Abstract
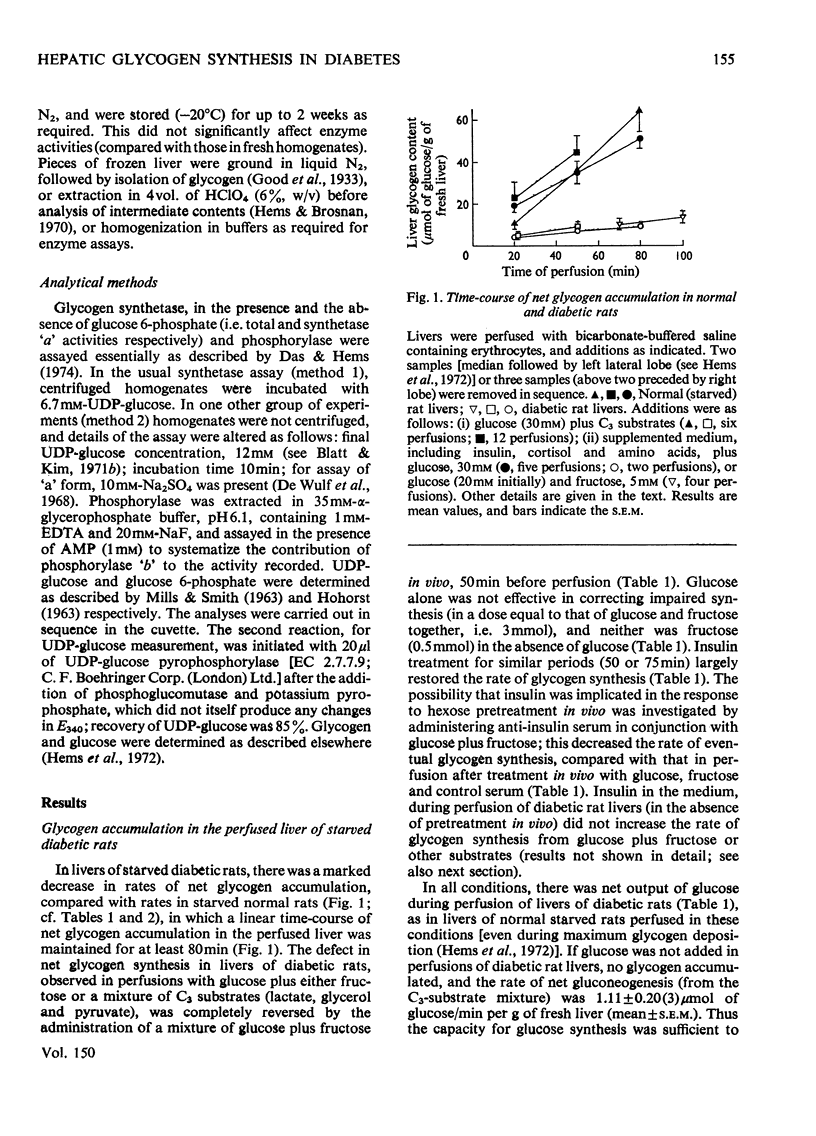
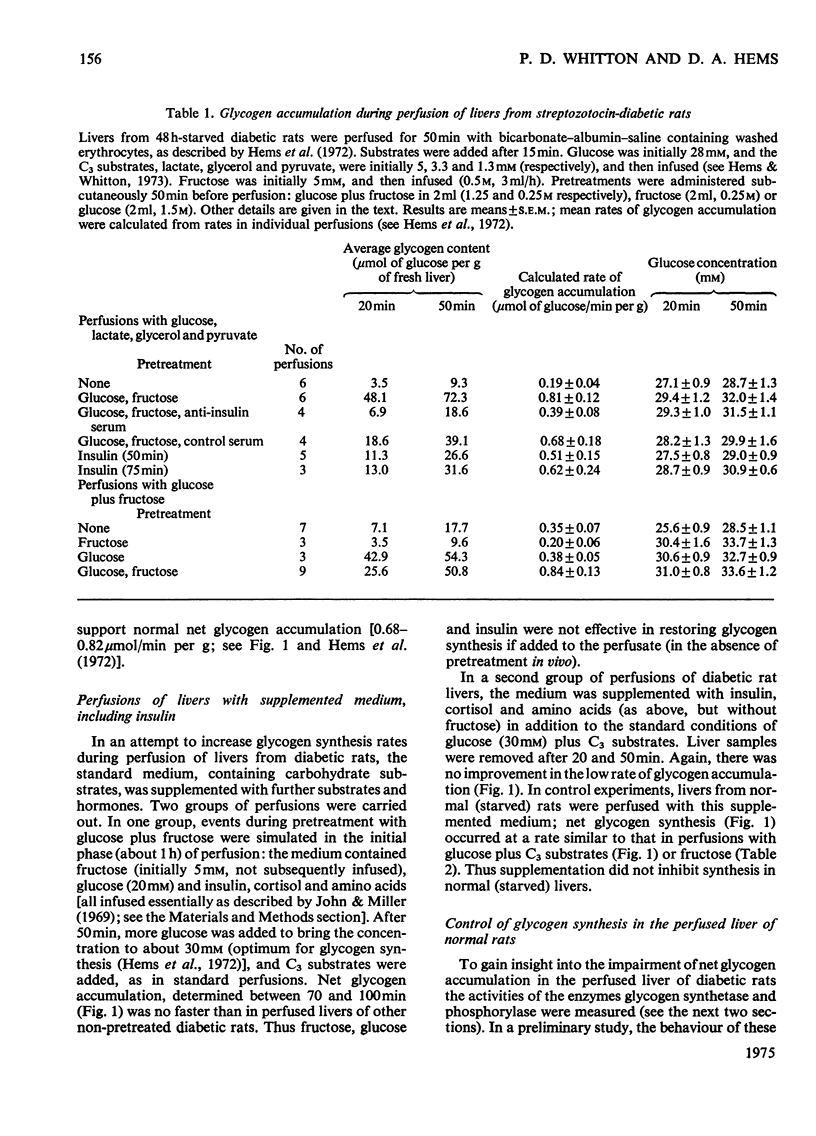
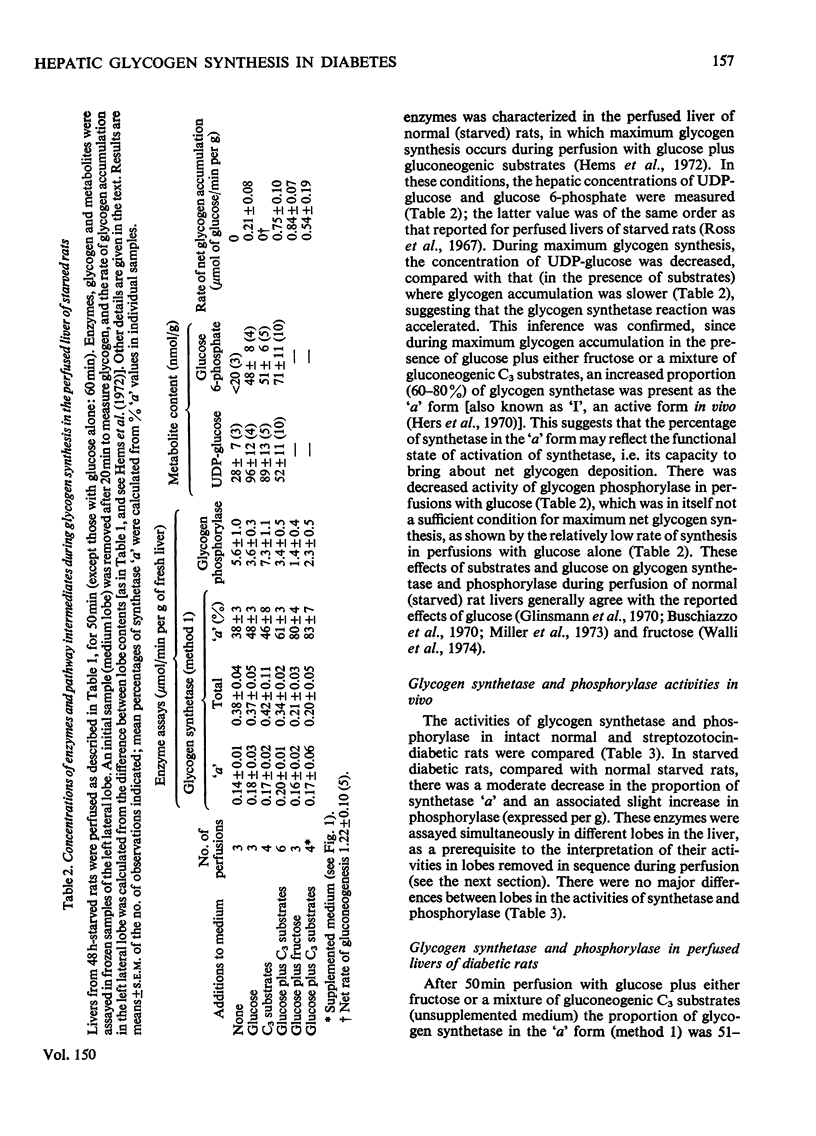
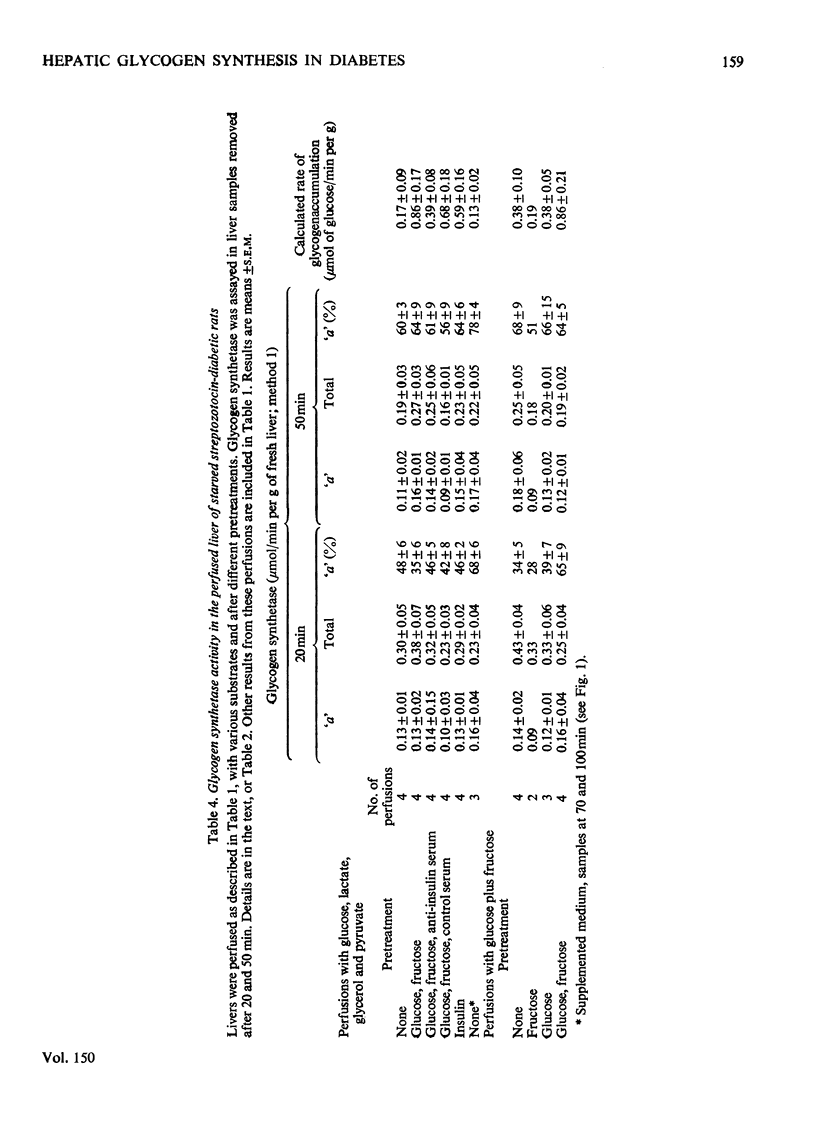
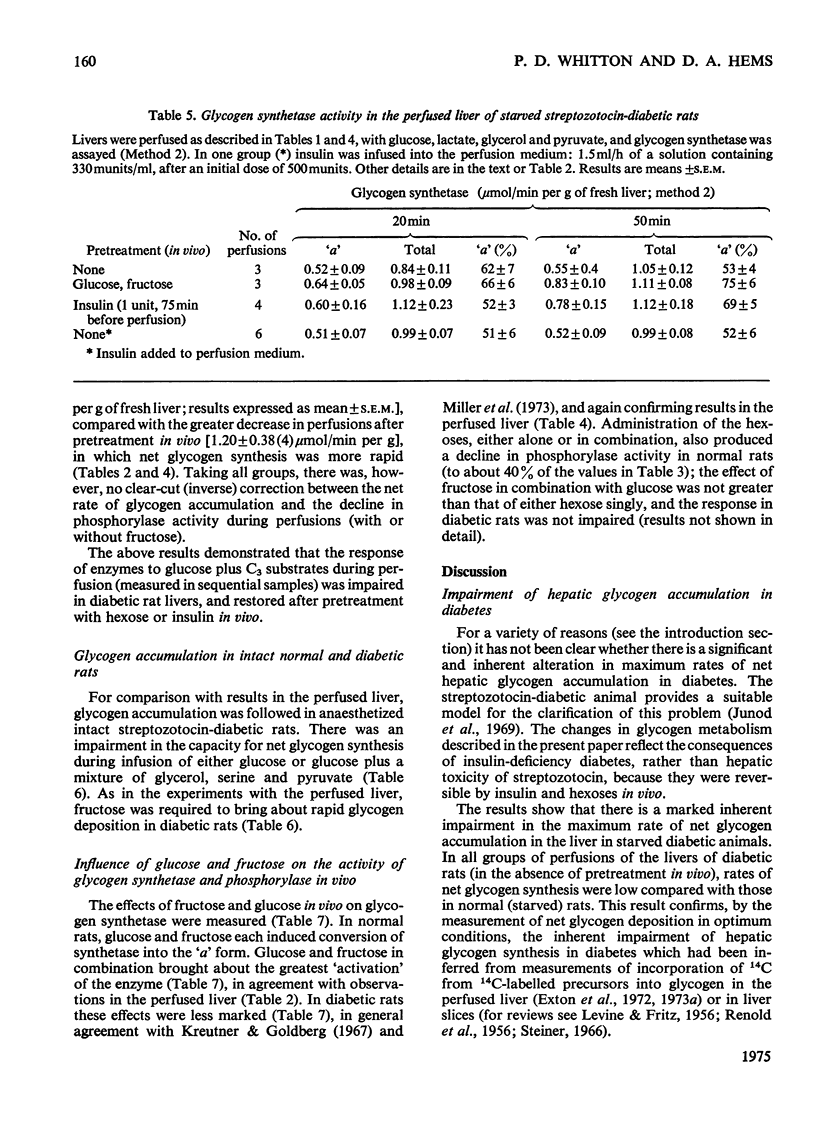
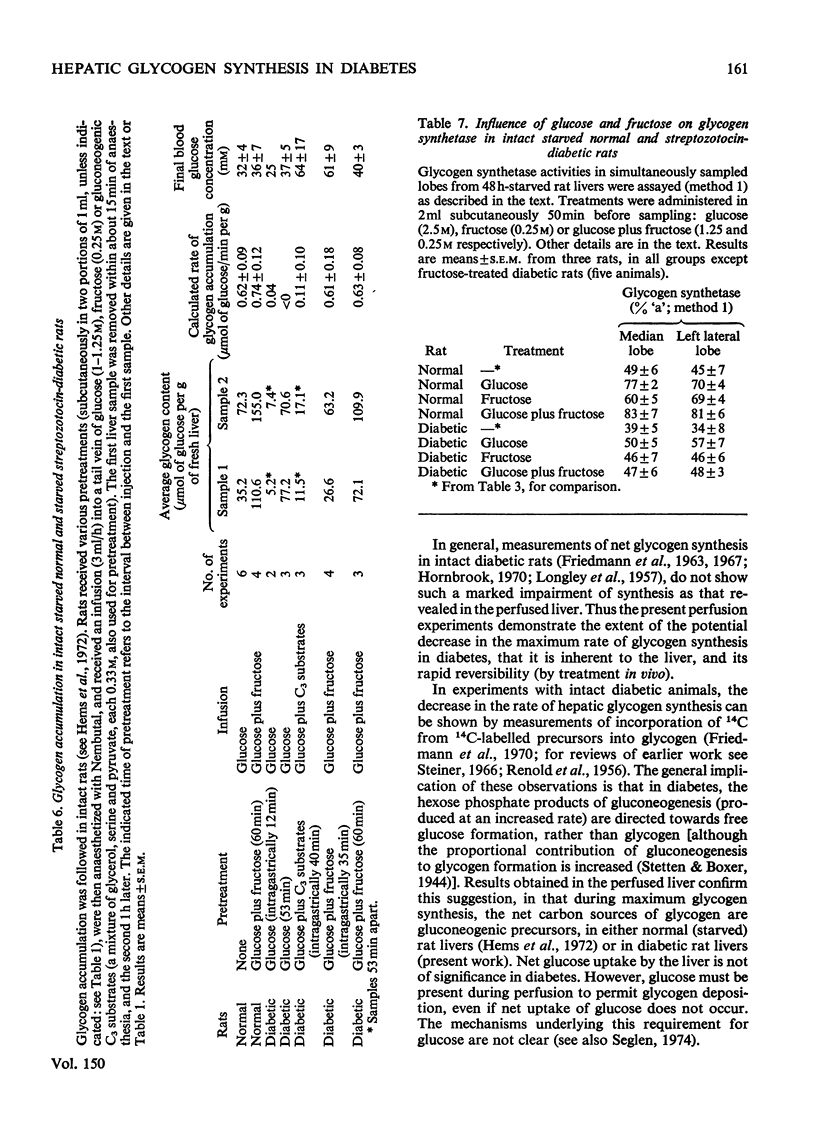
1. Net glycogen accumulation was measured in sequentially removed samples during perfusion of the liver of starved streptozotocin-diabetic rats, and shown to be significantly impaired, compared with rates in normal (starved) rats. 2. In perfusions of normal livers with glucose plus C3 substrates, there was an increase in the proportion of glycogen synthetase 'a', compared with that in the absence of substrates. This response to substrates, followed in sequential synthesis and enzymic sensitivity in the perfused liver of diabetic rats were reversed by pretreatment in vivo with glucose plus fructose, or insulin. Glucose alone did not produce this effect. 4. Glucose, fructose, insulin or cortisol added to e perfusion medium (in the absence of pretreatment in vivo) did not stimulate glycogen synthesis in diabetic rats. 5. In intact diabetic rats, there was a decline in rates of net hepatic glycogen accumulation, and the response of glycogen synthetase to substrates. The most rapid rates of synthesis were obtained after fructose administration. 