Abstract
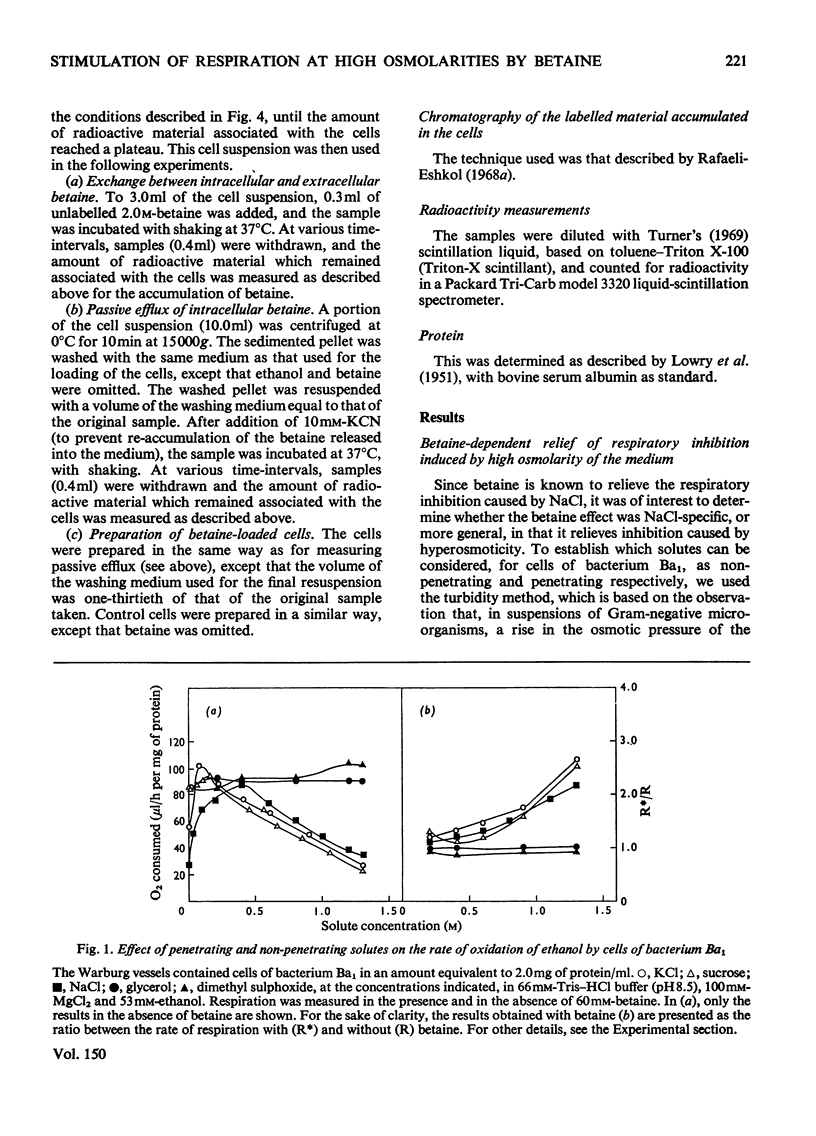
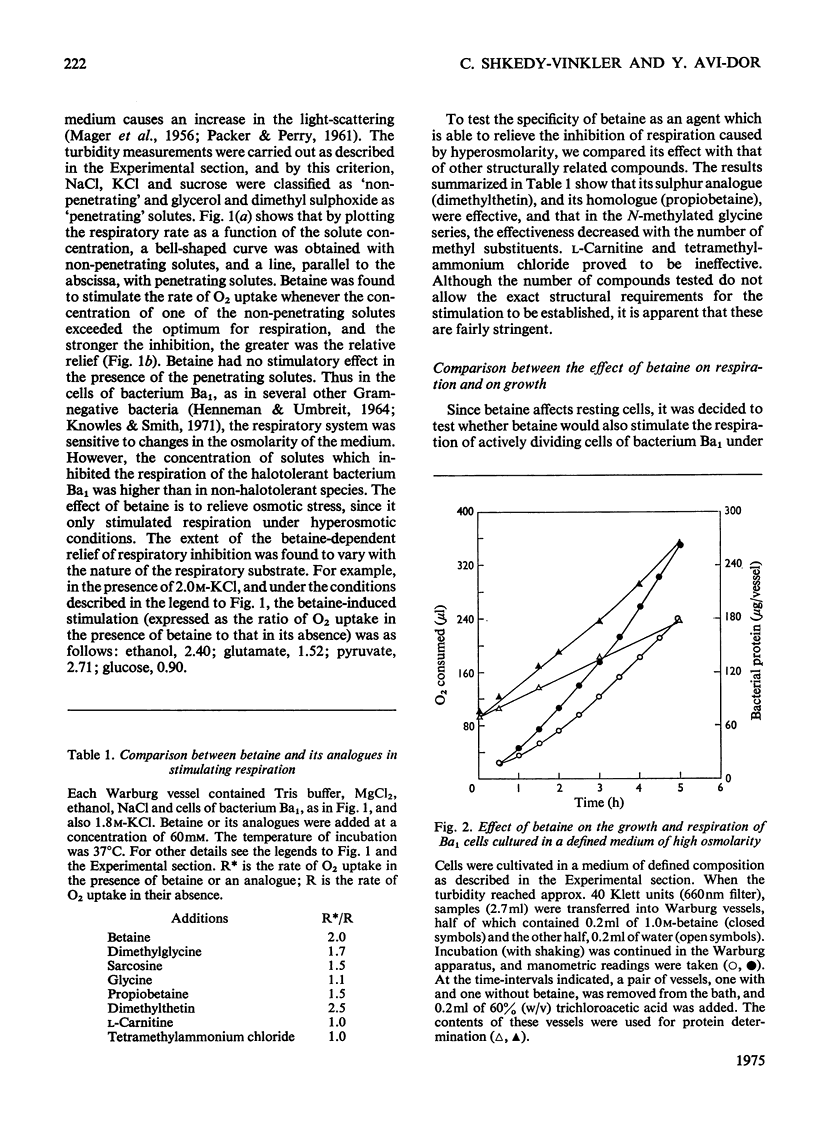
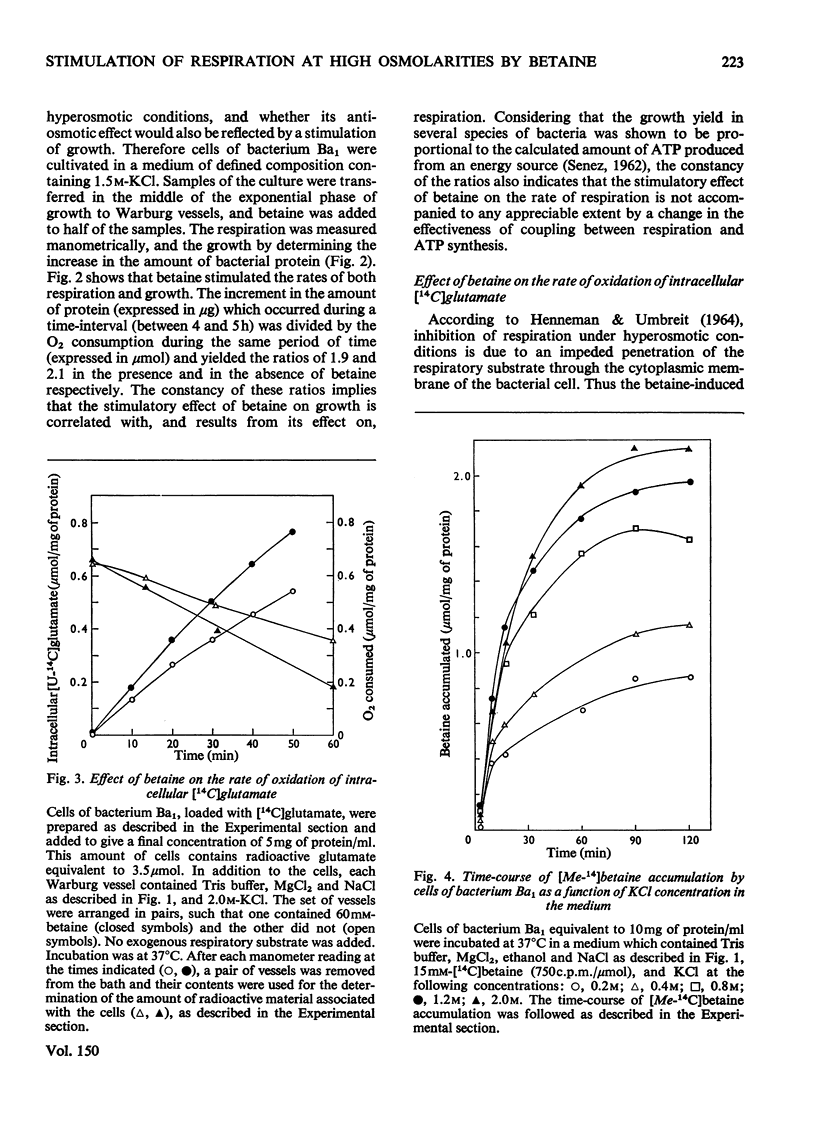
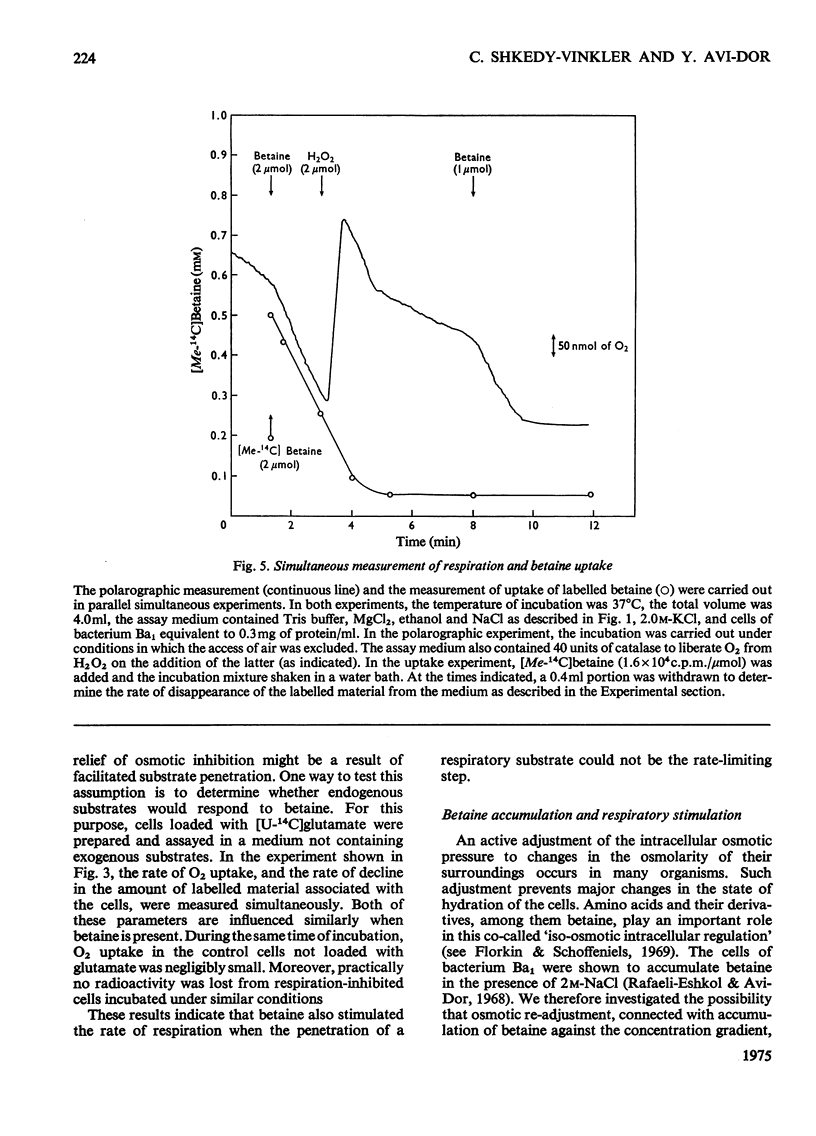
It was shown that non-penetrating solutes at high concentrations inhibit the respiration of the halotolerant bacterium Ba1. Betaine relieved the inhibition caused by osmotic stress and exhibited in this respect a considerable structural specificity. The rate of oxidation of various substrates was stimulated to different extents. It stimulated the rates of both respiration and growth to a similar extent, leaving the energy yield essentially unchanged. In cells pre-loaded with labelled glutamate, betaine also stimulated the rate of oxidation of this intracellular substrate. Betaine was accumulated by respiring cells, and the maximum amount taken up was correlated with the osmolarity of the medium. As judged