Abstract
Schistosomiasis is considered the most widespread parasitic infection. Both Schistosoma haematobium and Schistosoma mansoni are present, and as waterborne infections, their epidemiology is closely associated with proximity and exposure to freshwater sources. The objective of the current study is to estimate the pooled prevalence of schistosomiasis among the Sudanese population and examine any associated sociocultural risk factors. A systematic review was conducted in December 2022. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Sixty-seven research articles were recruited representing a total sample size of 813,571 participants. Schistosoma haematobium pooled prevalence was 24.83% (95% confidence interval (CI): 22.75, 26.92) among 700,337 participants tested, while S. mansoni pooled prevalence of 19.13% (95% CI: 18.70, 19.56) among 685,133 participants was originated. Moreover, schistosomiasis prevalence among school-age children was assessed in 45 included studies; the pooled prevalence of S. haematobium was 22.37 (95% CI: 20.12, 24.63), while S. mansoni pooled prevalence was 18.62 (95% CI: 13.14, 24.11). Furthermore, the highest Schistosoma prevalence (overall pooled prevalence: 41% (95% CI: 26.72, 55,29), S. haematobium pooled prevalence: 38.59 (95% CI: 21.03, 56.14), S. mansoni pooled prevalence: 25.85 (95% CI: 5.07, 46.63)) was found among Gezira State participants, based on a sample size of 5,712 individuals. Farming, male gender, no presence of latrines, canal and stream water sources, and swimming, playing, or bathing in the Nile River and canals were found to be significantly associated with schistosomiasis infection. The current findings are believed to serve as a cornerstone for designing strategies and preventive measures.
Keywords: africa, communicable diseases, developing countries, intestinal parasite, middle east
Introduction and background
Considering the ongoing political turmoil, marked by decades of war and hostility in Sudan, healthcare has largely been neglected, overshadowed by what the government may deem as more urgent concerns. The country is confronting a worsening humanitarian crisis, with almost eight million people facing severe challenges to their psychological and clinical well-being, including approximately 1.6 million internally displaced individuals and around one million refugees. Resources are limited, and the country's economic output dropped by almost 67% between 2017 and 2018, even before the current armed conflict. Healthcare infrastructure is inadequately resourced and unable to meet the increasing and neglected demands. To make matters worse, Sudan remains far from achieving the Sustainable Development Goals (SDGs). These sociopolitical and economic challenges may increase vulnerability to infectious diseases by disrupting healthcare infrastructure, limiting access to healthcare services, and creating conditions conducive to disease transmission. The primary communicable diseases contributing to morbidity in the country include malaria, tuberculosis, schistosomiasis, pneumonia, and diarrheal diseases, according to the WHO and the Sudan Health Observatory under the Federal Ministry of Health [1,2].
Schistosomiasis is recognized as the most common parasitic infection. Both Schistosoma haematobium and Schistosoma mansoni are present in the region, and as waterborne diseases, their distribution is closely linked to the availability and accessibility of natural freshwater sources. A recent nationwide survey involving over 100,000 school-age children has highlighted the widespread nature of this infection. The overall prevalence of S. haematobium was found to be 5.2%, while S. mansoni showed a prevalence of 0.06%. However, other studies have reported even higher localized prevalence rates; for instance, in certain schools within White Nile State, 46.5% of the children sampled were infected, with 45% infected with S. haematobium, 5.9% with S. mansoni, and 4.4% with mixed infections [3]. This study aims to estimate the pooled prevalence of schistosomiasis among the Sudanese population and identify related social and cultural risk factors. This objective is crucial due to the variability and limited scope of existing studies, which are often region-specific and hinder a cohesive understanding of national prevalence. By synthesizing diverse data, this study provides a reliable, comprehensive estimate and highlights sociocultural risk factors, contributing valuable insights to guide targeted, equitable disease control strategies across Sudan.
Review
Materials and methods
Search Strategy
To identify relevant studies, a systematic review of the literature was conducted in December 2022. The review was regulated in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [4]. A comprehensive search was conducted across Google Scholar, Scopus, PubMed, Embase, Directory of Open Access Journals (DOAJ), Index Copernicus, Elton B. Stephens Company (EBSCO)-Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane databases, without language restrictions (although studies in languages other than English were later excluded). To ensure relevance to the current situation, only studies published from 2010 onward were included. Additionally, studies with data collection prior to 2010 were excluded, except where data collection began in or before 2010 and continued into 2010 or beyond, as previously described [5].
Due to the limited availability of medical literature from Sudan in international databases and the variability in the reporting of sociocultural factors, these factors were not included in the formulation of keywords. Instead, relevant information was extracted from the studies that were included later on. The keywords used in PubMed were as follows: "Schistosomiasis" OR "Schistosoma mansoni" OR "Schistosoma haematobium" OR "Schistosoma japonicum" AND "Sudan*[tiab]", as previously described [6].
Furthermore, to refine the search process, manual searches of the reference lists from the included articles were conducted.
Study Selection and Data Extraction
Titles and abstracts were evaluated for initial eligibility. Full texts were obtained for all research articles that were available and tentatively approved for inclusion. Data abstraction followed a task separation approach; the methods and results sections of each study were abstracted separately on different occasions to minimize bias. Additionally, abstraction was performed without regard to the authors' qualifications or expertise. All authors carefully selected relevant studies from the literature, and any disagreements that arose during the process were resolved through thorough discussion and consensus. Each research article was examined for all pertinent information and recorded in a data extraction file (Microsoft Excel, Microsoft Corp., Redmond, WA). Data from each methods section were extracted using a predefined set of variables, including study characteristics, participant demographics, study population size, geographical region, methodology employed for prevalence or risk assessment, and study duration. Moreover, since risk factor-related keywords were not included in the search strategy, each study was thoroughly screened to identify the nature of the risks investigated. Studies that did not assess prevalence or sociocultural risks were subsequently excluded, as previously described [5].
Assessment of Quality and Risk of Bias
Each article included in the review was assessed using a structured framework designed for summarizing quality evaluations. The existing literature was examined, and a specific framework was developed to evaluate the representativeness of the studied population and assess the strength of the estimates reported. Each article was required to address five questions, with responses scored as follows: 1 point for "yes," 0 points for "no," and 0 points for "not available." The total score for risk of bias and quality was calculated by summing the scores across all five domains, yielding a score ranging from 0 to 5. A higher score indicates superior quality, and only studies with a quality score of 3 or above were included in the analysis, as previously described [5].
As outlined previously [5], the five criteria evaluated were as follows: is the study objective explicitly stated, is the study population well-defined and specified, is the study sample comprehensively identified, is the methodology robust, and is the data analysis robust?
Secondary Analysis
Among all the included studies that reported either prevalence or risk factor estimates, it was noted whether the standard error (SE) was provided. For studies that did not report the SE, it was calculated using the following formula: SE = √p (1-p)/n, where p represents prevalence. Regarding risk factors, each included study may have had different objectives, which influenced how results were presented (e.g., adjusted odds ratio (OR), unadjusted OR, or frequencies). For each sociocultural variable investigated, the odds ratio (OR) was calculated for individual categories whenever possible, allowing for univariate analysis of each category within the studied population, as previously described [5].
The categorization of variables was structured to enhance the population size for specific estimates. For instance, while most studies examining the sociocultural risks of schistosomiasis classified education levels as below secondary and secondary/above, the few studies that used a primary, secondary, and university classification were re-categorized to combine similar groups. This resulted in a new classification where "primary" was defined as below secondary, and "secondary and university" were combined into the secondary/above category, as previously described [5].
Quantitative Analysis
Meta-analysis was conducted using Review Manager software versions 5.3 and 5.4 (The Cochrane Collaboration, London, UK) whenever feasible. The software automatically calculated the confidence interval (CI) based on the provided standard error (SE), and if a CI was reported in a study, it was incorporated accordingly. The heterogeneity of each meta-analysis was also evaluated, with the random effects model preferred over the fixed effects model due to the expected variability between study populations. Sensitivity analysis was performed to assess the impact of studies conducted in populations thought to behave similarly or presumed to have low risk on the overall pooled data. Additionally, subgroup analyses were carried out when appropriate to determine prevalence or risk levels within specific states or populations. An outcome needed to be included in at least two studies to be considered for the meta-analysis. The trim-and-fill method was employed to evaluate the risk of publication bias in each meta-analysis performed, as previously described [5,7].
Results
Studies Included
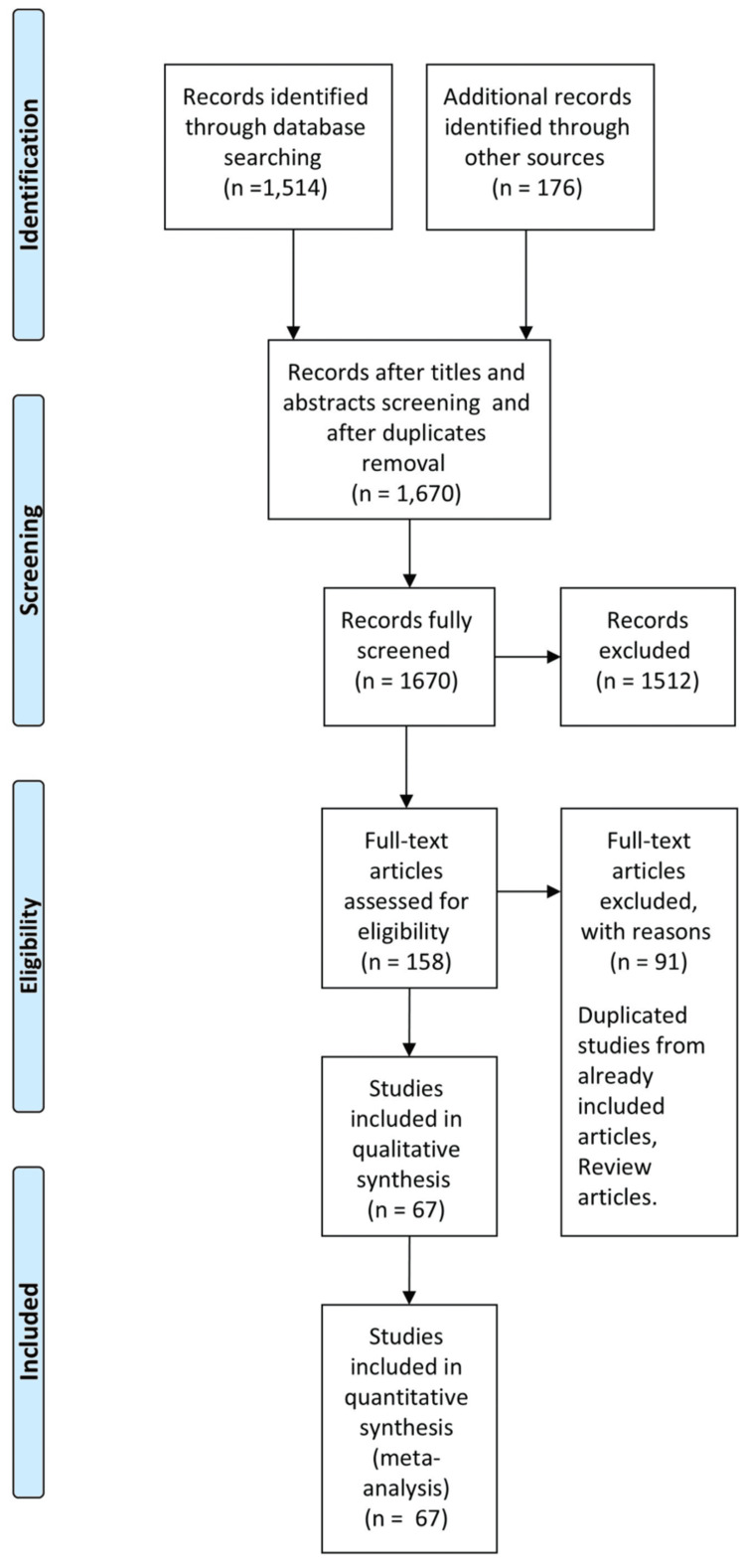
A total of 1,690 articles were identified using the search strategy, which included manual searches of reference lists from pertinent original research articles and reviews. Out of these, 1,512 articles were excluded. Subsequently, after screening the abstracts and full texts, 67 articles met our inclusion criteria and successfully passed the quality assessment. These articles provided information on prevalence in specific populations and/or associated risk factors. The PRISMA flow diagram and checklist are shown in Figure 1 and Appendices, respectively. The quality assessment and risk of bias of included studies is provided in the Appendices.