6. These results demonstrate that there is a marked inherent impairment in hepatic glycogen synthesis in starved diabetic rats, which can be rapidly reversed in vivo but no in perfusion. Thus hepatic glycogen synthesis does not appear to be sensitive to either the short-term direct action of insulin (added alone to perfusions) of to long-term insulin deprivation in vivo. The regulatory roles of substrates, insulin and glycogen synthetase in hepatic glycogen accumulation are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akpan J. O., Gardner R., Wagle S. R. Studies on the effects of insulin and acetylcholine on activation of glycogen synthase and on glycogenesis in hepatocytes. Biochem Biophys Res Commun. 1974 Nov 6;61(1):222–229. doi: 10.1016/0006-291x(74)90556-7. [DOI] [PubMed] [Google Scholar]
- BAKER N., CHAIKOFF I. L., SCHUSDEK A. Effect of fructose on lipogenesis from lactate and acetate in diabetic liver. J Biol Chem. 1952 Jan;194(1):435–443. [PubMed] [Google Scholar]
- BORTNICK R. J., LONGLEY R. W., ROE J. H. Rate of glycogenesis in liver of depancreatized rat after parenteral administration of glucose and fructose. Proc Soc Exp Biol Med. 1957 Jan;94(1):108–110. doi: 10.3181/00379727-94-22869. [DOI] [PubMed] [Google Scholar]
- Bishop J. S., Goldberg N. D., Larner J. Insulin regulation of hepatic glycogen metabolism in the dog. Am J Physiol. 1971 Feb;220(2):499–506. doi: 10.1152/ajplegacy.1971.220.2.499. [DOI] [PubMed] [Google Scholar]
