reeds (Phragmites australis). The average crude fiber content in this study was 5.27%, which is similar to the content reported by Ulziijargal & Mau (2011) (5.33%) but lower than that reported by Boadu et al. (2023) (9.09%). In summary, the current study demonstrates that mushrooms grown on BW are suitable for the health and nutritional needs of modern consumers.
Table 4. Nutritional content of Pleurotus ostreatus mushrooms from previous studies and this article.
| Crude protein (%) | Crude fat (%) | Ash (%) | Crude polysaccharide (%) | Crude fiber (%) | Reference |
|---|---|---|---|---|---|
| 19.08 | 1.60 | 5.49 | 5.00–6.00 | Li et al. (2023) | |
| 7.02 | 1.40 | 5.72 | Reis et al. (2012) | ||
| 23.85 | 2.16 | 7.59 | 5.33 | Ulziijargal & Mau (2011) | |
| 41.60 | 0.50 | 6.00 | Kirbağ & Akÿuz (2010) | ||
| 16.69 | 5.45 | 6.70 | Jaworska, Bernaś & Mickowska (2011) | ||
| 18.36 | 4.25 | 9.80 | 9.09 | Boadu et al. (2023) | |
| 23.47 | 0.60 | 6.88 | 6.77 | 5.27 | This article (T2) |
This article reports the first attempt at using BW for edible mushroom cultivation. We confirmed the feasibility and potential of BW for P. ostreatus mushroom cultivation. However, the experimental design has certain limitations, as it lacks a treatment group in which 100% of the wheat bran was replaced by BW, and therefore the results are not comprehensive. Despite this, the current study showed that the total yield was significantly reduced when the amount of BW was more than 7% (7% and 9% BW resulted in yields of 1,398.53 ± 7.50 and 1,346.81 ± 5.36 g bag−1, respectively). In future research, a complete replacement treatment group should be included to optimize the experimental design. In addition, the effects of BW on mushroom flavor, texture, and fruiting body morphology, as well as the mechanisms by which BW affects the growth and development of P. ostreatus, need further research.
Before BW can be widely used in edible mushroom cultivation, the following three issues need to be addressed. First, the safety and stability of BW needs to be ensured, and effective methods need to be developed to remove impurities and potentially harmful substances such as pesticide residues and heavy metals in BW. Second, the fermentation efficiency of BW and the quality of fermentation products need to be improved. The quality of fermentation materials plays an important role in the growth and development of mushrooms (Kong et al., 2020). In this study, secondary fermentation of BW was used to improve the quality of fermentation products using Aspergillus oryzae (Ruan, 2023). However, the BW fermentation process needs to be further optimized (such as fermentation time, temperature, humidity, ventilation, etc.) to improve the utilization rate and nutritional value of BW. Third, further research is needed on the adaptability of other edible mushroom varieties to BW fermentation products, as well as the feasibility and economic benefits of BW in mushroom production.
Conclusions
The effects of BW on the growth of mycelia, BE, yield, and nutritional value of P. ostreatus were evaluated. This study found that BW improved the BE, total yield, and nutritional value of P. ostreatus by affecting its accumulation of proteins and polysaccharides. Therefore, BW is a suitable nitrogen source for the cultivation of P. ostreatus. This provides a practical and sustainable use for BW, reducing the environmental impact of waste accumulation.
Supplemental Information
Acknowledgments
We wish to thank Wei Jiang and Haiyang Jiang from Raohe Northeast Black Bee Industry (Group) Co., Ltd. for their selfless assistance in providing the raw material of beeswax waste.
Funding Statement
This work was supported by the China Agriculture Research System (No. CARS44). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Chunlei Pan conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Chunge Sheng conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Kang Wang analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Yi Zhang analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Chunguang Liu performed the experiments, prepared figures and/or tables, and approved the final draft.
Zhihao Zhang performed the experiments, prepared figures and/or tables, and approved the final draft.
Liang Tao analyzed the data, prepared figures and/or tables, and approved the final draft.
Yang Lv performed the experiments, prepared figures and/or tables, and approved the final draft.
Fuchao Gao conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.
