Abstract
Metastases are the most common tumors of the spine. As an important increase in the annual incidence of spinal metastases (SMs) has been observed in the last decade, the aim of this study was to describe the epidemiology and histopathological types of SMs surgically treated in the Neurosurgery Clinics of a Regional Hospital in North-Eastern Romania over a period of five years, in order to define a certain tumor profile that would benefit from an early screening. We retrospectively evaluated 115 adult patients, searching for demographic data (gender and age of the patients), primary tumor characteristics (location and histological type), topography of the SMs, and the time interval between the diagnosis of the primary tumor and the surgery for the SMs. The patients were elderly (average age: 58.96 years), with a male predominance (67.82%). Main location of SMs was in thoracic region (44.34%), with multiple vertebral metastases in 30.43% of patients. Only 33.04% of the patients had a known cancer at the time of admission. Primary tumor was located mainly in lung (47.82%), gastrointestinal tract (15.65%), breast (11.30%), prostate (10.43%) and kidney (9.56%). SMs from lung cancer (LC) mostly expressed squamous cell carcinoma (19.13%), probably due to patients’ smoking habits, and those from the digestive system mostly exhibited a moderately/poor colorectal adenocarcinoma (8.69%). Our data suggest the need for close surveillance of patients diagnosed with LC and colorectal cancer because these malignancies most frequently develop SMs. Smoking prevention actions and screening programs for the detection and removal of precancerous colorectal lesions must be developed and expanded.
Keywords: spinal metastases , epidemiology , pathology , immunohistochemistry
Introduction
Spinal metastases (SMs) are common in oncology practice as they can occur in 70–90% of breast cancer (BC) and prostate cancer (PC) [1]. Metastases are the most common tumors of the spine, accounting for approximately 90–95% of the lesions identified on imaging investigations of this segment of the body [1, 2]. An important increase in the annual incidence of SMs has been observed in the last decade [3], especially of those with lung cancer (LC), BC, PC, urological cancer as a starting point, although no significant increases were found in the incidence of the same primary cancers. More significant is the fact that in the next two decades an even more obvious increase in the incidence of cancer has been estimated, so that in 2040 almost 30 million new cases will be diagnosed [4]. On the other hand, however, the possibilities of detection and treatment of a cancer will improve, so life expectancy will increase, but the possibility of developing metastases with various locations, including bones, will also increase. Bone is one of the most common sites where advanced solid tumors metastasize. But bone metastases (BMs) greatly affect patients’ quality of life (QoL), in addition to increasing healthcare costs and mortality risk [5].
Aim
To date there are quite few data on the epidemiology and pathological diagnosis of SMs [6], so that new data are needed in the conditions of the increase in the survival rate of these patients due to improving treatment option. As in the coming years there will be an increasing number of patients who will present BMs at a certain moment in the tumor evolution, the aim of this study was to describe the epidemiology and histopathological (HP) types of SMs hospitalized in the Neurosurgery Clinics of a Regional Hospital in North-Eastern Romania, in order to define a certain tumor profile that would benefit from an early screening.
Patients, Materials and Methods
We performed a retrospective study on 115 patients older than 18 years, with SMs that were diagnosed and surgically treated in the Neurosurgery Clinics of Prof. Dr. Nicolae Oblu Emergency Clinical Hospital, Iaşi, Romania, over a five-year period (January 2015–December 2019).
The institutional database (Electronic Clinical Observation Chart and the Pathology Registers) was searched to identify all patients who underwent surgery for SMs during that period.
We included only patients with a well-established pathological diagnosis of a vertebral metastasis based on the specimens collected intraoperatively.
Demographic data (gender and age of the patients), primary tumor characteristics (location and histological type), topography of the SM, medical history, including surgical pathology of the primary tumor when it was known, and the time period between the diagnosis of the primary tumor and the moment of the surgery for the SM were collected from electronic medical records.
During the period of this study, all the surgical specimens were submitted from the operating rooms to the Pathology Department of the same Hospital for processing. Initially, small representative tissue fragments were processed as intraoperative squash smear preparation, as well as frozen section on cryostat while still in an unfixed state; then, Toluidine Blue staining was performed on all slides. After that, the remaining tissues were transferred to 10% neutral buffered formalin for fixation overnight at 37°C. Next day, representative surgical specimens were identified and were processed routinely, namely dehydration in acetone and xylene, and then embedding into paraffin. Paraffin block was cut in 3 μm thickness sections and routine Hematoxylin–Eosin (HE) staining was performed. All slides were thoroughly evaluated by two pathologists using a Leica DM 2500 binocular microscope (Leica Microsystem GmbH, Germany). New histological sections with 3 μm thickness were obtained from a single representative paraffin block to realize histochemical staining, namely Mucicarmine and Alcian Blue stainings, for mucin identification, but also for immunohistochemical (IHC) staining. For IHC investigations, representative 3 μm sections were processed according to a two-step IHC staining technique, non-Avidin–Biotin method [EnVision+ Dual Link System–Horseradish peroxidase (HRP), Dako Corp.] according to the protocol provided by the manufacturer.
Table 1 documents the panel of all IHC antibodies that have been used in the present study. An IHC-positive reaction was considered in the presence of a brown membrane staining of tumoral cell for human epidermal growth factor receptor 2 (HER2), cytoplasm staining in tumoral cells for pan-cytokeratin (CK) AE1/AE3, CK7, CK20, CK8/18, CK19, vimentin, Melan A, and human melanoma black 45 (HMB45), and a brown nuclear staining of tumor cells for thyroid transcription factor-1 (TTF-1), caudal-related homeobox transcription factor 2 (CDX2), GATA3, estrogen receptor (ER), progesterone receptor (PR), and Ki67, cytoplasmic and nuclear staining for S100 protein.
Table 1.
Immunohistochemical antibodies that have been used in the present study
|
Antibody |
Clone |
Manufacturer |
Dilution |
Antigen retrieval |
|
Pan-CK AE1/AE3 |
Mouse monoclonal AE1/AE3 |
DAKO |
1/50 |
Citrate, pH 6 |
|
CK7 |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
CK20 |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
TTF-1 |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
CDX2 |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
GATA3 |
Mouse monoclonal |
Diagnostic BioSystems |
RTU |
Citrate, pH 6 |
|
CK8/18 |
Rabbit monoclonal |
Diagnostic BioSystems |
1/100 |
Citrate, pH 6 |
|
CK19 |
Mouse monoclonal |
DAKO |
1/100 |
Citrate, pH 6 |
|
ER |
Rabbit monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
PR |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
HER2 |
Polyclonal rabbit |
DAKO |
1/400 |
Citrate, pH 6 |
|
PSA |
Polyclonal rabbit |
DAKO |
1/50 |
Citrate, pH 6 |
|
Vimentin |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
S100 protein |
Polyclonal rabbit |
DAKO |
RTU |
Citrate, pH 6 |
|
HMB45 |
Mouse monoclonal |
DAKO |
RTU |
Citrate, pH 6 |
|
Ki67 |
Mouse monoclonal |
Novocastra |
1/200 |
Citrate, pH 6 |
CDX2: Caudal-related homeobox transcription factor 2; CK: Cytokeratin; ER: Estrogen receptor; HER2: Human epidermal growth factor receptor 2; HMB45: Human melanoma black 45; PR: Progesterone receptor; PSA: Prostate-specific antigen; TTF-1: Thyroid transcription factor-1; RTU: Ready-to-use
Ki67 labeling index (LI) was defined as the percentage of positive cells (having brown nuclear staining) counted among 100 tumor cells in the fields with the greatest number of positive cells.
The HP and IHC analysis of the samples was performed using a Leica DM 2500 binocular microscope (Leica Microsystem GmbH, Germany) by the same two pathologists for each case, to identify the histological type of the spinal tumor. The final diagnosis was established by consensus. All tumors were classified according to World Health Organization (WHO) classifications.