- Bishop J. S. Inability of insulin to activate liver glycogen transferase D phosphatase in the diabetic pancreatectomized dog. Biochim Biophys Acta. 1970 May 12;208(2):208–218. doi: 10.1016/0304-4165(70)90239-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. S., Larner J. Rapid activation-inactivation of liver uridine diphosphate glucose-glycogen transferase and phosphorylase by insulin and glucagon in vivo. J Biol Chem. 1967 Mar 25;242(6):1354–1356. [PubMed] [Google Scholar]
- Blatt L. M., Kim K. H. Regulation of hepatic glycogen synthetase. Stimulation of glycogen synthetase in an in vitro liver system by insulin bound to sepharose. J Biol Chem. 1971 Aug 25;246(16):4895–4898. [PubMed] [Google Scholar]
- Blatt L. M., Kim K. H. Regulation of rat liver glycogen synthetase. Relationship of the hormonal activation and the time-dependent in vitro activation. J Biol Chem. 1971 Dec 10;246(23):7256–7264. [PubMed] [Google Scholar]
- Buschiazzo H., Exton J. H., Park C. R. Effects of glucose on glycogen synthetase, phosphorylase, and glycogen deposition in the perfused rat liver. Proc Natl Acad Sci U S A. 1970 Feb;65(2):383–387. doi: 10.1073/pnas.65.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNICK S. S., CHAIKOFF I. L. Two blocks in carbohydrate utilization in the liver of the diabetic rat. J Biol Chem. 1951 Jan;188(1):389–396. [PubMed] [Google Scholar]
- Curry D. L., Curry K. P., Gomez M. Fructose potentiation of insulin secretion. Endocrinology. 1972 Dec;91(6):1493–1498. doi: 10.1210/endo-91-6-1493. [DOI] [PubMed] [Google Scholar]
