Abstract
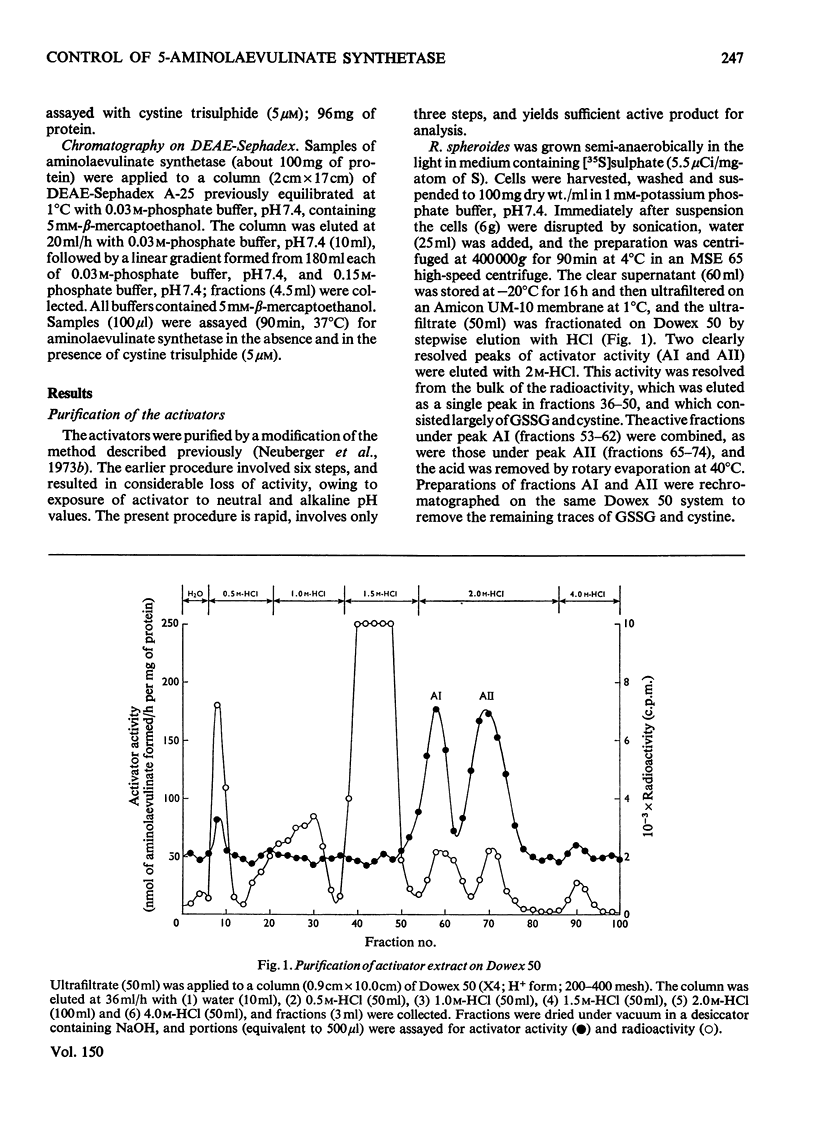
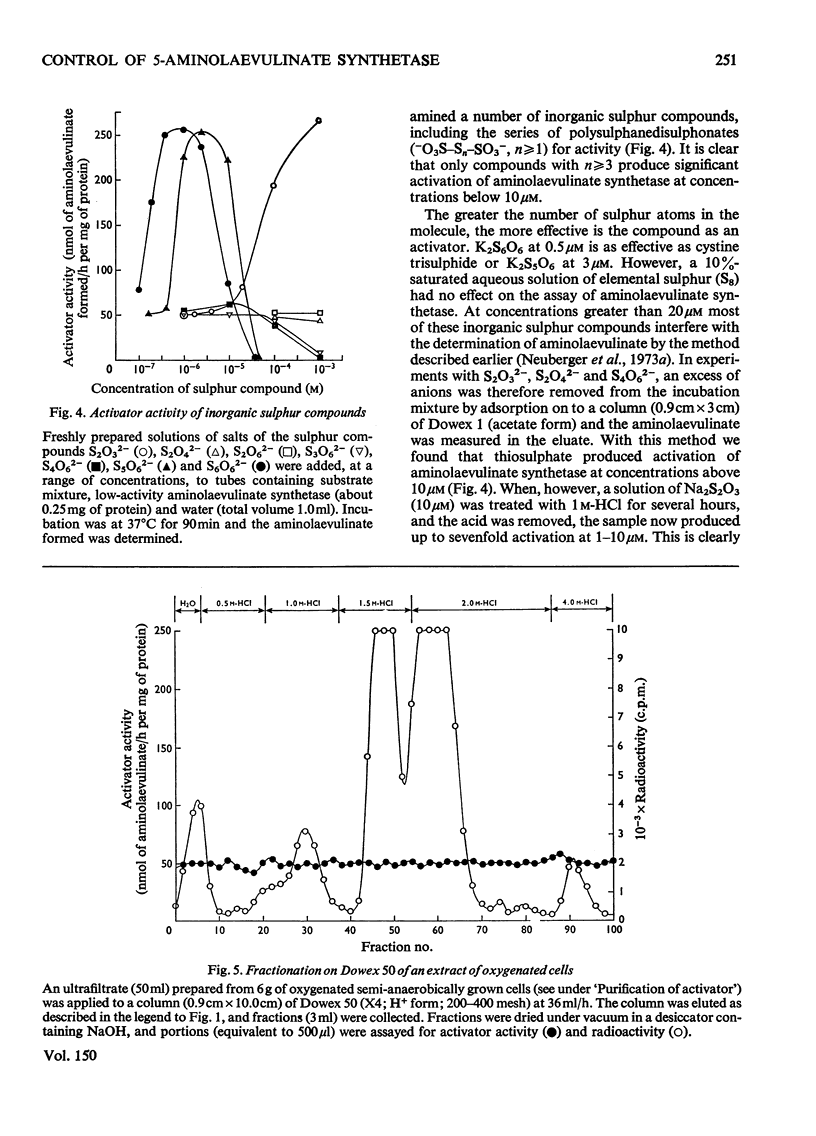
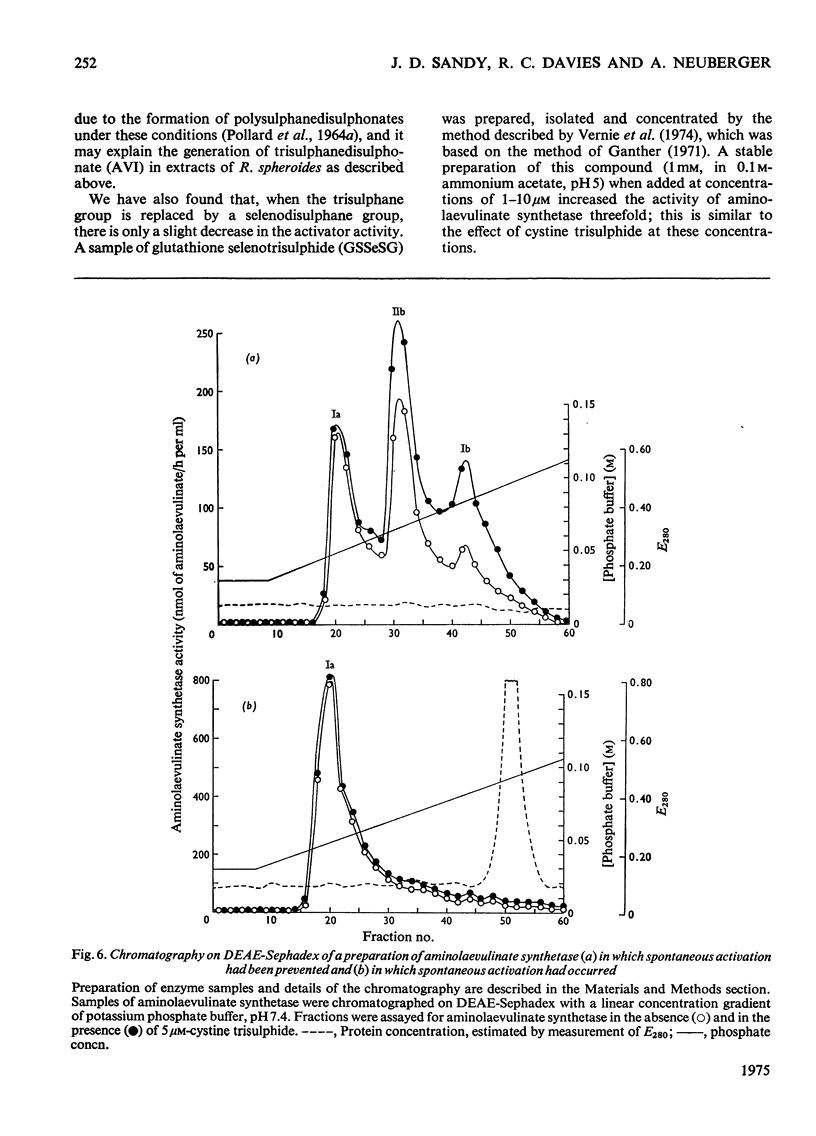
1. The aminolaevulinate synthetase activator of fresh extracts of semi-anaerobically grown Rhodopseudomonas spheroids was resolved into two fractions by ion-exchange chromatography. One fraction was identified as cystine trisulphide (CySSSCy). Analysis of the other fraction indicated the presence of about equal amounts of glutathione trisulphide (GSSSG) and the mixed trisulphide of glutathione and cystine (GSSSCy). 2. Four further fractions with activator activity were observed on ion-exchange chromatography of extracts prepared by methods similar to those described earlier [Neuberger et al. (1973)Biochem. J. 136,491-499]. These activators were generated by the extraction procedure. Two of them have been identified as trisulphanedisulphonate (S5O62-) and additional cystine trisulphide. 3. For the series of polysulphanedisulphonates (-O3S-Sn-SO3-, n greater than or equal to 1), activator activity at muM concentrations was exhibited only by compounds with n greater than 3. This, together with the results described above, indicates that for a compound R-Sn-R' (where R and R' are organic or inorganic groups) the only structural requirement for activity is n greater than or equal to 3. 4. Oxygenation of a semipanaerobic culture of R. spheroids for 1.5h before harvesting the cells produced a decrease of more than 90% in the cellular content of cystine trisulphide and glutathione trisulphides. 5. Chromatography on DEAE-Sephadex confirmed the presence of multiple forms of aminolaevulinate synthetase in extracts of R. spheroides [Tuboi et al. (1970) Arch.Biochem. Biophys. 