References
- Abou Fayssal et al. (2021).Abou Fayssal S, El Sebaaly Z, Alsanad MA, Najjar R, Bohme M, Yordanova MH, Sassine YN. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLOS ONE. 2021;16(9):e0255794.0255794. doi: 10.1371/journal.pone.0255794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal et al. (2003).Baysal E, Peker H, Yalinkilic MK, Temiz A. Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresource Technology. 2003;89:95–97. doi: 10.1016/S0960-8524(03)00028-2. [DOI] [PubMed] [Google Scholar]
- Boadu et al. (2023).Boadu KB, Nsiah-Asante R, Antwi RT, Obirikorang KA, Anokye R, Ansong M. Influence of the chemical content of sawdust on the levels of important macronutrients and ash composition in Pearl oyster mushroom (Pleurotus ostreatus) PLOS ONE. 2023;18(6):e0287532. doi: 10.1371/journal.pone.0287532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen (1979).Chen YH. Utilization of beeswax waste. Chinese Beekeeping. 1979;3:26. [Google Scholar]
- Chen et al. (2012).Chen LH, Zhang FX, Wu J, Siriwat W, Romanee S. Enlightenment of European apiculture development status to China. Journal of Agricultural Science and Technology. 2012;14(3):16–21. doi: 10.3969/j.issn.1008-0864.2012.03.03. [DOI] [Google Scholar]
- China Agricultural Press (2023).China Agricultural Press . Determination of crude polysaccharides in edible mushrooms by spectrophotometry. Beijing, China: China Agriculture Press; 2023. [Google Scholar]
- Das et al. (2014).Das D, Kadiruzzaman M, Adhikary S, Kabir M, Akhtaruzzaman M. Yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates. Bangladesh Journal of Agricultural Research. 2014;38(4):613–623. doi: 10.3329/bjar.v38i4.18946. [DOI] [Google Scholar]
- Dundar, Acay & Yildiz (2009).Dundar A, Acay H, Yildiz A. Effect of using different lignocellulosic wastes for cultivation of Pleurotus ostreatus (Jacq.) P. Kumm. on mushroom yield, chemical composition and nutritional value. African Journal of Biotechnology. 2009;8(4):662–666. [Google Scholar]
- Ejigu et al. (2022).Ejigu N, Sitotaw B, Girmay S, Assaye H. Evaluation of oyster mushroom (Pleurotus ostreatus) production using water hyacinth (Eichhornia crassipes) biomass supplemented with agricultural wastes. International Journal of Food Science. 2022;2022:9289043. doi: 10.1155/20C22/9289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisa et al. (2017).Elisa W, Silvia A, Özge T, Ulrike Z, Daniel K, Karl S, Wolfgang K. Wheat bran biodegradation by edible Pleurotus fungi-A sustainable perspective for food and feed. LWT-Food Science and Technology. 2017;86(43):123–131. doi: 10.1016/j.lwt.2017.07.051. [DOI] [Google Scholar]
- Elisashvili et al. (2008).Elisashvili V, Penninckx M, Kachlishvili E, Tsiklauri N, Metreveli E, Kharziani T, Kvesitadze G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresource Technology. 2008;99(3):457–462. doi: 10.1016/j.biortech.2007.01.011. [DOI] [PubMed] [Google Scholar]
- GB 5009.4 (2016).GB 5009.4 . National food safety standard—determination of ash content in food. Beijing, China: Standards Press of China; 2016. [Google Scholar]
- GB 5009.5 (2016).GB 5009.5 . National food safety standard—determination of protein in food. Beijing, China: Standards Press of China; 2016. [Google Scholar]
- GB 5009.6 (2016).GB 5009.6 . National food safety standard—determination of fat in food. Beijing, China: China Agriculture Press; 2016. [Google Scholar]
- GB/T 5009.10 (2003).GB/T 5009.10 . Determination of crude fiber in plant-based foods. Beijing, China: Standards Press of China; 2003. [Google Scholar]