- Das I., Hems D. A. Glycogen synthetase and phosphorylase activities in different tissues of genetically obese mice. Horm Metab Res. 1974 Jan;6(1):40–44. doi: 10.1055/s-0028-1093901. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Harper S. C. Control of gluconeogenesis in liver. V. Effects of fasting, diabetes, and glucagon on lactate and endogenous metabolism in the perfused rat liver. J Biol Chem. 1972 Aug 25;247(16):4996–5003. [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Harper S. C., Tucker A. L., Flagg J. L., Park C. R. Effects of adrenalectomy and glucocorticoid replacement on gluconeogenesis in perfused livers from diabetic rats. Biochim Biophys Acta. 1973 Nov 2;329(1):41–57. doi: 10.1016/0304-4165(73)90006-8. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Harper S. C., Tucker A. L., Ho R. J. Effects of insulin on gluconeogenesis and cyclic AMP levels in perfused livers from diabetic rats. Biochim Biophys Acta. 1973 Nov 2;329(1):23–40. doi: 10.1016/0304-4165(73)90005-6. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN B., GOODMAN E. H., Jr, WEINHOUSE S. LIVER GLYCOGEN SYNTHESIS IN INTACT ALLOXAN DIABETIC RATS. J Biol Chem. 1963 Sep;238:2899–2905. [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Dietary and hormonal effects on gluconeogenesis and glycogenesis from pyrute-3-14C, fructose-U-14C and glycerol-2-14C in the rat. Endocrinology. 1970 Jun;86(6):1264–1271. doi: 10.1210/endo-86-6-1264. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Effects of glucose feeding, cortisol, and insulin on liver glycogen synthesis in the rat. Endocrinology. 1967 Sep;81(3):486–496. doi: 10.1210/endo-81-3-486. [DOI] [PubMed] [Google Scholar]
