Abstract
Background
Extracardiac conduit Fontan procedure (ECFP) employing a Gore-Tex conduit has been widely used for patients with single ventricle physiology; however, the long-term status of the conduit is unknown. We investigated the changes in a Gore-Tex conduit after ECFP and the factors associated with its narrowing.
Methods
We conducted a retrospective analysis of 86 patients who underwent ECFP between January 1995 and December 2008 and had cardiac computed tomography (CT) during the follow-up period.
Results
The median patient age at ECFP was 2.8 years (range 1.6–9.7), and a cardiac CT was obtained at 13.1 ± 3.4 years later. The minimum conduit area decreased by approximately two-thirds of the original due to calcification, pseudointimal hyperplasia, thrombus, and luminal irregularity. The normalized minimum conduit area was influenced by the time interval from ECFP and normalized original conduit area at ECFP. An oversized conduit was associated with a narrowing of both its sides and a high frequency of pseudointimal hyperplasia or mural thrombus. The ratio of minimum conduit-to-inferior vena cava areas was lower in patients with chronic liver disease than in those with a normal liver. The maximum percent stenosis of the conduit correlated with oxygen pulse and heart rate during peak exercise.
Conclusions
Using a larger conduit at ECFP resulted in a larger minimum conduit area at follow-up. However, oversizing requires careful monitoring for stenosis near anastomotic sites and the occurrence of pseudointimal hyperplasia or thrombus.
Keywords: Fontan procedure, Univentricular heart, Polytetrafluoroethylene, Computed tomography
Highlights
-
•
Minimum conduit area reduced to 2/3 of the original 13 years after Fontan.
-
•
Using a larger conduit led to a larger minimum area at follow-up.
-
•
Oversized conduit linked to anastomosis stenosis, requiring careful monitoring.
-
•
Conduit stenosis in Fontan correlates with hepatic issues and exercise intolerance.
Abbreviations list
- ECFP
Extracardiac conduit Fontan procedure
- CT
Computed tomography
- MR
Magnetic resonance
- IVC
Inferior vena cava
- CLD
Chronic liver disease
- CPET
Cardiopulmonary exercise test
1. Introduction
The extracardiac conduit Fontan procedure (ECFP) is the most frequently used surgical method for patients with single ventricle physiology [1,2]. Due to its structural advantages, energy loss and incidence of late-onset atrial arrhythmia is low compared with atriopulmonary and lateral tunnel Fontan procedure [[3], [4], [5]]. However, as the conduit is a synthetic material with no growth potential, there are concerns regarding its inherent thrombogenicity and relatively small diameter for body growth. Significant conduit obstruction sometimes occurs, leading to chronic ascites and exercise intolerance [[6], [7], [8], [9]]. Cardiovascular intervention or surgery is necessary to alleviate this obstruction [6,[9], [10], [11]]. Nonetheless, the temporal changes in the conduit after ECFP and its associated factors remain unknown.
Cardiac computed tomography (CT) has high spatial resolution and an extremely short scanning time. It can provide an accurate assessment of cardiac and extracardiac structures in patients who are unable to undergo cardiac magnetic resonance (MR) imaging or have MR-incompatible devices or metallic implants [[12], [13], [14], [15]]. Advances in CT technology with low radiation dose protocol can reduce radiation exposure for patients with Fontan circulation. As homogenous opacification of extracardiac conduit can be achieved with delayed scanning or dual injection protocol, cardiac CT is useful for evaluating changes in the conduit after ECFP [16,17].
Therefore, our objective was to assess patients who have undergone cardiac CT after ECFP, examining changes in conduit shape, identifying factors influencing conduit narrowing, and exploring association between conduit changes and patients’ clinical status.
2. Methods
2.1. Patients
A total of 135 patients who underwent the ECFP at our institution between January 1, 1995, and December 31, 2008, were retrospectively reviewed, and patients who underwent ECFP with a Gore-Tex conduit and cardiac CT until December 31, 2019, were included. After excluding patients without cardiac CT data suitable to analyze the conduit, 86 patients were included (Supplementary Fig. 1). The cardiac CT images acquired before stent implantation or conduit replacement were analyzed in three cases: one patient who had percutaneous transcatheter stent implantation and two patients who underwent surgical conduit replacement.