138,147-154]. Oxygenation of a semi-anaerobic culture resulted in the disappearance of high-activity enzyme (a-form) and the accumulation of low-activity enzyme (b-form) in the cell. Spontaneous activation [Marriott et al. (1969) Biochem. J. 111,385-394] And activation by cystine trisulphide both resulted in the almost complete conversion of the b-form into the a-form.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrò A. F., Cannella C., Graziani M. T., Cavallini D. A possible role for rhodanese: The formation of 'labile' sulfur from thiosulfate. FEBS Lett. 1971 Aug 15;16(3):172–174. doi: 10.1016/0014-5793(71)80124-2. [DOI] [PubMed] [Google Scholar]
- Aminuddin M., Nicholas D. J. Sulphide oxidation linked to the reduction of nitrate and nitrite in Thiobacillus denitrificans. Biochim Biophys Acta. 1973 Oct 19;325(1):81–93. doi: 10.1016/0005-2728(73)90153-9. [DOI] [PubMed] [Google Scholar]
- Branzoli U., Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J Biol Chem. 1974 Jul 25;249(14):4346–4349. [PubMed] [Google Scholar]
- Fletcher J. C., Robson A. The occurrence of bis-(2-amino-2-carboxyethyl) trisulphide in hydrolysates of wool and other proteins. Biochem J. 1963 Jun;87(3):553–559. doi: 10.1042/bj0870553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganther H. E. Reduction of the selenotrisulfide derivative of glutathione to a persulfide analog by glutathione reductase. Biochemistry. 1971 Oct 26;10(22):4089–4098. doi: 10.1021/bi00798a013. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Tuboi S. Control of delta-aminolevulinate synthetase activity in Rhodopseudomonas spheroides. III. Partial purification of the fraction I activating enzyme and the occurrence of two forms of fraction II. J Biochem. 1974 Jul;76(1):157–168. doi: 10.1093/oxfordjournals.jbchem.a130541. [DOI] [PubMed] [Google Scholar]