- Glinsmann W., Pauk G., Hern E. Control of rat liver glycogen synthetase and phosphorylase activities by glucose. Biochem Biophys Res Commun. 1970 May 22;39(4):774–782. doi: 10.1016/0006-291x(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Gold A. H. The effect of diabetes and insulin on liver glycogen synthetase activation. J Biol Chem. 1970 Feb 25;245(4):903–905. [PubMed] [Google Scholar]
- Haft D. E. Studies of the metabolism of isolated livers of normal and alloxan-diabetic rats perfused with insulin. Diabetes. 1968 May;17(5):244–250. doi: 10.2337/diab.17.5.244. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., De Wulf H., Stalmans W., van den Berghe G. The control of glycogen synthesis in the liver. Adv Enzyme Regul. 1970;8:171–190. doi: 10.1016/0065-2571(70)90016-6. [DOI] [PubMed] [Google Scholar]
- Hoftiezer V., Carpenter A. M. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973 Jun;9(3):178–184. doi: 10.1007/BF01219780. [DOI] [PubMed] [Google Scholar]
- Hornbrook K. R. Synthesis of liver glycogen in starved alloxan diabetic rats. Diabetes. 1970 Dec;19(12):916–923. doi: 10.2337/diab.19.12.916. [DOI] [PubMed] [Google Scholar]
- Höstmark A. T. The effect of insulin on epinephrine and glucagon inactivated glycogen synthetase I in the isolated perfused rat liver. Acta Physiol Scand. 1973 Jun;88(2):248–255. doi: 10.1111/j.1748-1716.1973.tb05451.x. [DOI] [PubMed] [Google Scholar]