Figure 1. PRISMA flow diagram.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics of the Studies
The characteristics of the included studies are outlined in Table 1. Sixty-seven research articles were recruited [8-74], among which 63 research articles determined the prevalence of schistosomiasis among different study populations. The earliest was published in 2010, while the most recent articles were published in 2022. Seventeen studies were conducted in White Nile State, 13 in Khartoum State, 11 in Gezira State, eight in Kassala State, four in River Nile State, three in Sennar State, two in southern Kordofan State, and one in each of Gadarif, Northern State, South Darfur, and both of Khartoum and Kassala States. Moreover, two studies were conducted among all 18 states of Sudan. All included studies represent a total sample size of 813,571 participants. Moreover, 53 articles were conducted among both genders, seven studies were conducted among only males, one study was conducted among only females, and the remaining three studies did not specify the gender of their participants. Moreover, the majority of studies (45) focused on the prevalence/sociocultural risk factors among school-age children; several studies included the general population, encompassing both school-age children and others; two studies were conducted among patients and suspected patients; one study focused on pregnant women; and another study was toward fishermen. Publication bias assessment indicated no major asymmetry.
Table 1. Characteristics of the included studies.
PCR: polymerase chain reaction, ELISA: enzyme-linked immunosorbent assay, IHA: indirect hemagglutination assay
| Study ID | Publication year | Study design | State | Study population(s) | Assessment | Sample size | Gender | Participants' age (years) |
| Abakar et al. [8] | 2021 | Cross-sectional | Khartoum | Patients | Prevalence (parasitological methods) and risk factors | 150 | Both | 5-≥35 |
| Elfaki et al. [25] | 2015 | Cross-sectional | Kassala | General population | Prevalence (parasitological methods) and risk factors | 100 | Both | Mean: 19±13 |
| Abdalla et al. [40] | 2020 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 102 | Not determined | 6-20 |
| Abdalla [42] | 2013 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 1,257 | Not determined | 5-19 |
| Abdelgadir et al. [73] | 2012 | Cross-sectional | Gezira | Pregnant women | Prevalence (parasitological methods) | 292 | Females | Not determined |
| Abdelrhman et al. [9] | 2017 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 200 | Both | 6-≥15 |
| Abdo et al. [59] | 2015 | Cross-sectional | Gezira | School-age children and general population | Prevalence (parasitological methods) | 203 | Males | 10-55 |
| Abou-Zeid et al. [18] | 2012 | Cross-sectional | Southern Kordofan | General population | Prevalence (parasitological methods) and risk factors | 1,826 | Both | Not determined |
| Abou-Zeid et al. [70] | 2013 | Cross-sectional | Southern Kordofan | School-age children | Prevalence (parasitological methods) and risk factors | 2,302 | Both | <8-≥12 |
| Afifi et al. [51] | 2016 | Cross-sectional | Kassala | General population | Prevalence (parasitological methods) and risk factors | 2,433 | Both | 1-≥50 |
| Ahmed et al. [35] | 2012 | Cohort | Gezira | School-age children | Prevalence (parasitological methods) and risk factors | 2,741 | Both | 6-15 |
| Ahmed et al. [10] | 2012 | Cohort | Gezira | School-age children | Prevalence (parasitological methods) and risk factors | 420 | Both | 1-16 |
| Ahmed et al. [31] | 2015 | Cohort | Khartoum | Suspected patients | Prevalence (radiological methods) | 109 | Both | Mean: 58 |
| Al-Basheer et al. [16] | 2017 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 150 | Males | <11-≥11 |
| Alsanosi et al. [67] | 2019 | Cross-sectional | Khartoum | Children (general population) | Prevalence (parasitological methods) and risk factors | 240 | Both | ≤16 |
| Altijani et al. [63] | 2017 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) | 182 | Both | 5-14 |
| Amin et al. [48] | 2017 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological methods) | 500 | Both | 11-14 |
| Bakhit et al. [54] | 2019 | Cross-sectional | White Nile | General population | Prevalence (parasitological methods) | 1,029 | Both | Mean: 15 |
| Cha et al. [43] | 2019 | Cross-sectional | All 18 states of Sudan | School-age children | Prevalence (parasitological methods) and risk factors | 105,167 | Both | Not determined |
| Cha et al. [56] | 2020 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 2,784 | Both | ≤9-≥13 |
| Deribe et al. [69] | 2011 | Cross-sectional | South Darfur | School-age children and general population | Prevalence (parasitological methods) and risk factors | 811 | Both | ≤5->15 |
| El-amin et al. [36] | 2014 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological methods, radiological methods, and PCR) | 438 | Both | Mean: 11 |
| Elbasheir et al. [58] | 2020 | Longitudinal survey | Sennar | School-age children | Prevalence (parasitological methods) | 489 | Both | 5-15 |
| Elfadol et al. [38] | 2020 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 314 | Both | 7-18 |
| Elfaki et al. [66] | 2015 | Cross-sectional | Kassala | General population | Prevalence (parasitological methods) | 75 | Both | Mean: 17 |
| Elfaki et al. [37] | 2015 | Cross-sectional | Khartoum | General population | Prevalence (parasitological methods) and risk factor | 141 | Males | 15-55 |
| Elfaki et al. [64] | 2016 | Retrospective | Kassala | School-age children | Prevalence (parasitological methods and PCR) and risk factors | 234 | Both | 4-85 |
| Elfaki et al. [20] | 2020 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 160 | Both | Not determined |
| Elhag et al. [65] | 2011 | Cross-sectional | Gezira | General population | Prevalence (parasitological methods and ELISA) and risk factors | 208 | Both | 4-80 |
| Elmadani et al. [57] | 2013 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological and radiological methods) | 103 | Males | 7-20 |
| Elmadhoun et al. [41] | 2013 | Cross-sectional | River Nile | School-age children | Prevalence (parasitological methods) | 2,490 | Both | 8-19 |
| Elmekki et al. [50] | 2018 | Cross-sectional | Khartoum and Kassala | School-age children | Prevalence (parasitological methods) and risk factors | 770 | both | 4-85 |
| Elsammani et al. [55] | 2019 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) | 600 | Both | 6-15 |
| Elsiddig et al. [14] | 2019 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 385 | Both | 6-15 |
| Gasmelseed et al. [44] | 2012 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological and radiological methods) | 438 | Both | 6-20 |
| Gasmelseed et al. [26] | 2014 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological and radiological methods, and PCR) | 83 | Males | 6-20 |
| Hajissa et al. [33] | 2018 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 170 | Both | 6-17 |
| Hamad et al. [46] | 2018 | Cross-sectional | River Nile | School-age children | Prevalence (parasitological methods) | 200 | Not determined | Not determined |
| Hassan et al. [22] | 2019 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) and risk factors | 134 | Both | 6-14 |
| Ibrahim et al. [21] | 2014 | Cross-sectional | Sennar | School-age children | Prevalence (parasitological methods, ELISA, and IHA) | 214 | Both | 6-16 |
| Ibrahim et al. [62] | 2019 | Cross-sectional | Sennar | School-age children | Prevalence (parasitological methods) | 396 | Both | 9-16 |
| Ismail et al. [49] | 2014 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 338 | Both | 7-15 |
| Jin et al. [27] | 2022 | Cross-sectional | All 18 states of Sudan | School-age children | Prevalence (parasitological methods) and risk factors | 105,167 | Both | Mean: 11 |
| Jin et al. [19] | 2020 | Cohort | White Nile | School-age children | Prevalence (parasitological methods) | 1,286 | Both | 6-16 |
| Jin et al. [29] | 2021 | Cohort | White Nile | School-age children | Prevalence (parasitological methods) | 1,951 | Both | Mean: 9 |
| Kardaman et al. [74] | 2017 | Cross-sectional | Gezira | School-age children | Prevalence (parasitological methods) | 286 | Both | 3-14 |
| Kassar [39] | 2017 | Cross-sectional | North Kordofan | School-age children | Risk factors | 310 | Both | 8-16 |
| Kebayer et al. [34] | 2022 | Cross-sectional | Kassala | General population | Prevalence (parasitological methods) and risk factors | 190 | Both | 1-99 |
| Khalid et al. [13] | 2012 | Cross-sectional | Gezira | Pregnant women | Risk factors | 292 | Female | Not determined |
| Kim et al. [11] | 2016 | Cross-sectional | White Nile | School-age children and general population | Prevalence (parasitological and radiological methods) | 1,462 | Both | 1-80 |
| Lee et al. [23] | 2015 | Cross-sectional | White Nile | School-age children and general population | Prevalence (parasitological methods) | 561,517 | Both | Not determined |
| Lee et al. [45] | 2019 | Cross-sectional | White Nile | General population | Prevalence (parasitological methods) and risk factors | 1,138 | Both | 0-<30 |
| Mahgoub et al. [60] | 2010 | Cross-sectional | Kassala | School-age children | Prevalence (parasitological methods) and risk factors | 640 | Both | 8-18 |
| Mahmood [12] | 2016 | Case-control | Khartoum | School-age children | Risk factors | 768 | Both | 8-15 |
| Malik et al. [61] | 2021 | Case-control | White Nile | Fishermen | Prevalence (parasitological methods, ELISA, and immunological assays) | 119 | Males | 14-77 |
| Mohamed et al. [15] | 2013 | Cross-sectional | Kassala | General population | Prevalence (parasitological methods) | 770 | Both | 4-85 |
| Mohammed et al. [17] | 2018 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 475 | Both | 6-15 |
| Omer et al. [71] | 2020 | Cross-sectional | River Nile | School-age children | Prevalence (parasitological methods) and risk factors | 1,188 | Both | 6-18 |
| Osman et al. [28] | 2018 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) | 300 | Both | 5-13 |
| Osman et al. [68] | 2022 | Cross-sectional | Northern State | School-age children | Prevalence (parasitological methods) | 1,557 | Males | 6-13 |
| Salah et al. [52] | 2014 | Cross-sectional | Gedarif | School-age children | Prevalence (parasitological methods) and risk factors | 480 | Both | Mean: 18 |
| Sulieman et al. [30] | 2017 | Cross-sectional | River Nile | School-age children | Prevalence (parasitological methods) and risk factors | 385 | Both | 7-≥14 |
| Suliman et al. [72] | 2021 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 347 | Both | 10-17 |
| Taha et al. [47] | 2019 | Cross-sectional | Khartoum | School-age children | Prevalence (parasitological methods) | 1,205 | Both | 6-14 |
| Talab et al. [24] | 2018 | Cross-sectional | White Nile | School-age children | Risk factors | 420 | Both | 9-17 |
| Tamomh et al. [32] | 2018 | Cross-sectional | White Nile | School-age children | Prevalence (parasitological methods) and risk factors | 480 | Both | 5-≥12 |
| Tamomh et al. [53] | 2018 | Cross-sectional | White Nile | School-age children | Risk factors | 480 | Both | 5-≥12 |
Schistosomiasis Prevalence
Prevalence estimates were compiled to highlight the overall disease burden and assess the burden within specific subgroups based on the study population, causative agent, and geographic location, whenever feasible. Detailed pooled prevalence data is provided below, with a summary in Table 2.