Demographic data, such as sex, primary cardiac diagnosis, type of situs, cardiac position, ventricular dominance, presence of apicocaval juxtaposition, and presence of bilateral bidirectional cavopulmonary connection, were obtained from patients’ medical records. Perioperative details, including age, weight, body surface area, conduit diameter, creation of fenestration, and type of concomitant surgeries, were collected. The conduit was considered oversized when its diameter was at least 30% larger than the estimated inferior vena cava (IVC) diameter based on a regression equation against weight, noted in Steinberg et al. [18]. Age, weight, body surface area, use of antithrombotic drugs, and elapsed time since ECFP at the time of cardiac CT were also identified. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board of Seoul National University Hospital (IRB number: 2108-019-1241). The requirement for informed consent was waived due to the retrospective design.
2.2. Cardiac CT
Cardiac CT was performed using a dual-source CT scanner or multidetector CT scanner (SOMATOM Definition Flash, Siemens Healthineers, Erlangen, Germany [n = 73]; SOMATOM Definition, Siemens Healthineers [n = 11]; Philips iCT, Philips Healthcare, Amsterdam, Netherlands [n = 1]; or LightSpeed Ultra, General Electric Healthcare, Chicago, IL, USA [n = 1]) with following scanning parameters: slice thickness, 0.75−1.25 mm; increment, 0.5−1.0 mm; tube voltage, 80−120 kV (peak); and gantry rotation time, 0.285−0.886 s. We analyzed the conduit by using a delayed acquisition of cardiac CT between 2 and 4 min to achieve a homogeneous contrast enhancement of the conduit and IVC, aiming to reduce streaming artifacts. The parameters of the conduit and IVC were measured using a picture-archiving and communication system viewer (INFINITT PiViewSTAR, INFINITT Healthcare, Seoul, Republic of Korea) and available three-dimensional software (Xelis, INFINITT Healthcare).
The smallest cross-sectional area of the conduit and cross-sectional area of IVC were measured. The minimum conduit area was normalized to the body surface area. The maximum percent stenosis was calculated as a decrease in the minimum conduit area compared to the original conduit area. The location of the minimum conduit area was divided into upper, middle, and lower thirds. The aspect ratio quantified the deviation of an ellipse from a perfect circle; it was calculated as the ratio of minor axis to major axis diameter at the minimum conduit area. This calculation helped to assess the degree of conduit compression. Tortuosity, defined as a ratio of the total length of the conduit to the linear distance between the conduit extremes, was also calculated (Supplementary Fig. 2). Finally, the presence of luminal irregularity, calcification, pseudointimal hyperplasia, or mural thrombus was assessed in the cross-sectional image with the minimum conduit area.
2.3. Liver imaging studies
The results of the liver imaging studies, performed at the nearest time within 2 years of the cardiac CT, were investigated. Based on liver imaging, such as liver ultrasound, CT, MR, and medical records, the patients were determined to have a normal liver, chronic liver disease (CLD), or hepatocellular carcinoma, according to a prior study by Nandwana et al. [19].
2.4. Cardiopulmonary exercise test
The results of the cardiopulmonary exercise test (CPET), performed at the nearest time within 2 years of the cardiac CT, were investigated. CPET was carried out on treadmills or a cycle ergometer (General Electric T-2100, GE Healthcare, Chicago, IL, USA; VIAsprint 150P, Ergoline, Bitz, Germany). Expired gas was collected and analyzed using a metabolic cart (VMAX Encore 29, Carefusion, San Diego, CA, USA). The variables measured by the CPET, included for analysis, were peak oxygen consumption (VO2), peak ventilatory equivalent for carbon dioxide (VE/VCO2), oxygen pulse, resting and peak heart rate, resting and peak oxygen saturation, forced vital capacity, forced expiratory volume in 1 s, and maximal work. The tests with maximal effort, defined as a respiratory exchange ratio of 1.05 or higher, were included in the analysis.
2.5. Statistical analysis
Descriptive variables are presented as mean ± SD or median (range), depending on the normality of distribution. Categorical variables are presented as numbers and percentages. Chi-square test or Fisher exact test was performed for categorical variables. Student's t-test, Mann–Whitney U test, one-way analysis of variance, Kruskal-Wallis test, rank analysis of covariance (ANCOVA), Pearson's correlation analysis, or Spearman's rank correlation analysis was performed for continuous variables where appropriate. Univariate and multivariable linear regression analyses were performed to determine the predictors of normalized minimum conduit area. P < 0.05 was considered statistically significant. Data manipulation and statistical analyses were performed using SPSS 25.0 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
The demographic and clinical data are depicted in Table 1. A total of 86 patients with extracardiac Gore-Tex conduit were followed up for 15.4 ± 2.8 years. A quarter of the patients were female, and the most common cardiac diagnosis was complete atrioventricular septal defect. The median patient age at ECFP was 2.8 years (range 1.6–9.7), and a cardiac CT were performed at 13.1 ± 3.4 years after ECFP.
