Abstract
Neuronal oscillations refer to rhythmic and periodic fluctuations of electrical activity in the central nervous system that arise from the cellular properties of diverse neuronal populations and their interactions. Specifically, gamma oscillations play a crucial role in governing the connectivity between distinct brain regions, which are essential in perception, motor control, memory, and emotions. In this context, we recapitulate various current stimulation methods to induce gamma entrainment. These methods include sensory stimulation, optogenetic modulation, photobiomodulation, and transcranial electrical or magnetic stimulation. Simultaneously, we explore the association between abnormal gamma oscillations and central nervous system disorders such as Alzheimer’s disease, Parkinson’s disease, stroke, schizophrenia, and autism spectrum disorders. Evidence suggests that gamma entrainment-inducing stimulation methods offer notable neuroprotection, although somewhat controversial. This review comprehensively discusses the functional role of gamma oscillations in higher-order brain activities from both physiological and pathological perspectives, emphasizing gamma entrainment as a potential therapeutic approach for neuropsychiatric disorders. Additionally, we discuss future opportunities and challenges in implementing such strategies.
Keywords: Gamma entrainment, γ oscillations, Brain stimulation, Memory, Neurological function, Alzheimer’s disease
Introduction
Brain oscillations refer to rhythmic brain activity [1]. Endogenous brain oscillations occur at different frequencies, including delta (δ, 1–4 Hz), theta (θ, 4–12 Hz), beta (β, 15–30), and gamma (γ, 30–80 Hz) bands [2, 3] (Fig. 1). Additionally, alterations in oscillatory power are observed across a broad frequency range (80–250 Hz), known as the high-gamma band [4]. Gamma rhythms in various brain regions are believed to be integral to information storing and processing [5]. In the hippocampal CA1 region, these frequency bands specifically manifest during distinct phases of hippocampal coding, suggesting that they facilitate the routing of information originating from various brain areas to CA1 [6]. Especially, the low γ rhythms emanating from the primary visual cortex tend to process higher spatial frequency information [7]. Moreover, γ oscillations have been extensively investigated in the cortex, hippocampus, amygdala, olfactory bulb, striatum, and brainstem and found to play a critical role in sensory processing [8], perceptual integration [9], recognition, working memory [10], locomotion [11], and emotion [12]. In contrast, disrupted γ oscillations induce aberrant neural activity and brain dysfunction (Table 1) [13]. For example, disrupted γ oscillations cause dysregulation of neural circuits involved in cognitive function, exacerbating Alzheimer’s disease (AD) pathology [14, 15]. Furthermore, in an animal model of depression-like behaviors, including Flinders sensitive line (FSL) rats and mice expressing the truncated Disrupted-in-schizophrenia 1 (Disc1) mutation, γ oscillation abnormalities are observed [16, 17]. Emerging evidence suggests that the abnormal γ oscillations could be a biomarker for major depression [18].
Fig. 1.
Diagram illustrating brain oscillation at different frequencies, including delta (δ, 1–4 Hz), theta (θ, 4–12 Hz), beta (β, 15–30), and gamma (γ, 30–80 Hz) oscillations. The γ oscillation is associated with heightened perception, learning, problem-solving tasks, and cognitive processing
Table 1.
Aberrant γ oscillations in central nervous system diseases
| Subject | Pathophysiology | Neural oscillations | Characteristics | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease | ||||
| TgF344-AD rats |
Synaptic dysfunction Neuronal hyperexcitability |
SWR power ↑ SWR duration ↓ θ-γ coupling↓ |
Cognitive impairment | [139] |
| C57BL/6, PV-Cre knock-in mice, SST-IRES-Cre knock-in mice | Aβo1-42 causes synapse-specific dysfunctions in PV and SST interneurons |
θ-nested γ oscillations↓ LTP↓ |
Memory encoding dysfunction | [140, 143] |
| (APP)/PS1 mice | Dysfunction of reciprocal dendrodendritic synapses between GCs and MCs |
LFP↑ Aberrant increase in γ oscillations↑ |
Olfactory impairment preceding learning defect |
[152] |
| APP/PS1 and 3xTg mice | Decrease in the excitatory responses of M/T cells | Ability of M/T cells to trigger interneuron GABA release↓ | Olfactory dysfunction | [153] |
| Human apoE4-KI C57BL/6 mice | ApoE4-induced GABAergic interneuron loss | SWR-associated slow gamma power in the hippocampus↓ | Learning and memory deficits | (14, 207) |
| C57BL/6 mice | Loss of tau homeostasis in hilar astrocytes of the dentate gyrus; Altered mitochondrial dynamics and function | Gamma oscillations and the number of neurons expressing PV in the dentate gyrus↓ | Spatial memory impairments | [150] |
| Parkinson’s disease | ||||
| PD patients |
Dopamine depletion The basal ganglia function disruption |
Recruitment of fast gamma bursts during movement ↓ | Bradykinesia | [169] |
| PD patients | LTP-like plasticity capacity in M1↓ | γ oscillations within the basal ganglia-thalamocortical network↓ | Locomotor dysfunction | [123] |
| C57BL/6 mice | Dopamine depletion selectively disrupts interactions between striatal neuron subtypes and LFP oscillations | Striatal transient high-γ (60–100 Hz) power ↑ | Movement initiation and rotation impairment | (208) |
| Stroke | ||||
| C57/BL6J mice | Enduring depolarization and interneuron function impairment | The activity of adjacent excitatory neurons↓ | Vascular and behavioral dysfunction | [29] |
| Two-vessel occlusion (2VO) rat model | Reduction of the theta-gamma cross-frequency coupling strength in the hippocampus | Short and long-term potentiation impairment | Cognitive dysfunction | [179] |
| Schizophrenia | ||||
| Sdy mice | Dysbindin-1 mutation-induced defective mitochondrial fission | Gamma range integrated power in CA3 ↓ | Cognitive impairment | [186] |
| Dlx5/6(+/-) mice | Abnormalities in GABAergic interneurons | FSINs generate gamma oscillations↓ | Disrupt PFC-dependent cognition | [68] |
| Autism spectrum disorder | ||||
| ASD patients |
The number of interneurons↓ Dysregulation in GABA receptor subunit expression |
Imbalance between excitatory and inhibitory signaling | Impairments in activities of daily living | [37] |
| ASD patients | Spontaneous gamma activity in frontal, temporal, and right-lateral regions↓ | Task-related gamma power↓; Long- and short-range gamma connectivity↓ | Sensory abnormalities | [190] |
SWR, sharp wave-ripple; PV, parvalbumin interneurons; SST, somatostatin interneurons; LTP, long-term potentiation; GCs, Granule cells; MCs, mitral cells; LFP, local field potential; OSNs, olfactory sensory neurons; EOG, electro-olfactogram; M/T cells, mitral/tufted cells; M1, primary motor cortex; FSINs, fast-spiking interneurons; PFC, prefrontal cortex
Multiple studies demonstrate the beneficial effects of γ oscillation stimulation (Table 2) [19–22]. Currently, γ stimulations are conducted using a variety of methods, including non-invasive techniques such as sound [23], light [24], electricity [25], and magnetism [26], as well as invasive methods like optogenetic stimulation [27]. Promisingly, γ stimulation produced by non-invasive or invasive approaches has been shown to exert potent neuroprotective effects in brain disorders [24, 28, 29]. Parvalbumin-expressing (PV+) interneurons, which innervate the perisomatic regions of pyramidal neurons, are believed to be pivotal in regulating and sustaining γ oscillations within the brain [30]. Substantial evidence supports the notion that modulating γ oscillations affects neurocircuit function and behavior [11, 20, 31, 32]. Therefore, this review provides an overview of current research progress on the potential therapeutic effects of γ oscillations in various brain disorders. Furthermore, this review focuses on moderating γ activity in the brain through external stimulation, particularly on 40 Hz γ activity.
Table 2.
The effect of gamma entrainment in central nervous system diseases
| Method | Protocol | Subject | Outcome | Behavior | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cognitive disorders | |||||||
| Optogenetic activation of PV and SST interneurons | 5 Hz | C57BL/6 mice, PV-Cre knock-in mice, SST-IRES-Cre knock-in mice | Restores theta-nested gamma oscillations and oscillation-induced spike timing-dependent LTP |
Memory encoding↑ The execution of cognitive function↑ |
[140] | ||
| Auditory or audiovisual stimulation | 40 Hz, 2 h/day, 14 days | ApoE4 Knock-In Mice | Amyloid protein levels↓ Neuronal apoptosis↓ cholinergic transmission↑ |
Cognitive performance↑ Neuropathology↓ |
[158] | ||
|
Optogenetic stimulation of parvalbumin neurons |
40 Hz |
J20-APP AD mouse 5XFAD mice |
Slow gamma oscillations amplitude and phase-amplitude coupling↑ Aβ deposition↑ |
Spatial memory↑ | [2, 106] | ||
| Optogenetic stimulation of FS-PV interneurons | 40 Hz, 1 h/day | 5XFAD mice |
Aβ1–40 and Aβ1–42 isoforms level↓ Microglial Aβ uptake ↑ |
Cognitive function↑ | [48] | ||
| Visual stimulation | 40 Hz, 1 h/day, 7 days | 5XFAD mice |
Aβ levels↓ Microglial Aβ uptake↑ |
Cognitive function↑ | [48] | ||
| Chronic daily gamma visual entrainment | 40 Hz, 1 h/day, 22 days |
Tau P301S mice CK-p25 mice |
Neuronal loss↓ DNA damage↓ Synaptic function ↑ Neuroprotective factors↑ |
Learning and spatial memory ↑ Neurodegeneration↓ |
[21] | ||
| Combined visual and auditory stimulation | 40 Hz, 1 h/d, for 7 days | 5XFAD mice |
Aβ levels↓ Tau phosphorylation↓ Reactive astrocytes and microglia↑ |
Recognition and spatial memory↑ | [20] | ||
| Transcranial focused ultrasound | 40 Hz | 5XFAD mice |
Microglia activation↑ Aβ plaque clearance↑ |
Learning and memory↑ | [159] | ||
| Transcranial alternating current stimulation | 40 Hz, 1 h/day, 4 weeks | Patients with mild-to-moderate dementia (AD) | p-Tau burden temporal lobe regions↓ | Cognitive function↑ | [166] | ||
| Visual stimulation |
30–50 Hz, 1 h/day, 14 days |
two-vessel occlusion (2VO) rat model | Reinstated the synchronization of phase-amplitude coupling with theta oscillations |
Degeneration↓ Cognitive function↑ |
[49] | ||
| Mental disorders | |||||||
| Visual stimulation | 40 Hz, 1 h/day, 30 days | APP/PS1 AD mouse |
Aβ deposition↓ Clock proteins expression↑ |
Circadian rhythm disorders↓ | [22] | ||
| Chronic multisensory gamma stimulation | 40 Hz, 20 min per session, 3 sessions per block | C57BL/6 PD mice |
p-α-Syn deposition↓ Stress-related ACTH and corticosterone levels↓ |
Depressive behaviors↓ | [173] | ||
| Visual stimulation | 40 Hz, 2 h/d, for 21 days | C57BL/6 stroke mice |
Anxiety susceptibility to stress exposure ↓ Microglia activation ↓ |
anxiety-like behaviors↓ | [78] | ||
| Motor disorders | |||||||
| iTBS-γ tACS costimulation | 70 Hz (γ-tACS) and 20 Hz (β-tACS) | PD patients |
LTP-like plasticity↑ Facilitation of MEPs↑ |
Motor function↑ | [123] | ||
| Sensory stimulation | 40 Hz, 2 h/day, 1 month | C57BL/6 PD mice |
α-Syn clearance↑ Cell apoptosis in M1↓ |
Neuromuscular strength↑ | [173] | ||
| Vibration at gamma frequency |
40 Hz, 25 min/day, 12 weeks |
PD patients |
Tremor↓ Rigidity↓ Bradykinesia↓ |
Motor symptoms↑ | [174] | ||
| Deep brain stimulation | 160 Hz | PD patients | The cross-frequency interactions between finely tuned gamma oscillations↑ | Motor performance ↑ Beta power ↓ Gamma power ↑ | (209) | ||
| Optogenetic stimulation of interneurons | 40 Hz, 1 h/d | C57/BL6J stroke mice | Spreading depolarizations Cerebral blood flow ↑ |
Motor performance ↑ Brain swelling and lesion volume ↓ |
[29] | ||
| Optogenetic stimulation of the nucleus basalis | 20 Hz | Thirty-five adult ChAT-Cre/Ai32(ChR2-YFP) |
Acetylcholine↑ Improved recovery of reaching and movement scores |
Functional recovery↑ Motor behavior↑ |
[176] | ||
PV, parvalbumin interneurons; SST, somatostatin interneurons; LTP, long-term potentiation; FS-PV, fast-spiking, parvalbumin-positive interneurons; VC, visual cortex; AC, auditory cortex; mPFC, medial prefrontal cortex; ACTH, adreno-cortico-tropic-hormone; MEPs, motor-evoked potentials;
Gamma oscillations
Gamma oscillations are rhythmic fluctuations across multiple brain regions, characterized by local field potential changes and interareal coherence, and aid in sensory information processing, attentional selection, and memory operations [33, 34]. For example, enhanced γ activity is observed in the neocortex and hippocampus during sensory information transmission and in interareal coherence [34]. As mentioned above, gamma rhythms are categorized into narrowband gamma (i.e., gamma oscillations) and broadband γ (i.e., high gamma), which exert different biophysical effects [35]. Whereas narrowband gamma represents a “true” gamma oscillation, broadband gamma often represents a non-oscillatory or “aperiodic” electroencephalography (EEG) phenomenon [35]. Mechanistically, the emergence of γ oscillations has been attributed to γ-aminobutyric acid type A (GABAA) receptor-mediated inhibition involving interactions between fast-spiking and PV + interneurons [36, 37]. Furthermore, functional differences in PV + interneurons are observed in multiple disorders, disrupting the excitation/inhibition balance and causing abnormalities in γ oscillations [38, 39]. Empirical evidence has elucidated that optogenetic stimulation of PV + interneurons amplifies oscillatory γ activity, while inhibition of PV + interneurons diminishes γ oscillations [40]. For instance, therapeutics designed to target PV + interneurons specifically have been shown to restore normal γ oscillation patterns, thereby enhancing the cognitive function of the J20-APP AD mouse model through optogenetic interventions [2].
