Abstract
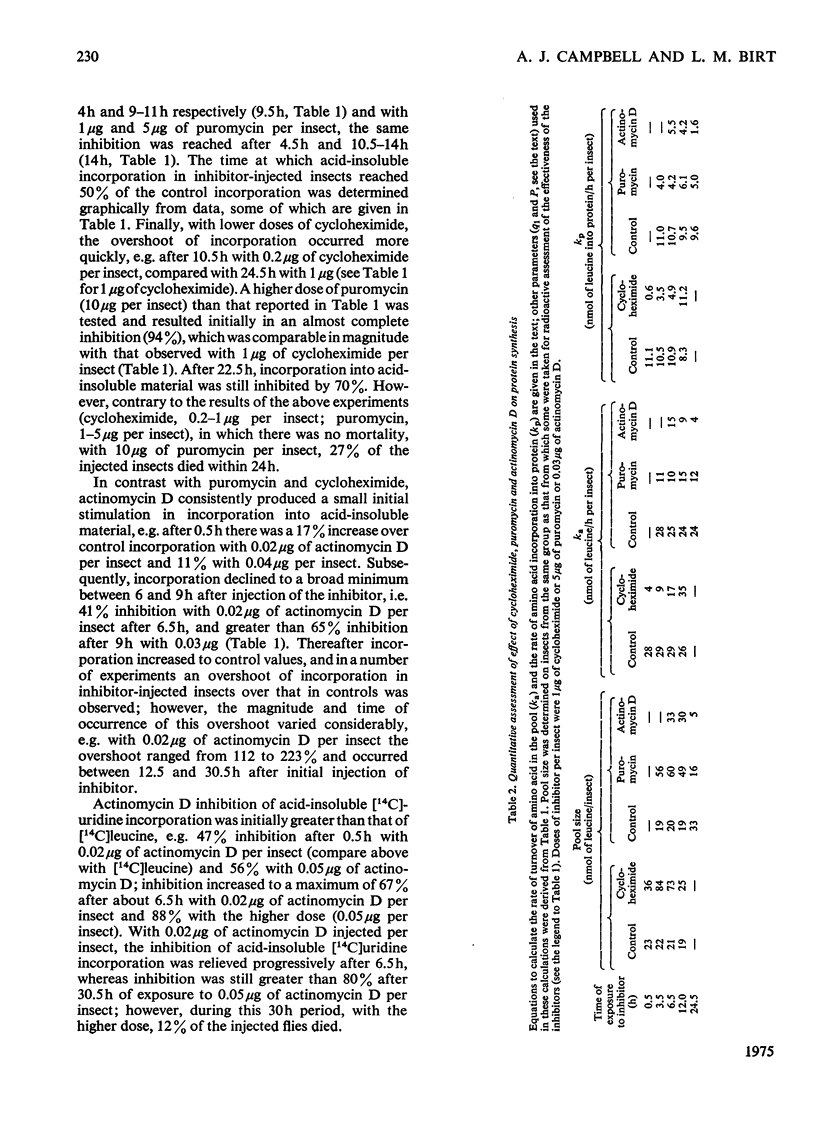
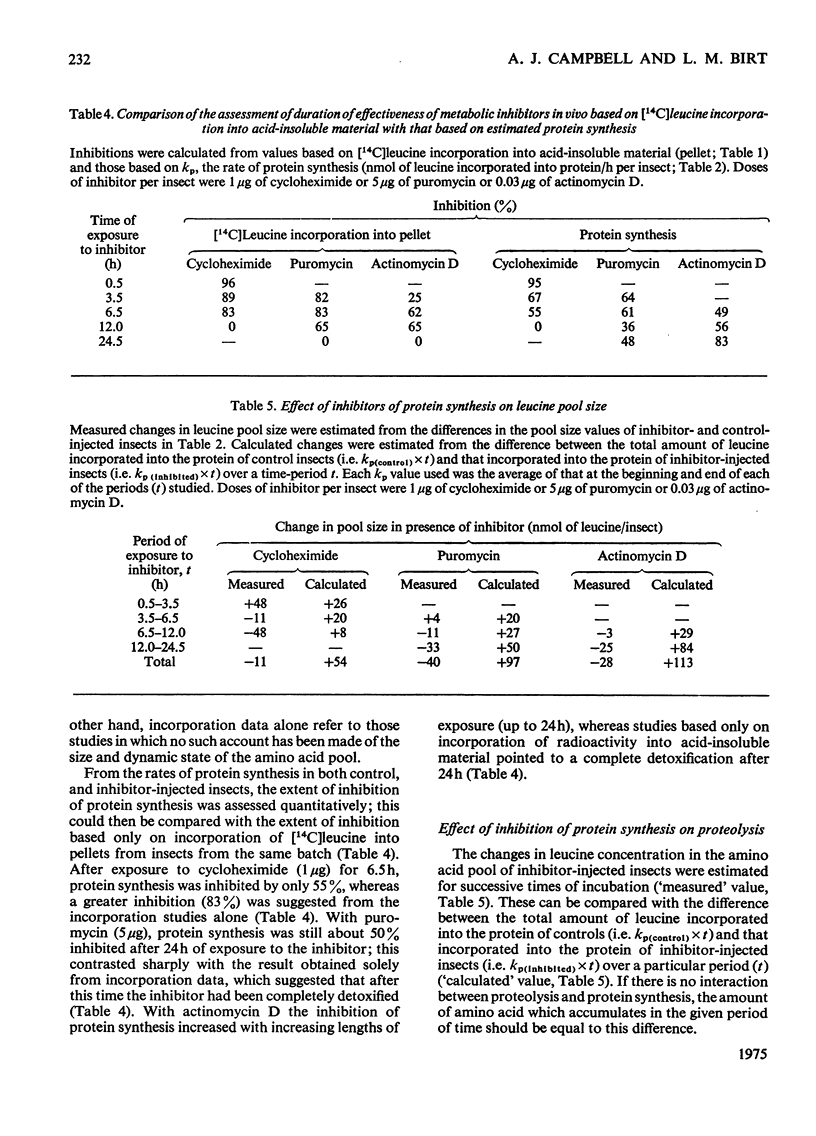
1. The rates of detoxification of cycloheximide (33 mug/g fresh wt.), puromycin (167 mug/g fresh wt.) and actinomycin D (1 mug/g fresh wt.) were assessed in vivo on the basis of acid-insoluble [14C]leucine incorporation in the sheep blowfly, Lucilla cuprina; these were compared with quantitative estimates which took account not only of incorporation data but also of leucine pool size and turnover. Quantitatively, cycloheximide and puromycin were still at least 50% effective in inhibiting protein synthesis after 6.5 and 24.5h of exposure respectively, whereas values based only on incorporation data suggested that cycloheximide was 83% effective and puromycin completely ineffective after the respective periods. Quantitative estimates also showed that actinomycin D effectiveness increased with increasing exposure over 24.5h, in contrast with values based only on incorporation data, which suggested that it was completely ineffective after 24h.2. All inhibitors affected the dynamic state of the amino acid pool; there was a marked decrease in the rate of leucine-pool turnover as well as an increase in the half-life of leucine in the pool. 3. Inhibition of protein synthesis resulted in changes in leucine-pool size; the most pronounced increase occurred with cycloheximide and puromycin and the most pronounced decreases with actinomycin D. 4. Evidence is presented which suggests that proteolysis is functionally linked to protein synthesis, which determines its rate indirectly.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Martin D., Jr, Tompkins G. Control of degradation and synthesis of induced tyrosine aminotransferase studied in hepatoma cells in culture. Nature. 1969 Nov 22;224(5221):806–808. doi: 10.1038/224806b0. [DOI] [PubMed] [Google Scholar]