Table 1.
Baseline characteristics, operative data, and conduit parameters.
| Variable | Value |
|---|---|
| Female | 22 (25.6) |
| Cardiac position | |
| Levocardia | 69 (80.2) |
| Mesocardia or Dextrocardia | 17 (19.8) |
| Cardiac situs | |
| Solitus | 66 (76.7) |
| Inversus | 5 (5.8) |
| Ambiguous | 15 (17.5) |
| Dominant ventricle | |
| Left ventricle | 26 (30.2) |
| Right ventricle | 42 (48.8) |
| Biventricle | 18 (21.0) |
| Apicocaval juxtaposition | 25 (29.1) |
| Primary cardiac diagnosis | |
| Complete atrioventricular septal defect | 18 (20.9) |
| Tricuspid atresia | 12 (13.9) |
| Double inlet right ventricle | 10 (11.6) |
| Mitral atresia | 10 (11.6) |
| Double inlet left ventricle | 9 (10.5) |
| Double outlet right ventricle | 8 (9.3) |
| Criss-cross heart | 4 (4.7) |
| Hypoplastic left heart syndrome | 4 (4.7) |
| Pulmonary atresia with intact ventricular septum | 3 (3.5) |
| Corrected transposition of the great arteries | 2 (2.3) |
| Other | 6 (7.0) |
| Operative data | |
| Age at Fontan procedure, year | 2.8 (1.6–9.7) |
| Weight at Fontan procedure, kg | 13.7 (10.0–25.7) |
| Body surface area at Fontan procedure, m2 | 0.59 (0.47–0.97) |
| Bilateral bidirectional cavopulmonary connection | 21 (24.4) |
| Conduit diameter (area) | |
| 16 mm (803.8 mm2) | 4 (4.6) |
| 18 mm (1017.4 mm2) | 16 (18.6) |
| 20 mm (1256.0 mm2) | 41 (47.7) |
| 22 mm (1519.8 mm2) | 24 (27.9) |
| 24 mm (1808.6 mm2) | 1 (1.2) |
| Normalized original conduit area, mm2/m2 | 540.3 (261.7–694.0) |
| Creation of fenestration | 28 (32.6) |
| Diameter of fenestration, mm | 4.0 (4.0–6.0) |
| Concomitant surgeries | 34 (39.5) |
| Conduit parameters on cardiac CT | |
| Interval from Fontan procedure, year | 13.1 ± 3.4 |
| Weight on cardiac CT, kg | 55.2 ± 16.9 |
| Body surface area on cardiac CT, m2 | 1.61 (0.76–2.22) |
| Minimum conduit area, mm2 | 201.8 ± 49.6 |
| Normalized minimum conduit area, mm2/m2 | 126.3 (32.6–278.4) |
| Maximum percent stenosis, % | 33.6 (6.9–79.1) |
| Minimum conduit area/IVC, % | 45.6 (10.8–95.2) |
| Aspect ratio | 1.43 (1.04–8.98) |
| Tortuosity, % | 104.5 (100.3–131.5) |
| Location of the minimum conduit area | |
| Upper third | 16 (18.6) |
| Middle third | 42 (48.8) |
| Lower third | 28 (32.6) |
| Mechanism for narrowing of the conduit | |
| Luminal irregularity | 20 (23.3) |
| Calcification | 64 (74.4) |
| Pseudointimal hyperplasia or mural thrombus | 58 (67.4) |
Data are expressed as mean ± standard deviation, median (range), or number (%).
CT = computed tomography, IVC = inferior vena cava.
3.2. Cardiac CT
The median minimum conduit area normalized for the body surface area was 126.3 (32.6–278.4) mm2/m2, and the minimum absolute conduit area decreased to approximately two-thirds of the original conduit area. The normalized minimum conduit area was correlated with the ratio of the minimum conduit-to-IVC areas (Rs = 0.708, p < 0.001), maximum percent stenosis (Rs = −0.419, p < 0.001), and aspect ratio (Rs = −0.295, p = 0.006) (Supplementary Fig. 3). Tortuosity was correlated with the aspect ratio (Rs = 0.380, p < 0.001), but not with the other parameters. The most prevalent location of the minimum conduit area was the middle third. The normalized original conduit area was smaller in the middle third group than in the other two groups (Supplementary Table 1). Presence of calcification was the most common mechanism for the narrowing of the conduit area (64, 74.4%) (Supplementary Fig. 4).