With evidence highlighting the critical role of γ oscillations in sensory and cognitive processes, researchers have investigated the presence of abnormal γ oscillations in neurological and neuropsychiatric conditions [41, 42]. Indeed, γ-frequency oscillations are disrupted in various brain disorders, including AD [14], Parkinson’s disease (PD) [43], stroke [44], Schizophrenia (SCZ) [45], and autism spectrum disorder (ASD) [46]. These disrupted γ-frequency oscillations impair neuronal encoding and sensory and/or cognitive information transformation [30, 35]. Research suggests that γ oscillations may serve as potential biomarkers for neural imbalances or interneuron dysfunction, reflecting the underlying pathophysiological mechanisms of essential neural functions in neuropsychiatric diseases [40, 47, 48]. Thus, reinstating normal γ activity is a potential therapy for improving higher-order cognition, sensory-motor integration, working memory, attention, perceptual binding, and network synchronization.
Sensory stimulation methods to induce gamma oscillations
Various stimulation modalities are currently used to induce γ entrainment, including sensory stimulation, optogenetics, transcranial electrical or magnetic stimulation, and deep brain electrical stimulation (Fig. 2) [40].
Fig. 2.
Gamma entrainment using sensory stimuli (GENUS). GENUS encompasses a range of methodologies, including visual stimulation, auditory simulation, audiovisual combined stimulation, and somatosensory stimulation. Feasible clinical advantages stemming from γ sensory stimulation emanate from alterations in neural function, neural circuitry, and immune signaling pathways
Gamma entrainment using sensory stimuli
Various studies on animal models and human diseases have investigated Gamma Entrainment Using Sensory stimuli (GENUS), primarily involving auditory and visual entrainment [20, 32, 49, 50]. The potential clinical benefits of γ sensory stimulation are likely derived from flicker-induced changes in neural function, circuitry, and immune signaling pathways.
Visual stimulation
The magnitude of visual oscillations is influenced by the frequency, chromaticity, and luminance of the light stimulus [40]. During experiments, participants wear portable opaque eye masks and earplugs while undergoing scalp-EEG recording. They are exposed to flickering light in the γ band to evoke γ oscillations [51, 52]. In animal models, the visual aspect of GENUS involves moving the animals from the holding room to a flicker cage, where light-emitting diodes (LEDs) deliver flickering light at the desired frequency [21].
Brain oscillatory activity is one of the fundamental mechanisms supporting cognitive processes. Present studies indicate that exposure to γ light stimulation leads to functional reorganization across diverse brain regions and modulates functional connectivity within relevant neural networks [53, 54]. EEG results demonstrate γ wave light flicker augments the power of brain oscillations in healthy individuals, emphasizing enhancing activity in the occipital regions bilaterally [55]. Additionally, microglia exhibit a notable affinity for PV + neurons and can restructure perineuronal nets (PNNs), which are crucial for regulating critical period plasticity in the adult cerebral cortex [56, 57]. Evidence shows that exposure to γ wave light flicker reduces PNN coverage in the healthy adult brain and promotes juvenile-like plasticity [56]. In parallel, γ oscillations elicited by light flicker stimulation have been shown to benefit cognitive function and synaptic plasticity in animal models [58, 59]. Prolonged exposure to γ visual flicker drives the reorganization of stress-related neural circuits and enhances hippocampal neuroplasticity in wild-type mice [59]. Visual stimulation with low γ light flicker induces slow γ oscillations in the hippocampal CA1 region, thereby alleviating cognitive impairments in the mouse two-vessel occlusion (2VO) model of cerebral ischemia [58]. Similar investigations in Tau P301S and CK-p25 mice also demonstrated that chronic γ flickering light stimulation enhances functional neuronal connectivity across brain areas, ameliorates neuronal loss, reduces DNA double-strand breaks, offers neuroprotection, and improves spatial memory [21].
Previous studies have shown that flickering light stimulation induces neuronal spiking activity, significantly reducing β-amyloid (Aβ) plaque burden in the visual cortex of 5XFAD mice and facilitating microglial morphological transformation [60]. Furthermore, visual gamma entrainment reduces phosphorylated tau levels in tauopathy mouse models, including P301S and CK-p25, while inducing microglial responses similar to those observed in 5XFAD mice [21]. However, in elderly C57BL/6J mice, γ oscillations induced by visual stimulation did not significantly alter microglial transition to a phagocytic state, microglial quantity, or neuroinflammatory markers [21]. Similarly, in an animal model of ischemic stroke, microglial responses to GENUS appear limited, suggesting that its effects on microglia may depend on disease status or genetic background [13, 58]. As a result, the precise mechanisms and implications of microglial alterations induced by γ wave visual stimulation remain to be determined. In addition, γ stimulation positively modulates neuroimmune biochemical signaling. Exposure to γ flickering lights in wild-type mice upregulates cytokines such as IL-6, and IL-4, enhances microglial phagocytosis, and increases the expression of chemokines, including macrophage colony-stimulating factor and monokines induced by interferon-γ [61]. This neuroimmune activation is mediated by γ-induced phosphorylation of proteins in the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase pathways [61].
Auditory simulation
Consistent exposure to auditory stimulation has been shown to maintain magnetic field amplitudes in the auditory cortex and induce progressive changes in synaptic efficacy and sensory input, thereby influencing neuronal activity [62]. In animal models, the auditory component of GENUS is administered by exposing animals to tones flickering at the target frequency in a dimly lit, soundproofed room [20]. In humans, auditory stimulation is provided through earphones that emit tones at the specified frequency, while participants wear LED goggles [52, 63]. Previously, auditory cue-triggered neuronal synchronization was discovered and termed Auditory Steady-State Response [64, 65]. It was previously believed that auditory-driven gamma oscillations were restricted to the temporal/auditory cortex [65, 66]. However, recent findings propose that auditory-driven γ oscillations instead encompass the entirety of the cortical mantle [66]. This widespread cortical distribution of auditory-driven γ oscillations is supported by diverse research methods, including whole-head EEG, Magnetoencephalography (MEG), invasive recordings, and electrocorticography (ECoG), which collectively confirm that γ auditory exposure induces γ synchronization across the entire cortical surface [67–70].
Cerebral blood flow (CBF) and vascular changes associated with auditory stimulation-induced γ oscillations have also been investigated [20, 71]. Previous findings suggested that auditory evoked entrainment in healthy humans elicited increased regional cerebral blood flow (rCBF) in the cortex of the posterior aspect of both cerebellar hemispheres [71]. Recent immunostaining studies revealed increased vasodilation and blood vessel diameter in the auditory cortex and CA1 region following chronic auditory stimulation-induced γ entrainment in 5XFAD mice [20]. However, the underlying mechanisms of the interplay between blood flow and γ entrainment remain largely unknown. Interestingly, in a mouse cerebral ischemia model, light flicker failed to increase CBF and blood vessel density [58]. Therefore, further studies are needed to determine whether γ oscillations evoked by auditory entrainment could offer therapeutic benefits for impaired blood supply and vascular damage.
Audiovisual combined stimulation
Audiovisual stimulation (AVS) is a neurostimulation technique that induces a cerebral response by synchronizing visual and auditory inputs [72]. Specifically, flashing lights are presented to the eyes while pulsed tones are administered to the ears at frequencies associated with brain wave activity, which can be recorded by EEG [73].
According to a recent EEG study, GENUS audiovisual stimulation effectively entrains both cortical sensory regions and deeper brain areas such as the hippocampus, amygdala, insula, and gyrus rectus, noticeably amplifying the power spectral density of frontal and occipital neuron oscillations [74]. In addition, entraining γ oscillations using simultaneous auditory and visual stimulation also influence functional brain connectivity, triggering a change toward normal function [49, 74]. Chronic (8 weeks of daily) audiovisual γ stimulation strengthened functional connectivity between the PCC and PCUN nodes in the DMN of AD patients [49]. PCC-PCUN functional connection strength was positively correlated with cognitive performance [75]. In contrast, another study showed that audiovisual stimulation (3 months daily) did not result in connectivity changes within the DMN but led to a significant increase in mean functional connectivity in the MVN in mild AD patients [74]. However, the authors deemed the observed augmentation in MVN functional connectivity due to the regular use of GENUS light and sound stimulation less probable [74].
In addition to the human study, the beneficial effects of audiovisual combined stimulation have also been demonstrated in a 5XFAD mouse model. Audiovisual stimulation may exert more widespread effects than auditory or visual stimulation. Audiovisual stimulation modality uniquely elicited microglial clustering responses in the auditory cortex, hippocampus, and medial prefrontal cortex and reduced amyloid burden not only in these specific regions but also across the entire neocortex [20]. Furthermore, altered immune factors and cytokines in the cerebrospinal fluid of Alzheimer’s patients following audiovisual γ flicker include downregulation of TGF-α (astrocyte activator), IL-5 (microglial proliferation), MIP-1β (microglial motility), and TWEAK (apoptosis inducer) [49]. Therefore, long-term audiovisual stimulation therapy may attenuate potentially harmful cytokines involved in the activation of microglia and astrocytes. Notedly, TWEAK regulates key immune signaling cascades, including NF‐κB, matrix metalloproteinase, and cellular responses, and results in the disruption of the permeability of the neurovascular unit and blood-brain barrier [76]. Moreover, inhibition of TWEAK may have therapeutic potential in several degenerative diseases [77]. Thus, TWEAK may be a new target for treating neurological diseases through audiovisual stimulation combined with γ oscillations.
Somatosensory stimulation
The primary modality of γ somatosensory stimulation involves the use of vibrotactile stimuli [78, 79]. The delivery of vibrotactile stimulation was facilitated by an acoustic system that converts γ wave electrical sinusoidal signals to corresponding vertical vibrations [78]. The animal was placed inside a cage on top of a speaker connected to an audio amplifier [78]. In human studies, the participants underwent vibrations while sitting on a vibrating platform chair [79].
External passive γ tactile stimulation induces neural oscillations in the somatosensory cortex [80]. In a clinical study with healthy adult participants, a functional whole-body vibration exercise platform was associated with widespread changes in oxygenated hemoglobin concentration in multiple cortices [81]. Animal studies have corroborated these results following several weeks of daily whole-body γ wave vibrotactile stimulation, which triggered neural activity in the primary somatosensory cortex (SSp) and primary motor cortex (M1), resulting in improved motor performance [78]. Furthermore, after vibration stimulation, the SSp and M1 regions showed decreased phosphorylated tau, synaptic protein loss, DNA damage, and neurodegeneration [78]. Daily vibrotactile stimulation sessions improved anxiety-like behavior, motor performance, and spatial memory in aged rats [82]. In addition, physical exercise combined with γ wave light flickering improves Ca2+ homeostasis, reduces reactive oxygen species (ROS), and enhances cognitive performance, mitochondrial function, and neuroplasticity in the 3xTg mouse model [83, 84].
Based on current findings, evidence suggests that inducing γ oscillation stimulation can potentially ameliorate several neuropathologies [85, 86]. However, a significant concern is whether health-related risks occur in the neural circuits of long-term frequent visual flickering γ oscillations [85, 86]. A recent study proposes a novel γ visual entrainment method using Invisible Spectral Flicker (ISF) [87]. Compared to interventions with stroboscopic flicker, ISF induces lower γ amplitude oscillations but exhibits a similar spatial distribution, primarily localized in the posterior electrodes near the visual cortex [87]. Consequently, ISF presents an opportunity for future randomized placebo-controlled clinical trials that substantially reduce the potential for discomfort [87]. In addition, multiple studies have confirmed that GENUS is safe with no serious adverse events and effectively induced γ entrainment with the treatment [49, 74, 88].
Photobiomodulation
Photobiomodulation (PBM) refers to using low-power light in the visible and near-infrared spectra to induce beneficial biological processes in cells and tissues. Monochromatic wavelengths evoke distinct colors of light on the short-wavelength end of the visible spectrum, including violet (360–400 nm), blue (400–580 nm), and green (560–650 nm) [89].