Data concerning patients’ age and gender, location and pathological diagnosis of the primary tumor as well as of the SMs were included into a Microsoft Excel spreadsheet, thus obtaining absolute and percentage frequencies. The results were illustrated and compared using the above-mentioned software charting capabilities.
This study was approved by the Ethics Committee of Prof. Dr. Nicolae Oblu Emergency Clinical Hospital, Iaşi, by Decision No. 8/05.06.2024 and written informed consent was obtained from each patient at the time of admission to Hospital.
Results
Socio-demographic characteristics of patients
In the present study, 115 patients were included, of which 67.82% (n=78) were males and 32.15% (n=37) were females (Figure 1), with a male-to-female (M:F) ratio of 2.1. The average age of the entire studied group was 58.96 years (range: 27 to 84 years). The mean age of female patients was 58.56 years (range: 29 to 79 years) and the mean age of male patients was 59.15 years (range: 27 to 84 years).
Figure 1.
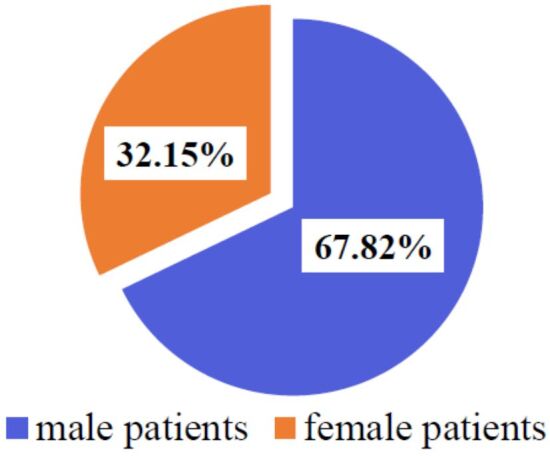
Gender distribution of spinal metastases
Topography of spinal metastasis
The location of the spinal metastatic tumor was at the cervical level in 10.43% (n=12) of cases, with a M:F ratio of 1; in the thoracic region in 44.34% cases (n=51), with a M:F ratio of 2.4; 39.13% (n=45) of cases were located in the lumbar region, with a M:F ratio of 2; 6.08% (n=7) of all cases were registered in the sacral region, with a M:F ratio of 1.33 (Figure 2). There were multiple vertebral metastases in 30.43% (n=35) of patients (Figure 3).
Figure 2.
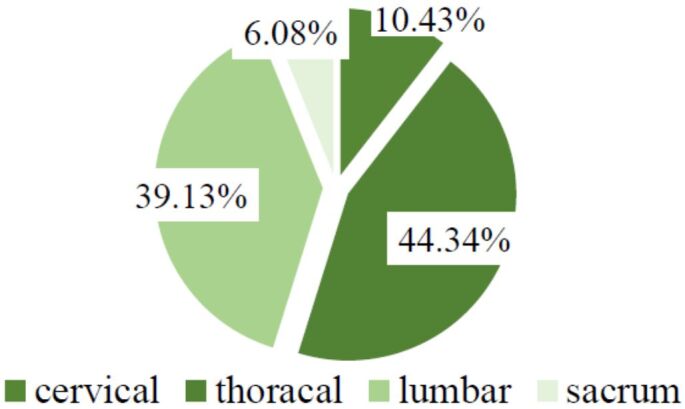
Topography of spinal metastases
Figure 3.
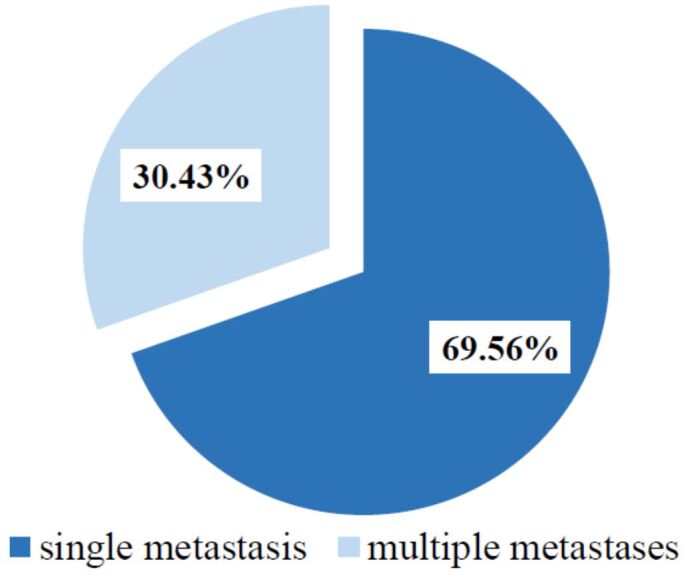
Patients’ distribution according to number of spinal metastases
Only in 33.04% (n=38) of patients the diagnosis of cancer was known at the time of admission to Neurosurgery Clinics (Figure 4), but the location of the primary tumor was established in all (n=115) patients after the surgical intervention due to pathological diagnosis, which included both HE staining, histochemical and IHC stainings.
Figure 4.
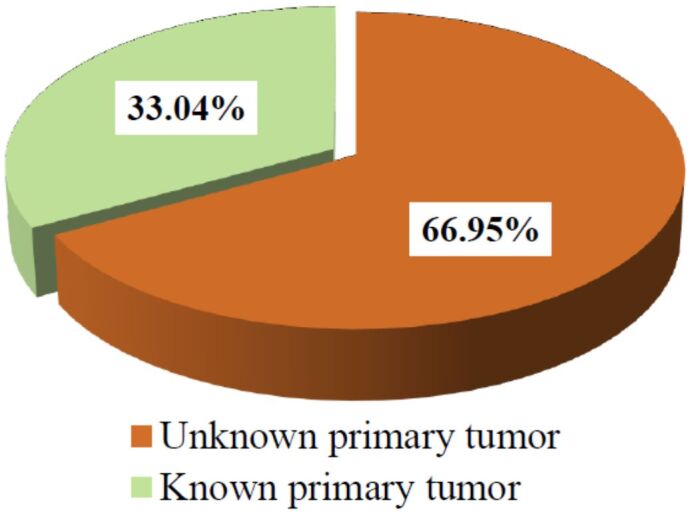
Patients’ distribution according to known/unknown pathological diagnosis of primary tumor before the spinal surgery.
Location and histological type of the primary tumor
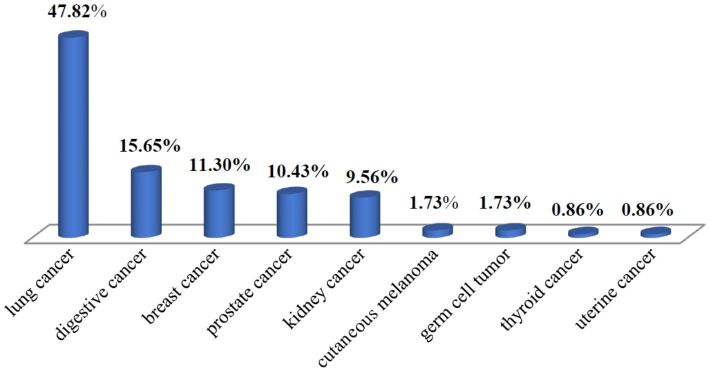
Regarding the location of the primary tumor, vertebral BMs originated in a LC in 47.82% (n=55) of patients, gastrointestinal (GI) cancer in 15.65% (n=18) of patients, BC in 11.30% (n=13) of patients, PC in 10.43% (n=12) of patients, kidney cancer (KC) in 9.56% (n=11) of patients, skin melanoma in 1.73% (n=2) of patients, germ cell tumors (GCTs) in 1.73% (n=2) of patients, thyroid cancer (TC) and endometrial carcinoma in 0.86% of all patients for each case (n=1) (Figure 5).