Table 2. Summary of prevalence estimates synthesized from the included studies.
CI: confidence interval
| Prevalence | Assessed in (state) | Assessed among | Total sample size | Pooled prevalence | 95% CI |
| Prevalence of schistosomiasis | All 18 states of Sudan | General population, school-age children, suspected patients, farmers, pregnant women, and fishermen | 812,801 | 26.86 | 24.71, 29.02 |
| Prevalence of S. haematobium | Khartoum, Gezira, River Nile, Sennar, Gadarif, Northern State, South Darfur, and Kassala | School-age children, general population, suspected patients, and fishermen | 700,337 | 24.83% | 22.75, 26.92 |
| Prevalence of S. mansoni | All 18 states of Sudan | General population, school-age children, and pregnant women | 685,133 | 19.13 | 18.70, 19.56 |
| Prevalence among the general population | White Nile, Khartoum, Gezira, Kassala, River Nile, Sennar, Southern Kordofan, Gadarif, Northern State, and South Darfur | General population | 812,131 | 25.75 | 23.53, 27.97 |
| Prevalence among school-age children | White Nile, Khartoum, Gezira, Sennar, River Nile, Kassala State, Gadarif, Southern Kordofan, and Northern State | School-age children | 240,228 | 24.46% | 22.78, 26.13 |
| Prevalence in Khartoum State | Khartoum | School-age children, general population, and suspected patients | 3,775 | 20.66% | 11.74, 29.57 |
| Prevalence in Gezira State | Gezira | Pregnant women, students, and general population | 5,712 | 41.00% | 26.72, 55.29 |
| Prevalence in Kassala State | Kassala | General population and school-age children | 5,212 | 30.33% | 19.15, 41.51 |
| Prevalence in River Nile State | River Nile | School-age children | 4,263 | 17.33% | 6.44, 28.22 |
| Prevalence in Sennar state | Sennar | School-age children | 1,099 | 28.60% | 20.52, 36.68 |
| Prevalence in White Nile State | White Nile | School-age children, general population, and fishermen | 575,430 | 27.94% | 22.96, 32.93 |
Schistosomiasis Prevalence Among Different Populations
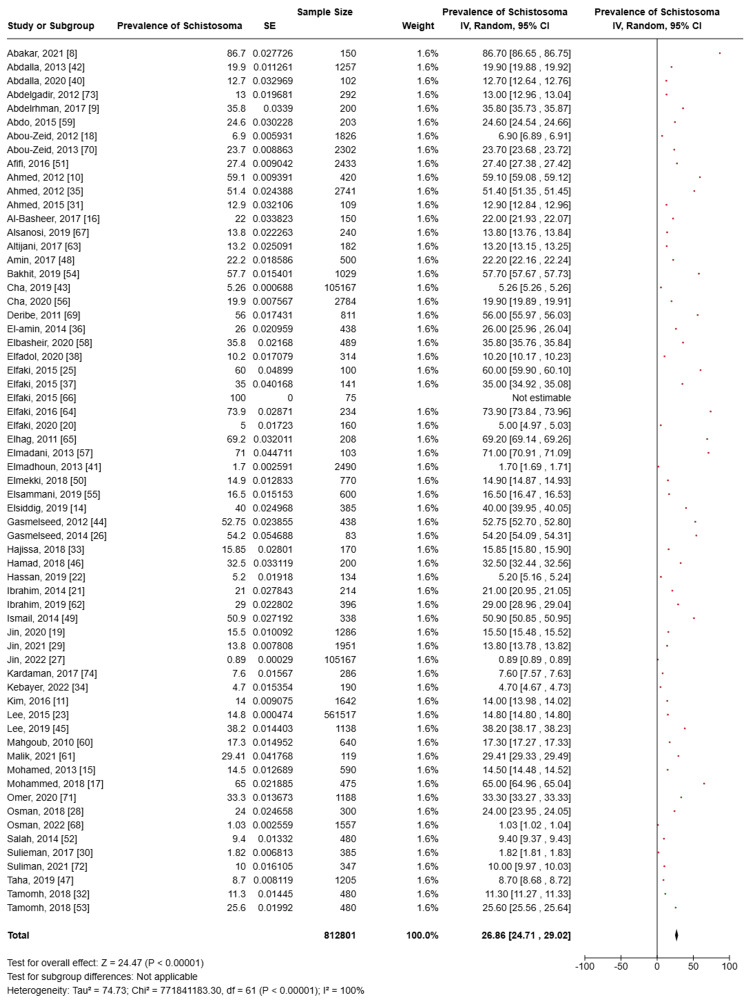
Among 63 included studies to quantify the burden of the disease among the Sudanese population, despite the causative agent, and based on a total sample size of 812,801 participants of different populations as well as geographical locations, the pooled prevalence of schistosomiasis was 26.86% (95% confidence interval (CI): 24.71, 29.02). Heterogeneity was high (I2 = 100%) (Figure 2). The characteristics of all included studies are presented in Table 1.
Figure 2. Meta-analysis of the prevalence of schistosomiasis among the participants of the included studies.
Prevalence of S. haematobium
Forty-eight research articles determined the prevalence of S. haematobium [8,9,11,14,16-20,23,26,28-33,35,37,38,40-47,49-52,54-57,59,61-63,65,67-72,74]. Sixteen studies were conducted in White Nile State, 12 in Khartoum State, seven in Gezira State, four in River Nile State, three in Sennar State, two in southern Kordofan State, and one research article in each of Kassala, Gadarif, Northern State, South Darfur, and one in both Khartoum and Kassala States. Also, one study covered all 18 states in Sudan, representing a total sample size of 700,337 participants. Moreover, 38 articles were conducted among both genders, seven studies were conducted among males only, and the other three studies did not report the gender of their participants. Additionally, 39 studies were focused on the prevalence or risk factors among school-age children, 11 were toward the general population of different ages, two were conducted among patients and suspected patients, and one was conducted on fishermen. The pooled prevalence was 24.83% (95% CI: 22.75, 26.92). Heterogeneity was high (I2 = 100%).
Prevalence of S. mansoni
Twenty-eight research articles determined the prevalence of S. mansoni [10,15,18,20-23,25,33,34,36,43-45,48-51,53,55,58-60,62,64-66,70]. Seven studies were established in Kassala State, seven in Gezira State, and four in each of Khartoum and White Nile States. Moreover, three related articles were conducted in Sennar State, two in southern Kordofan State, one in both Khartoum and Kassala States, and one in all 18 states of Sudan, resulting in a total sample size of 685,133. Moreover, 27 articles recruited both genders, one study was conducted among males only, and one was conducted on females only. Furthermore, 20 studies were concerned with the prevalence or risk factors among school-age children. Additionally, nine studies were toward the general population, while one study was toward pregnant women. The pooled prevalence was 19.13% (95% CI: 18.70, 19.56). Heterogeneity was high (I2 = 100%).
Schistosoma Prevalence Among the General Population
Fifty-eight studies determined their participants as the general population (i.e., not being hospital outpatients or proposed to be at specific risk). Sixteen studies were conducted in White Nile, 11 in Khartoum, 10 in Gizera, eight in Kassala, four in River Nile, three in Sennar, two in Southern Kordofan, one in Gadarif, one in Northern State, and one in South Darfur State, resulting in a total sample size of 812,131. Fifty-one studies recruited both genders, six were among males, and three did not identify the gender of their participants. Age among the participants ranged from 0 to 99 years. The pooled prevalence was 25.75% (95% CI: 23.53, 27.97). Moreover, among the same population (general population), S. haematobium pooled prevalence was 22.84 (95% CI: 20.74, 24.95), while S. mansoni pooled prevalence was 19.37 (95% CI: 18.93, 19.82). Heterogeneity was high in all meta-analyses (I2 = 100%).