Current literature suggests that γ rhythm violet optical stimulation through the eyes significantly increases alpha-gamma coupling oscillations, enhancing attention, perception, and memory [54]. Interestingly, exposure to violet light (360–400 nm) has been found to upregulate myopia suppressive gene (EGR-1) expression. EGR-1 is a transcriptional regulator that controls the distribution of methylation sites on brain DNA, which is crucial for neuronal plasticity and memory formation [90, 91]. However, whether specific γ rhythm violet optical stimulation triggers an increase in EGR-1 expression or improves related cognitive functions remains unclear and warrants further investigation. In addition, blue light regulates brain activity patterns more broadly than violet light [89]. Human functional magnetic resonance imaging (fMRI) reveals distinct neural activation patterns in response to γ rhythm blue light exposure through the eyes during a recognition memory task [92]. Furthermore, the γ rhythm visual stimulation-induced neural response exhibits a stronger link to regulating core components within the memory-related network, such as the hippocampus, than exposure to non-flickering natural light [92].
Red-to-infrared light therapy within the 600–1070 nm wavelength range, particularly the near-infrared range, is recognized as a safe and potent therapeutic approach for arresting neuronal degeneration [93]. The application of 1070 nm light stimulation through the scalp and skull at a θ rhythm pulse frequency (10 Hz) activates microglia, leading to morphological changes and enhanced co-localization of microglia with Aβ in APP/PS1 mice, thereby ultimately improving memory ability [28]. Moreover, applying a 1064 nm laser results in significant amplifications of the spectral power strength of electrophysiological oscillations within the alpha (8–13 Hz) and beta (13–30 Hz) bands, observed across a wide range of scalp regions in the human brain [94]. Consequently, employing selective pulse frequencies to manipulate brain oscillations closely linked to specific memory functions may represent a promising strategy to optimize the benefits of light intervention for regulating cognitive function.
Genetic modifications or optogenetic stimulation
Optogenetic stimulation, a genetic technique that uses genetically engineered cells expressing photosensitive proteins, allows precise activation or inhibition of specific neuronal populations [95]. Moreover, modern optogenetics represents a pivotal milestone in neuroscience, enabling profound insights into the complex orchestration of neural circuitry and behavioral mechanisms while overcoming the limitations of most other methods [96, 97]. Previous research has demonstrated that optogenetic stimulation induces γ rhythms and activates excitatory neurons [98, 99]. For example, constant optogenetic stimulation activates channel rhodopsin 2 (ChR2)-expressing interneurons in the sensory cortex and produces γ band activity in anesthetized cats [27]. In addition, optogenetic stimulation applied to the peri-infarct zone has been shown to effectively restore neuronal activity after stroke in motor and parietal association areas. This also helps attenuate vascular and behavioral dysfunction [29].
Indeed, mounting evidence suggests that optogenetic manipulation of γ oscillations affects neurocircuit function and behavior. For instance, optogenetic activation of γ oscillations in the prefrontal cortex during a goal-directed attentional task improved attentional behavior [100]. Furthermore, optogenetic stimulation of parvalbumin interneurons in the mPFC effectively improved social novelty preference and rescued the social novelty deficit in autism [101]. Similarly, optogenetic stimulation targeting fast-spiking interneurons (FSINs) to induce γ oscillations in the basolateral amygdala has been shown to enhance contextual memory consolidation [102]. The crucial role of the Dlx5/6 gene in the development of GABAergic interneurons provides further evidence supporting the impact of γ stimulation on circuit function and behavioral flexibility, as demonstrated in experiments with Dlx5/6+/− mice [69, 103]. In these mice, the abnormality of FSINs occurs during adolescence, coinciding with the onset of cognitive inflexibility and compromised task-evoked γ oscillations [69]. However, optogenetic induction of γ oscillations in the PFC effectively restored cognitive flexibility in Dlx5/6+/− mice, enabling them to perform the task consistently over an extended duration [69].
Although optogenetic stimulation has demonstrated neuroprotective effects, the mechanisms by which γ entrainment in various brain regions affects Aβ deposition remain unclear. For example, optogenetic manipulation of γ oscillations in CA1 neurons has been linked to reduced Aβ levels in both 5XFAD and APP/PS1 mouse models [60]. Conversely, a separate study found that optogenetic stimulation of PV + neurons in the basal forebrain of 5XFAD mice increased amyloid burden in the frontal cortical region [104]. Similarly, optogenetic stimulation of medial septal PV + neurons rescue the amplitude of hippocampal low-frequency γ oscillations and enhances spatial memory performance despite significant plaque deposition [2]. Hence, it is hypothesized that divergent stimulation modalities elicit distinct molecular and cellular responses. These responses may involve different action mechanisms, potentially entraining γ oscillations within a complex neurocircuit that spans multiple brain regions [13]. Along with meticulously designed clinical trials, further investigations are warranted to elucidate these limitations, ascertaining whether induction methods have potentially positive or harmful impacts on pathology.
Transcranial electrical stimulation
Transcranial electrical stimulation (TES) is a non-invasive technique that delivers controlled electric fields to the scalp to directly modulate cerebral activity through low voltage constant or alternating currents [105]. TES encompasses a range of methodologies, including transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS), transcranial alternating temporal interference (tTIS), and transcranial alternating current stimulation (tACS) [106–108]. Specifically, tDCS modulates cortical areas by delivering low-intensity direct current, which induces bidirectional, polarity-dependent changes in spontaneous neuronal activity [109]. Meanwhile, tDCS exhibits remarkable tolerability in humans, allowing for a comprehensive assessment of neuropsychological, physiological, and motor effects in clinical research [109]. In comparison, tRNS delivers low-intensity, randomly alternating biphasic current directly to the scalp and elucidates the modulatory effects of cortical excitability on motor learning and perceptual processing [110]. Furthermore, tTIS is a non-invasive method for achieving focal and steerable deep brain stimulation, which involves applying high-frequency alternating currents at distinct scalp sites [106]. Finally, tACS entails the administration of sinusoidal alternating electric currents with specific frequencies in pre-defined cerebral regions across the scalp to primarily impact endogenous oscillatory activity in the brain [111]. Additionally, tACS is intended to modulate cerebral function and influence cognitive processes by entraining brain oscillations and enhancing neural communication [112]. Although tDCS and tRNS effectively modulate cortical excitability and plasticity, tACS uniquely targets frequency-specific modulation of oscillatory dynamics. Meanwhile, current literature insinuates that tACS applied at γ frequencies effectively modulates various cerebral functions [19, 113]. Thus, this section will investigate the theoretical and practical applications of tACS.
In a randomized, double-blind, sham-controlled crossover pilot study, the impact of transcranial alternating current stimulation at γ frequency (γ-tACS) or sham tACS was meticulously explored in patients with mild cognitive impairment [19]. Notably, the active γ-tACS intervention involves a solitary 60-minute treatment session, precisely targeting the Pz region (an area overlying the medial parietal cortex and the precuneus), which is known to play a pivotal role in the episodic memory network [114]. Compared to the sham exposure, γ-tACS yielded significant improvements in memory performance and reinstated intracortical connectivity measures of cholinergic neurotransmission [19]. A subsequent study examined the effects of γ-tACS on episodic memory and cholinergic transmission in patients with Alzheimer’s [113]. The 60-minute treatment targeted the precuneus with either γ-tACS or a sham intervention. Results showed a significant correlation between improvements in episodic memory and indirect measures of cholinergic neurotransmission following active γ-tACS [113]. Pre- and post-EEG assessments revealed increased γ-power activity in posterior brain regions, indicating the localized impact of γ-tACS on the precuneus, posterior parietal cortex, and cognitive function [113].
Recent investigations have unveiled a causal nexus between γ oscillations and preparatory and execution stages of movement [115]. Targeting the application of γ-tACS within the M1 enhances the velocity and acceleration of visually triggered movements, contrasting with the negligible impact of beta-tACS or sham stimulation [115]. These improvements induced by γ-tACS are significantly associated with the altered blood oxygenation level-dependent activity localized to the stimulated M1 region and task-specific modulation of neural activity in the distant dorsomedial prefrontal cortex [115]. Additionally, γ-tACS is related to the motor performance of tasks requiring motor control, like visuomotor performance [116]. Applying 70 Hz tACS over the M1 and cerebellar cortex significantly improved performance on an isometric force task involving visuomotor control of the right index finger, particularly in healthy individuals with suboptimal baseline motor performance [116]. Similarly, stimulation at a high γ frequency (80 Hz) enhances motor performance during a visuomotor coordination task [117]. Thus, the involvement of high-frequency motor cortex γ oscillations in complex visuomotor tasks involves abrupt adjustments to motor planning and execution [117]. In addition, γ oscillations in cortical motor areas reflect synaptic activity and contribute to plasticity [118]. Previous studies indicate that γ-tACS combined with intermittent θ burst stimulation (iTBS) induces LTP-like plasticity in the M1 of healthy individuals [119]. Clinical research also shows that γ entrainment (70 Hz) via tACS improves motor impairment in PD patients and modulates GABAA activity in M1 [43, 118]. Specifically, γ-tACS reverses LTD-like effects and enhances LTP-like plasticity by inhibiting GABAergic interneurons in M1 [43]. Thus, γ-tACS can potentially reverse LTD-like plasticity in the human M1. In addition, working memory is a complex cognitive function involved in temporary information storage and manipulation, making it a target for neurorehabilitation [120].
Several studies suggest that tDCS also modulates γ activity. During a visual task, administration of tDCS to the occipital cortices results in augmented local γ oscillation amplitude [121]. Remarkably, tDCS also unravels network-level ramifications, characterized by heightened γ oscillations in the prefrontal cortex, parietal cortex, and various visual attention regions [121]. Similarly, anodal tDCS applied to the dorsolateral prefrontal cortex significantly increases γ power and improves working memory performance in patients with SCZ [122]. In addition, vagus nerve stimulation (VNS), another γ-band stimulation methodology, involves the modulation of the vagus nerve through electrical impulses [123]. The vagus nerve traverses the neck, forming a neural pathway that links peripheral organs with lower regions of the brain [123]. Vagal nerve branches intricately innervate anatomical structures associated with human memory processing within complex neuronal networks [123]. γ entrainment using transcutaneous auricular vagus nerve stimulation (γ-taVNS) efficiently reduces hippocampal amyloid load in APP/PS1 mice [124]. Furthermore, γ-taVNS elicits microglial phagocytosis and regulates microglial pyroptosis by effectively suppressing the P2 × 7R/NLRP3/caspase-1 pathway in the hippocampus [124]. Additionally, γ-taVNS exerts inhibitory effects on the hippocampal NF-κB pathway, increasing neuroprotection, spatial memory, and learning [124].
Transcranial magnetic stimulation
Transcranial Magnetic Stimulation (TMS) is a non-invasive medical procedure that uses magnetic fields to stimulate nerve cells in the brain [105]. It involves placing a coil near the scalp, generating magnetic pulses that pass through the skull and penetrate targeted brain regions [105]. Recent studies show that periodic electromagnetic force engendered through rhythmic TMS modulates brain function [125]. Notably, rhythmic TMS fosters the regulation of brain oscillations by perturbing and realigning ongoing oscillatory activities [125]. The most commonly used TMS method is repetitive transcranial magnetic stimulation (rTMS), capable of inducing time-varying magnetic fields within the cerebral cortex [126]. These evoked magnetic fields generate action potentials within specific neurons of targeted brain regions by eliciting electric currents in rhythmic patterns [126]. Recently, γ-band rTMS treatment amplified power in the γ frequency band within the left temporoparietal cortex, improving cognitive and executive functions by facilitating local, long-range, and dynamic connectivity within the brain regions, promoting information flow and integration [127].
Interestingly, all patients maintained favorable health status, without any documented unwanted reactions during therapy, indicating the safety and feasibility of γ-rTMS intervention [127]. Enhancing γ oscillatory activity through rTMS applied to the dorsolateral prefrontal cortex has emerged as a promising cognitive enhancement strategy for neuropsychiatric disorders characterized by cognitive impairments [128]. Compelling evidence highlights the ability of rTMS to target the dorsolateral prefrontal cortex and to induce normalizing excessive gamma oscillations in individuals with schizophrenia and ASD [129, 130]. Furthermore, rTMS elicits plasticity-like changes in cortical function and behavior, improving language function in healthy individuals and various aspects of memory in patients with severe depression [128].
Gamma brain stimulation for Alzheimer’s disease
AD is one of the most prevalent neurodegenerative diseases, pathologically characterized by excessive extracellular Aβ accumulation and intracellular tau hyperphosphorylation [131, 132]. Although numerous studies have been conducted over the past decades to treat AD by targeting Aβ and abnormal tau, nearly all clinical trials targeting Aβ and tau hyperphosphorylation have failed [133]. Therefore, the Aβ and tau hypotheses have been questioned in recent years [134].