by chromatography, accumulated intracellular betaine underwent no chemical modification, and this accumulated betaine could not be exchanged with the betaine in the medium or released by passive efflux when respiration was inhibited. Intracellular betaine caused no stimulation of respiration, whereas betaine added to the medium increased the respiratory rate to the same extent in cells pre-loaded with betaine as that in the nonloaded cells. The above observations suggest that iso-economic adjustment is not involved in the anti-osmotic effect of betaine, and that betaine exerts its action on the cellular membrane from the outside.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ERICSON L. E., WILLIAMS J. N., Jr, ELVEHJEM C. A. Studies on partially purified betaine-homocysteine transmethylase of liver. J Biol Chem. 1955 Feb;212(2):537–544. [PubMed] [Google Scholar]
- HENNEMAN D. H., UMBREIT W. W. INFLUENCE OF THE PHYSICAL STATE OF THE BACTERIAL CELL MEMBRANE UPON THE RATE OF RESPIRATION. J Bacteriol. 1964 Jun;87:1274–1280. doi: 10.1128/jb.87.6.1274-1280.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JELLINEK M., STRENGTH D. R., THAYER S. A. Isolation and identification of the products of the oxidation of choline. J Biol Chem. 1959 May;234(5):1171–1173. [PubMed] [Google Scholar]
- Knowles C. J., Smith L. Effect of osmotic pressure of the medium on the volume of intact cells of Azotobacter vinelandii and on the rate of respiration. Biochim Biophys Acta. 1971 Apr 6;234(1):144–152. doi: 10.1016/0005-2728(71)90139-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- MAGER J., KUCZYNSKI M., SCHATZBERG G., AVI-DOR Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956 Feb;14(1):69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- PACKER L., PERRY M. Energy-linked light-scattering changes in Escherichia coli. Arch Biochem Biophys. 1961 Nov;95:379–388. doi: 10.1016/0003-9861(61)90163-1. [DOI] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D., Avi-Dor Y. Studies on halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem J. 1968 Oct;109(4):687–691. doi: 10.1042/bj1090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D. Studies on halotolerance in a moderately halophilic bacterium. Effect of growth conditions on salt resistance of the respiratory system. Biochem J. 1968 Oct;109(4):679–685. doi: 10.1042/bj1090679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]
- SHIEH H. S. AEROBIC DEGRADATION OF CHOLINE. II. SOME PROPERTIES OF WHOLE CELLS AND CELL-FREE EXTRACTS OF ACHROMOBACTER CHOLINOPHAGUM. Can J Microbiol. 1965 Apr;11:375–379. doi: 10.1139/m65-045. [DOI] [PubMed] [Google Scholar]