Schistosoma Prevalence Among School-Age Children
Schistosomiasis prevalence among school-age children was assessed in 45 included studies [9,10,14,16,17,19-22,26-30,32,33,35,36,38,41-44,46-50,52,53,55-58,60,62-64,67,68,70-72,74]. Twelve studies were conducted in White Nile, 10 in Khartoum, eight in Gezira, three in Sennar, four in River Nile, two in Kassala, one in Khartoum and Kassala, one in Gadarif, one in Southern Kordofan, and one in Northern State. Two studies covered all 18 states of Sudan, representing a total sample size of 240,228 participants. Thirty-eight research articles targeted both genders with participants of up to 20 years old. Four studies were conducted among males only, while the remaining three studies did not determine their participants' gender. The pooled prevalence was 24.46% (95% CI: 22.78, 26.13). Moreover, among the same population (school-age children), S. haematobium pooled prevalence was 22.37 (95% CI: 20.12, 24.63), while S. mansoni pooled prevalence was 18.62 (95% CI: 13.14, 24.11). Heterogeneity was high (I2 = 100%).
Schistosoma Prevalence in Khartoum State
Schistosoma prevalence in Khartoum State was investigated in 13 included studies [8,16,20,22,28,31,33,37,38,40,47,55,67]. The related studies were focused on school-age children, the general population, and suspected patients, resulting in a total sample size of 3,775 participants from two genders in the majority of studies. The pooled prevalence was 20.66% (95% CI: 11.74, 29.57). Moreover, among the same population (Khartoum States' residents), S. haematobium pooled prevalence was 21.55 (95% CI: 12.04, 31.07), while S. mansoni pooled prevalence was 2.47 (95% CI: 0.95, 4.00). Heterogeneity was high (I2 = 100%).
Schistosoma Prevalence in White Nile State
Seventeen included studies determined Schistosoma prevalence among White Nile State participants, representing a total sample size of 575,430 participants [9,11,14,17,19,23,29,32,42,45,49,53,54,56,61,63,72]. Sixteen studies were toward the general population, and one study was among fishermen. The majority of studies were toward both genders, one study was conducted among males only, and one study did not identify the age of their participants. The age of the participants ranged from 18 to 50 years. The pooled prevalence was 27.94% (95% CI: 22.96, 32.93). Moreover, among the same population (White Nile State residents), S. haematobium pooled prevalence was 27.49 (95% CI: 22.30, 32.69), while S. mansoni pooled prevalence was 8.77 (95% CI: 4.77, 12.78). Heterogeneity was high in all meta-analyses (I2 = 100%).
Schistosoma Prevalence in Gezira State
Eleven included studies determined Schistosoma prevalence among Gezira State participants, representing a total sample size of 5,712 participants [10,26,35,38,44,48,57,59,65,73,74]. Eight studies were toward school-age children or pregnant women. Seven studies recruited both genders; three studies were toward males, and one study was toward females of all ages. The pooled prevalence was 41% (95% CI: 26.72, 55,29). Moreover, among the same population (Gezira State residents), S. haematobium pooled prevalence was 38.59 (95% CI: 21.03, 56.14), while S. mansoni pooled prevalence was 25.85 (95% CI: 5.07, 46.63). Heterogeneity was high in all meta-analyses (I2 = 100%).
Schistosoma Prevalence in Kassala State
Schistosoma prevalence in Kassala State was examined in seven studies targeting the general population of school-age children, comprising a total sample size of 5,212 participants of various ages and both genders [15,25,34,51,60,64,66]. The pooled prevalence was found to be 32.97 (95% CI: 19.46, 46.47). Additionally, within the same population of Kassala State residents, it was not possible to determine the pooled prevalence for S. haematobium due to the inclusion of only one related study. However, the pooled prevalence for S. mansoni was calculated to be 30.33 (95% CI: 19.15, 41.51) based on the results from two included studies. Heterogeneity was high (I2 = 100%).
Schistosoma Prevalence in River Nile State
The prevalence of schistosomiasis among residents of River Nile State was evaluated in four included studies [30,41,46,71]. Three of these studies focused on school-age children, with a combined total sample size of 4,263 participants; only one study did not specify the gender of its participants. The pooled prevalence was determined to be 17.33% (95% CI: 6.44, 28.22). Furthermore, within the same population of River Nile State residents, the pooled prevalence for S. haematobium was 17.33% (95% CI: 6.44, 28.22). However, it was not possible to calculate the pooled prevalence for S. mansoni as this prevalence was not reported in any of the four included studies. Heterogeneity was high (I2 = 100%).
Schistosoma Prevalence in Sennar State
Schistosoma prevalence among residents of Sennar State was assessed in three included studies [21,58,62]. These studies focused on school-age children and comprised a total sample size of 1,099 participants of both genders. The pooled prevalence was calculated to be 28.60% (95% CI: 20.52, 36.68). Moreover, among the same population (Sennar State residents), S. haematobium pooled prevalence was available as S. haematobium prevalence was reported only in one related study, while S. mansoni pooled prevalence was 19.73 (95% CI: -2.86, 42.33) based on findings of two studies. Heterogeneity was high (I2 = 100%).
Sociocultural Factors Associated With Schistosomiasis
Sex: Sex was examined as a potential risk factor for schistosomiasis in 27 included studies. Participants comprised the general population and school-age children from all 18 states of Sudan. Among 67,531 male participants, the pooled odds ratio for male infection was 1.70 (95% CI: 1.39, 2.08), with a significant p-value of z = 5.22 (P < 0.00001). In contrast, there were 56,490 female participants from the same populations, with a pooled odds ratio for female infection of 0.59 (95% CI: 0.45, 0.76) and a significant p-value of z = 4.02 (P < 0.0001). Results are illustrated in Table 3.
Table 3. Summary of sociocultural risk factor estimates synthesized from the included studies.
OR: odds ratio, CI: confidence interval
| Risk | Assessed in (state) | Assessed among | Total sample size | Pooled OR (95% CI) | Test for overall effect (Z score) |
| Male gender | All 18 states of Sudan | General population and school-age children | 67,531 | 1.70 (1.39, 2.08) | 5.22 (P < 0.00001) |
| Female gender | All 18 states of Sudan | General population and school-age children | 56,490 | 0.59 (0.45, 0.76) | 4.02 (P < 0.0001) |
| Illiteracy | Southern Kordofan, Kassala, North Kordofan White Nile, Gezira, and Khartoum | General population, pregnant women, and school-age children | 1,496 | 0.26 (0.03, 2.07) | 1.28 (P = 0.20) |
| Farming | All 18 states of Sudan | General population and school-age children | 3,935 | 2.18 (1.12, 4.26) | 2.29 (P = 0.02) |
| Fishing | All 18 states of Sudan | School-age children | 652 | 1.51 (0.23, 9.81) | 0.43 (P = 0.67) |
| Latrines | All 18 states of Sudan | School-age children and general population | 81,940 | 0.62 (0.44, 0.88) | 2.70 (P = 0.007) |
| No latrines | All 18 states of Sudan | School-age children and general population | 24,301 | 1.62 (1.25, 2.09) | 3.69 (P = 0.0002) |
| Canal and stream water source | Khartoum, South Kordofan, Kassala, North Kordofan, and White Nile | School-age children and general population | 2,347 | 2.10 (1.07, 4.10) | 2.17 (P = 0.03) |
| Donkey cart and tanker water source | Eastern Sudan, Kassala, Khartoum, and White Nile | School-age children and general population | 167 | 0.59 (0.55, 0.64) | 13.50 (P < 0.00001) |
| Pipe, tape, and hand pump water source | Khartoum, Eastern Sudan, South Kordofan, Kassala, North Kordofan, and White Nile | School-age children and general population | 3,092 | 0.62 (0.34, 1.11) | 1.61 (P = 0.11) |
| Swimming, playing, bathing, planting crops, and contact with water | Khartoum, South Kordofan, White Nile, Eastern Sudan, and River Nile | School-age children and general population | 33,516 | 2.48 (1.81, 3.39) | 5.67 (P < 0.00001) |
| No contact to water | All 18 states of Sudan | School-age children and general population | 63,054 | 0.46 (0.28, 0.74) | 3.15 (P = 0.002) |
Education level: Illiteracy was examined as a possible risk factor for schistosomiasis across eight studies. The participants included individuals from the general population, pregnant women, and school-age children from Southern Kordofan, Kassala, North Kordofan, White Nile, Gezira, and Khartoum States, comprising a total sample size of 1,496. The pooled odds ratio for illiterate individuals being infected was 0.26 (95% CI: 0.03, 2.07); however, the p-value was not significant, with z = 1.28 (P = 0.20). Results are illustrated in Table 3.
Occupation: Farming occupation was examined as a possible risk factor for schistosomiasis across eight included studies. The participants included individuals from the general population and school-age children from all 18 states of Sudan. There were 3,935 farmers, and the pooled odds ratio of their infection was 2.18 (95% CI: 1.12, 4.26), with a significant p-value of z = 2.29 (P = 0.02). Moreover, fishing occupation was investigated among young 652 fishermen from different states; the pooled odds ratio of them being infected was 1.51 (95% CI: 0.23, 9.81), with an insignificant p-value of z = 0.43 (P = 0.67). The results are illustrated in Table 3.
Sanitation: The availability of latrines was assessed as a potential risk factor for schistosomiasis in nine studies. Participants included individuals from the general population and school-age children across all 18 states of Sudan. Among the 81,940 participants who reported having access to latrines, the pooled odds ratio for infection was 0.62 (95% CI: 0.44, 0.88), with a significant p-value of z = 2.70 (P = 0.007). In contrast, 24,301 participants from the same populations reported no access to latrines. The pooled odds ratio for this group being infected was 1.62 (95% CI: 1.25, 2.09), with a significant p-value of z = 3.69 (P = 0.0002). All results are presented in Table 3.
Water source: The use of canals and streams as water sources was examined as a potential risk factor for schistosomiasis in seven studies. Participants included individuals from the general population and school-age children in Khartoum, Southern Kordofan, Kassala, North Kordofan, and White Nile States, totaling 2,347 participants. The pooled odds ratio for infection in this group was 2.10 (95% CI: 1.07, 4.10), with a significant p-value of z = 2.17 (P = 0.03).
Additionally, the use of donkey carts (small tank vehicles pulled by donkeys, used for delivering water sourced mostly from wells in rural and semi-urban areas) and tankers was investigated in three studies involving participants from Eastern Sudan, Kassala, and White Nile States, including school-age children and the general population, with a total sample size of 167 participants. The pooled odds ratio for infection in this group was 0.59 (95% CI: 0.55, 0.64), with a significant p-value of z = 13.50 (P < 0.00001).