The pathological buildup of amyloid-beta oligomers (Aβo) disrupts the synchronized generation of action potentials in pyramidal cells and disturbs the balance of excitatory and inhibitory processes within the hippocampal network [135]. This disruption results in impaired hippocampal theta-gamma phase-amplitude coupling and compromised long-term potentiation (LTP), which are crucial for memory encoding and cognitive function [136, 137]. PV + and somatostatin-positive (SST) interneurons represent the prominent subtypes of interneurons in the hippocampus, playing a pivotal role in θ -nested γ oscillogenesis and LTP induction [137, 138]. Specifically, PV + interneurons selectively modulate γ oscillations, while SST + interneurons modulate θ oscillations [60, 139]. Dysfunction in SST + and PV + interneurons contributes to impairments in θ and γ oscillations observed in an AβO-injected mouse model of AD [140]. Thus, Aβo causes synapse-specific dysfunction in PV + and SST + interneurons, likely contributing to impaired hippocampal γ oscillations and synaptic plasticity in AD [137]. In AD mouse models, the regulatory capacity of inhibitory interneurons to maintain oscillatory rhythms and network synchrony crucial for cognitive function is compromised [48, 141]. Notably, the dysfunction of Nav1.1-dependent interneurons is functionally significant in the pathogenesis of AD-associated cognitive impairments [141]. Efforts to restore the normal levels of Nav1.1 facilitate the enhancement of γ-oscillatory activities, mitigate excessive network synchrony, and alleviate cognitive decline in hAPP mice [142].
The alteration of neuronal network activity may predate the onset of AD, potentially occurring before the deposition of Aβ and leading to changes within the hippocampal network [143, 144]. In the early stages of the disease, abnormal slow γ oscillations are observed during hippocampal sharp wave ripples (SWRs) in AD mouse models [60]. The gradual decline in slow γ activity initiated by interneurons during SWRs significantly contributes to apoE4-mediated learning and memory impairments [14]. SWRs originate in hippocampus and are triggered by synchronized activation of CA3 pyramidal neurons, leading to high-frequency oscillations in the local field potential recorded from the CA1 region [145]. During SWRs, slow gamma oscillations are elevated, and the increased gamma synchrony between CA3 and CA1 is associated with more coordinated neuronal firing [146]. Restoring slow γ oscillations during SWRs is critical for modulating memory retrieval. In addition, accumulation of the 1N3R isoform of tau within astrocytic processes in the dentate gyrus of AD patients triggered mitochondrial relocation and impaired motility in hilus astrocytes, diminishing γ oscillations and PV-expressing neurons, resulting in spatial memory impairments [147]. On the other hand, before the deposition of Aβ plaques and the onset of cognitive impairments, individuals with AD exhibit olfactory dysfunction characterized by an inability to perceive and identify odors [148]. With advancing age, Aβ aggregation induces the dysfunction of reciprocal dendrodendritic synapses between granule cells and mitral cells, consequently leading to aberrantly enhanced γ oscillations and olfactory impairment [149]. Thus, considering γ oscillations as potential biomarkers for preclinical AD is rational (Fig. 3) [150].
Fig. 3.
The beneficial effect of gamma stimulation in AD. Gamma stimulation confers various benefits on AD, including enhancing brain-inter-area communication, improving Aβ and p-tau clearance, regulating glial cell function, preserving respiratory chain enzyme activity, alleviating cognitive symptoms, and enhancing clock protein expression
Mounting evidence suggests γ-band oscillations (especially 40 Hz) are critical for multiple sensory and cognitive processes [3, 151, 152]. Previous studies confirm that cognitive activity induces 40 Hz event-related potential in humans [153]. Similarly, researchers discovered that compared with healthy people, Alzheimer’s patients had reduced 40 Hz brainwaves in the cortical component [154]. In the 5XFAD mouse model, exposure to flicker stimulation at various frequencies (including 8 Hz, 40 Hz, 80 Hz, random stimulation, and no stimulation) revealed that 40 Hz (1 h per day, 7 days) flicker significantly reduced Aβ plaque burden in the visual cortex [60]. Similarly, the application of 40 Hz (1 h/d day, 7 days) γ visual stimulation enhanced gamma power among several brain areas, including the visual cortex, hippocampus, prefrontal cortex, and somatosensory cortex, leading to improvements in associated cognitive symptoms and neurodegeneration [21, 60]. Interestingly, random stimulation resulted in increased Aβ levels, suggesting that different types of visual stimuli may elicit distinct effects [60]. Similarly, auditory or audiovisual stimulation at 40 Hz (2 h/day, 14 days) improves cognitive performance and mitigates neuropathological alterations in apoE4 knock-in mice while also reducing neuronal apoptosis and enhancing cholinergic transmission in the hippocampus [155]. Another study used transcranial-focused ultrasound pulsed at 40 Hz, decreasing Aβ plaque deposition in 5XFAD mouse models [156]. Notably, 40 Hz flickering also improves mitochondrial function. In the Aβ1–42 toxicity condition (as an AD model), utilizing a 40 Hz flickering white LED has been shown to improve the structural and functional integrity of ion channels, particularly mitoBKCa channel, and promote mitochondrial respiratory chain enzyme activity, specifically complex I and IV [157, 158]. Furthermore, evidence suggests that 40 Hz light simulation enhances mitochondrial membrane potential (ΔΨm) and mitigates ROS production in mouse models of AD [157].
Sleep and circadian dysfunction commonly occur in AD patients, partly contributing to the progression of neurodegeneration [159, 160]. A recent study demonstrates that 40 Hz (1 h/day, 30 days) light simulation ameliorates circadian rhythm disturbance in the APP/PS1 AD mouse model, restoring the hypothalamus electrophysiological changes, reducing Aβ deposition in the hypothalamus, and enhancing rhythmic expression of clock proteins, including BMAL1, CLOCK, and PER2 [22, 161]. Specifically, pretreatment with 40 Hz (1 h/day, 30 days) flickering light alleviated disrupted circadian rhythms, improved the ratio of nighttime to total activity, and corrected fragmented rest periods in AD mice [22]. Furthermore, after 30 days of 40 Hz flickering light treatment, no adverse effects on body weight, blood glucose levels, heart rate, or biological rhythms were observed in the mice [22]. Overall, 40 Hz entrainment exhibited positive outcomes across various AD pathology animal models (including 5XFAD, Tau P301S, APP/PSI, and CK-p25), indicating that the effects are not model-specific [162, 163].
However, despite these promising results, it is important to note that other studies could not replicate these outcomes. Specifically, a recent study showed that both acute (10-minute baseline followed by one-hour stimulation) and chronic (one hour per day for seven days) 40 Hz visual flickering failed to entrain deeper brain structures in APP/PS1 and 5XFAD models [31]. Only a small fraction of neurons responded to light stimulation, with no detectable effects on intrinsic γ oscillations [31]. Furthermore, the results revealed no overt reliably reduced Aβ load in the neocortex or hippocampus or alteration in microglial morphology within the experimental animals [31]. Optogenetic stimulation was employed to selectively activate medial septal PV neurons at different γ-band frequencies in the cortex of J20-APP animal models [2, 164]. Although 40 Hz stimulation successfully restored hippocampal slow γ oscillation amplitude and phase-amplitude coupling, effectively rescuing spatial memory deficits, Aβ plaque deposition persisted [2]. Likewise, 40 Hz (1 h/day, 4 weeks) tACS did not substantially impact Aβ burden but did reduce p-Tau levels within the specific temporal lobe area in AD patients [165]. Given the constraints of small sample sizes, varying treatment protocols, and diverse evaluation criteria in clinical trials, large-scale studies are needed to establish a robust therapeutic phenotype for γ entrainment in AD pathology.
Gamma brain stimulation for Parkinson’s disease
PD is characterized by dopaminergic neuron depletion, α-synuclein (α-Syn) misfolding and aggregation, mitochondrial dysfunction, neuroinflammation, and oxidative stress [166]. PD is clinically manifested by motor symptoms such as resting tremor, bradykinesia, rigidity, and postural instability, as well as non-motor symptoms like REM sleep disorder, anosmia, cognitive impairment, and depression [167]. Pharmacological interventions, such as dopamine replacement therapy, remain the predominant treatment modality. However, these treatments show diminishing efficacy over time, potentially leading to motor complications [166]. Increasing investigations into non-invasive neurostimulation and neuromodulation techniques have emerged as alternative strategies to address PD pathology.
Under the decreased burst rate of the hypodopaminergic state, a deficiency in regulating subcortical γ signaling may contribute to the pathomechanism underlying bradykinesia in PD (Fig. 4) [168]. Furthermore, dopamine loss disrupts the basal ganglia, a brain structure responsible for regulating motor function [168]. Enhancing gamma oscillations restores synaptic plasticity in the cortical motor regions [43, 169]. Recent neurophysiological studies show reduced long-term potentiation (LTP)-like plasticity in M1 and diminished γ oscillations within the basal ganglia-thalamo-cortical network in PD patients [43, 170]. The specific γ oscillatory activity ranging from 60 to 90 Hz is relevant to the motor network and exhibits correlated changes with movement execution [171]. The combination of tACS delivered over the cortical motor areas at 70 Hz and intermittent θ burst stimulation demonstrates that driving γ oscillations restores LTP-like plasticity in patients with PD [43]. Furthermore, a double-blind, randomized controlled trial suggests that 40 Hz vibration (25 min/day, 12 weeks) through psychoacoustic therapy improves tremor, rigidity, bradykinesia, posture, and gait in PD patients [172].
Fig. 4.
The beneficial effect of gamma stimulation in PD. Gamma stimulation improves basal ganglia normalization, preserves synaptic plasticity, alleviates the increase in stress-related hormones, and improves behavioral changes
Additionally, GENUS has the potential to facilitate aberrant protein clearance and treat non-motor symptoms in PD animal models. Prolonged multisensory 40 Hz (2 h/day, 1 month) stimulation effectively reduces p-α-Syn deposition in the cortex and striatum [173]. However, 40 Hz (2 h/day, 1 month) audiovisual stimulation ameliorates neuromuscular strength, spatial working memory, and depressive behaviors in A53T PD mice [173]. Thus, γ stimulation has the potential to modify PD progression.
Gamma brain stimulation for stroke
Stroke is a prominent cause of mortality and functional impairment that results from a transient or lasting decrease in cerebral perfusion [174]. After a stroke, neurons may undergo persistent depolarization, worsened by impaired interneuron function, which typically inhibits adjacent excitatory neurons [29]. During a stroke, rapid and extensive deterioration occurs within the neuronal structure and function, with limited restoration during reperfusion [175]. Moreover, the delicate balance between excitatory and inhibitory processes is disrupted, leading to reduced cerebral activity that impedes the dynamic reorganization of functions after the stroke [175]. However, the persistence of γ oscillations in the affected hemisphere is positively correlated with rehabilitation progress in stroke patients, suggesting that γ oscillations are integral to the post-stroke recovery process [44]. Hence, γ oscillation synchronization is strongly associated with clinical outcomes in stroke rehabilitation survivors [44].
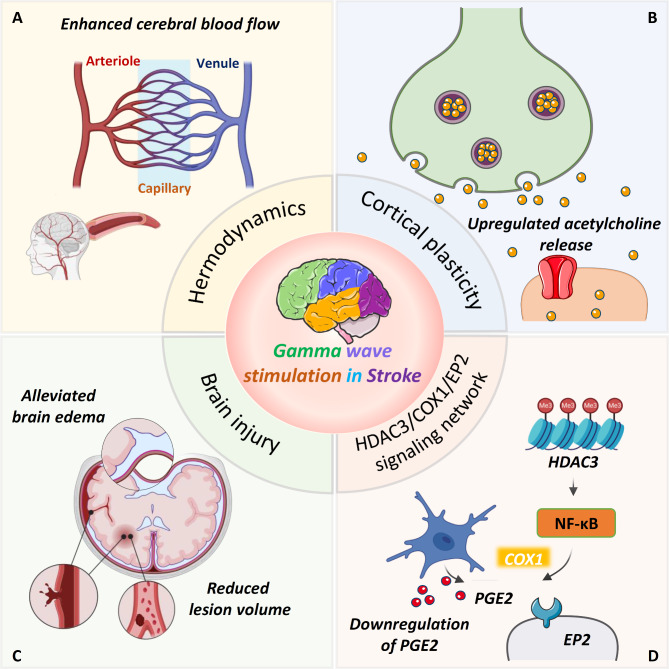
Recent findings suggest modulating cortical oscillatory dynamics during the acute phase may offer neuroprotection against stroke (Fig. 5) [29]. In the acute phase following stroke, optogenetic stimulation of fast-spiking interneurons at 40 Hz in the lesioned hemisphere activates inhibitory interneurons in the M1, reducing the incidence of spreading depolarizations [29]. Subsequently, activation of interneurons at 40 Hz alleviates brain edema and lesion volume, enhances cerebral blood flow, and improves behavioral outcomes of post-stroke mice [29]. In addition, the cholinergic neurons of the basal forebrain exert influence over an array of functions, including cortical plasticity, attention, and sensorimotor behavior [175]. Research indicates that acetylcholine (ACh) regulates cortical plasticity during the acute phase after stroke, playing a key role in recovery and compensation [176]. Thus, ACh innervation in the neocortex is thought to play a significant role in post-stroke recovery [176]. Optogenetic stimulation of the nucleus basalis during the post-stroke period increases ACh release, improving functional recovery and motor behavior in the photothrombotic stroke mouse model [175].
Fig. 5.