Figure 5.
Location of the primary tumor among the patients with spinal metastases in the present study
From a pathological point of view, a SM from LC expressed the following histological types: adenocarcinoma (solid, acinar, papillary, and colloid) in 17.39% (n=20) of cases, squamous cell carcinoma (SCC; moderate/poorly differentiated) in 19.13% (n=22) of patients, neuroendocrine tumors (small cell carcinoma and large cell neuroendocrine carcinoma) in 9.56% (n=11) of cases, pleomorphic carcinoma and adenosquamous carcinoma, each of them in only 0.86% (n=1) of LC cases (Table 2; Figure 6).
Table 2.
Histopathological types of spinal metastases starting from lung cancer
|
Histological type |
No. of cases |
Percentage |
|
Adenocarcinoma |
20 |
17.39% |
|
Solid |
9 |
7.82% |
|
Acinar |
7 |
6.08% |
|
Papillary |
3 |
2.60% |
|
Colloid |
1 |
0.86% |
|
Squamous cell carcinoma (moderate/poorly differentiated) |
22 |
19.13% |
|
Neuroendocrine tumors |
11 |
9.56% |
|
Small cell carcinoma |
8 |
6.95% |
|
Large cell neuroendocrine carcinoma |
3 |
2.60% |
|
Pleomorphic carcinoma |
1 |
0.86% |
|
Adenosquamous carcinoma |
1 |
0.86% |
|
Total |
55 |
47.82% |
Figure 6.
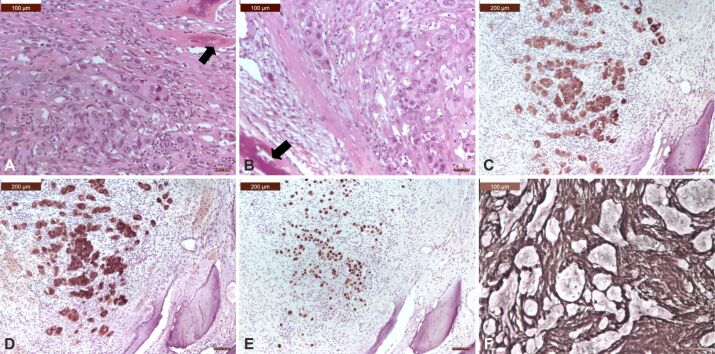
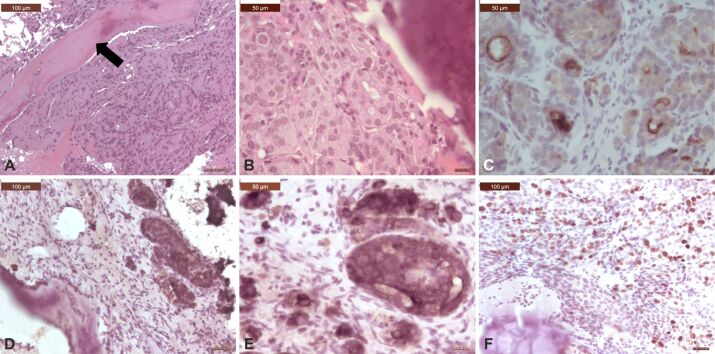
Microscopic view of a spinal metastasis from a solid adenocarcinoma of the lung: (A and B) Tumor was made of solid small nests of poorly-differentiated non-small cells, with abundant cytoplasm, well-defined cell borders, vesicular nuclei, and prominent nucleoli; there were osteosclerotic changes of the vertebral bone (arrows); (C) Tumor cells showed strong cytoplasmic positivity for CK AE1/AE3; (D) Tumor cells showed strong cytoplasmic positivity for CK7; (E) Tumor cells showed strong nuclear TTF-1 immunoreactivity; (F) There were numerous reticulin fibers around small islands of tumor cells. HE staining: (A and B) ×200. Anti-CK AE1/AE3 antibody immunomarking: (C) ×100. Anti-CK7 antibody immunomarking: (D) ×100. Anti-TTF-1 antibody immunomarking: (E) ×100. Silver impregnation: (F) ×200. CK: Cytokeratin; HE: Hematoxylin–Eosin; TTF-1: Thyroid transcription factor-1
Those SMs originating from the digestive system presented various histological types depending on the location of the primary tumor, namely: SCC (moderate/poorly differentiated) from mucosa of the oral cavity in 1.73% (n=2) of cases, signet-ring carcinoma from gastric cancer in 0.86% (n=1) of cases, adenocarcinoma (moderate/poor differentiated) from colorectal cancer in 8.69% (n=10) of cases, trabecular hepatocellular carcinoma (HCC) from liver cancer in 3.47% (n=4) of cases, cholangiocarcinoma from a biliary tree cancer in 0.86% (n=1) of all cases (Table 3; Figure 7).
Table 3.
Histopathological types of spinal metastases originating in gastrointestinal cancer
|
Location |
Histological type |
No. of cases |
Percentage |
|
Mucosa of the oral cavity |
Squamous cell carcinoma (moderate/poorly differentiated) |
2 |
1.73% |
|
Stomach |
Signet-ring cell carcinoma |
1 |
0.86% |
|
Colorectal |
Adenocarcinoma (moderate/poorly differentiated) |
10 |
8.69% |
|
Liver |
Trabecular hepatocellular carcinoma (moderate differentiated) |
4 |
3.47% |
|
Biliary tree |
Cholangiocarcinoma |
1 |
0.86% |
|
Total |
18 |
15.65% |
Figure 7.
Microscopic view of a spinal metastasis from a moderately differentiated adenocarcinoma of the right colon: (A) At the edge of the vertebral tumor there were small areas of carcinoma made of moderately differentiated gland with marked desmoplasia, osteolytic changes of the vertebral bone in the upper right corner; (B) In the center of the tumor there were sheets of cells with a cribriform pattern; small gland lumen was filled with necrotic debris (dirty necrosis) (arrow); (C) Tumor cells exhibited strong cytoplasmic positivity for CK AE1/AE3; (D) Tumor cells exhibited strong cytoplasmic positivity for CK20; (E) Tumor cells exhibited strong nuclear positivity for p53; (F) Tumor cells exhibited a very high Ki67 LI demonstrating an aggressive evolution (anti-Ki67 antibody, x40). HE staining: (A) ×100; (B) ×200. Anti-CK AE1/AE3 antibody immunomarking: (C) ×400. Anti-CK20 antibody immunomarking: (D) ×100. Anti-p53 antibody immunomarking: (E) ×400. Anti-Ki67 antibody immunomarking: (F) ×400. CK: Cytokeratin; HE: Hematoxylin–Eosin; LI: Labeling index
SMs originating in BCs exhibited two histological types: infiltrating ductal carcinoma, not otherwise specified (NOS) in 10.43% (n=12) of all cases and oncocytic carcinoma in 0.86% (n=1) of cases (Table 4; Figure 8).
Table 4.
Histopathological types of spinal metastases starting from breast cancer
|
Histological type |
No. of cases |
Percentage |
|
Infiltrating ductal carcinoma, NOS |
12 |
10.43% |
|
Oncocytic carcinoma |
1 |
0.86% |
|
Total |
13 |
11.30% |
NOS: Not otherwise specified
Figure 8.