- Marriott J., Neuberger A., Tait G. H. Activation of delta-aminolaevulate synthetase in extracts of Rhodopseudomonas spheroides. Biochem J. 1970 Apr;117(3):609–613. doi: 10.1042/bj1170609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott J., Neuberger A., Tait G. H. Control of delta-aminolaevulate synthetase activity in Rhodopseudomonas spheroides. Biochem J. 1969 Feb;111(4):385–394. doi: 10.1042/bj1110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr, Palmer G. The presence of S degrees-containing impurities in commercial samples of oxidized glutathione and their catalytic effect on the reduction of cytochrome c. Biochem Biophys Res Commun. 1971 Feb 19;42(4):730–738. doi: 10.1016/0006-291x(71)90548-1. [DOI] [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. The involvement of sulphur metabolism. Biochem J. 1973 Nov;136(3):477–490. doi: 10.1042/bj1360477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. The purification and properties of an endogenous activator of the enzyme. Biochem J. 1973 Nov;136(3):491–499. doi: 10.1042/bj1360491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of aminolaevulinate synthetase in Rhodopseudomonas spheroides. Enzyme. 1973;16(1):79–85. doi: 10.1159/000459365. [DOI] [PubMed] [Google Scholar]
- Petering D., Fee J. A., Palmer G. The oxygen sensitivity of spinach ferredoxin and other iron-sulfur proteins. The formation of protein-bound sulfur-zero. J Biol Chem. 1971 Feb 10;246(3):643–653. [PubMed] [Google Scholar]