Furthermore, the use of pipes, taps, and hand pumps as water sources was studied in seven studies with participants from Khartoum, Eastern Sudan, Southern Kordofan, Kassala, North Kordofan, and White Nile States, encompassing both school-age children and the general population. The total sample size was 3,092 participants, and the pooled odds ratio for infection was 0.62 (95% CI: 0.34, 1.11), with an insignificant p-value of z = 1.61 (P = 0.11). All results are presented in Table 3.
Water contact: Contact with water through activities such as swimming, playing, or bathing was examined as a potential risk factor for schistosomiasis in 10 studies. Participants included school-age children and individuals from the general population in Khartoum, Southern Kordofan, White Nile, Eastern Sudan, and River Nile States, totaling 33,516 participants. The pooled odds ratio for infection in this group was 2.48 (95% CI: 1.81, 3.39), with a significant p-value of z = 5.67 (P < 0.00001).
Conversely, the absence of water contact was investigated across all 18 states of Sudan in five studies, involving school-age children and the general population, with a total sample size of 63,054 participants. The pooled odds ratio for this group was 0.46 (95% CI: 0.28, 0.74), with a significant p-value of z = 3.15 (P = 0.002). All results are presented in Table 3.
Discussion
To our knowledge, this review is the first attempt to evaluate the overall prevalence of schistosomiasis and its associated sociocultural risk factors in Sudan. The study utilized a thorough search across various published databases and employed a meticulous methodology for screening and selecting relevant studies.
In the current study, the pooled prevalence of schistosomiasis was 26.86% among 812,801 participants from all 18 states of Sudan. This finding is almost similar in comparison to a study done in Uganda (25.6%, 95% CI: 22.3, 29.0) [75]; however, a much lower estimate has been reported in the Philippines (8.4%, 95% CI: 3.5, 14.0) [76]. These differences may be attributed to social demographics and diagnostic protocols.
Moreover, the prevalence of S. haematobium was found to be 24.83% among 700,337 participants from different states. An even higher estimate has been reported in Zambia (35.5%) [77]. Furthermore, the prevalence of S. mansoni was 19.13% among 685,133 participants from all 18 states of Sudan. Higher estimates have been concluded in the literature as well. In neighboring Ethiopia, a prevalence of 26.3% was reported [78], while 34.9% was reported in Zambia [77]. These differences may be attributed to social demographics, study designs, and diagnostics protocols.
In regard to schistosomiasis prevalence among school-age children, the current study calculated the prevalence of schistosomiasis among 240,228 school-age children from different states as 24.46%. Higher estimates have been reported in neighboring Ethiopia (28.77%) [79] and Mozambique (52.8%) [80]. Such differences may be attributed to several factors, such as age-specific exposure patterns, school-based health programs, or social practices affecting water contact.
Moreover, S. haematobium prevalence among school-age children was 22.37. This finding is lower than the finding of a study conducted in Mozambique, which found the prevalence of S. haematobium to be 47% [80], as well as Zambia with 32.2% among the same population [77]. Nevertheless, the current finding is higher than the finding concluded in neighboring Kenya (14.8%) [81]. On the other hand, S. mansoni prevalence was 18.62 in the current study, which almost agrees with the finding concluded in a meta-analysis conducted among Zambians (18.1%) [77] but very much higher than the prevalence reported in Mozambique (1%) [80] and Kenya (1.2%) [81].
Furthermore, the current study found that males are linked to a higher rate of schistosomiasis infection in comparison with female gender. This finding is in alignment with a systematic review conducted earlier in Africa [82], as well as studies conducted in the Philippines and Ethiopia [76,79]. On the contrary, a study conducted in South Africa indicated that the female gender has a higher infection rate [83]. These differences may be attributed to variations in sociocultural characteristics among the study populations, such as gender-specific roles in water collection, which may expose males more frequently to contaminated water sources. Additionally, differing levels of access to healthcare and preventive measures, as well as variations in health-seeking behaviors between genders, could also contribute to these contradictory findings.
Regarding sanitation, the significant pooled odds ratio of participants being infected when latrines are unavailable was 1.62 (95% CI: 1.25, 2.09) in the current study. This finding is in agreement with the finding of a recent meta-analysis, as the authors stated that the odds ratio of schistosomiasis infection among participants with poor sanitation status is significantly increased [84].
Additionally, farming was indicated as significantly correlated to higher odds of schistosomiasis, which comes in agreement with the WHO's recent evidence [3]. Furthermore, being a fisherman was investigated as a potential risk factor in the current study. However, a 1.51 odds ratio was concluded with no significant difference. This finding opposed several reports [3,76,85,86]. Notably, the smaller sample size of fishermen in the current study (652 participants among three included studies) is to be considered when interpreting results.
Lastly, the current study indicated a significant association between water contact, such as bathing, washing clothes, collecting water for household use, fishing, and washing cars, and Schistosoma infection, which was previously reported in the literature [75].
Strengths and Limitations
The strengths of this review include the systematic identification and inclusion of relevant studies from 2010 to 2022. Additionally, a meta-analysis was conducted to generate pooled prevalence estimates from the included studies. Furthermore, a quality assessment was performed using criteria specifically designed to evaluate the quality of the selected studies.
Nevertheless, several limitations are to be considered when interpreting study results. Grey literature evidence was not assessed. Moreover, African journals that are not indexed in the screened databases were not considered for inclusion as well. Although all included studies are of good quality, several decent studies might have been missed. Furthermore, the heterogeneity was high in the meta-analysis conducted. Lastly, a potential limitation to acknowledge in this review is the impact of the current armed conflict in Sudan, which may influence the generalizability of the findings. Although the data included in the review was collected prior to the conflict, the sociopolitical instability, including the breakdown of healthcare infrastructure, interruptions in disease surveillance programs, and challenges in access to clean water and sanitation, may exacerbate the conditions for schistosomiasis transmission. The displacement of large populations and the potential for overcrowding in refugee camps or small villages further intensify the risk. Therefore, the findings should be interpreted with caution, considering the rapidly evolving situation, which may affect both disease transmission dynamics and access to preventive or therapeutic interventions.
Conclusions
Schistosoma haematobium pooled prevalence was 24.83% (95% CI: 22.75, 26.92) among 700,337 participants tested, while S. mansoni pooled prevalence of 19.13% (95% CI: 18.70, 19.56) among 685,133 participants was found. Moreover, the highest Schistosoma prevalence (overall pooled prevalence: 41% (95% CI: 26.72, 55,29)) was found among Gezira State participants. Furthermore, farming, male sex, no presence of latrines, canal and stream water sources, and swimming, playing, or bathing in rivers and canals were found to be significantly associated with schistosomiasis infection. These findings serve as a cornerstone for designing targeted containment strategies and preventive measures, particularly in high-prevalence areas. Future interventions could focus on improving sanitation, promoting safe water practices, and raising awareness among vulnerable populations.
Appendices
Table 4 shows the PRISMA checklist of the included studies.
Table 4. PRISMA checklist of the included studies.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PICOS: participants, interventions, comparisons, outcomes, and study design
| Section/topic | # | Checklist item | Reported on page # |
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS. | 3 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | NA |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe the method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing the risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 5 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 6 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 8 |
| Risk of bias within studies | 19 | Present data on the risk of bias of each study and, if available, any outcome level assessment (see Item 12). | 15 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 16 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 16 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 16 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)). | 17 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policymakers). | 47 |
| Limitations | 25 | Discuss limitations at the study and outcome level (e.g., risk of bias) and the review level (e.g., incomplete retrieval of identified research, reporting bias). | 48 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research. | 49 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data), and the role of funders for the systematic review. | 50 |
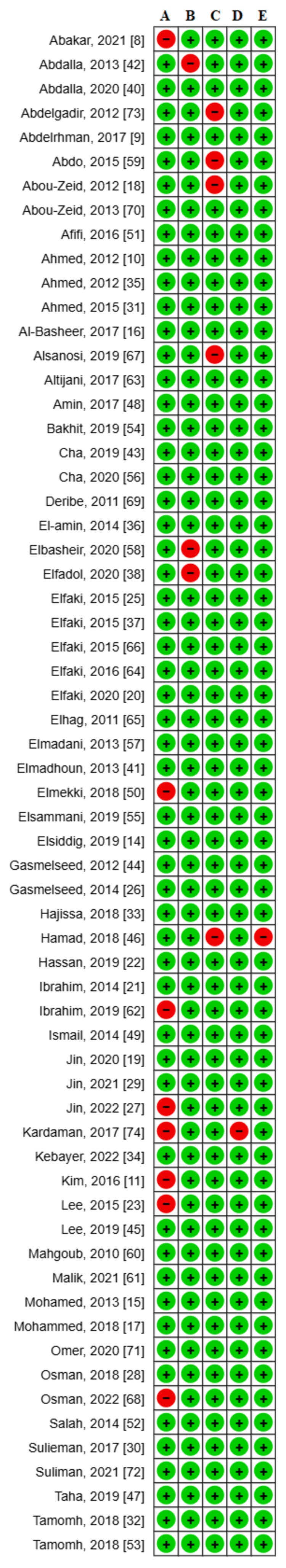
The quality assessment and risk of bias of the included studies are shown in Figure 3.
Figure 3. Risk of bias summary of the included studies.
A: Is the study objective clearly defined? B: Is the study sample completely determined? C: Is the study population clearly defined and specified? D: Is the methodology rigorous? E: Is the data analysis rigorous?