- John D. W., Miller L. L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969 Nov 25;244(22):6134–6142. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutner W., Goldberg N. D. Dependence on insulin of the apparent hydrocortisone activation of hepatic glycogen synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1515–1519. doi: 10.1073/pnas.58.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R., FRITZ I. B. The relation of insulin to liver metabolism. Diabetes. 1956 May-Jun;5(3):209-19; discussion, 219-22. doi: 10.2337/diab.5.3.209. [DOI] [PubMed] [Google Scholar]
- MILLER M., DRUCKER W. R., OWENS J. E., CRAIG J. W., WOODWARD H., Jr Metabolism of intravenous fructose and glucose in normal and diabetic subjects. J Clin Invest. 1952 Jan;31(1):115–125. doi: 10.1172/JCI102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansford K. R. Influence of anti-insulin serum on glucose metabolism. II. In the perfused heart. Diabetes. 1967 Jul;16(7):475–477. doi: 10.2337/diab.16.7.475. [DOI] [PubMed] [Google Scholar]
- Miller T. B., Jr, Hazen R., Larner J. An absolute requirement for insulin in the control of hepatic glycogenesis by glucose. Biochem Biophys Res Commun. 1973 Jul 17;53(2):466–474. doi: 10.1016/0006-291x(73)90685-2. [DOI] [PubMed] [Google Scholar]
- Miller T. B., Jr, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973 May 25;248(10):3483–3488. [PubMed] [Google Scholar]