- SEGEL I. H., JOHNSON M. J. Synthesis and characterization of sodium cysteine-S-sulfate monohydrate. Anal Biochem. 1963 Apr;5:330–337. doi: 10.1016/0003-2697(63)90085-x. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Control of -aminolevulinate synthetase activity in Rhodopseudomonas spheroides. I. Partial purification of the inactive form of fraction I. Arch Biochem Biophys. 1971 Sep;146(1):282–290. doi: 10.1016/s0003-9861(71)80065-6. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Control of -aminolevulinate synthetase activity in Rhodopseudomonas spheroides. II. Requirement of a disulfide compound for the conversion of the inactive form of fraction I to the active form. Arch Biochem Biophys. 1972 Jun;150(2):690–697. doi: 10.1016/0003-9861(72)90087-2. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Interconversion between the active and inactive forms of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides. Enzyme. 1973;16(1):86–93. doi: 10.1159/000459366. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Kim H. J., Kikuchi G. Occurrence and properes of two types of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 May;138(1):147–154. doi: 10.1016/0003-9861(70)90293-6. [DOI] [PubMed] [Google Scholar]
- VILLAREJO M., WESTLEY J. MECHANISM OF RHODANESE CATALYSIS OF THIOSULFATE-LIPOATE OXIDATION-REDUCTION. J Biol Chem. 1963 Dec;238:4016–4020. [PubMed] [Google Scholar]
- Vernie L. N., Bont W. S., Emmelot P. Inhibition of in vitro amino acid incorporation by sodium selenite. Biochemistry. 1974 Jan 15;13(2):337–341. doi: 10.1021/bi00699a018. [DOI] [PubMed] [Google Scholar]
- WALEY S. G. Acidic peptides of the lens. 5. S-Sulphoglutathione. Biochem J. 1959 Jan;71(1):132–137. doi: 10.1042/bj0710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley J. Rhodanese. Adv Enzymol Relat Areas Mol Biol. 1973;39:327–368. doi: 10.1002/9780470122846.ch5. [DOI] [PubMed] [Google Scholar]