- Grimm & Wösten (2018).Grimm D, Wösten HAB. Mushroom cultivation in the circular economy. Applied Microbiology and Biotechnology. 2018;102(18):7795–7803. doi: 10.1007/s00253-018-9226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2024).Guo HZ, Lu XX, Liu YD, Jin RX, Zou M. Effects of different bran content on the growth, quality and texture of Pleurotus ostreatus. North Horticulture. 2024;2:109–118. doi: 10.11937/bfyy.20232119. [DOI] [Google Scholar]
- Gupta et al. (2013).Gupta A, Sharma S, Saha S, Walia S. Yield and nutritional content of Pleurotus sajor caju on wheat straw supplemented with raw and detoxified mahua cake. Food Chemistry. 2013;141(4):4231–4239. doi: 10.1016/j.foodchem.2013.06.126. [DOI] [PubMed] [Google Scholar]
- Han et al. (2021).Han ML, Yang J, Liu ZY, Wang CR, Chen SY, Han N, Hao WY, An Q, Dai YC. Evaluation of laccase activities by three newly isolated fungal species in submerged fermentation with single or mixed lignocellulosic wastes. Frontiers in Microbiology. 2021;12:682679. doi: 10.3389/fmicb.2021.682679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa, Wang & Wang (2015).Hoa HT, Wang CL, Wang CH. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus) Mycobiology. 2015;43(4):423–434. doi: 10.5941/myco.2015.43.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2022).Hu YR, Ni ZY, Zhang JX, Yu HY, Qi YC, Shen JW, Wen Q. Effects of nitrogen content and C: N Ratio on growth and nutritional components of Pleurotus ostreatus. Acta Edulis Fungi. 2022;29(1):36–40. doi: 10.16488/j.cnki.1005-9873.2022.01.005. [DOI] [Google Scholar]
- Jaworska, Bernaś & Mickowska (2011).Jaworska G, Bernaś E, Mickowska B. Effect of production process on the amino acid content of frozen and canned Pleurotus ostreatus mushrooms. Food Chemistry. 2011;125(3):936–943. doi: 10.1016/j.foodchem.2010.09.084. [DOI] [Google Scholar]
- Kirbağ & Akÿuz (2010).Kirbağ S, Akÿuz M. Nutritive value of wild edible and cultured mushrooms. Turkish Journal of Biology. 2010;34:97–102. doi: 10.3906/biy-0805-17. [DOI] [Google Scholar]
- Kong et al. (2020).Kong WL, Sun B, Zhang JY, Zhang YT, Gu LK, Bao LJ, Liu SX. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus. Bioresource Technology. 2020;306(5):123156. doi: 10.1016/j.biortech.2020.123156. [DOI] [PubMed] [Google Scholar]
- Koutrotsios et al. (2014).Koutrotsios G, Mountzouris KC, Chatzipavlidis I, Zervakis GI. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—assessment of their effect on the final product and spent substrate properties. Food Chemistry. 2014;161(1):127–135. doi: 10.1016/j.foodchem.2014.03.121. [DOI] [PubMed] [Google Scholar]
- Kuforiji & Fasidi (2009).Kuforiji OO, Fasidi IO. Influence of light and spawn quantity on the growth of nigerian mushroom Pleurotus tuber-regium. Journal of Environmental Biology. 2009;30(4):605–608. [PubMed] [Google Scholar]
- Li (2023).Li TC. More than 1.34 million bee colonies estimated in Heilongjiang province in 2023. 2023. https://www.moa.gov.cn/xw/qg/202303/t20230314_6422974.htm. [10 March 2024]. https://www.moa.gov.cn/xw/qg/202303/t20230314_6422974.htm
- Li et al. (2023).Li XY, Chen GS, Li XJ, Yao FJ. Three Pleurotus mushroom species cultivated in a mixed Phragmites australis substrate differ in nutrient utilization capacity. Journal of Food Composition and Analysis. 2023;115(4):104924. doi: 10.1016/j.jfca.2022.104924. [DOI] [Google Scholar]