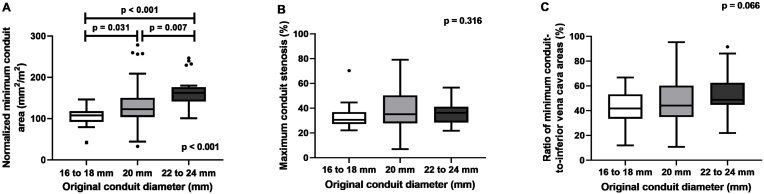
Age at the time of ECFP in the 22–24 mm group was higher than that in the 16–18 mm (p < 0.001) and 20 mm groups (p = 0.037) (Supplementary Table 2). The body surface area at ECFP in the 22–24 mm group was larger than that in the 16–18 mm group (p = 0.027). With a larger original conduit diameter, both the normalized original and normalized minimum conduit area at follow-up cardiac CT were larger (Fig. 1). There were no significant differences in the maximum percent stenosis and ratio of the minimum conduit-to-IVC areas among the 16–18 mm, 20 mm, and 22–24 mm groups.
Fig. 1.
Comparison of conduit parameters based on the original conduit diameter.
(A) normalized minimum conduit area (B) maximum percent stenosis, and (C) ratio of minimum conduit-to-inferior vena cava areas.
Body weight at ECFP was lower in the oversized group than in the non-oversized group, although the age at ECFP was similar in both groups (Table 2). Both the normalized original and normalized minimum conduit area at follow-up cardiac CT were larger in the oversized group than in the non-oversized group. The location of the minimum conduit area differed between the two groups; the most prevalent location was the lower third in the oversized group and the middle third in the non-oversized group. Although the frequency of luminal irregularity and calcification did not differ between the two groups, pseudointimal hyperplasia or mural thrombus were more common in the oversized group than in the non-oversized group (84.1% [37/44] vs. 50.0% [21/42], p = 0.001).
Table 3.
Univariate and multivariable linear regression analyses for different predictors of the normalized minimum conduit area.
| Variable | Univariate regression analysis |
Multivariable regression analysis |
||
|---|---|---|---|---|
| Slope (95% CI) | p value | Slope (95% CI) | p value | |
| Female | 3.67 (−20.67 to 28.01) | 0.150 | – | |
| Mesocardia or dextrocardia | 1.83 (−24.86 to 28.51) | 0.892 | – | |
| Isomerism | −11.33 (−39.22 to 16.57) | 0.422 | – | |
| Simple cardiac situs and position | 5.29 (−17.58 to 28.16) | 0.647 | ||
| Dominant ventricle | ||||
| Biventricle | Reference | |||
| Left ventricle | −19.14 (−49.07 to 10.80) | 0.207 | – | |
| Right ventricle | −21.85 (−49.35 to 5.66) | 0.118 | – | |
| Apicocaval juxtaposition | −13.77 (−36.71 to 9.18) | 0.236 | – | |
| Bilateral bidirectional cavopulmonary connection | −18.78 (−43.18 to 5.62) | 0.130 | – | |
| Use of antithrombotic drugs | ||||
| No medication | Reference | |||
| Aspirin | −15.76 (−86.10 to 54.57) | 0.657 | – | |
| Warfarin | 3.55 (−70.96 to 78.06) | 0.925 | – | |
| Age on Fontan procedure, year | 5.18 (−2.55 to 12.91) | 0.186 | – | |
| Weight at Fontan procedure, kg | −0.70 (−5.10 to 3.71) | 0.754 | – | |
| Age on cardiac CT, year | −5.59 (−8.54 to −2.64) | <0.001 | ||
| Normalized original conduit area, mm2/m2 | 0.22 (0.11–0.33) | <0.001 | 0.23 (0.13–0.32) | <0.001 |
| Fenestration | −9.89 (−32.47 to 12.69) | 0.386 | – | |
| Follow-up duration, year | −3.07 (−6.81 to 0.67) | 0.067 | – | |
| Interval from Fontan procedure, year | −6.43 (−9.29 to −3.57) | <0.001 | −6.65 (−9.20 to −4.10) | <0.001 |
CT = computed tomography.