- Mondon C. E., Burton S. D. Factors modifying carbohydrate metabolism and effect of insulin in perfused rat liver. Am J Physiol. 1971 Mar;220(3):724–734. doi: 10.1152/ajplegacy.1971.220.3.724. [DOI] [PubMed] [Google Scholar]
- Nichols W. K., Goldberg N. D. The relationship between insulin and apparent glucocorticoid-promoted activation of hepatic glycogen synthetase. Biochim Biophys Acta. 1972 Sep 15;279(2):245–259. doi: 10.1016/0304-4165(72)90140-7. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- RENOLD A. E., ASHMORE J., HASTINGS A. B. Regulation of carbohydrate metabolism in isolated tissues. Vitam Horm. 1956;14:139–185. doi: 10.1016/s0083-6729(08)60300-3. [DOI] [PubMed] [Google Scholar]
- RENOLD A. E., HASTINGS A. B., NESBETT F. B., ASHMORE J. Studies on carbohydrate metabolism in rat liver slices. IV. Biochemical sequence of events after insulin administration. J Biol Chem. 1955 Mar;213(1):135–146. [PubMed] [Google Scholar]
- RENOLD A. E., TENG C. T., NESBETT F. B., HASTING A. B. Studies on carbohydrate metabolism in rat liver slices. II. The effect of fasting and of hormonal deficiencies. J Biol Chem. 1953 Oct;204(2):533–546. [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman B. E., Whelan W. J. New aspects of glycogen metabolism. Adv Enzymol Relat Areas Mol Biol. 1971;34:285–443. doi: 10.1002/9780470122792.ch6. [DOI] [PubMed] [Google Scholar]