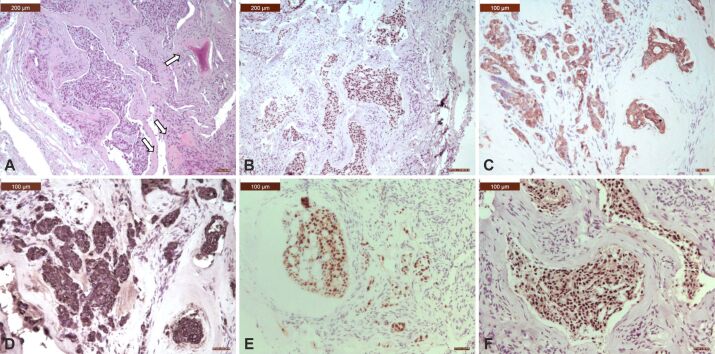
Microscopic view of a spinal metastasis from an adenocarcinoma of the breast: (A) Mostly infiltrative large and solid nests of cells with tubule formation in 30% to 40% of the tumor; tumor cells showed moderately enlarged nuclei; there is an osteoid matrix with new osteoid production, which was partially mineralization, due to osteolytic metastasis (arrows); (B) Tumor cells exhibited strong nuclear positivity for GATA3; (C) Tumor cells exhibited strong cytoplasmic positivity for CK7; (D) Tumor cells exhibited strong cytoplasmic positivity for CK19; (E) Tumor cells exhibited strong nuclear positivity for PR; (F) Tumor cell exhibited strong nuclear positivity for ER. HE staining: (A) ×100. Anti-GATA3 antibody immunomarking: (B) ×100. Anti-CK7 antibody immunomarking: (C) ×200. Anti-CK19 antibody immunomarking: (D) ×200. Anti-PR antibody immunomarking: (E) ×200. Anti-ER antibody immunomarking: (F) ×200. CK: Cytokeratin; ER: Estrogen receptor; HE: Hematoxylin–Eosin; PR: Progesterone receptor
SMs originating in PC exhibited an acinar adenocarcinoma as its histological type in 10.43% (n=12) of all cases (Table 5; Figure 9). When primary tumor was located in the kidney, then SMs presented the phenotype of a renal clear cell carcinoma in 9.56% (n=11) of all cases. SMs from cutaneous melanoma exhibited the same histological type and was identified in 1.73% (n=2) of all cases. A microscopical exam of SMs originating in GCTs revealed two histological types: seminoma and embryonal carcinoma, each of them being identified in 0.86% (n=1) of all cases. Uterine tumors metastasizing in vertebrae showed endometrial endometrioid carcinoma as its unique histological type and represented 0.86% (n=1) of all cases. SMs from TC expressed follicular carcinoma as its only histological type and also represented 0.86% (n=1) of all cases (Table 5).
Table 5.
|
Location |
Histological type |
No. of cases |
Percentage |
|
Prostate |
Acinar adenocarcinoma |
12 |
10.43% |
|
Kidney |
Renal clear cell carcinoma |
11 |
9.56% |
|
Skin cancer |
Melanoma |
2 |
1.73% |
|
Germ cell |
Embryonal carcinoma |
1 |
0.86% |
|
Seminoma |
1 |
0.86% |
|
|
Uterus |
Endometrial endometrioid carcinoma |
1 |
0.86% |
|
Thyroid |
Follicular carcinoma |
1 |
0.86% |
|
Total |
29 |
25.21% |
Figure 9.
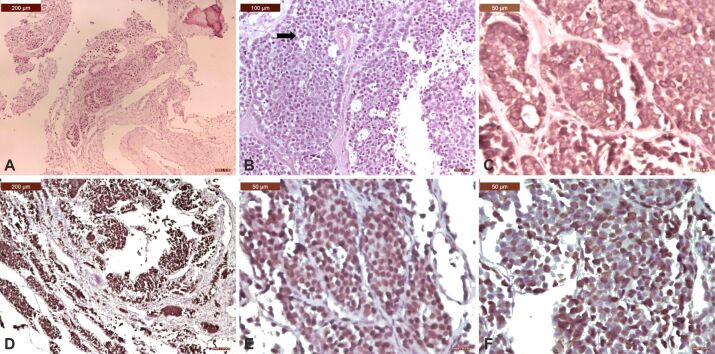
Microscopic view of a spinal metastasis from an acinar adenocarcinoma of the prostate: (A) Metastatic tumor showed proliferation of small, compact, malignant glands without the basal layer and with an infiltrative pattern among osseous lamellae (arrow); (B) With a higher objective, it could be seen a complicated glandular proliferation made of tumor cells with amphophilic cytoplasm and round, monomorphic, nuclei with prominent nucleoli; (C) Metastatic tumor expressed luminal PSA immunopositivity; (D) Tumor cells exhibited strong cytoplasmic positivity for CK8/18; (E) Tumor cells exhibited strong cytoplasmic positivity for CK19; (F) Tumor cells exhibited a very high Ki67 LI demonstrating an aggressive evolution. HE staining: (A) ×200; (B) ×400. Anti-PSA antibody immunomarking: (C) ×400. Anti-CK8/18 antibody immunomarking: (D) ×200. Anti-CK19 antibody immunomarking: (E) ×400. Anti-Ki67 antibody immunomarking: (F) ×200. CK: Cytokeratin; HE: Hematoxylin–Eosin; LI: Labeling index; PSA: Prostate-specific antigen
Time interval between the diagnosis of the primary tumor and the appearance of the primary metastasis
For cases in which the primary tumor was known, the time between initial diagnosis and diagnosis of surgical intervention for SMs averaged 20.06 months, with variations depending on the location of the primary tumor. Thus, the time interval between these two significant moments in the evolution of the analyzed malignancies was 17.1 months for LC, 19.56 months for GI cancer, with variation between 38 months for HCC and eight months for SCC of the oral cavity mucosa, 23 months for BC, 33 months for PC, 20.75 months for KC, 26 months for cutaneous melanoma and one month for GCTs (Table 6).
Table 6.
Time interval [months] between the initial diagnosis and the diagnosis of spinal metastasis according to the location of the primary tumor
|
Location of primary tumor |
Time interval [months] |
|
Lung |
17.1 |
|
Gastrointestinal system |
19.56 |
|
Colon |
22.2 |
|
Liver/biliary tree |
38 |
|
Pancreas |
Unknown |
|
Mucosa of the oral cavity |
8 |
|
Breast |
23 |
|
Prostate |
33 |
|
Kidney |
20.75 |
|
Cutaneous melanoma |
26 |
|
Germ cell tumors |
1 |
|
Thyroid |
Unknown |
|
Uterus |
Unknown |
Discussions
Over time, various theories have appeared regarding the mechanisms of SMs. Thus, various authors assumed that they could develop either by hematogenous, venous or arterial dissemination, by direct tumor extension, by lymphatic dissemination, and by subarachnoid and leptomeningeal seeding [7,8]. Recently, a new theory has emerged, i.e., that the center of the vertebral body is the primary niche for the development of complex interactions between metastatic cancer cells and the vertebral bone environment, with subsequent posterior dissemination via pedicles [1].
The age of SMs patients varies in the literature, depending on the type of patients included in the study group. When only adults (≥18 years) are included, as in the present study, the mean age is in the seventh decade of life. Thus, Truong et al. [9] reported for their series a mean age of 60.91±9.72 years, and the M:F ratio was in favor of the male patients (1.15:1). Similar research [10] with the present study but conducted in Brazil on 51 consecutive patients with SMs who were evaluated over a period of three years (2010–2012), identified a predominance of male patients (68.62%). The mean age was 61.07±11.78 years for females and 62.74±10.17 years for males. Another epidemiological study [11] that investigated the age and gender of 1196 patients with SMs found out a male predominance (59.95%), with a M:F ratio of 1.50:1. Also, most (63.71%) patients were elderly, as their age ranged from 50 to 69 years. The mean age was 58.6±11.6 years (range: 13 to 89 years), and the median age was 59.0 years. The mean age of males was 59.4±11.9 years (range: 16 to 89 years) and the mean age of females was 57.4±11.1 years (range: 13 to 83 years), thus showing that the time of onset of SMs was two years earlier in females than in males. These data differ from those obtained in the present paper in terms of age because those authors also included child patients (age ≤18 years).