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Yousef Alsaafin, Ayman Omer, Osama Felemban, Sarra Modawi, Maydolin Ibrahim, Abdullah Mohammed, Ammar Elfaki, Ahmed Abushara, Maryam A. SalahEldin
Critical review of the manuscript for important intellectual content: Yousef Alsaafin, Ayman Omer, Osama Felemban, Sarra Modawi, Maydolin Ibrahim, Abdullah Mohammed, Ammar Elfaki, Ahmed Abushara, Maryam A. SalahEldin
Acquisition, analysis, or interpretation of data: Maryam A. SalahEldin
Drafting of the manuscript: Maryam A. SalahEldin
Supervision: Maryam A. SalahEldin
References
- 1.Federal Ministry of Health, Department of Health Information, Research & Evidence, Sudan Health Observatory. [ Mar; 2023 ]. 2021. http://www.sho.gov.sd/ http://www.sho.gov.sd/
- 2.The state of emergency care in the Republic of the Sudan. A-Rahman NH, Jacquet GA. African J Emerg Med. 2014;4:55–60. [Google Scholar]
- 3.World Health Organization (WHO): Schistosomiasis. [ Apr; 2024 ]. 2023. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 4.Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. PLoS Med. 2009;6:0. [PMC free article] [PubMed] [Google Scholar]
- 5.Tuberculosis in Sudan: systematic review and meta analysis. Badawi MM, SalahEldin MA, Idris AB, Idris EB, Mohamed SG. BMC Pulm Med. 2024;24:51. doi: 10.1186/s12890-024-02865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A systematic review and meta-analysis on the rate of human schistosomiasis reinfection. Zacharia A, Mushi V, Makene T. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0243224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Duval S, Tweedie R. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 8.Risk factors of schistosomiasis and the health effect among patients attending Alkalakla Health Centre - Khartoum State-Sudan (2018) Abakar HI, Idris SM. https://scholar.archive.org/work/edcqjj532bg2tpkbciinoeczd4/access/wayback/https://sudorj.com/wp-content/uploads/2021/01/6ME.pdf Sudan Online Res J. 2021;2:35–41. [Google Scholar]
- 9.Assessment of Schistosoma haematobium prevalence among pupils in um Hani village at Kosti locality, White Nile State (Sudan) 2011-2012. Abdelrhman A, Ali M, Elbashir H, Samira A, Nour Nour. https://www.researchgate.net/publication/317000006_The_Prevalence_of_Schistosoma_haematobium_among_pupils_at_Um_Hani_village_in_Kosti_locality_White_Nile_State_Sudan_2011-2012 Adv Res J Multi-Disciplinary Discov. 2017;16:57–60. [Google Scholar]
- 10.Schistosoma haematobium infections among schoolchildren in central Sudan one year after treatment with praziquantel. Ahmed AM, Abbas H, Mansour FA, Gasim GI, Adam I. Parasit Vectors. 2012;5:108. doi: 10.1186/1756-3305-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Significance of echogenic snow sign as an ultrasonography finding for diagnosis of urogenital schistosomiasis. Kim MJ, Ryu K, Jin Y, et al. Am J Trop Med Hyg. 2016;95:842–848. doi: 10.4269/ajtmh.16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood KH. University of Gezira. University of Gezira; 2016. The effect of comprehensive school health education programme on the control and prevention of schistosomiasis in Jabel Awlia locality, Khartoum state, Sudan (2013-2015) [Google Scholar]
- 13.Schistosoma mansoni infection among prenatal attendees at a secondary-care hospital in central Sudan. Khalid A, Abdelgadir MA, Ashmaig A, Ibrahim AM, Ahmed AA, Adam I. Int J Gynaecol Obstet. 2012;116:10–12. doi: 10.1016/j.ijgo.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Prevalence of urinary schistosomiasis among schoolchildren in White Nile state, Sudan. Elsiddig HA, Khider E, Nour SM, Makhawi AM, Mogadam MB. https://eric.ed.gov/?id=EJ1208341 African Educ Res J. 2019;7:29–32. [Google Scholar]
- 15.Prevalence rate of intestinal schistosomiasis with interaction of other factors in new halfa City-Eastern Sudan. Mohamed TE, Goreish IA. https://search.emarefa.net/en/detail/BIM-440436-prevalence-rate-of-intestinal-schistosomiasis-with-interacti SUST J Nat Med Sci. 2013;14:1–10. [Google Scholar]
- 16.Urinary schistosomiasis among primary school children at Al-Takamul area, eastern Khartoum state-Sudan: an example for urban schistosomiasis. Al-Basheer BS, Aljafari AS. https://www.researchgate.net/profile/Alfatih-Aljafari/publication/317818852_Urinary_schistosomiasis_among_primary_school_children_at_Al-Takamul_area_eastern_Khartoum_state-Sudan_An_example_for_urban_schistosomiasis/links/5a1d284f0f7e9b2a5316ee6f/Urinary-schistosomiasis-among-primary-school-children-at-Al-Takamul-area-eastern-Khartoum-state-Sudan-An-example-for-urban-schistosomiasis.pdf Ann Trop Med Public Heal. 2017;10:353–356. [Google Scholar]
- 17.Prevalence, risk factors and effect of urinary schistosomiasis on academic performance of school children age 6-15 years in Asalaya Locality, White Nile State, Sudan. Mohammed MK, Halaly S, Awadalla H, Abdelrahman A, Balla S. J Adv Med Med Res. 2018;28:1–7. [Google Scholar]
- 18.Schistosomiasis and soil-transmitted helminths among an adult population in a war affected area, Southern Kordofan state, Sudan. Abou-Zeid AH, Abkar TA, Mohamed RO. Parasit Vectors. 2012;5:133. doi: 10.1186/1756-3305-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parasitological observation in schoolchildren with urogenital schistosomiasis following treatment with three different brands of praziquantel. Jin Y, Cha S, Lee J, Elhag MS, Hong ST, Lee YH. J Korean Med Sci. 2020;35:0. doi: 10.3346/jkms.2020.35.e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevalence of schistosomiasis among school aged children in Altakamol area, Khartoum state, Sudan. Elfaki TM, Hamad MN, Zarrug E, et al. https://www.researchgate.net/profile/Mosab-Nouraldein-Mohammed-Hamad/publication/344454238_Prevalence_of_schistosomiasis_among_school_aged_children_in_Altakamol_area_Khartoum_state_Sudan/links/5f77592d458515b7cf62a444/Prevalence-of-schistosomiasis-among-school-aged-children-in-Altakamol-area-Khartoum-state-Sudan.pdf J Microbiol Exp. 2020;8:167–169. [Google Scholar]
- 21.Evaluation of microscopical and serological techniques in the diagnosis of Schistosoma mansoni infection at Sennar State, Central Sudan. Ibrahim AM, Ibrahim ME. Asian Pac J Trop Dis. 2014;4:8–13. [Google Scholar]
- 22.Frequencies of gastrointestinal parasites among students of primary school in Al Kalakla Locality, Khartoum State, Sudan: a cross-sectional study. Hassan HA, Abd Alla AB, Elfaki TE, Saad MB. F1000Research. 2020;8:1719. doi: 10.12688/f1000research.20610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reduction of urogenital schistosomiasis with an integrated control project in Sudan. Lee YH, Jeong HG, Kong WH, et al. PLoS Negl Trop Dis. 2015;9:0. doi: 10.1371/journal.pntd.0003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assessment of diagnostic methods for urinary schistosomiasis, Assalya, White Nile State, Sudan. Talab H, Kardaman M, Alhidai S, Eissa M, Bayoumi M. https://www.researchgate.net/profile/Magdi-Bayoumi/publication/322628666_Assessment_of_diagnostic_methods_for_urinary_schistosomiasis_Assalya_White_Nile_State_Sudan/links/5a643f3d4585158bca4eeb13/Assessment-of-diagnostic-methods-for-urinary-schistosomiasis-Assalya-White-Nile-State-Sudan.pdf Eur Acad Res. 2018;5:5446–5456. [Google Scholar]
- 25.Faecal examination in Schistosoma mansoni patients in New Halfa City-Eastern Sudan. Elfaki TE, Abass WD, AbdAlla AB. SUST J Nat Med Sci. 2015;16:69–74. [Google Scholar]
- 26.Genetic diversity of Schistosoma haematobium parasite IS NOT associated with severity of disease in an endemic area in Sudan. Gasmelseed N, Karamino NE, Abdelwahed MO, Hamdoun AO, Elmadani AE. BMC Infect Dis. 2014;14:469. doi: 10.1186/1471-2334-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association between the prevalence of schistosomiasis in elementary school students and their parental occupation in Sudan. Jin Y, Cha S, Kim Y, et al. Korean J Parasitol. 2022;60:51–56. doi: 10.3347/kjp.2022.60.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prevalence of Schistosoma haematobium among school children in al-Lamab Bahar Abiad area, Khartoum State, Sudan 2017: a cross sectional study. Osman RO, Eisa NS, Nasr AA, Ali OO, Siddig EE, Mohamed NS. https://www.researchgate.net/publication/326262574_Prevalence_of_Schistosoma_haematobium_among_School_Children_in_Al-Lamab_Bahar_Abiad_Area_Khartoum_State_Sudan_2017_A_Cross_Sectional_Study EC Microbiol. 2018;14:454–459. [Google Scholar]
- 29.Transmission dynamics of Schistosoma haematobium among school-aged children: a cohort study on prevalence, reinfection and incidence after mass drug administration in the White Nile state of Sudan. Jin Y, Lee YH, Cha S, Choi IU, Ismail HA, Elhag MS, Hong ST. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph182111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epidemiology of urinary schistosomiasis among school children in the Alsaial Alsagair Village, River Nile state, Sudan. SU Y, EL RE, PE T, AF A, ZA MA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527040/ Iran J Parasitol. 2017;12:284–291. [PMC free article] [PubMed] [Google Scholar]
- 31.A comparison between transabdominal ultrasonographic and cystourethroscopy findings in adult Sudanese patients presenting with haematuria. Ahmed FO, Hamdan HZ, Abdelgalil HB, Sharfi AA. Int Urol Nephrol. 2015;47:223–228. doi: 10.1007/s11255-014-0869-9. [DOI] [PubMed] [Google Scholar]