Table 2.
Comparison of conduit parameters between patients with oversized and non-oversized conduits.
| Variable | Oversized (n = 44) | Non-oversized (n = 42) | p value |
|---|---|---|---|
| Age at Fontan procedure, year | 2.8 (1.6–5.8) | 2.7 (1.8–9.7) | 0.766 |
| Weight at Fontan procedure, kg | 13.0 (10.4–18.2) | 14.1 (10.0–25.7) | 0.010 |
| Body surface area at Fontan procedure, m2 | 0.58 (0.49–0.73) | 0.60 (0.47–0.97) | 0.056 |
| Simple cardiac situs and position | 29 (65.9) | 30(71.4) | 0.581 |
| Apicocaval juxtaposition | 13 (29.5) | 12 (28.6) | 0.921 |
| Bilateral bidirectional cavopulmonary connection | 12 (27.3) | 9 (21.4) | 0.528 |
| Normalized original conduit area, mm2/m2 | 600.4 (524.0–694.0) | 475.1 (261.7–556.9) | <0.001 |
| Interval from Fontan procedure, year | 13.0 ± 3.6 | 13.1 ± 3.2 | 0.0871 |
| Minimum conduit area, mm2 | 227.1 ± 38.2 | 175.2 ± 46.5 | <0.001 |
| Normalized minimum conduit area, mm2/m2 | 148.6 (88.6–278.4) | 109.9 (32.6–208.6) | <0.001 |
| Maximum percent stenosis, % | 34.5 (20.3–56.7) | 33.1 (6.9–79.1) | 0.809 |
| Minimum conduit area/IVC area, % | 47.7 (21.9–95.2) | 41.0 (10.8–73.3) | 0.010 |
| Aspect ratio | 1.41 (1.04–2.30) | 1.46 (1.11–8.98) | 0.766 |
| Tortuosity, % | 103.8 (100.3–115.8) | 104.9 (100.3–131.5) | 0.063 |
| Location of the minimum conduit area | 0.019 | ||
| Upper third | 11 (25.0) | 5 (11.9) | |
| Middle third | 15 (34.1) | 27 (64.3) | |
| Lower third | 18 (40.9) | 10 (23.8) | |
| Mechanism for narrowing of the conduit | |||
| Luminal irregularity | 11 (25.0) | 9 (21.4) | 0.695 |
| Calcification | 32 (72.7) | 32 (76.2) | 0.713 |
| Pseudointimal hyperplasia or mural thrombus | 37 (84.1) | 21 (50.0) | 0.001 |
Data are expressed as mean ± standard deviation, median (range), or number (%).
n = number; IVC = inferior vena cava.
Results of the univariate and multivariable regression analyses for predictors of normalized minimum conduit area are shown in Table 3. Both the normalized original conduit area and the time interval between ECFP and cardiac CT were independent predictors of a normalized minimum conduit area. Cardiovascular anatomic variables, aspirin or warfarin use, and timing of ECFP were not associated with the normalized minimum conduit area.
3.3. Association of the conduit with clinical status
Liver imaging studies were performed in 74 patients within 2 years of the cardiac CT. Sixty-three patients were found to have radiologic findings of CLD, while 11 patients had normal findings. No patients were diagnosed with hepatocellular carcinoma. Patients with normal radiological findings had a larger normalized minimum conduit area and a higher minimum conduit-to-IVC area ratio than patients with CLD (Supplementary Table 3). After rank ANCOVA with the time interval between ECFP and liver imaging studies, the ratio of the minimum conduit-to-IVC areas remained higher in patients with normal radiologic findings than in patients with CLD (p = 0.016).
Among the 54 patients who completed a CPET, 43 achieved maximal effort (Supplementary Table 4). The parameters of the conduit were not correlated with peak VO2 or VE/VCO2. The maximum percent stenosis was correlated with oxygen pulse (%predicted) (Rs = −0.315, p = 0.039) and peak heart rate (bpm) (Rs = 0.358, p = 0.018). The maximum conduit stenosis tended to be greater in the 27 patients with an oxygen pulse of <80% at peak exercise than in the 16 patients with ≥80%; however, this was not statistically significant (36.7 [23.7–79.1] versus 29.9 [23.9–45.7], p = 0.053).