- Bartelink A. K., De Kort C. A. Synthesis of mitochondrial protein in the flight muscles of the Colorado beetle. Differentiation in vivo between extra- and intra-mitochondrial contributions to the amino acid incorporation into mitochondrial protein. Biochem J. 1973 Nov;136(3):795–802. doi: 10.1042/bj1360795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R. A., Hernandez T. Catabolic effects of cycloheximide in the living reptile. Comp Biochem Physiol B. 1971 Nov 15;40(3):741–749. doi: 10.1016/0305-0491(71)90149-0. [DOI] [PubMed] [Google Scholar]
- Cowtan E. R., Yoda B., Israels L. G. Cycloheximide enhanced porphyrin synthesis in chick embryo liver: association with an increase in the hepatic glycine pool. Arch Biochem Biophys. 1973 Mar;155(1):194–202. doi: 10.1016/s0003-9861(73)80021-9. [DOI] [PubMed] [Google Scholar]
- Dinamarca M. L., Levenbook L. Oxidation, utilization, and incorporation into protein of alanine and lysine during metamorphosis of the blowfly Phormia regina (Meigen). Arch Biochem Biophys. 1966 Oct;117(1):110–119. doi: 10.1016/0003-9861(66)90133-0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Webb T. E. Regulation of tyrosine transaminase in the isolated perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4684–4688. [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C. Microtechnique for determining the specific activity of radioactive intracellular leucine and applications to in vivo studies of protein synthesis. J Biol Chem. 1971 Nov;246(21):6480–6488. [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun. 1964 Mar 26;15(3):214–219. doi: 10.1016/0006-291x(64)90148-2. [DOI] [PubMed] [Google Scholar]
- Selman K., Kafatos F. C. Transdifferentiation in the labial gland of silk moths: is DNA required for cellular metamorphosis? Cell Differ. 1974 Jul;3(2):81–94. doi: 10.1016/0045-6039(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. The turnover of proteins and the usage of information. J Theor Biol. 1972 Nov;37(2):321–334. doi: 10.1016/0022-5193(72)90026-4. [DOI] [PubMed] [Google Scholar]
- Williams K. L., Smith E., Shaw D. C., Birt L. M. Studies of the levels and synthesis of cytochrome c during adult development of the blowfly Lucilia cuprina. J Biol Chem. 1972 Oct 10;247(19):6024–6030. [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]