- STEINER D. F. MECHANISMS OF REGULATION OF HEPATIC GLYCOGEN SYNTHESIS. Nature. 1964 Dec 19;204:1171–1173. doi: 10.1038/2041171a0. [DOI] [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Effect of diabetes on the concentration of amino acids in plasma and heart muscle of rats. Biochem J. 1966 Apr;99(1):173–178. doi: 10.1042/bj0990173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F. Insulin and the regulation of hepatic biosynthetic activity. Vitam Horm. 1966;24:1–61. doi: 10.1016/s0083-6729(08)60202-2. [DOI] [PubMed] [Google Scholar]
- Thurston J. H., Jones E. M., Hauhart R. E. Decrease and inhibition of liver glycogen phosphorylase after fructose. An experimental model for the study of hereditary fructose intolerance. Diabetes. 1974 Jul;23(7):597–604. doi: 10.2337/diab.23.7.597. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Glucagon and diabetes mellitus. Adv Metab Disord. 1972;6:73–98. doi: 10.1016/b978-0-12-027306-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G., Hue L., Hers H. G. Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J. 1973 Jun;134(2):637–645. doi: 10.1042/bj1340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walli A. K., Siebler G., Zepf E., Schimassek H. Glycogen metabolism in isolated perfused rat liver. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):353–362. doi: 10.1515/bchm2.1974.355.1.353. [DOI] [PubMed] [Google Scholar]
- de Wulf H., Hers H. G. The influence of inorganic phosphate, adenosine triphosphate and glucose 6-phosphate on the activity of liver glycogen synthetase. Eur J Biochem. 1968 Dec 5;6(4):545–551. doi: 10.1111/j.1432-1033.1968.tb00479.x. [DOI] [PubMed] [Google Scholar]