The beneficial effect of gamma stimulation in stroke. Gamma stimulation confers various benefits on stroke, including preserving synaptic plasticity, alleviating lesion volume, and maintaining cerebral blood flow. Additionally, γ stimulation downregulates the HDAC3/COX1/EP2 network and alleviates deficits in behavioral changes
Deficits in specific hippocampal oscillation frequencies are closely linked to cognitive dysfunction in the ischemic brain. Previous studies suggest that reduced cross-frequency coupling between θ and γ rhythms in hippocampal local field potentials is associated with impaired short- and long-term potentiation in the 2VO rat model [177]. Additionally, a persistent reduction in low γ oscillations has been identified in an anesthetized mouse model of unilateral hippocampal ischemia [178]. Visual stimulation at low γ frequency (30–50 Hz, 1 h/day, 14 days) restores phase-amplitude coupling with θ oscillations and rescues cognitive dysfunction in the 2VO mouse model [58]. Mechanistically, γ frequency sensory entrainment enhances synaptic plasticity via RGS12-regulated N-type CaV2.2 voltage-gated calcium channels (N-VGCC) [58].
Most evidence indicates that post-stroke phobic anxiety is widely prevalent, impeding the rehabilitation of patients and disrupting their usual activities [179]. Post-stroke anxiety is mediated by the up-regulation of histone deacetylase 3 (HDAC3) in activated microglia residing within the ischemic cortex, which facilitates the deacetylation process, subsequently leading to the nuclear translocation of p65 and activation of the NF-κB pathway [13, 180]. The activation of the NF-κB pathway further upregulates downstream target genes involved in prostaglandin synthesis, including cyclooxygenase-1 (COX1) and prostaglandin E2 (PGE2) [13, 180]. Subsequently, the interaction between PGE2 and EP2 receptors in the amygdala enhances anxiety and depression susceptibility to stress following ischemic stroke [13, 180]. Importantly, it is worth noting that γ flicker stimulation has shown efficacy in inhibiting the activation of cortical microglia, down-regulating the HDAC3/COX1/EP2 signaling network, and alleviating anxiety-like behaviors in the photothrombotic stroke mouse model [180].
Gamma brain stimulation for schizophrenia
SCZ presents as a prominent psychotic disorder with manifestations of positive symptoms (hallucinations and delusions), negative symptoms (avolition and anhedonia), and impairments in the prefrontal cortex-dependent cognitive domains, encompassing attention, cognitive flexibility, working memory, and social cognition [181]. Cognitive dysfunction is a fundamental characteristic of SCZ [182]. Cognitive impairments persist continuously throughout the illness, which is strongly correlated with long-term functional prognosis and frequently preceding the onset of overt psychosis [182, 183]. Regrettably, existing antipsychotic treatments exhibit only marginal efficacy in addressing cognitive symptoms [183]. Dysbindin-1, a protein containing a coiled-coil domain, exhibits reduced levels within the cerebral cortex of individuals afflicted with SCZ [184]. Gamma-frequency neuronal firing facilitates the translocation of dysbindin-1 into mitochondria, where it interacts with Drp1 and related receptors, inducing the formation of oligomeric Drp1 complexes that promote mitochondrial fission [184, 185]. As a result, Drp1 deficiency may diminish mitochondrial fission and disrupt γ oscillations in mouse models [185]. However, the augmentation of mitochondrial fission using a light-responsive mitochondrial fission system offers a potential solution to restore the integrity of the γ rhythm [185].
Disrupted GABAergic signaling and diminished activity of NMDA receptors are pivotal components in the pathophysiology of SCZ, disrupting the balance between excitation and inhibition in cortical and subcortical networks leading to abnormal neural oscillations [181]. While performing tasks requiring cognitive control, individuals with SCZ exhibit observable deviations in the PFC γ activity and concomitant impairments in PV + neuron functionality [45, 103]. Therefore, the pathological mechanisms that influence PV + neurons detrimentally affect γ oscillations and the synchronization of cortical neural activity, contributing to the cognitive dysfunction observed in SCZ [45].
Previous findings demonstrate that optogenetic stimulation effectively overcomes the inherent cognitive impairment in the SCZ mouse model, resulting in long-lasting cognitive flexibility improvements (Fig. 6) [69]. Remarkably, cognitive benefits from interneuron stimulation occur only when γ-frequency stimulation is applied at 40–60 Hz, not with stimulation protocols combining higher and lower frequencies [69]. Hence, γ-frequency activity originating from prefrontal interneurons is crucial in cognitive functions central to SCZ [69]. Nevertheless, future investigations must explore the mechanisms by which interneuron-driven γ oscillations facilitate cognitive enhancement. Additionally, patients diagnosed with SCZ exhibit impairments in high-frequency γ (≥ 60 Hz) oscillations, particularly during visual processing [186], suggesting that gamma entrainment techniques could offer a promising therapeutic intervention for these visual processing abnormalities.
Fig. 6.
The beneficial effect of gamma stimulation in SCZ and ASD. Gamma stimulation confers various benefits on SCZ and ASD, including preserving GABAergic signaling, alleviating mitochondrial fission, enhancing prefrontal interneuron activity, improving cognitive flexibility and control, maintaining prefrontal activity dynamic balance modulation, preserving cerebral cortex excitatory-inhibitory equilibrium and parvalbumin cell function, and alleviating behavioral changes
Gamma brain stimulation for autism spectrum disorder
ASD is a multifactorial condition influenced by genetic and environmental factors, leading to persistent deficiencies in social engagement and communication, sensory abnormalities, restricted interests, and repetitive behaviors [187]. ASD often co-occurs with disorders such as anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and obsessive-compulsive disorder (OCD), contributing to significant impairments in activities of daily living (ADLs) in both pediatric and adult populations [46]. Research shows that ASD is characterized by disrupted neuronal interactions within local networks, leading to aberrant γ-frequency brainwave activity patterns [188]. In patients with ASD, reduced interneuron numbers and dysregulated GABA receptor subunit expression reflect an imbalance between excitatory and inhibitory signaling, primarily mediated by the GABAergic pathway [38]. Similarly, several studies in ASD patients have found reduced spontaneous γ activity in frontal, temporal, and right-lateral regions, reduced left-hemispheric MEG steady-state γ responses, reduced task-related γ power, and reduced long- and short-range γ connectivity [46]. Deviant patterns of evoked and induced γ oscillations elicited by sensory tasks have likewise been documented in individuals with ASD within the visual domain [189] and the auditory domain [190]. Given the various body of evidence, we posit that abnormal γ frequency activity should be regarded as an integral component within the expansive pathophysiological construct of ASD (Fig. 6) [126, 191].
Many scholarly investigations within the medical domain substantiate a robust association linking the functionality of PV cells, γ oscillations, and impairments in social cognition [126]. Disruptions in inhibitory feedback, mediated by fast-spiking interneurons, lead to imbalances in excitation and inhibition within prefrontal circuits among young individuals, resulting in reduced coherence in evoked γ frequency synchronization [192]. These extensive alterations at structural and functional levels culminate in attenuated cognitive capabilities and impaired social proficiency [192]. Consequently, dynamic modulation of prefrontal activity during the early stages of neurodevelopment plays a pivotal role in governing the cognitive competence of adults, potentially exerting a critical impact on the manifestation of cognitive symptoms in neuropsychiatric disorders [192]. Manipulation of γ oscillations, particularly within the dorsolateral prefrontal cortex, correlates with enhancements in cognitive abilities, corrections of the excitatory-inhibitory balance within the cerebral cortex, and improvement of social deficits [126, 129]. Recent literature suggests that TMS therapy over the dorsolateral prefrontal cortex in ASD patients normalizes γ band irregularities, enhances cognitive functioning, and improves socio-behavioral impairments [187].
Dysfunction in synaptic neurotransmission may underlie intricate modifications in neural circuits, contributing to behavioral phenotypes in ASD [193, 194]. Deletion of the autism-associated Cntnap2 gene disrupts the density of PV + interneurons within the hippocampus, leading to imbalances in inhibitory neurotransmission in the perisomatic region [193]. Reduction in PV + interneuron density leads to decreased inhibition of CA1 pyramidal cells, resulting in deficits in spatial discrimination and alterations in frequency-dependent circuit dynamics in the hippocampus, such as disrupted γ oscillations, sharp-wave ripples, and theta-gamma modulation [193]. Current evidence suggests that the real-time modulation of the excitation-inhibition balance in the prefrontal cortex of Cntnap2-null mutant mice effectively alleviates social behavior deficits reminiscent of autism phenotypes [194]. Currently, γ entrainment of medial septal PV + interneurons restores aberrant low-frequency γ oscillation amplitudes and theta-gamma phase-amplitude coupling within the hippocampus, ameliorating spatial memory deficits [2]. Consequently, harnessing the potential of γ entrainment to enhance hippocampal circuit dynamics in ASD might yield similar benefits and warrants further investigation.
Discussion and future directions
Gamma oscillations are essential for sensory processing, memory consolidation, and cognitive function and are attenuated in neurodegenerative diseases and other brain disorders. Various techniques for brain stimulation have been shown to induce gamma oscillations. Importantly, synchronized light and sound stimulation at 40 Hz effectively induces corresponding brain activity at the same frequency [13, 195]. Overall, 40 Hz GENUS is associated with reduced neuroinflammation, enhanced synaptic transmission, and increased expression of genes related to synaptic plasticity. These effects lead to improvements in cognitive function [21, 23, 24, 58, 83, 196, 197]. Furthermore, 40 Hz GENUS leads to an increase in the expression of cytokines in microglia, normalization of circadian rhythms, and a reduction in Aβ plaque burden [61, 198, 199]. Additionally, other studies have demonstrated that multisensory 40 Hz stimulation enhances the glymphatic clearance rate of Aβ [200]. Despite these promising findings, some studies have failed to replicate these results. Another study has demonstrated that 40 Hz optogenetic stimulation effectively modulated spatial memory, while plaque loads were not altered [2]. Additionally, several studies have reported failures to replicate the natural γ oscillations, Aβ reduction, and microglial activation observed with 40 Hz GENUS [31]. The complex pathological changes associated with AD may lead to variability in the accuracy and effectiveness of the 40 Hz stimulation protocol, depending on different stages of the pathogenesis of AD [63]. Thus, possible reasons for the discrepancies above lie in the variations in stimulation modalities and assay time relative to stimulation. To better understand the discrepancies between these results and previously reported findings, it is essential to investigate specific parameters (such as optimal color, intensity, and frequency) that can effectively induce gamma entrainment. Research on flicker light stimulation for γ wave entrainment in humans indicates that pure white light at a brightness level of 400 cd/m² and a flicker frequency of 34–38 Hz may represent the most effective strategy for achieving γ entrainment [86]. In addition to stimulation parameters, another potential contributing factor to the discrepancies observed between studies may be individual variances among animals or patients and their specific responses to the stimulation. Animals or patients exhibit slight differences in their processing of visual sensory stimuli between dark and light cycles, which can result in distinct behavioral responses [55]. For example, a study that applied 40 Hz visual stimulation during the dark phase observed an increase in anxiety-like behaviors in 5XFAD mice, potentially due to differences in brain states and neuroregulatory systems associated with circadian rhythms [31]. Furthermore, it is crucial to investigate whether the presence of aversive behaviors could diminish the gamma entrainment effect and impede the clearance of amyloid proteins. Other studies reporting positive outcomes performed light stimulation during non-aversive phases [60, 74]. Therefore, establishing appropriate control groups to examine the influence of environmental factors on the effectiveness of GENUS interventions is imperative. In fact, non-invasive acoustic stimulation experiments demonstrate that, in both animals and humans, the application of slow oscillatory sound stimuli during sleep can enhance γ oscillations, potentially improving circadian mechanisms and sleep quality [22, 198].
The neuroprotective effects of induced gamma activity, particularly through 40 Hz GENUS, are promising. However, several questions remain about the underlying molecular pathways and the roles of different cell types, such as neurons and glial cells. Future investigations are essential to clarify the cellular mechanisms that regulate brain oscillations, thereby enhancing understanding of the neuroprotective mechanisms that mitigate disease progression. While the pronounced neuroprotective effects of 40 Hz GENUS in various neurodegenerative disease models are noteworthy, several unresolved questions remain and warrant further exploration. Notably, most existing studies concentrate on the early stages of pathological changes, leaving unanswered whether 40 Hz GENUS can reverse substantial neuronal loss once damage has occurred. In addition, different diseases or disorders may have specific frequency characteristics [186, 201]. For instance, the 60–90 Hz frequency range is associated with bradykinesia [202], while γ wave anomalies in the range of 40 Hz to 100 Hz are related to the spectrum of SCZ [203]. Consequently, future research should recognize that variations in gamma frequency across different diseases necessitate disease-specific applications of sensory entrainment. Additionally, it is crucial to assess whether acute or chronic interventions with GENUS result in greater improvements in brain function. While GENUS has demonstrated safety and feasibility in humans and positive outcomes in various animal studies, previous research has been limited by small sample sizes [49, 74, 198, 204]. Therefore, large-scale clinical trials are indispensable for rigorously assessing the efficacy of GENUS in improving disease outcomes. Additionally, determining how long the neuroprotective effects of GENUS last after cessation is crucial, as this information could inform long-term treatment strategies for sustained therapeutic benefit.
Conclusions
Natural gamma and 40 Hz sensory-induced steady-state oscillations likely engage distinct neurobiological mechanisms. Thus, elucidating the mechanisms underlying spontaneous, sensory-evoked, and optogenetically induced gamma entrainment could provide critical insights into the nature of brain oscillations. In summary, the 40 Hz GENUS, with its ability to modulate higher-order emotional and cognitive processing via multiple pathways, exerts pervasive effects on the brain, potentially mitigating pathological states. Thus, further investigation into the neurobiological mechanisms behind induced gamma activity could lead to novel therapeutic strategies for treating neurological disorders.