- 32.Urinary schistosomiasis among basic school children in a new irrigated sugar scheme area, White Nile State, Sudan. Tamomh AG, Abakar AD, Nour BY. https://www.researchgate.net/profile/Abdelhakam-Tamomh/publication/324137323_Urinary_schistosomiasis_among_basic_school_children_in_a_new_irrigated_sugar_scheme_area_White_Nile_State_Sudan/links/5ac0a2e7aca27222c75a28bb/Urinary-schistosomiasis-among-basic-school-children-in-a-new-irrigated-sugar-scheme-area-White-Nile-State-Sudan.pdf J Microbiol Exp. 2018;6:93–96. [Google Scholar]
- 33.Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. Hajissa K, Muhajir AE, Eshag HA, et al. BMC Res Notes. 2018;11:779. doi: 10.1186/s13104-018-3871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prevalence of intestinal schistosomiasis and associated risk factors in New Halfa City, Kassala State, Sudan. Kebayer MH, Abdo HM, Alla Gabo MN, et al. https://www.infectiousjournal.com/wp-content/uploads/sites/6/2022/10/e872.pdf Infect Dis Trop Med. 2022;8:0. [Google Scholar]
- 35.High levels of Schistosoma mansoni infections among schoolchildren in central Sudan one year after treatment with praziquantel. Ahmed AM, El Tash LA, Mohamed EY, Adam I. J Helminthol. 2012;86:228–232. doi: 10.1017/S0022149X11000290. [DOI] [PubMed] [Google Scholar]
- 36.Prevalence of Schistosoma mansoni using different diangnostic techniques in the Gezira State, Sudan. Lana E, Nagla G, Nagla G, Ahmed M. https://d1wqtxts1xzle7.cloudfront.net/51567825/Prevalence_of_Schistosoma_Mansoni_Using_20170131-3967-rzv1o1-libre.pdf?1485865793=&response-content-disposition=inline%3B+filename%3DPrevalence_of_Schistosoma_Mansoni_Using.pdf&Expires=1728649136&Signature=EuMQGhSilE7GVVT-mKEB0ZrQsofhxGvMpkOb4hctDcgoFYI1Oe88eSUxmSgva9IcLgAJy-5nZVZju7LSulMjsLqjCtbz1wof3GEI~tlJK-4rWK6kKKI8ivB1LGxnT1F1VDgYw5u8YfCn9~UN6VqTovNOrXIS6Q7MoUuv-c76iqndc9ck~5Zrg9ZcBJlOwQC95wP1FEuIDzFNzzp7G3x8ntuNlZH4M2er~moQ31Po3VWAZHhyGCrVU7xkv306yKs1WV1MhpwxOcIBn3AUbLYGeToGfl5IYW-rGkpcQkW3Y9idA2~r4AcMCh3xlp1zueB5ZRdRempbnrjQ4E~qMcHJUA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA SUST J Nat Med Sci. 2014;15:79–85. [Google Scholar]
- 37.Association between urinary schistosomiasis and prostate cancer in Al-Shajara area Khartoum, Sudan. Elfaki TE, Kebayer MH, Elsayid M. https://www.noveltyjournals.com/upload/paper/Association%20between%20Urinary-407.pdf IJNRHN. 2015;2:91–97. [Google Scholar]
- 38.Frequency of urinary schistosomiasis among school -aged children in al-Lamab area, Khartoum -Sudan. Elfadol ME, Eltayeb MM, Hamad MN. https://www.researchgate.net/profile/Mosab-Nouraldein-Mohammed-Hamad/publication/344360442_Frequency_of_Urinary_Schistosomiasis_Among_School_-Aged_Children_in_Al-Lamab_Area_Khartoum_-Sudan/links/5f6c17f0a6fdcc008638552a/Frequency-of-Urinary-Schistosomiasis-Among-School-Aged-Children-in-Al-Lamab-Area-Khartoum-Sudan.pdf MAR Microbiol. 2020;1:2. [Google Scholar]
- 39.Effect of integrated school program in control of urinary schistosomiasis among basic schools children in Elrahad town, Elrahad locality, North Kordofan state, Sudan (2014 - 2017) Kassar AS, Eldin ME, HajAli OH, Mohammed KE. EPRA Int J Multidiscip Res. 2019;5 [Google Scholar]
- 40.Prevalence of Schistosoma haematobium infection among students at Al-Agali Islamic complex in Al-Kalakela area, Khartoum State-Sudan. Abdalla EA, Youssouf AM, Ahmed BM. Int J Community Med Public Heal. 2020;7:3796. [Google Scholar]
- 41.Situation analysis of schistosomiasis and soil-transmitted helminthes in River Nile State, Sudan. Elmadhoun WM, Msmar AH, Elnoby OA, Noor SK, Suliman AA, Bushara SO. Trans R Soc Trop Med Hyg. 2013;107:195–199. doi: 10.1093/trstmh/trs088. [DOI] [PubMed] [Google Scholar]
- 42.The epidemiology of Schistosoma haematobium in rural surrounding area of Duiem district, White Nile, Sudan. Abdalla MA. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=afc633ac751c5cd70257071cea5458e5d2c9e8ab J Basic Appl Sci Res. 2013;3:1–7. [Google Scholar]
- 43.Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Cha S, Elhag MS, Lee YH, Cho DS, Ismail HA, Hong ST. Parasit Vectors. 2019;12:429. doi: 10.1186/s13071-019-3689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evaluation of different diagnostic methods for Schistosoma haematobium infection in an endemic area, Gezira State Sudan. Nagla Gasmelseed LM, Elbalal M, Monis A. https://www.researchgate.net/publication/265273017_Evaluation_of_different_dignositc_Methods_for_Schistosoma_haematobium_infection_in_Endemic_area_Gezira_State_Sudan Gezira J Heal Sci. 2012;8:47. [Google Scholar]
- 45.Epidemiological survey on schistosomiasis and intestinal helminthiasis among village residents of the rural river basin area in White Nile state, Sudan. Lee YH, Lee JS, Jeoung HG, Kwon IS, Mohamed AA, Hong ST. Korean J Parasitol. 2019;57:135–144. doi: 10.3347/kjp.2019.57.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prevalence of urinary schistosomiasis among school aged children in Khelawa Village, River Nile State, Sudan, 2017. Hamad MN, Said Ahmed EA, Omer HA, et al. https://www.researchgate.net/profile/Mosab-Nouraldein-Mohammed-Hamad/publication/323813061_Prevalence_of_Urinary_Schistosomiasis_among_School_Aged_Children_in_Khelawa_Village_River_Nile_State_Sudan_2017/links/5aac332b458515ecebe5c6dd/Prevalence-of-Urinary-Schistosomiasis-among-School-Aged-Children-in-Khelawa-Village-River-Nile-State-Sudan-2017.pdf Res Med Eng Sci. 2017;3 [Google Scholar]
- 47.Distribution of urinary schistosomiasis among school children at elkeriab and tayba elkababish villages, East Nile Locality, Khartoum State, Sudan. Taha QH, Elshazali OH, Ahmed AI. https://d1wqtxts1xzle7.cloudfront.net/114391182/JPNC-09-00390-libre.pdf?1715360551=&response-content-disposition=inline%3B+filename%3DDistribution_of_urinary_schistosomiasis.pdf&Expires=1728649932&Signature=IUoyf9AFj5Tm2I56~aNOLRkzo-8MDyzbyq3M-q-msWeJF0qfM4XKFCDuTwXd98O6hgcdpNUEpdiodj1HtzcroX3seJHo2trjx8jrUpH~1ukBJGd7tqiB2Sob9hakDLKJ3LoPpQ-MmZ3jpDEVgh8cNNbaWbbl4fA0kCkqX2w3nD0eR9kFYKbqjikvwsB5swofapPZZJHrfV4mfGZpPrQ3J-IpODLWuOqz-NWvLPvxbhRcigqQF~YoYG4FGoAoWiiAYtR~LiBOoWor5tMi7U8Scbj43hoqFcDGd3frMRK2T6I5U48P~8iBL4Hktau9zC9h-0jkBBYHdUMyUKi2u8rqcg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA J Pediatr Neonatal Care. 2019;9:117–119. [Google Scholar]
- 48.Evaluation of cathodic antigen urine tests for diagnosis of Schistosoma mansoni infection in Sudan. Amin MA, Elsadig AM, Osman HA. Saudi J Med Med Sci. 2017;5:56–61. doi: 10.4103/1658-631X.194257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Ismail HA, Hong ST, Babiker AT, et al. Parasit Vectors. 2014;7:478. doi: 10.1186/s13071-014-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prevalence and intensity of infection of Schistosomiasis in two endemic areas in Sudan. Elmekki MA. https://d1wqtxts1xzle7.cloudfront.net/56851182/N1702166975-libre.pdf?1529733905=&response-content-disposition=inline%3B+filename%3DPrevalence_and_Intensity_of_Infection_of.pdf&Expires=1728650090&Signature=FiM26oVdUb37g78dGkaMlTdOH-5V4Q~3T2Tm7kq1vB4m8iP0r1Ck3pupyci2jGmFE0tfKwbNj7vjvO-bhb0gdioMj-kALQylYNzURjhKZ-pTaTt9jKkgibG8Kx10meH~zw1kplcsMB5BH4qN1qOhr2ogzkQumh7A~YN-HOKq5kckuqu2RF5RUQNG7udNqxgZnUV5YlRuGFfq5f52iMHzfSew0tzXVJLuRwcuFrvhgSENPsEsg9wi3TtqG-s7GdlTPCGtio-9fUWwyNUKsUIndfeoLU29H7LbQnWFXwOGOIsYDjyzztmPXakjxzzf3qso0k4ThBxLwEZAgogm7~rugg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA IORS J Dent Med Sci. 2017;17:69–75. [Google Scholar]
- 51.Epidemiology of schistosomiasis among villagers of the New Halfa agricultural scheme, Sudan. AF A, AH AA, SU Y, PE T. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4835460/ Iran J Parasitol. 2016;11:110–115. [PMC free article] [PubMed] [Google Scholar]
- 52.Urinary schistosomiasis in Gedarif: an endemic new focus in Eastern Sudan. Salah ET, Elmadhoun WM. https://www.ajol.info/index.php/sjms/article/view/114287 Sudan J Med Sci. 2014;9:163–168. [Google Scholar]
- 53.Prevalence of intestinal schistosomiasis among basic school children in White Nile sugar scheme a new irrigated project, White Nile state, Sudan. Tamomh AG, Yousfi SR, Abakar AD, et al. Biol Med. 2018;10:1–4. [Google Scholar]
- 54.Comparison between chromogenic colour Agar and biochemical tests in the identification of causative organism of bacteriuria associated with urinary schistosomiasis, in Abu RUKBA village, White Nile State (Sudan) Bakhit HA, Elsafi SS, Saad MB, et al. Int J Curr Microbiol Appl Sci. 2019;8:175–180. [Google Scholar]
- 55.Prevalence of schistosomiasis among school children in Bahri locality, Sudan. Elsammani NA, Ali Adam A, Mater AA, Elamin MO. Int J Res Granthaalayah. 2019;7:299–306. [Google Scholar]
- 56.Comparison of the change in the prevalence and intensity of Schistosoma haematobium infection between high and low prevalence areas of White Nile state, Sudan. Cha S, Hong ST, Lee JS, et al. Korean J Parasitol. 2020;58:421–430. doi: 10.3347/kjp.2020.58.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ultrasound findings in urinary shistosomaisis infection in school children in the Gezira State Central Sudan. Elmadani AE, Hamdoun AO, Monis A, Karamino NE, Gasmelseed N. Saudi J Kidney Dis Transpl. 2013;24:162–167. doi: 10.4103/1319-2442.106362. [DOI] [PubMed] [Google Scholar]