- Mahari et al. (2020).Mahari WAW, Peng WX, Nam WL, Yang H, Lee XY, Lee YK, Liew RK, Ma NL, Mohammad A, Sonne C, Van Le Q, Show PL, Chen WH, Lam SS. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. Journal of Hazardous Materials. 2020;400:123156. doi: 10.1016/j.jhazmat.2020.123156. [DOI] [PubMed] [Google Scholar]
- Mandeel, Al-Laith & Mohamed (2005).Mandeel QA, Al-Laith AA, Mohamed SA. Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World Journal of Microbiology & Biotechnology. 2005;21(4):601–607. doi: 10.1007/s11274-004-3494-4. [DOI] [Google Scholar]
- Marzluf (1997).Marzluf GA. Genetic regulation of nitrogen metabolism in the fungi. Microbiology and Molecular Biology Reviews. 1997;61(1):17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonmoon et al. (2011).Moonmoon M, Shelly NJ, Khan Md A, Uddin Md N, Hossain K, Tania M, Ahmed S. Effects of different levels of wheat bran, rice bran and maize powder supplementation with sawdust on the production of shiitake mushroom (Lentinus edodes (Berk.) Singer) Saudi Journal of Biological Sciences. 2011;18(4):323–328. doi: 10.1016/j.sjbs.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi, Pourghasemian & Naghizadeh (2019).Moradi R, Pourghasemian N, Naghizadeh M. Effect of beeswax waste biochar on growth, physiology and cadmium uptake in saffron. Journal of Cleaner Production. 2019;229(2):1251–1261. doi: 10.1016/j.jclepro.2019.05.047. [DOI] [Google Scholar]
- Nam et al. (2018).Nam WL, Phang XY, Su MH, Liew RK, Ma NL, Rosli M, Lam SS. Production of bio-fertilizer from microwave vacuum pyrolysis of palm kernel shell for cultivation of oyster mushroom (Pleurotus ostreatus) Science of the Total Environment. 2018;624(2):9–16. doi: 10.1016/j.scitotenv.2017.12.108. [DOI] [PubMed] [Google Scholar]
- Naraian, Narayan & Srivastava (2014).Naraian R, Narayan OP, Srivastava J. Differential response of oyster shell powder on enzyme profile and nutritional value of oyster mushroom Pleurotus florida PF05. BioMed Research International. 2014;2014:386265. doi: 10.1155/2014/386265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraian et al. (2008).Naraian R, Sahu RK, Kumar S, Garg SK, Singh CS, Kanaujia RS. Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corncob substrate. The Environmentalist. 2008;29:1–7. doi: 10.1007/s10669-008-9174-4. [DOI] [Google Scholar]
- National Bureau of Statistics (2024).National Bureau of Statistics Honey output of China. 2024. https://data.stats.gov.cn/search.htm?s=%E6%88%91%E5%9B%BD%E8%9C%82%E7%BE%A4. [12 March 2024]. https://data.stats.gov.cn/search.htm?s=%E6%88%91%E5%9B%BD%E8%9C%82%E7%BE%A4
- Obodai, Cleland-Okine & Vowotor (2003).Obodai M, Cleland-Okine J, Vowotor KA. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. Journal of Industrial Microbiology & Biotechnology. 2003;30(3):146–149. doi: 10.1007/s10295-002-0021-1. [DOI] [PubMed] [Google Scholar]
- Pourghasemian, Moradi & Iriti (2023).Pourghasemian N, Moradi R, Iriti M. Assessing anti-transpiration potential of beeswax waste on Calendula officinalis under drought stress conditions. Scientia Horticulturae. 2023;315(17):111987. doi: 10.1016/j.scienta.2023.111987. [DOI] [Google Scholar]
- Reis et al. (2012).Reis FS, Barros L, Martins A, Ferreira ICFR. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food and Chemical Toxicology. 2012;50:191–197. doi: 10.1016/j.fct.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Ruan (2023).Ruan LC. Analysis of extracellular enzyme secretion mechanism of Aspergillus oryzae and its regulatory application. D. Phil. Thesis. Tianjin University of Science and Technology. 2023.