Acknowledgements
Not applicable.
Abbreviations
- AD
Alzheimer’s disease
- EEG
Electroencephalography
- GABA
γ-aminobutyric acid
- PD
Parkinson’s disease
- SCZ
Schizophrenia
- ASD
Autism spectrum disorder
- GENUS
Gamma ENtrainment Using Sensory stimuli
- Aβ
Amyloid-beta
- NF-κB
Nuclear factor κ-light-chain-enhancer of activated B cells
- LTD
Long-term depression
- PNN
Perineuronal nets
- ROS
Reactive oxygen species
- MEG
Magnetoencephalography
- CBF
Cerebral blood flow
- PCC
Posterior cingulate cortex
- DMN
Default mode network
- ISF
Invisible Spectral Flicker
- PBM
Photobiomodulation
- FSINs
Fast-spiking interneurons
- TES
Transcranial electrical stimulation
- tDCS
Transcranial direct current stimulation
- tRNS
Transcranial random noise stimulation
- tTIS
Transcranial alternating temporal interference
- tACS
Transcranial alternating current stimulation
- TMS
Transcranial Magnetic Stimulation
- SWR
Sharp wave-ripple
- α-Syn
α-synuclein
- LTP
Long-term potentiation
Author contributions
QD reviewed the literature and drafted the manuscript. QD, CW, and LY prepared the figures and tables. EP, LY, and RD edited the manuscript. JZ and TCL provided feedback on the manuscript. LY and RD supervised the writing process. All authors read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32100918 and 32300959), the project funded by China Postdoctoral Science Foundation (2021M690060 and 2022T150227), Guangzhou Scientific Research Grant (SL2022B04J00013 and SL2024A04J00578), and the SCNU Young Faculty Development Program (22KJ04).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interest exists.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Duan, Email: duanrui@m.scnu.edu.cn.
Luodan Yang, Email: luodanyang@m.scnu.edu.cn.
References
- 1.Mencarelli L, Monti L, Romanella S, Neri F, Koch G, Salvador R, et al. Local and distributed fMRI Changes Induced by 40 hz Gamma tACS of the bilateral Dorsolateral Prefrontal Cortex: a pilot study. Neural Plast. 2022;2022:6197505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun. 2019;10(1):5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole SR, Voytek B. Brain oscillations and the importance of waveform shape. Trends Cogn Sci. 2017;21(2):137–49. [DOI] [PubMed] [Google Scholar]
- 4.Takaura K, Tsuchiya N, Fujii N. Frequency-dependent spatiotemporal profiles of visual responses recorded with subdural ECoG electrodes in awake monkeys: differences between high- and low-frequency activity. NeuroImage. 2016;124(Pt A):557–72. [DOI] [PubMed] [Google Scholar]
- 5.Lasztoczi B, Klausberger T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 2014;81(5):1126–39. [DOI] [PubMed] [Google Scholar]
- 6.Struber M, Sauer JF, Jonas P, Bartos M. Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nat Commun. 2017;8(1):758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han C, Wang T, Yang Y, Wu Y, Li Y, Dai W, et al. Multiple gamma rhythms carry distinct spatial frequency information in primary visual cortex. PLoS Biol. 2021;19(12):e3001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan LL, Oswald MJ, Heinl C, Retana Romero OA, Kaushalya SK, Monyer H, et al. Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat Commun. 2019;10(1):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Headley DB, Pare D. In sync: gamma oscillations and emotional memory. Front Behav Neurosci. 2013;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, et al. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex. 2015;25(6):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra A, Colella D, Giangrosso M, Cannavacciuolo A, Paparella G, Fabbrini G, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson’s disease. Brain. 2022;145(1):224–36. [DOI] [PubMed] [Google Scholar]
- 12.Headley DB, Kyriazi P, Feng F, Nair S, Pare D. Gamma oscillations in the basolateral amygdala: localization, microcircuitry, and behavioral correlates. J Neurosci. 2021;41(28):6087–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan A, Wang S, Huang A, Qiu C, Li Y, Li X, et al. The role of gamma oscillations in central nervous system diseases: mechanism and treatment. Front Cell Neurosci. 2022;16:962957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie AK, Jones EA, Lin YH, Karlsson MP, Kay K, Yoon SY, et al. Apolipoprotein E4 causes age-dependent disruption of slow Gamma oscillations during hippocampal Sharp-Wave ripples. Neuron. 2016;90(4):740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stam CJ, van Cappellen AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer’s Disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19(6):562–74. [DOI] [PubMed] [Google Scholar]
- 16.Sauer JF, Struber M, Bartos M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife. 2015;4. [DOI] [PMC free article] [PubMed]
- 17.Voget M, Rummel J, Avchalumov Y, Sohr R, Haumesser JK, Rea E, et al. Altered local field potential activity and serotonergic neurotransmission are further characteristics of the Flinders sensitive line rat model of depression. Behav Brain Res. 2015;291:299–305. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald PJ, Watson BO. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry. 2018;8(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benussi A, Cantoni V, Cotelli MS, Cotelli M, Brattini C, Datta A, et al. Exposure to gamma tACS in Alzheimer’s disease: a randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 2021;14(3):531–40. [DOI] [PubMed] [Google Scholar]
- 20.Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, et al. Multi-sensory Gamma Stimulation ameliorates Alzheimer’s-Associated Pathology and improves cognition. Cell. 2019;177(2):256–71. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DN, et al. Gamma Entrainment binds higher-order brain regions and offers neuroprotection. Neuron. 2019;102(5):929–43. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Ying Y, Deng Q, Zhang W, Zhu H, Lin Z, et al. Non-invasive 40-Hz light flicker ameliorates Alzheimer’s-Associated Rhythm Disorder via regulating Central Circadian Clock in mice. Front Physiol. 2020;11:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jirakittayakorn N, Wongsawat Y. Brain responses to 40-Hz binaural beat and effects on emotion and memory. Int J Psychophysiol. 2017;120:96–107. [DOI] [PubMed] [Google Scholar]
- 24.Singer AC, Martorell AJ, Douglas JM, Abdurrob F, Attokaren MK, Tipton J, et al. Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nat Protoc. 2018;13(8):1850–68. [DOI] [PubMed] [Google Scholar]
- 25.Zhou D, Li A, Li X, Zhuang W, Liang Y, Zheng CY, et al. Effects of 40 hz transcranial alternating current stimulation (tACS) on cognitive functions of patients with Alzheimer’s disease: a randomised, double-blind, sham-controlled clinical trial. J Neurol Neurosurg Psychiatry. 2022;93(5):568–70. [DOI] [PubMed] [Google Scholar]
- 26.Legros A, Modolo J, Brown S, Roberston J, Thomas AW. Effects of a 60 hz magnetic field exposure up to 3000 muT on human brain activation as measured by functional magnetic resonance imaging. PLoS ONE. 2015;10(7):e0132024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni J, Wunderle T, Lewis CM, Desimone R, Diester I, Fries P. Gamma-Rhythmic Gain Modulation Neuron. 2016;92(1):240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Liu Q, Zhang F, Fu Y, Zhu X, Weng X, et al. Microglia modulation with 1070-nm light attenuates Abeta burden and cognitive impairment in Alzheimer’s disease mouse model. Light Sci Appl. 2021;10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balbi M, Xiao D, Jativa Vega M, Hu H, Vanni MP, Bernier LP, et al. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep. 2021;34(5):108696. [DOI] [PubMed] [Google Scholar]
- 30.Antonoudiou P, Tan YL, Kontou G, Upton AL, Mann EO. Parvalbumin and Somatostatin Interneurons Contribute to the generation of hippocampal Gamma oscillations. J Neurosci. 2020;40(40):7668–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soula M, Martin-Avila A, Zhang Y, Dhingra A, Nitzan N, Sadowski MJ, et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat Neurosci. 2023;26(4):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manippa V, Palmisano A, Filardi M, Vilella D, Nitsche MA, Rivolta D, et al. An update on the use of gamma (multi)sensory stimulation for Alzheimer’s disease treatment. Front Aging Neurosci. 2022;14:1095081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramlakhan JU, Ma M, Zomorrodi R, Blumberger DM, Noda Y, Barr MS. The role of Gamma oscillations in the pathophysiology of Substance Use disorders. J Pers Med. 2020;11(1). [DOI] [PMC free article] [PubMed]
- 34.Adaikkan C, Tsai LH. Gamma Entrainment: impact on neurocircuits, Glia, and Therapeutic opportunities. Trends Neurosci. 2020;43(1):24–41. [DOI] [PubMed] [Google Scholar]
- 35.Bartoli E, Bosking W, Chen Y, Li Y, Sheth SA, Beauchamp MS, et al. Functionally distinct Gamma Range Activity revealed by stimulus tuning in human visual cortex. Curr Biol. 2019;29(20):3345–58. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic inhibitory interneurons. Physiol Rev. 2017;97(4):1619–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. [DOI] [PubMed] [Google Scholar]
- 38.Hashemi E, Ariza J, Rogers H, Noctor SC, Martinez-Cerdeno V. The number of parvalbumin-expressing interneurons is decreased in the Prefrontal Cortex in Autism. Cereb Cortex. 2017;27(3):1931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juarez P, Martinez Cerdeno V. Parvalbumin and parvalbumin chandelier interneurons in autism and other psychiatric disorders. Front Psychiatry. 2022;13:913550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Struber D, Herrmann CS. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: a review and perspective. Int J Psychophysiol. 2020;152:15–25. [DOI] [PubMed] [Google Scholar]
- 41.Basar E. A review of gamma oscillations in healthy subjects and in cognitive impairment. Int J Psychophysiol. 2013;90(2):99–117. [DOI] [PubMed] [Google Scholar]
- 42.Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol. 2018;52:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerra A, Asci F, D’Onofrio V, Sveva V, Bologna M, Fabbrini G, et al. Enhancing Gamma oscillations restores primary motor cortex plasticity in Parkinson’s Disease. J Neurosci. 2020;40(24):4788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrino G, Arcara G, Cortese AM, Weis L, Di Tomasso S, Marioni G, et al. Cortical gamma-synchrony measured with magnetoencephalography is a marker of clinical status and predicts clinical outcome in stroke survivors. Neuroimage Clin. 2019;24:102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77(12):1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayarian FB, Jannati A, Rotenberg A, Santarnecchi E. Targeting Gamma-related pathophysiology in Autism Spectrum Disorder using Transcranial Electrical Stimulation: opportunities and challenges. Autism Res. 2020;13(7):1051–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016;29(3):202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17(12):777–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Q, Colon-Motas KM, Pybus AF, Piendel L, Seppa JK, Walker ML, et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7(1):e12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clements-Cortes A, Ahonen H, Evans M, Freedman M, Bartel L. Short-term effects of rhythmic sensory stimulation in Alzheimer’s Disease: an exploratory pilot study. J Alzheimers Dis. 2016;52(2):651–60. [DOI] [PubMed] [Google Scholar]
- 51.Khachatryan E, Wittevrongel B, Reinartz M, Dauwe I, Carrette E, Meurs A, et al. Cognitive tasks propagate the neural entrainment in response to a visual 40 hz stimulation in humans. Front Aging Neurosci. 2022;14:1010765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Attokaren MK, Jeong N, Blanpain L, Paulson AL, Garza KM, Borron B et al. BrainWAVE: a flexible method for noninvasive stimulation of brain rhythms across species. eNeuro. 2023;10(2). [DOI] [PMC free article] [PubMed]
- 53.Jaeger C, Nuttall R, Zimmermann J, Dowsett J, Preibisch C, Sorg C, et al. Targeted rhythmic visual stimulation at individual participants’ intrinsic alpha frequency causes selective increase of occipitoparietal BOLD-fMRI and EEG functional connectivity. NeuroImage. 2023;270:119981. [DOI] [PubMed] [Google Scholar]
- 54.Noda Y, Takano M, Hayano M, Li X, Wada M, Nakajima S et al. Photobiological neuromodulation of resting-state EEG and steady-state visual-evoked potentials by 40 Hz Violet Light Optical Stimulation in healthy individuals. J Pers Med. 2021;11(6). [DOI] [PMC free article] [PubMed]
- 55.Zhang Y, Zhang Z, Luo L, Tong H, Chen F, Hou ST. 40 hz light flicker alters human brain Electroencephalography microstates and Complexity implicated in Brain diseases. Front Neurosci. 2021;15:777183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venturino A, Schulz R, De Jesus-Cortes H, Maes ME, Nagy B, Reilly-Andujar F, et al. Microglia enable mature perineuronal nets disassembly upon anesthetic ketamine exposure or 60-Hz light entrainment in the healthy brain. Cell Rep. 2021;36(1):109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L, Yu M, Lin R, Wang Y, Zhuo Z, Cheng N, et al. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat Commun. 2020;11(1):3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian T, Qin X, Wang Y, Shi Y, Yang X. 40 hz light flicker promotes Learning and Memory via Long Term Depression in Wild-Type mice. J Alzheimers Dis. 2021;84(3):983–93. [DOI] [PubMed] [Google Scholar]