- 58.Evaluation of a rapid diagnostic test for Schistosoma mansoni infection based on the detection of circulating cathodic antigen in urine in Central Sudan. Elbasheir MM, Karti IA, Elamin EM. PLoS Negl Trop Dis. 2020;14:0. doi: 10.1371/journal.pntd.0008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frequent carriage of invasive Salmonellae amongst patients infected with schistosomiasis in Sudan. Abdo KS, Naser EB, Mutasim EI. African J Microbiol Res. 2015;9:543–548. [Google Scholar]
- 60.Schistosoma mansoni infection as a predictor of severe anaemia in schoolchildren in eastern Sudan. Mahgoub HM, Mohamed AA, Magzoub M, Gasim GI, Eldein WN, Ahmed AA, Adam I. J Helminthol. 2010;84:132–135. doi: 10.1017/S0022149X09990368. [DOI] [PubMed] [Google Scholar]
- 61.Prevalence and immunological aspects of Schistosoma haematobium among non-infected fishermen in White Nile State, Sudan. Malik AH, Al-Sayed SE, Elfath M, Musa HA, Abdelalim AO, Hassan Y, Saeed MI. J Heal Transl Med. 2021;24:45–49. [Google Scholar]
- 62.Prevalence of Schistosomiasis infections among school children in Sinnar State, Central Sudan. Ibrahim AM, Fadl OF, Eisa IM, Elbasheir MM. Merit Res J Med Med Sci. 2019;7:275–280. [Google Scholar]
- 63.Prevalence of Schistosoma haematobium among pupils_ El-Tawella village Kosti-White Nile state- Sudan (2012) Abdelrhman AA, Ali M, Elbashir A, Samira A, Bakri N. https://www.researchgate.net/publication/317000006_The_Prevalence_of_Schistosoma_haematobium_among_pupils_at_Um_Hani_village_in_Kosti_locality_White_Nile_State_Sudan_2011-2012 Adv Res J Multi-Disciplinary Discov. 2012;18:11–14. [Google Scholar]
- 64.Multivariable regression analysis in Schistosoma mansoni-infected individuals in the Sudan reveals unique immunoepidemiological profiles in uninfected, egg+ and non-egg+ infected individuals. Elfaki TE, Arndts K, Wiszniewsky A, et al. PLoS Negl Trop Dis. 2016;10:0. doi: 10.1371/journal.pntd.0004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Detection of schistosomiasis antibodies in urine patients as a promising diagnostic maker. Elhag SM, Abdelkareem EA, Yousif AS, Frah EA, Mohamed AB. Asian Pac J Trop Med. 2011;4:773–777. doi: 10.1016/S1995-7645(11)60192-2. [DOI] [PubMed] [Google Scholar]
- 66.Association between intestinal schistosomiasis and enteric fever in New Halfa City, Sudan. Elfaki TE, Abdalla EJ. https://www.noveltyjournals.com/upload/paper/Association%20between%20Intestinal-360.pdf Int J Novel Res Healthc Nurs. 2015;2:45–52. [Google Scholar]
- 67.Infection with Schistosoma haematobium among children and urination pattern in Alshajarah district, Khartoum, Sudan. Alsanosi K, Sulieman A, Obaid M, Elawad M. https://www.researchgate.net/profile/Mohammed-Elawad/publication/337603820_Infection_with_Schistosoma_haematobium_among_Children_and_Urination_Pattern_inAlshajarah_District/links/5ddfd828a6fdcc2837f3bc8f/Infection-with-Schistosoma-haematobium-among-Children-and-Urination-Pattern-inAlshajarah-District.pdf J Nurs Health Sci. 2019;8:30–32. [Google Scholar]
- 68.Prevalence of Schistosoma haematobium among schoolchildren in new foci in Merowe locality, North State, Sudan. Osman AM, Ahmed AA, Osman A, MohamedAhmed KA, Ali YY, Mukhtar M, Abakar AD. https://www.researchgate.net/profile/Khalid-Mohamedahmed/publication/360182337_Prevalence_of_Schistosoma_haematobium_among_Schoolchildren_in_New_Foci_in_Merowe_Locality_North_State_Sudan/links/62671845ee24725b3ec620e4/Prevalence-of-Schistosoma-haematobium-among-Schoolchildren-in-New-Foci-in-Merowe-Locality-North-State-Sudan.pdf Jouf Univ Med J. 2022;9:37–44. [Google Scholar]
- 69.High prevalence of urinary schistosomiasis in two communities in South Darfur: implication for interventions. Deribe K, Eldaw A, Hadziabduli S, et al. Parasit Vectors. 2011;4:14. doi: 10.1186/1756-3305-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schistosomiasis infection among primary school students in a war zone, Southern Kordofan State, Sudan: a cross-sectional study. Abou-Zeid AH, Abkar TA, Mohamed RO. BMC Public Health. 2013;13:643. doi: 10.1186/1471-2458-13-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haematobium schistosomiasis prevalence among school age children in irrigated schemes at Shendi locality, River Nile state, Sudan: implication of behavior and risk factors. Omer EO, Goja AM, Elhag KI, Gadalrap OM. https://www.mjhs-mu.org/fulltext/176-1538392885.pdf Majmaah J Heal Sci. 2020;8:23–35. [Google Scholar]
- 72.Prevalence and risk factors of schistosomiasis and intestinal helminthes infection among school children in White Nile State, Sudan. Suliman MA, Magboul AM, Mohammed HY, Tamomh AG, Daleel ZI, Al ajup AM. Microbes Infect Dis. 2021;2:177–182. [Google Scholar]
- 73.Epidemiology of anaemia among pregnant women in Geizera, central Sudan. Abdelgadir MA, Khalid AR, Ashmaig AL, Ibrahim AR, Ahmed AA, Adam I. J Obstet Gynaecol. 2012;32:42–44. doi: 10.3109/01443615.2011.617849. [DOI] [PubMed] [Google Scholar]
- 74.Follow-up of treated school and pre-school children for schistosomiasis at Kamleen locality, Gezira State, Sudan. Kardaman M, Swar M, Homeida M, et al. https://www.researchgate.net/publication/320559325_International_Journal_of_Current_Medical_and_Pharmaceutical_Research_FOLLOW-UP_OF_TREATED_SCHOOL_AND_PRE-SCHOOL_CHILDREN_FOR_SCHISTOSOMIASIS_AT_KAMLEEN_LOCALITY_GEZIRA_STATE_SUDAN Int J Curr Med Pharm Res. 2017;3:1409–1413. [Google Scholar]
- 75.The prevalence of schistosomiasis in Uganda: a nationally representative population estimate to inform control programs and water and sanitation interventions. Exum NG, Kibira SP, Ssenyonga R, et al. PLoS Negl Trop Dis. 2019;13:0. doi: 10.1371/journal.pntd.0007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prevalence of soil-transmitted helminth infections, schistosomiasis, and lymphatic filariasis before and after preventive chemotherapy initiation in the Philippines: a systematic review and meta-analysis. Delos Trinos JP, Wulandari LP, Clarke N, Belizario V Jr, Kaldor J, Nery SV. PLoS Negl Trop Dis. 2021;15:0. doi: 10.1371/journal.pntd.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.A meta-analysis of changes in schistosomiasis prevalence in Zambia: implications on the 2020 elimination target. Kalinda C, Mutengo M, Chimbari M. Parasitol Res. 2020;119:1–10. doi: 10.1007/s00436-019-06534-0. [DOI] [PubMed] [Google Scholar]
- 78.Prevalence of intestinal parasites versus knowledge, attitude and practices (KAPs) with special emphasis to Schistosoma mansoni among individuals who have river water contact in Addiremets town, Western Tigray, Ethiopia. Gebreyohanns A, Legese MH, Wolde M, Leta G, Tasew G. PLoS One. 2018;13:0. doi: 10.1371/journal.pone.0204259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prevalence of Schistosomiasis (S. mansoni and S. haematobium) and its association with gender of school age children in Ethiopia: a systematic review and meta-analysis. Woldeyohannes D, Sahiledengle B, Tekalegn Y, Hailemariam Z. Parasite Epidemiol Control. 2021;13:0. doi: 10.1016/j.parepi.2021.e00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geographic distribution and prevalence of schistosomiasis and soil-transmitted helminths among schoolchildren in Mozambique. Augusto G, Nalá R, Casmo V, Sabonete A, Mapaco L, Monteiro J. https://d1wqtxts1xzle7.cloudfront.net/102626021/09e092e3b27295dbde88493de48406369a06-libre.pdf?1684996414=&response-content-disposition=inline%3B+filename%3DGeographic_Distribution_and_Prevalence_o.pdf&Expires=1728659795&Signature=JdSlHDhQjfit~Z0d1awAkpC0K56VmjiGN5CqxxZCLTaXO~rZGYrHuBhzEIy9grevu0JR9K11r09aVG62Nh2vhIgG7IIee8991O7FoEgfzwZHsBQo6kbhP~c-HXdvfLaX3L5P~BxiV6Ge2kjGhkDse897ZOWTuRYMlOjXm1jdHrjP7L9XzgXVVRKLEcsfnyxR4Bf-AhVbIbAM8XtbmpkmCCMpcBkAYu4oV7XExtoT1VTXFggvbIP1IN5NzLbKNrLO43K6sNbaGvENcv5U0IwU-Wqtu7d0sjJidoHYAbV3tKaTbTE4QKLUcXQphhVUselXeoEQsOSwK37IT~~HeWlmeg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA. Am J Trop Med Hyg. 2009;81:799–803. doi: 10.4269/ajtmh.2009.08-0344. [DOI] [PubMed] [Google Scholar]
- 81.Monitoring and evaluating the impact of national school-based deworming in Kenya: study design and baseline results. Mwandawiro CS, Nikolay B, Kihara JH, et al. Parasit Vectors. 2013;6:198. doi: 10.1186/1756-3305-6-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: a systematic review and meta-analysis. Ayabina DV, Clark J, Bayley H, Lamberton PH, Toor J, Hollingsworth TD. PLoS Negl Trop Dis. 2021;15:0. doi: 10.1371/journal.pntd.0009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. South African J Infect Dis. 2017;32:67–72. [Google Scholar]
- 84.The impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Freeman MC, Garn JV, Sclar GD, et al. Int J Hyg Environ Health. 2017;220:928–949. doi: 10.1016/j.ijheh.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Prevalence and risk factors for schistosomiasis among schoolchildren in two settings of Côte d’Ivoire. Angora EK, Boissier J, Menan H, et al. Trop Med Infect Dis. 2019;4 doi: 10.3390/tropicalmed4030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Assessment of knowledge, attitude and practices and the analysis of risk factors regarding schistosomiasis among fishermen and boatmen in the Dongting Lake Basin, the People's Republic of China. Guan Z, Dai SM, Zhou J, et al. Parasit Vectors. 2020;13:273. doi: 10.1186/s13071-020-04157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]