Cerqueira et al. [2] also included both children and adults with SMs in their study, so the mean age was 51.9 years, and the median age was 54 years. Anyway, also in their study, most (33.3%) patients were in their seventh decade of life (60–69 years).
A study conducted in Korea [5] identified that SMs predominantly affects male patients (53.05%), mostly elderly (51.97%), aged over 60 years. However, in BC and PC, SMs occur predominantly in the corresponding gender, and the incidence increases with age in PC and decreases with age in BC. In cases with genitourinary cancers, SMs developed mostly in female patients. Lu et al. [12] reported that their female patients presented a median age of 64 years (range: 36 to 88 years), but their male patients had a median age of 71 years (range: 26 to 92 years) at the time of SMs diagnosis.
On the other hand, in a previous personal work on SMs, but focused only on those with a GI cancer starting point [13], we also identified an average age of the patients of 66.42 years, with variations between 35 and 80 years and a male predominance (75%).
Regarding the topography of SMs, there is unanimity among researchers, namely that these lesions are more frequently found in the thoracic region, followed by the lumbar and sacral regions, while the cervical region is the least frequently affected by these malignancies [1, 2, 9, 11]. As all these authors, we also found out that the thoracic region was the most affected by SM. A study similar to the present one [9], conducted on 191 patients aged ≥18 years who underwent surgery for at least one SM, reported that the lesion was predominantly located at thoracic level (50.26%), followed by the lumbar (25.13%) and cervical (24.60%) levels. Univariate analysis, however, did not identify a prognostic role of the region affected by SMs, either in terms of patient survival or improvement in patient motor function after surgery.
The literature states that usually, at the time of diagnosis, SMs are most often multiple, meaning that two or more levels are affected. Wang et al. [11] reported the presence of multiple SMs in 36.12% of their cases. In the present study, multiple SMs were present in two-thirds of the cases, thus demonstrating that the addressability of oncological patients in neurosurgery clinics for symptoms determined by SMs is delayed. This finding may be due to either a lack of medical education or a lower economic-social status, but a greater aggressiveness of primary tumors that disseminate at the spinal level must also be considered.
In the present research, two-thirds of the cases admitted to the Neurosurgery Clinics did not have any primary tumor identified before surgery. The reasons for not knowing the primary tumor location could be the relatively small size of the tumor, as can be the case of LC, which can escape imaging detection, or the lacking of specific symptoms, as in the case of colon cancer, liver and biliary tree cancers, TCs or KCs, or the primary tumor location in the pelvis, such as uterine or PC, for which patients usually delay seeing the doctor for personal reasons.
The identification of the primary tumor is very important in the management of SMs because it is of great value in selecting the best treatment option to obtain the longest possible patient survival, especially in cases with unknown primary tumor [14]. Especially in these situations, but even in cases with already known cancer, without surgery there is no therapy available, and the patient’s survival will be very low. As a result, the diagnosis of SMs can be obtained with the help of at least one biopsy that ensures the sampling of tumor tissue. The biopsy or surgical specimens ensure the identification of the site, and the histological type of primary tumor based on HP investigations, supplemented with IHC and/or histochemical stainings, especially due to the fact that SMs are moderately or poorly differentiated compared to the morphological appearance of the corresponding primary tumor.
Over time, there have been widely varying reports regarding the primary tumor pathology that presents the highest incidence of SMs. An earlier study, from 1997 [15], analyzed 71 patients with SMs to identify the importance of primary tumor location in determining preoperative prognosis. The authors found that 47.88% of the analyzed patients had TC, 39.43% had KC, and in the remaining 12.67% of patients the site of the primary tumor was unknown at the time of surgery. The authors concluded that, when the primary tumor is unknown, the median survival period is significantly shorter than in patients with known primary tumors at the time of SMs treatment. In our study, two-thirds of the patients did not have a known primary tumor before surgery for SMs and this fact could be related with the lack of health education of the Romanian population, but also with the inadequate implementation of screening programs, especially aimed at the early detection of LCs and colon cancers.
A prospective clinical study [16], carried out in 2000 on 153 patients with SMs identified the site and histology of the primary tumor, as follows: BC (37%), PC (28%), LC (18%), which included non-small cell lung carcinoma (NSCLC) in 12% of cases and small cell lung carcinoma (SCLC) in 6% of cases. In 17% of all cases, other solid tumors were identified. A magnetic resonance imaging (MRI) study [17] performed on 280 patients identified the locations of primary tumors in patients with SMs, as follows: lung (25.71%), breast (23.21%), prostate (20.35%), hematological cells (8.21%), urinary tract (7.5%), GI system (4.64%), unknown (4.28%), and others (6.07%). In their series of 134 clinically and MRI-investigated oncological patients with SMs, Lu et al. [12] identified primary cancer in equal percentages (24%) at the breast and lung level, 15% of all cases were from the prostate, 10% were hematological neoplasms, and 26% of cases had other primary locations. In the series of Chaichana et al. [18], primary cancer diagnoses for their 162 included patients were diverse, reported as follows: hematopoietic cancers (17%), LC (16%), BC (16%), KC (13%), and PC (12%). Botelho et al. [10] reported that, of the 51 patients with SMs analyzed in their study, 23.52% were diagnosed with primary breast tumors, 23.52% with PC, 13.72% had a hematological malignancy (lymphoma or multiple myeloma), 7.84% of patients had LC and 5.88% had colon cancer. Bladder cancers, KCs and larynx cancers were reported in 1.96% of cases each. Zhang et al. [19] used pathological examination to identify the primary tumor in patients with SMs. The first three primary tumor sites were as follows: breast (26.6% of cases), lung (21.7% of cases), and prostate (19.2% of all cases), but they also found lymphoma, multiple myeloma, and kidney as fewer common locations.
A survey conducted in the United States on SMs annually diagnosed identified that 16.3% had LC as their starting point, 14% were derived from BC, 13.1% from renal cancer, 6.8% from PC, 4.1% from cutaneous melanoma, and 2.3% from primary TC [20].
Correlated with the fact that they studied both adult and child patients, but also with the fact that the analyses were performed on patients diagnosed with SMs based either on clinical symptoms, radiographic examinations and/or HP diagnosis and that hematological malignancies (myeloma and lymphoma) were also included, the study realized by Wang et al. [11] reported similar, but also different data from the above studies. The most common primary tumor causing SMs was LC (36.54%), followed by unknown origin (16.22%), childhood cancer (6.52%), BC (6.35%), liver/biliary cancer (6.27%), GI cancer (4.43%), myeloma (4.43%), PC (4.43%), TC (3.09%), sarcoma (2.76%), and less common primary neoplasia (8.95%), such as esophageal cancer, lymphoma, and cervical cancer.
In 2021, an author from Turkey [21] investigated 156 patients with SMs and found that among the primary tumors that spread to the spine, respiratory system cancer was the first (47.44%), BC was the second (21.15%), and both were followed by PC (11.54%), GI tract cancer (10.26%), urinary tract (6.41%) and gynecological cancer (3.21%).
Although in the present series the same locations of the primary tumors leading to the onset of SMs appear as in other studies, their order differs. Thus, in the present study, LC was the most common primary tumor that determined SMs, being identified in 47.82% of non-hematological tumor cases and representing three times more cases than the second primary tumor, respectively GI cancers.