- 60.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540(7632):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garza KM, Zhang L, Borron B, Wood LB, Singer AC. Gamma Visual Stimulation induces a Neuroimmune Signaling Profile distinct from Acute Neuroinflammation. J Neurosci. 2020;40(6):1211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auksztulewicz R, Barascud N, Cooray G, Nobre AC, Chait M, Friston K. The cumulative effects of predictability on synaptic Gain in the Auditory Processing Stream. J Neurosci. 2017;37(28):6751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahu PP, Tseng P. Gamma sensory entrainment for cognitive improvement in neurodegenerative diseases: opportunities and challenges ahead. Front Integr Neurosci. 2023;17:1146687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses. Hear Res. 1996;101(1–2):62–74. [DOI] [PubMed] [Google Scholar]
- 65.Ross B, Borgmann C, Draganova R, Roberts LE, Pantev C. A high-precision magnetoencephalographic study of human auditory steady-state responses to amplitude-modulated tones. J Acoust Soc Am. 2000;108(2):679–91. [DOI] [PubMed] [Google Scholar]
- 66.Schuler AL, Ferrazzi G, Colenbier N, Arcara G, Piccione F, Ferreri F, et al. Auditory driven gamma synchrony is associated with cortical thickness in widespread cortical areas. NeuroImage. 2022;255:119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farahani ED, Wouters J, van Wieringen A. Brain mapping of auditory steady-state responses: a broad view of cortical and subcortical sources. Hum Brain Mapp. 2021;42(3):780–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pellegrino G, Arcara G, Di Pino G, Turco C, Maran M, Weis L, et al. Transcranial direct current stimulation over the sensory-motor regions inhibits gamma synchrony. Hum Brain Mapp. 2019;40(9):2736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron. 2015;85(6):1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tada M, Kirihara K, Ishishita Y, Takasago M, Kunii N, Uka T, et al. Global and parallel cortical Processing based on auditory Gamma Oscillatory responses in humans. Cereb Cortex. 2021;31(10):4518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 hz. J Neurosci. 2002;22(23):10501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saiu S, Grosso E. Controlled audio-visual stimulation for anxiety reduction. Comput Methods Programs Biomed. 2022;223:106898. [DOI] [PubMed] [Google Scholar]
- 73.Teplan M, Krakovska A, Stolc S. Direct effects of audio-visual stimulation on EEG. Comput Methods Programs Biomed. 2011;102(1):17–24. [DOI] [PubMed] [Google Scholar]
- 74.Chan D, Suk HJ, Jackson BL, Milman NP, Stark D, Klerman EB, et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: results of feasibility and pilot studies. PLoS ONE. 2022;17(12):e0278412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, Liang P, Jia X, Jin G, Song H, Han Y, et al. The baseline and longitudinal changes of PCC connectivity in mild cognitive impairment: a combined structure and resting-state fMRI study. PLoS ONE. 2012;7(5):e36838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation. J Neurosci. 2005;25(44):10094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yepes M, Winkles JA. Inhibition of TWEAK activity as a new treatment for inflammatory and degenerative diseases. Drug News Perspect. 2006;19(10):589–95. [DOI] [PubMed] [Google Scholar]
- 78.Suk HJ, Buie N, Xu G, Banerjee A, Boyden ES, Tsai LH. Vibrotactile stimulation at gamma frequency mitigates pathology related to neurodegeneration and improves motor function. Front Aging Neurosci. 2023;15:1129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boerema AS, Heesterbeek M, Boersma SA, Schoemaker R, de Vries EFJ, van Heuvelen MJG, et al. Beneficial effects of whole body vibration on brain functions in mice and humans. Dose Response. 2018;16(4):1559325818811756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jamali S, Ross B. Sustained changes in somatosensory gamma responses after brief vibrotactile stimulation. NeuroReport. 2014;25(7):537–41. [DOI] [PubMed] [Google Scholar]
- 81.Choi DS, Lee HJ, Shin YI, Lee A, Kim HG, Kim YH. Modulation of cortical activity by high-frequency whole-body vibration Exercise: an fNIRS Study. J Sport Rehabil. 2019;28(7):665–70. [DOI] [PubMed] [Google Scholar]
- 82.Oroszi T, de Boer SF, Nyakas C, Schoemaker RG, van der Zee EA. Chronic whole body vibration ameliorates hippocampal neuroinflammation, anxiety-like behavior, memory functions and motor performance in aged male rats dose dependently. Sci Rep. 2022;12(1):9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SS, Park HS, Kim CJ, Kang HS, Kim DH, Baek SS, et al. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park SS, Park HS, Kim CJ, Baek SS, Park SY, Anderson C, et al. Combined effects of aerobic exercise and 40-Hz light flicker exposure on early cognitive impairments in Alzheimer’s disease of 3xTg mice. J Appl Physiol (1985). 2022;132(4):1054–68. [DOI] [PubMed] [Google Scholar]
- 85.Hermes D, Kasteleijn-Nolst Trenite DGA, Winawer J. Gamma oscillations and photosensitive epilepsy. Curr Biol. 2017;27(9):R336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee K, Park Y, Suh SW, Kim SS, Kim DW, Lee J, et al. Optimal flickering light stimulation for entraining gamma waves in the human brain. Sci Rep. 2021;11(1):16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agger MP, Carstensen MS, Henney MA, Hansen LS, Baandrup AO, Nguyen M, et al. Novel invisible spectral flicker induces 40 hz neural entrainment with similar spatial distribution as 40 hz stroboscopic light. J Alzheimers Dis. 2022;88(1):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agger MP, Danielsen ER, Carstensen MS, Nguyen NM, Horning M, Henney MA, et al. Safety, Feasibility, and potential clinical efficacy of 40 hz invisible spectral flicker versus placebo in patients with mild-to-moderate Alzheimer’s Disease: a Randomized, Placebo-Controlled, Double-Blinded, Pilot Study. J Alzheimers Dis. 2023;92(2):653–65. [DOI] [PubMed] [Google Scholar]
- 89.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS ONE. 2007;2(11):e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74(4):183–211. [DOI] [PubMed] [Google Scholar]
- 92.Lin Z, Hou G, Yao Y, Zhou Z, Zhu F, Liu L, et al. 40-Hz blue light changes hippocampal activation and functional connectivity underlying Recognition Memory. Front Hum Neurosci. 2021;15:739333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front Neurosci. 2015;9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X, Dmochowski JP, Zeng L, Kallioniemi E, Husain M, Gonzalez-Lima F, et al. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. Neurophotonics. 2019;6(2):025013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18(4):222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mirzayi P, Shobeiri P, Kalantari A, Perry G, Rezaei N. Optogenetics: implications for Alzheimer’s disease research and therapy. Mol Brain. 2022;15(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obi-Nagata K, Temma Y, Hayashi-Takagi A. Synaptic functions and their disruption in schizophrenia: from clinical evidence to synaptic optogenetics in an animal model. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(5):179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164(1–2):208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao W, Lin S, Xia QQ, Du YL, Yang Q, Zhang MY, et al. Gamma Oscillation Dysfunction in mPFC leads to Social deficits in Neuroligin 3 R451C Knockin mice. Neuron. 2018;97(6):1253–60. e7. [DOI] [PubMed] [Google Scholar]
- 102.Kanta V, Pare D, Headley DB. Closed-loop control of gamma oscillations in the amygdala demonstrates their role in spatial memory consolidation. Nat Commun. 2019;10(1):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson CA, Fouda S, Sakata S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer’s disease pathology. Sci Rep. 2020;10(1):15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu A, Voroslakos M, Kronberg G, Henin S, Krause MR, Huang Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018;9(1):5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rampersad S, Roig-Solvas B, Yarossi M, Kulkarni PP, Santarnecchi E, Dorval AD, et al. Prospects for transcranial temporal interference stimulation in humans: a computational study. NeuroImage. 2019;202:116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M, Cheng I, Sasegbon A, Dou Z, Hamdy S. Exploring parameters of gamma transcranial alternating current stimulation (tACS) and full-spectrum transcranial random noise stimulation (tRNS) on human pharyngeal cortical excitability. Neurogastroenterol Motil. 2021;33(9):e14173. [DOI] [PubMed] [Google Scholar]
- 108.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electr Fields Cell. 2017;169(6):1029–e4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moret B, Donato R, Nucci M, Cona G, Campana G. Transcranial random noise stimulation (tRNS): a wide range of frequencies is needed for increasing cortical excitability. Sci Rep. 2019;9(1):15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tavakoli AV, Yun K. Transcranial Alternating Current Stimulation (tACS) mechanisms and protocols. Front Cell Neurosci. 2017;11:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elyamany O, Leicht G, Herrmann CS, Mulert C. Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. 2021;271(1):135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benussi A, Cantoni V, Grassi M, Brechet L, Michel CM, Datta A, et al. Increasing Brain Gamma Activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s Disease. Ann Neurol. 2022;92(2):322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moisa M, Polania R, Grueschow M, Ruff CC. Brain Network mechanisms Underlying Motor Enhancement by Transcranial Entrainment of Gamma oscillations. J Neurosci. 2016;36(47):12053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miyaguchi S, Otsuru N, Kojima S, Saito K, Inukai Y, Masaki M, et al. Transcranial Alternating Current Stimulation with Gamma Oscillations over the Primary Motor Cortex and Cerebellar Hemisphere Improved Visuomotor performance. Front Behav Neurosci. 2018;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santarnecchi E, Biasella A, Tatti E, Rossi A, Prattichizzo D, Rossi S. High-gamma oscillations in the motor cortex during visuo-motor coordination: a tACS interferential study. Brain Res Bull. 2017;131:47–54. [DOI] [PubMed] [Google Scholar]
- 118.Guerra A, Suppa A, Bologna M, D’Onofrio V, Bianchini E, Brown P, et al. Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimul. 2018;11(4):734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guerra A, Suppa A, Asci F, De Marco G, D’Onofrio V, Bologna M, et al. LTD-like plasticity of the human primary motor cortex can be reversed by gamma-tACS. Brain Stimul. 2019;12(6):1490–9. [DOI] [PubMed] [Google Scholar]
- 120.Park J, Lee C, Lee S, Im CH. 80 hz but not 40 hz, transcranial alternating current stimulation of 80 hz over right intraparietal sulcus increases visuospatial working memory capacity. Sci Rep. 2022;12(1):13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson TW, McDermott TJ, Mills MS, Coolidge NM, Heinrichs-Graham E. tDCS modulates visual Gamma oscillations and basal alpha activity in Occipital cortices: evidence from MEG. Cereb Cortex. 2018;28(5):1597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoy KE, Bailey NW, Arnold SL, Fitzgerald PB. The effect of transcranial direct current stimulation on gamma activity and working memory in schizophrenia. Psychiatry Res. 2015;228(2):191–6. [DOI] [PubMed] [Google Scholar]
- 123.Broncel A, Bocian R, Klos-Wojtczak P, Kulbat-Warycha K, Konopacki J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull. 2020;155:37–47. [DOI] [PubMed] [Google Scholar]
- 124.Yu Y, Jiang X, Fang X, Wang Y, Liu P, Ling J, et al. Transauricular Vagal Nerve Stimulation at 40 Hz Inhibits Hippocampal P2X7R/NLRP3/Caspase-1 Signaling and Improves Spatial Learning and Memory in 6-Month-Old APP/PS1 Mice. Neuromodulation. 2023;26(3):589–600. [DOI] [PubMed] [Google Scholar]
- 125.Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21(14):1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Casanova MF, Shaban M, Ghazal M, El-Baz AS, Casanova EL, Opris I et al. Effects of Transcranial Magnetic Stimulation Therapy on Evoked and Induced Gamma oscillations in children with Autism Spectrum Disorder. Brain Sci. 2020;10(7). [DOI] [PMC free article] [PubMed]
- 127.Liu C, Han T, Xu Z, Liu J, Zhang M, Du J, et al. Modulating Gamma oscillations promotes Brain Connectivity to improve cognitive impairment. Cereb Cortex. 2022;32(12):2644–56. [DOI] [PubMed] [Google Scholar]
- 128.Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2009;34(11):2359–67. [DOI] [PubMed] [Google Scholar]
- 129.Farzan F, Barr MS, Sun Y, Fitzgerald PB, Daskalakis ZJ. Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Ann N Y Acad Sci. 2012;1265:25–35. [DOI] [PubMed] [Google Scholar]
- 130.Casanova MF, Baruth JM, El-Baz A, Tasman A, Sears L, Sokhadze E. Repetitive Transcranial Magnetic Stimulation (rTMS) modulates event-related potential (ERP) indices of attention in Autism. Transl Neurosci. 2012;3(2):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang L, Wu C, Parker E, Li Y, Dong Y, Tucker L, et al. Non-invasive photobiomodulation treatment in an Alzheimer Disease-like transgenic rat model. Theranostics. 2022;12(5):2205–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang L, Wu C, Li Y, Dong Y, Wu CY, Lee RH, et al. Long-term exercise pre-training attenuates Alzheimer’s disease-related pathology in a transgenic rat model of Alzheimer’s disease. Geroscience. 2022;44(3):1457–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Korczyn AD. Why have we failed to cure Alzheimer’s disease? J Alzheimers Dis. 2012;29(2):275–82. [DOI] [PubMed] [Google Scholar]