- Santa-Rose et al. (2018).Santa-Rose PS, Souza AL, Roque RA, Andrade EV, Astolfi-Filho S, Mota AJ, Nunes-Silve CG. Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology. 2018;31(1):84–92. doi: 10.1016/j.ejbt.2017.11.005. [DOI] [Google Scholar]
- Shen et al. (2010).Shen JW, Wu QT, Zhuang QL, Gao YQ, Qi YC, Qiu LY, Ma BJ. Extracellular enzyme production by three Pleurotus ostreatus strains at different stages of the cultivation cycle. Acta Edulis Fungi. 2010;17(2):56–59. [Google Scholar]
- Shen et al. (2021).Shen M, Yuan Y, Song XY, Wang BJ, Zheng QP, Wang RS, Zhang M, Yao XT. Analysis of nutrient composition of 6 kinds of forest and fruit trees sawdust and its effect on the growth of edible fungi. Acta Agriculturae Shanghai. 2021;37(4):74–80. doi: 10.15955/j.issn1000-3924.2021.04.14. [DOI] [Google Scholar]
- Sheng et al. (2024).Sheng CG, Wang YF, Pan CL, Shi L, Wang YH, Ma YP, Wang JH, Zhao J, Zhang P, Liu ZT, Yu HY, Wang F, Dong XM, Yan SH. Evaluation of rice straw, corncob, and soybean straw as substrates for the cultivation of Lepista sordida. Life. 2024;14(1):101. doi: 10.3390/life14010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher, Al-Yemeni & Khan (2011).Sher H, Al-Yemeni M, Khan K. Cultivation of the oyster mushroom (Pleurotus ostreatus (jacq.) p. kumm.) in two different agroecological zones of Pakistan. African Journal of Biotechnology. 2011;10(2):183–188. [Google Scholar]
- Ulziijargal & Mau (2011).Ulziijargal E, Mau JL. Nutrient composition of culinary-medicinal mushroom fruiting bodies and mycelia. International Journal of Medicinal Mushrooms. 2011;13(4):343–349. doi: 10.1615/IntJMedMushr.v13.i4.40. [DOI] [PubMed] [Google Scholar]
- Wang (2023).Wang RB. Report of bee products in Heilongjiang province. China Apiculture. 2023;74(11):50–51. [Google Scholar]
- Wang et al. (2015).Wang SX, Xu F, Li ZM, Zhao S, Song S, Rong CB, Geng XL, Liu Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Scientia Horticulturae. 2015;186:217–222. doi: 10.1016/j.scienta.2015.02.028. [DOI] [Google Scholar]
- Wen et al. (2018).Wen Q, Zou M, Jin G, Guo DD, Wei ZF, Shen JW. Cloning and prokaryotic expression analysis of urease gene Pourease from Pleurotus ostreatus. Mycosystema. 2018;37(11):1498–1506. doi: 10.13346/j.mycosystema.180172. [DOI] [Google Scholar]
- Wu, Liang & Liang (2020).Wu CY, Liang CH, Liang ZC. Evaluation of using spent mushroom sawdust wastes for cultivation of Auricularia polytricha. Agronomy. 2020;10:1892. doi: 10.3390/agronomy10121892. [DOI] [Google Scholar]
- Xu et al. (2016).Xu F, Li ZM, Liu Y, Rong CB, Wang SX. Evaluation of edible mushroom Oudemansiella canarii cultivation on different lignocellulosic substrates. Saudi Journal of Biological Sciences. 2016;23:607–613. doi: 10.1016/j.sjbs.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang (2019).Yang J. Analysis on the potential of edible Fungi industry for poverty alleviation based on diamond model. Chinese Journal of Agricultural Resources and Regional Planning. 2019;40(4):34–39. doi: 10.7621/cjarrp.1005-9121.20190406. [DOI] [Google Scholar]
- Yang, Guo & Wan (2013).Yang WJ, Guo FL, Wan ZJ. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi Journal of Biological Sciences. 2013;20(4):333–338. doi: 10.1016/j.sjbs.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakil et al. (2021).Zakil FA, Xuan LH, Zaman N, Alan NI, Salahutheen NAA, Sueb MSM, Isha R. Growth performance and mineral analysis of Pleurotus ostreatus from various agricultural wastes mixed with rubber tree sawdust in Malaysia. Bioresource Technology Reports. 2021;17(2022):e100873. doi: 10.1016/j.biteb.2021.100873. [DOI] [Google Scholar]
- Zhang & Yang (2017).Zhang YF, Yang XB. Comparison of growth of Grifola frondsa mycelium on substrate with different ratios of carbon to nitrogen. North Horticulture. 2017;3:151–155. doi: 10.11937/bfyy.201703035. [DOI] [Google Scholar]
- Zhao & Zhao (1991).Zhao XC, Zhao SJ. Animal fuel-beeswax waste. Apiculturcal Sciences and Technology. 1991;3:36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.