Similar aspects were identified by another study carried out in Romania by Bratu et al. [22]. These Romanian researchers retrospectively analyzed, from imaging and pathological points of view, 309 cases of SMs diagnosed at a hospital in Bucharest between 2010 and 2014. In 44.33% of cases, the patients presented SMs from a hematological neoplasia, and 55.66% from another type of cancer. Those 171 patients with SMs of non-hematological origin presented the following primary tumor: LC (44.44%), BC (25.14%), PC (8.18%), KC (4.09%), TC (4.09%), GI tract cancer (3.50%), bladder cancer (2.92%), malignant melanoma (2.92%), pancreatic cancer (2.33%), ovarian cancer (1.75%), and neck squamous cell cancer (1.16%).
In the present series, we identified the highest prevalence of LCs as a starting point for SMs, probably because, as reported by other authors [3], LC not only is the most frequent, but also presents a high risk of dissemination to vertebrae. Also, patients with NSCLC mostly disseminate to the spinal column as this site is the most common site for BM [23].
Even though there are studies in the literature stating that patients with GI cancer have the lowest risk of spinal dissemination [3], we found out in the present series that SMs with a GI cancer as a starting point, especially colorectal cancer, ranks second. As far as we know, this aspect has not been identified by another study. The cause may be the fact that such patients do not undergo imaging of the spine to detect SMs unless clinical signs appear [24]. Also, in an earlier personal study [13], we also reported the epidemiological and pathological findings of 40 patients with GI cancers and SMs diagnosed and treated during a period of nine years in the same hospital, among which the colorectal adenocarcinoma was the most frequent histological type (40%). These data demonstrate that the prevalence of GI carcinomas have increased significantly in Romania in the last two decades. These data are confirmed by a recently published article [25], which studied all colorectal cancer cases reported by all hospitals to the National Diagnosis-Related Group (DRG) System, during a period of three years (2016–2018). The authors identified a colorectal cancer mortality almost twice higher than the European average, which also means an increase in the corresponding incidence of colorectal cancer in Romania compared to the other European countries. It is worth mentioning that, even in a country like England, with well-established national screening programs, the majority of colorectal cancers were diagnosed in the late stages, mainly T3 and T4 [26], probably because these tumors are growing with few symptoms.
Regarding the histological type of SMs originating in colorectal cancer, we have found out that all cases expressed a moderate/poorly differentiated adenocarcinoma. The same findings were reported by another group of researchers from Romania [27].
Although some authors found out that 21.15–42.3% of SMs originate from BC [21, 28], placing this primary tumor in the first or second place among all primary tumors that cause spinal dissemination, in our series BC ranks third. In addition, in the case of PC, the present study identified SMs with this starting point in fourth place, although this type of malignancy is the most common form of cancer affecting men. It is well known that this type of neoplasia presents a particular tropism for BM. In 2007, approximately 350 000 patients were diagnosed with BMs in the United States [29]. On the other hand, an autopsy study demonstrated that approximately 90% of men with metastatic PC also had BMs at the time of death [30]. Furthermore, more than 80% of therapeutically castrated PC patients experienced SMs [31].
In the present series, new entities of malignant tumors that disseminated at the vertebral level, such as GCT, also appeared, but in a very small percentage. Some other studies have also shown the rarity of this medical condition. Jamal-Hanjani et al. [32] analyzed 2550 patients with GCTs among which they found only 0.74% of cases with BMs, most of them (88.23%) at the vertebral level.
The data obtained in the present research are also confirmed regarding the prevalence of SMs originating from a cutaneous melanoma. If in the present study this type of cancer was identified as the starting point of SMs in only 1.73% of cases, the literature reports similar data (1.63–4.1% of cases) [5, 20], thus demonstrating that patients with cutaneous melanoma rarely disseminate to the vertebrae. As such, it can be concluded that this type of dissemination appears as a late event in the evolution of a melanoma [33].
All these data demonstrate that there is a great variation between studies regarding the starting point of SMs, probably due to the pattern of development of primary tumors in a given population as a result of specific genetics and as a result of the temporal trends followed by each tumor type. For Romania, the fact that LC ranks first among the primary tumors that cause SMs demonstrates the significant increase in the incidence of this type of cancer in the last 25 years, both in women and in men. Also, the prevalence and long-term survival of GI cancer, especially colorectal type, have increased significantly in recent years. Thus, although in previous studies it was considered that this type of cancer rarely causes SMs, currently we found a representative number of cases.
Regarding the histological subtypes of LCs causing SMs, in the present series there were mostly squamous cell LCs and lung adenocarcinomas, the former being slightly more frequent. These aspects support previously published data regarding the HP types of LC in Romania. Compared to other studies, from Asia or Latin America, which reported that lung adenocarcinoma was the primary origin of SMs in 58.3–69.6% of cases, and SCC was associated with a much lower risk of BM, being identified only in 13–17% of cases [34, 35], statistical analyzes in Romania show that 48% of LC patients have squamous cell LC, 29% have adenocarcinoma, 7% have large cell carcinoma and 16% have SCLCs [36]. These statistical data are also confirmed by the present study, in which the histological subtype of LC that metastasizes at spinal level most frequently was SCC. Similar aspects were identified by a study from Turkey [37], where the HP exam of 168 SMs revealed the same hierarchy of histological types of LC: SCC (48%), adenocarcinoma (31%), small cell carcinoma (15%), and large cell carcinoma (6%).
In our series, SMs from TC were most often of the follicular type, an aspect that was similar with other studies. Enkaoua et al. [15] reported, in addition to the follicular type, other histological types of TC that have disseminated to the spine, such as the papillary type and, more rarely, medullary carcinoma with amyloid in the stroma.
In the present series, SMs from renal cancer exhibited only clear cell renal carcinoma histological type and the same histological aspect was identified by Enkaoua et al. [15].
From a prognostic point of view, recent research identified that patients with SMs from SCLC have a median overall survival of only 6.3 months, in contrast to those with NSCLC, which have a survival of 8.9 months. Notably, within the NSCLC subgroup, patients diagnosed with adenocarcinoma showed the most prolonged survival, with a survival of 25.3 months [38].
The time interval from the diagnosis of the primary tumor to the diagnosis of SMs varies widely, depending on the histological type of the primary neoplasia. A study conducted in Korea [5] evaluated the time interval from primary tumor diagnosis to BM for various primary solid malignancies. They observed that LC had the shortest mean time to BM (9.0±15.2 months), followed by BC (14.9 months) and PC (17.4 months). Conversely, in the case of colorectal cancer they identified the longest average time to BM (28.9±25.5 months). Overall, the median time from primary cancer diagnosis to surgery for BM was 18.9 months.
Van den Brande et al. [39] also reported that patients with LC had the shortest time interval (about nine months), while those with BC and PC have a significantly longer interval of 14.9 and 17.4 months, respectively. A group of researchers from Brazil [40] found that the time interval between the diagnosis of the primary tumor and surgery for the treatment of SMs was on average 9.6 months, with minimum and maximum values between four days and 3183 days (8.84 years), respectively.
In the present series, the mean time interval between the diagnosis of the primary tumor and surgery for SMs for the entire group of patients was similar to that reported by Hong et al. [5] in Korea (20.06 months versus 18.9 months). However, given the fact that in the present series we identified new types of primary tumors as the starting point of SMs, the obtained data differ from those published by other authors [5, 39, 40]. The longest interval of time between those two moments in the evolution of neoplasia was in the case of liver cancer (38 months) and the shortest was for GCTs (one month).
As demonstrated by the present study, many of the patients with SMs are elderly and have neurological deficits due to involvement of several vertebral regions, with spine instability. Quantification of vertebral involvement, neurological status, general health, and primary tumor histology are important factors to consider for surgical planning and therapeutic targeting.