- 134.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s Disease. Front Neurosci. 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kurudenkandy FR, Zilberter M, Biverstal H, Presto J, Honcharenko D, Stromberg R, et al. Amyloid-beta-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. J Neurosci. 2014;34(34):11416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van den Berg M, Toen D, Verhoye M, Keliris GA. Alterations in theta-gamma coupling and sharp wave-ripple, signs of prodromal hippocampal network impairment in the TgF344-AD rat model. Front Aging Neurosci. 2023;15:1081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park K, Lee J, Jang HJ, Richards BA, Kohl MM, Kwag J. Optogenetic activation of parvalbumin and somatostatin interneurons selectively restores theta-nested gamma oscillations and oscillation-induced spike timing-dependent long-term potentiation impaired by amyloid beta oligomers. BMC Biol. 2020;18(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, et al. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory deficits in an Alzheimer’s Disease Model. Neuron. 2016;92(1):114–25. [DOI] [PubMed] [Google Scholar]
- 139.Mikulovic S, Restrepo CE, Siwani S, Bauer P, Pupe S, Tort ABL, et al. Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat Commun. 2018;9(1):3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chung H, Park K, Jang HJ, Kohl MM, Kwag J. Dissociation of somatostatin and parvalbumin interneurons circuit dysfunctions underlying hippocampal theta and gamma oscillations impaired by amyloid beta oligomers in vivo. Brain Struct Funct. 2020;225(3):935–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a mouse model of Alzheimer’s Disease. Neuron. 2018;98(1):75–e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klein AS, Donoso JR, Kempter R, Schmitz D, Beed P. Early cortical changes in Gamma oscillations in Alzheimer’s Disease. Front Syst Neurosci. 2016;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot JG, Quirion R, et al. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Abeta overproduction in a mouse model of Alzheimer’s disease. Eur J Neurosci. 2013;37(12):1896–902. [DOI] [PubMed] [Google Scholar]
- 145.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14(2):147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75(4):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richetin K, Steullet P, Pachoud M, Perbet R, Parietti E, Maheswaran M, et al. Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat Neurosci. 2020;23(12):1567–79. [DOI] [PubMed] [Google Scholar]
- 148.Yan Y, Aierken A, Wang C, Song D, Ni J, Wang Z, et al. A potential biomarker of preclinical Alzheimer’s disease: the olfactory dysfunction and its pathogenesis-based neural circuitry impairments. Neurosci Biobehav Rev. 2022;132:857–69. [DOI] [PubMed] [Google Scholar]
- 149.Li W, Li S, Shen L, Wang J, Wu X, Li J, et al. Impairment of dendrodendritic inhibition in the olfactory bulb of APP/PS1 mice. Front Aging Neurosci. 2019;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen M, Chen Y, Huo Q, Wang L, Tan S, Misrani A, et al. Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models. Mol Neurodegener. 2021;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bosman CA, Lansink CS, Pennartz CM. Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur J Neurosci. 2014;39(11):1982–99. [DOI] [PubMed] [Google Scholar]
- 152.Cannon J, McCarthy MM, Lee S, Lee J, Borgers C, Whittington MA, et al. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci. 2014;39(5):705–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981;78(4):2643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88(24):11037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jung H, Lee Y, Lee SH, Sohn JH. Auditory or Audiovisual Stimulation ameliorates cognitive impairment and neuropathology in ApoE4 Knock-In mice. Int J Mol Sci. 2023;24(2). [DOI] [PMC free article] [PubMed]
- 156.Bobola MS, Chen L, Ezeokeke CK, Olmstead TA, Nguyen C, Sahota A, et al. Transcranial focused ultrasound, pulsed at 40 hz, activates microglia acutely and reduces Abeta load chronically, as demonstrated in vivo. Brain Stimul. 2020;13(4):1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nazari M, Vajed-Samiei T, Torabi N, Fahanik-Babaei J, Saghiri R, Khodagholi F, et al. The 40-Hz White Light-Emitting Diode (LED) improves the structure-function of the Brain mitochondrial KATP Channel and respiratory chain activities in amyloid Beta toxicity. Mol Neurobiol. 2022;59(4):2424–40. [DOI] [PubMed] [Google Scholar]
- 158.Nazari M, Jafari A, Torabi N, Vajed-Samiei T, Ghasemi R, Fahanik-Babaei J, et al. The Effect of 40-Hz white LED therapy on structure-function of Brain mitochondrial ATP-Sensitive Ca-Activated large-conductance Potassium Channel in amyloid Beta toxicity. Neurotox Res. 2022;40(5):1380–92. [DOI] [PubMed] [Google Scholar]
- 159.Homolak J, Mudrovcic M, Vukic B, Toljan K. Circadian Rhythm and Alzheimer’s Disease. Med Sci (Basel). 2018;6(3). [DOI] [PMC free article] [PubMed]
- 160.Phan TX, Malkani RG. Sleep and circadian rhythm disruption and stress intersect in Alzheimer’s disease. Neurobiol Stress. 2019;10:100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vanderheyden WM, Lim MM, Musiek ES, Gerstner JR. Alzheimer’s Disease and Sleep-Wake disturbances: amyloid, astrocytes, and animal models. J Neurosci. 2018;38(12):2901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oblak AL, Lin PB, Kotredes KP, Pandey RS, Garceau D, Williams HM, et al. Comprehensive evaluation of the 5XFAD mouse model for preclinical testing applications: a MODEL-AD study. Front Aging Neurosci. 2021;13:713726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Traikapi A, Konstantinou N. Gamma oscillations in Alzheimer’s Disease and their potential therapeutic role. Front Syst Neurosci. 2021;15:782399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531(7595):508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dhaynaut M, Sprugnoli G, Cappon D, Macone J, Sanchez JS, Normandin MD, et al. Impact of 40 hz transcranial Alternating Current Stimulation on Cerebral Tau Burden in patients with Alzheimer’s Disease: a Case Series. J Alzheimers Dis. 2022;85(4):1667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808. [DOI] [PubMed] [Google Scholar]
- 167.Jagadeesan AJ, Murugesan R, Vimala Devi S, Meera M, Madhumala G, Vishwanathan Padmaja M, et al. Current trends in etiology, prognosis and therapeutic aspects of Parkinson’s disease: a review. Acta Biomed. 2017;88(3):249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lofredi R, Neumann WJ, Bock A, Horn A, Huebl J, Siegert S et al. Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson’s disease. Elife. 2018;7. [DOI] [PMC free article] [PubMed]
- 169.Nowak M, Hinson E, van Ede F, Pogosyan A, Guerra A, Quinn A, et al. Driving Human Motor cortical oscillations leads to behaviorally relevant changes in local GABA(A) inhibition: a tACS-TMS study. J Neurosci. 2017;37(17):4481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Udupa K, Chen R. Motor cortical plasticity in Parkinson’s disease. Front Neurol. 2013;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T, et al. Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci. 2012;32(31):10541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mosabbir A, Almeida QJ, Ahonen H. The Effects of Long-Term 40-Hz Physioacoustic Vibrations on Motor Impairments in Parkinson’s Disease: A Double-Blinded Randomized Control Trial. Healthc (Basel). 2020;8(2). [DOI] [PMC free article] [PubMed]
- 173.Liu Y, Liu H, Lu Y, Yin X, Lu W, Lian X, et al. Non-invasive auditory and visual stimulation attenuates alpha-synuclein deposition and improves motor and non-motor symptoms in PD mice. Exp Neurol. 2023;364:114396. [DOI] [PubMed] [Google Scholar]
- 174.Hakon J, Quattromani MJ, Sjolund C, Tomasevic G, Carey L, Lee JM, et al. Multisensory stimulation improves functional recovery and resting-state functional connectivity in the mouse brain after stroke. Neuroimage Clin. 2018;17:717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mirza Agha B, Akbary R, Ghasroddashti A, Nazari-Ahangarkolaee M, Whishaw IQ, Mohajerani MH. Cholinergic upregulation by optogenetic stimulation of nucleus basalis after photothrombotic stroke in forelimb somatosensory cortex improves endpoint and motor but not sensory control of skilled reaching in mice. J Cereb Blood Flow Metab. 2021;41(7):1608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for Rehabilitation after Stroke. Front Neurosci. 2019;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu X, Zheng C, Zhang T. Reduction in LFP cross-frequency coupling between theta and gamma rhythms associated with impaired STP and LTP in a rat model of brain ischemia. Front Comput Neurosci. 2013;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barth AM, Mody I. Changes in hippocampal neuronal activity during and after unilateral selective hippocampal ischemia in vivo. J Neurosci. 2011;31(3):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Knapp P, Dunn-Roberts A, Sahib N, Cook L, Astin F, Kontou E, et al. Frequency of anxiety after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2020;15(3):244–55. [DOI] [PubMed] [Google Scholar]
- 180.Zhu H, Guo Y, Huang A, Shen H, Chen Y, Song J, et al. HDAC3-Regulated PGE2 production by Microglia induces phobic anxiety susceptibility after stroke and pointedly exploiting a Signal-targeted Gamma Visual Stimulation New Therapy. Front Immunol. 2022;13:845678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Maas DA, Eijsink VD, Spoelder M, van Hulten JA, De Weerd P, Homberg JR, et al. Interneuron hypomyelination is associated with cognitive inflexibility in a rat model of schizophrenia. Nat Commun. 2020;11(1):2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Minzenberg MJ, Carter CS. Developing treatments for impaired cognition in schizophrenia. Trends Cogn Sci. 2012;16(1):35–42. [DOI] [PubMed] [Google Scholar]
- 184.Wang H, Xu J, Lazarovici P, Zheng W. Dysbindin-1 involvement in the etiology of Schizophrenia. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed]
- 185.Zhao J, Zhu H, Duan K, Petralia RS, Wang YX, Gu Q, et al. Dysbindin-1 regulates mitochondrial fission and gamma oscillations. Mol Psychiatry. 2021;26(9):4633–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grutzner C, Wibral M, Sun L, Rivolta D, Singer W, Maurer K, et al. Deficits in high- (> 60 hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci. 2013;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Casanova MF, Sokhadze EM, Casanova EL, Li X. Transcranial Magnetic Stimulation in Autism Spectrum disorders: neuropathological underpinnings and clinical correlations. Semin Pediatr Neurol. 2020;35:100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Casanova MF, Shaban M, Ghazal M, El-Baz AS, Casanova EL, Sokhadze EM. Ringing decay of Gamma oscillations and Transcranial Magnetic Stimulation Therapy in Autism Spectrum Disorder. Appl Psychophysiol Biofeedback. 2021;46(2):161–73. [DOI] [PubMed] [Google Scholar]
- 189.Snijders TM, Milivojevic B, Kemner C. Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. Neuroimage Clin. 2013;3:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun L, Grutzner C, Bolte S, Wibral M, Tozman T, Schlitt S, et al. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci. 2012;32(28):9563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rojas DC, Wilson LB. Gamma-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8(3):353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bitzenhofer SH, Popplau JA, Chini M, Marquardt A, Hanganu-Opatz IL. A transient developmental increase in prefrontal activity alters network maturation and causes cognitive dysfunction in adult mice. Neuron. 2021;109(8):1350–64. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paterno R, Marafiga JR, Ramsay H, Li T, Salvati KA, Baraban SC. Hippocampal gamma and sharp-wave ripple oscillations are altered in a Cntnap2 mouse model of autism spectrum disorder. Cell Rep. 2021;37(6):109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I et al. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med. 2017;9(401). [DOI] [PMC free article] [PubMed]
- 195.Blanco-Duque C, Chan D, Kahn MC, Murdock MH, Tsai LH. Audiovisual gamma stimulation for the treatment of neurodegeneration. J Intern Med. 2024;295(2):146–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li KT, Liang J, Zhou C. Gamma oscillations facilitate effective learning in excitatory-inhibitory balanced neural circuits. Neural Plast. 2021;2021:6668175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ross B, Fujioka T. 40-Hz oscillations underlying perceptual binding in young and older adults. Psychophysiology. 2016;53(7):974–90. [DOI] [PubMed] [Google Scholar]
- 198.Cimenser A, Hempel E, Travers T, Strozewski N, Martin K, Malchano Z, et al. Sensory-evoked 40-Hz Gamma Oscillation improves Sleep and Daily Living activities in Alzheimer’s Disease patients. Front Syst Neurosci. 2021;15:746859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prichard A, Garza KM, Shridhar A, He C, Bitarafan S, Pybus A, et al. Brain rhythms control microglial response and cytokine expression via NF-kappaB signaling. Sci Adv. 2023;9(32):eadf5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Murdock MH, Yang CY, Sun N, Pao PC, Blanco-Duque C, Kahn MC, et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature. 2024;627(8002):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brazhnik E, Novikov N, McCoy AJ, Ilieva NM, Ghraib MW, Walters JR. Early decreases in cortical mid-gamma peaks coincide with the onset of motor deficits and precede exaggerated beta build-up in rat models for Parkinson’s disease. Neurobiol Dis. 2021;155:105393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, et al. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s Disease. J Neurosci. 2016;36(24):6445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hirano Y, Uhlhaas PJ. Current findings and perspectives on aberrant neural oscillations in schizophrenia. Psychiatry Clin Neurosci. 2021;75(12):358–68. [DOI] [PubMed] [Google Scholar]
- 204.Krolak-Salmon P, Henaff MA, Tallon-Baudry C, Yvert B, Guenot M, Vighetto A, et al. Human lateral geniculate nucleus and visual cortex respond to screen flicker. Ann Neurol. 2003;53(1):73–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.