SMs can be treated by chemotherapy, radiotherapy, and surgical treatment. Patients with SMs are difficult to treat surgically, because the metastases represent an advanced stage of the oncological disease and therefore the postoperative prognosis can be very poor [10]. Surgical treatment aims to improve QoL by achieving pain control and improving neurological deficits [41]. Conventional surgery in SMs is highly invasive and requires a long hospital stay to stabilize the spine and remove nerve compression caused by the tumor. The most commonly used is decompression or “detachment surgery”, in which the tumor is resected so as to achieve decompression of the spinal cord [41]. Preoperative embolization can also be used to reduce the risk of hemorrhage and improve outcomes with low complication rates [42].
For patients with solitary SM without invasion of the vertebral canal and a good general status of health, with a long-life expectancy because the primary tumor has a slow growth rate, curative surgical interventions, such as metastasectomy or en bloc resection of tumors [43] or en bloc spondylectomy/total vertebrectomy), must be considered. Vertebral resection should be followed by spinal reconstruction and appropriate instrumentation [44]. When the prognosis is poor, local control must be obtained in the medium term, intralesional excision methods such as piecemeal excision or “eggshell” curettage can be used. For patients with the poorest prognosis, i.e., in the advanced phase of metastatic disease, palliative surgery is recommended, such as spinal cord decompression with stabilization, or only supportive care [42].
Since 2005, in Japan, minimally invasive spine stabilization with percutaneous pedicle screw fixation of the spine is used for patients with SMs and advanced metastatic disease, in order to reduce pain and allow the oncological patient to be able to carry out his daily activities related to personal care. At the same time, this treatment method of SMs can prevent vertebral pathological fractures [45]. A multivariate analysis of the risk factors for poor prognosis of patients with SMs surgically treated [46] found out that age ≥65 years at surgery, presence of extra-SMs and poor performance scores were associated with 180-day mortality. For these reasons, the authors considered that multidisciplinary discussions about the benefits and risks of surgery in patients with these risk factors are necessary.
Conclusions
The present study provides a detailed description of the epidemiological and pathological characteristics of SMs, which could help orthopedic surgeons understand the clinical characteristics of SMs and is of great importance in guiding scientific research. Our findings have direct implications for the allocation of resources necessary for the care of these patients, but also for health policy. In the coming years, healthcare systems will face a growing population of elderly patients with SMs, for whom direct healthcare costs will be high. Moreover, our data suggests the need for close surveillance of patients diagnosed with LC and colorectal cancer because these malignancies most frequently develop SMs. It is becoming clear that smoking prevention actions and screening programs for the detection and removal of precancerous colorectal lesions must be developed and expanded.
Conflict of interests
The authors declare that they have no conflict of interests.
Source of funding
This research was funded by Romania’s National Recovery and Resilience Plan (PNRR), Pylon III, section I5. Establishment and operationalization of Competence Centers PNRR-III-C9-2022 – I5, project “Creation, Operational and Development of the National Center of Competence in the field of Cancer”, acronym CNCC, code 14.
References
- 1. Ziu E , Viswanathan VK , Mesfin FB . StatPearls [Internet] Treasure Island, FL, USA : StatPearls Publishing ; 2023 . Spinal metastasis . [PubMed] [Google Scholar]
- 2.Cerqueira BP, dos Santos, de Barros. Epidemiological characterization of patients with spinal tumors in Alagoas, Brazil [Perfil epidemiológico de pacientes com tumores da coluna vertebral em Alagoas, Brasil] J Bras Neurocirur. 2023;34(3):291–296. [Google Scholar]
- 3.Shakil H, Malhotra AK, Badhiwala JH, Karthikeyan V, Essa A, He Y, Fehlings MG, Sahgal A, Dea N, Kiss A, Witiw CD, Redelmeier DA, Wilson JR. Contemporary trends in the incidence and timing of spinal metastases: a population-based study. Neurooncol Adv. 2024;6(1):vdae051–vdae051. doi: 10.1093/noajnl/vdae051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . WHO Report on Cancer: setting priorities, investing wisely and providing care for all . Geneva, Switzerland : WHO Global Report ; 2020 . [Google Scholar]
- 5.Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM. Bone metastasis and skeletal-related events in patients with solid cancer: a Korean nationwide health insurance database study. PLoS One. 2020;15(7):e0234927–e0234927. doi: 10.1371/journal.pone.0234927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortez PR. Spinal metastasis: diagnosis, treatment and prognosis - integrative review from 2012 to 2017 [Metástases da coluna vertebral: diagnóstico, tratamento e prognóstico - revisão integrativa de 2012 a 2017] Coluna/Columna. 2020;19(1):58–66. [Google Scholar]
- 7.Esperança-Martins M, Roque D, Barroso T, Abrunhosa-Branquinho A, Belo D, Simas N, Costa L. Multidisciplinary approach to spinal metastases and metastatic spinal cord compression - a new integrative flowchart for patient management. Cancers (Basel) 2023;15(6):1796–1796. doi: 10.3390/cancers15061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fomchenko EI, Bayley JC, Alvarez-Breckenridge C, Rhines LD, Tatsui CE. Spinal metastases and the evolving role of molecular targeted therapy, chemotherapy, and immunotherapy. Neurospine. 2022;19(4):978–993. doi: 10.14245/ns.2244290.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong VT, Al-Shakfa F, Phan P, Newman N, Boubez G, Shedid D, Yuh SJ, Wang Z. Does the region of the spine involved with metastatic tumor affect outcomes of surgical treatments. World Neurosurg. 2021;156:e139–e151. doi: 10.1016/j.wneu.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Botelho RV, de Oliveira, Rotta JM. Quantification of vertebral involvement in metastatic spinal disease. Open Orthop J. 2013;7:286–291. doi: 10.2174/1874325001307010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Zhang H, Yang L, Yang XG, Zhang HR, Li JK, Qiao RQ, Hu YC. Epidemiological characteristics of 1196 patients with spinal metastases: a retrospective study. Orthop Surg. 2019;11(6):1048–1053. doi: 10.1111/os.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305–312. [PubMed] [Google Scholar]
- 13.elaru Ş, Sava A, Scripcariu DV, Costea CF, Dumitrescu AM, Costăchescu B, Dumitrescu GF, Ciupilan C, Vatavu R, Haba RM, Poroch V, Dima-Cozma LC, Vornicu V, Stan CI. Epidemiological and pathological characteristics of spinal metastases from gastrointestinal cancers - a series of 40 cases. Rom J Morphol Embryol. 2023;64(2):225–234. doi: 10.47162/RJME.64.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candido PBM, Pinheiro RP, Peria FM, Toledo VN, Costa HRT, Defino HLA. Unknown primary tumor sites in spinal metastasis [Metástase vertebral em tumor primário de localização desconhecida] Coluna/Columna. 2021;20(1):64–67. [Google Scholar]
- 15.Enkaoua EA, Doursounian L, Chatellier G, Mabesoone F, Aimard T, Saillant G. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine (Phila Pa 1976) 1997;22(19):2293–2298. doi: 10.1097/00007632-199710010-00020. [DOI] [PubMed] [Google Scholar]
- 16.Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163–1169. doi: 10.1016/s0360-3016(99)00333-8. [DOI] [PubMed] [Google Scholar]
- 17.Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15–23. doi: 10.1259/bjr.74.877.740015. [DOI] [PubMed] [Google Scholar]
- 18.Chaichana KL, Pendleton C, Wolinsky JP, Gokaslan ZL, Sciubba DM. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurgery. 2009;65(2):267–274. doi: 10.1227/01.NEU.0000349919.31636.05. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Cai F, Liu L, Liu XD. Pathological investigation of vertebral tumor metastasis from unknown primaries - a systematic analysis. Asian Pac J Cancer Prev. 2015;16(3):1047–1049. doi: 10.7314/apjcp.2015.16.3.1047. [DOI] [PubMed] [Google Scholar]
- 20.Wright E, Ricciardi F, Arts M, Buchowski JM, Chung CK, Coppes M, Crockard A, Depreitere B, Fehlings M, Kawahara N, Lee CS, Leung Y, Martin-Benlloch A, Massicotte E, Mazel C, Oner C, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Ulbricht C, Verlaan JJ, Wang M, Choi D. Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg. 2018;114:e809–e817. doi: 10.1016/j.wneu.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 21.Başdelioğlu K. Features of spinal metastases: a retrospective view. Int J Spine Surg. 2021;15(1):119–129. doi: 10.14444/8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bratu AM, Raica VP, Sălcianu IA, Zaharia C, Popa VB, Lupu AR, Ştefănescu V, Dobrea CM, Iana G, Marinescu AN. MRI differential diagnosis: bone metastases versus bone lesions due to malignant hemopathies. Rom J Morphol Embryol. 2017;58(4):1217–1228. [PubMed] [Google Scholar]
- 23.Amelot A, Terrier LM, Cristini J, Buffenoir K, Pascal-Moussellard H, Carpentier A, Bonaccorsi R, Le Nail, Mathon B. Spinal metastases from lung cancer: survival depends only on genotype, neurological and personal status, scarcely of surgical resection. Surg Oncol. 2020;34:51–56. doi: 10.1016/j.suronc.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Portales F, Thézenas S, Samalin E, Assenat E, Mazard T, Ychou M. Bone metastases in gastrointestinal cancer. Clin Exp Metastasis. 2015;32(1):7–14. doi: 10.1007/s10585-014-9686-x. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu EM, Tieranu CG, Maftei D, Grivei A, Olteanu AO, Arbanas T, Calu V, Musat S, Mihaescu-Pintia C, Cucu IC. Colorectal cancer trends of 2018 in Romania - an important geographical variation between northern and southern lands and high mortality versus European averages. J Gastrointest Cancer. 2021;52(1):222–228. doi: 10.1007/s12029-020-00382-3. [DOI] [PubMed] [Google Scholar]
- 26.Ilie DS, Şerbanescu MS, Ionovici N, Busuioc CJ, Mogoantă L. Colorectal cancer in County Durham-England a clinical and statistical study. Curr Health Sci J. 2021;47(3):338–347. doi: 10.12865/CHSJ.47.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florescu-Ţenea RM, Kamal AM, Mitruţ P, Mitruţ R, Ilie DS, Nicolaescu AC, Mogoantă L. A statistical analysis of risk groups in colorectal cancer patients. Curr Health Sci J. 2019;45(2):179–184. doi: 10.12865/CHSJ.45.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhoobasarova D, Sadykova A, Muratov Z, Abdraeva F, Aitieva A, Aitieva Z, Zheenbekova D, Ismailova F, Tazhibaeva U, Kyzy AM, Abdullaeva Z, Kochkorbaeva Z, Maksatbek T, Keneshbaev B, Kadyrberdieva M, Sherieva N. Optimization diagnosis of breast cancer vertebral metastases. Adv Breast Cancer Res. 2021;10(4):156–164. [Google Scholar]
- 29.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 30.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 31.Crnalic S, Löfvenberg R, Bergh A, Widmark A, Hildingsson C. Predicting survival for surgery of metastatic spinal cord compression in prostate cancer: a new score. Spine (Phila Pa 1976) 2012;37(26):2168–2176. doi: 10.1097/BRS.0b013e31826011bc. [DOI] [PubMed] [Google Scholar]
- 32.Jamal-Hanjani M, Karpathakis A, Kwan A, Mazhar D, Ansell W, Shamash J, Harper P, Rudman S, Powles T, Chowdhury S. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int. 2013;112(2):176–181. doi: 10.1111/bju.12218. [DOI] [PubMed] [Google Scholar]
- 33.Zheng DX, Soldozy S, Mulligan KM, Levoska MA, Cohn EF, Finberg A, Alsaloum P, Cwalina TB, Hanft SJ, Scott JF, Rothermel LD, Nambudiri VE. Epidemiology, management, and treatment outcomes of metastatic spinal melanoma. World Neurosurg X. 2023;18:100156–100156. doi: 10.1016/j.wnsx.2023.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira MBDR, Souza LC, Sampayo EJG, Carvalho GS, Mello FCQ, Paschoal MEM. The impact of lung carcinoma histology on the frequency of bone metastases. Rev Bras Ortop (Sao Paulo) 2019;54(5):524–530. doi: 10.1016/j.rbo.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai S, Hu P, Liu X, Li Z, Wang B, Zhou H, Liu Z, Liu X, Li Y, Wei F. Prognostic analysis of spinal metastasis secondary to lung cancer after surgeries: a unicentric, large-cohort, retrospective study. Orthop Surg. 2023;15(1):70–78. doi: 10.1111/os.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciuleanu TE. Research and standard of care: lung cancer in Romania. Am Soc Clin Oncol Educ Book. 2012;32(1):437–441. doi: 10.14694/EdBook_AM.2012.32.437. [DOI] [PubMed] [Google Scholar]
- 37.Aydinli U, Ozturk C, Bayram S, Sarihan S, Evrensel T, Yilmaz HS. Evaluation of lung cancer metastases to the spine. Acta Orthop Belg. 2006;72(5):592–597. [PubMed] [Google Scholar]
- 38.Groszman L, Hubermann JA, Kooner P, Alamiri N, Bozzo A, Aoude A. The impact of adjunct medical therapy on survival after spine metastasis: a systematic review and pooled data analysis. Cancers (Basel) 2024;16(7):1425–1425. doi: 10.3390/cancers16071425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van den, Cornips EM, Peeters M, Ost P, Billiet C, Van de. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: a systematic review. J Bone Oncol. 2022;35:100446–100446. doi: 10.1016/j.jbo.2022.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filho ESV, Tardini R, de Abreu, Motter BV, Adami F, Rodrigues LMR. Epidemiological study of 55 patients with symptomatic metastatic spinal disease in Santo André - SP, Brazil [Estudo epidemiológico de 55 pacientes portadores de doença vertebral metastática sintomática em Santo André - SP, Brasil] Coluna/Columna. 2013;12(1):32–35. [Google Scholar]
- 41.Hong SH, Chang BS, Kim H, Kang DH, Chang SY. An updated review on the treatment strategy for spinal metastasis from the spine surgeon’s perspective. Asian Spine J. 2022;16(5):799–811. doi: 10.31616/asj.2022.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Perna, Cofano F, Mantovani C, Badellino S, Marengo N, Ajello M, Comite LM, Palmieri G, Tartara F, Zenga F, Ricardi U, Garbossa D. Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol. 2020;25:100320–100320. doi: 10.1016/j.jbo.2020.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato S, Demura S, Shinmura K, Yokogawa N, Shimizu T, Murakami H, Kawahara N, Tomita K, Tsuchiya H. Surgical metastasectomy in the spine: a review article. Oncologist. 2021;26(10):e1833–e1843. doi: 10.1002/onco.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al Farii, Aoude A, Al Shammasi, Reynolds J, Weber M. Surgical management of the metastatic spine disease: a review of the literature and proposed algorithm. Global Spine J. 2023;13(2):486–498. doi: 10.1177/21925682221146741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi K, Uchino K, Watanabe S, Misaki K, Iba H. Effect of minimally invasive spine stabilization in metastatic spinal tumors. Medicina (Kaunas) 2022;58(3):358–358. doi: 10.3390/medicina58030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knapp B, Govindan A, Patel SS, Pepin K, Wu N, Devarakonda S, Buchowski JM. Outcomes in patients with spinal metastases managed with surgical intervention. Cancers (Basel) 2024;16(2):438–438. doi: 10.3390/cancers16020438. [DOI] [PMC free article] [PubMed] [Google Scholar]