4. Discussion
This study demonstrated several findings regarding long-term change to the conduit after ECFP using cardiac CT. First, the minimum conduit cross-sectional area decreased to approximately two-thirds of the original conduit area by calcification, pseudointimal hyperplasia, thrombus, and luminal irregularity. Second, the normalized original conduit area and elapsed time after ECFP affected the normalized minimum conduit area during long-term follow-up. Third, an oversized conduit was associated with narrowing of upper and lower conduit sides and high frequency pseudointimal hyperplasia or mural thrombus. Fourth, several associations between conduit stenosis and chronic liver disease and exercise intolerance were demonstrated.
This study demonstrated that the minimum conduit cross-sectional area decreased to approximately two-thirds of the original conduit area, consistent with findings from previous studies [20,21]. Patel and colleagues reported a median percentage decrease in the minimum conduit cross-sectional area, as measured by cardiac MR or angiography, of 33% (IQR 25–41%) during a mean follow-up of 9.6 years [21]. In a study by Lee and colleagues, the mean percentage decrease in the mid-conduit cross-sectional area was 14.3% at an average of 36.1 months after the Fontan procedure, with the limitation that the measurement was not taken at its narrowest point [20]. The mechanisms underlying conduit stenosis is complex and not yet fully understood. Calcification, thrombus, pseudointimal hyperplasia, and luminal irregularity were observed at the minimum conduit area in this study. Since Gore-Tex is a prosthetic material, the likelihood of pseudointimal peel formation in the conduit has been reported [22]. Inherent thrombogenicity is an additional concern; the prevalence of silent thromboembolism in the conduit was found to be 13% [23]. However, this study found no significant relationship between the thromboprophylaxis method and conduit stenosis. This result is in line with several studies that have also recognized that antiplatelet agents have an anti-thrombotic effect comparable to that of anticoagulation therapy [[24], [25], [26]]. Compression by surrounding structures can also distort the shape of the conduit, given that the aspect ratio of the conduit correlated with the normalized minimum conduit area.
The normalized minimum conduit area at follow-up was affected by the normalized original conduit area and the elapsed time after ECFP. As the maximum percent stenosis does not depend on the original conduit size in this study and previous studies, the normalized original conduit area affected the normalized minimum conduit area [[20], [21], [22]]. This result should not be overgeneralized to suggest that a large conduit is superior to a smaller one, as functional hemodynamics were not evaluated in this study. Itatani and colleagues reported that a conduit diameter of 16 mm and 18 mm is optimal for Fontan patients with a mean age of 36 months, considering energy loss and stagnation volume [27]. However, Rjinberg and colleagues suggested that a 16–20 mm conduit become undersized for adolescent Fontan patients, showing a significantly smaller mean conduit cross-sectional area normalized for conduit flow rate than other surrounding vessel and reporting blood flow acceleration from the IVC toward the conduit [[28], [29], [30]]. The effect of the elapsed time after ECFP on conduit stenosis was also interpreted cautiously because it was likely to be correlated with body surface area, not with minimum conduit area. As the patients grew, the median body surface area increased from 0.59 m2 at ECFP to 1.61 m2 at cardiac CT. The association with elapsed time after ECFP and conduit stenosis was controversial in previous studies [[20], [21], [22],31,32]. The decrease in the minimum conduit cross-sectional area at mean follow up of 9.6 years were not associated with elapsed time after ECFP [21]. There was no significant difference in the mean cross-sectional area of the conduit at 1 month and 5.2 years after ECFP [32]. Fogel and colleagues demonstrated that the ratio of the minimum conduit area per the average conduit area increased over time in adolescents aged over 13 years [31].
The minimum conduit area in an oversized conduit is more commonly located on both sides than in the middle. Moreover, surgical anastomosis between an oversized conduit and a relatively small IVC or pulmonary artery was difficult to achieve, leaving these sites vulnerable to postoperative stenosis. An oversized conduit was associated with a high incidence of pseudointimal hyperplasia and thrombus in this study. An oversized conduits have unfavorable hemodynamics stemming from a size discrepancy, such as turbulence and stagnation, associated with conduit thrombosis [27,33]. Thus, the Fontan pathway in patients with an oversized conduit should be monitored during follow-up for potential anastomotic stenosis and the occurrence of pseudointimal hyperplasia and thrombus.
Even after correcting the time elapsed after ECFP, which was significantly associated with Fontan-associated liver disease, patients with radiological findings of CLD had a lower ratio of minimum conduit-to-IVC areas [34,35]. Resistance of the conduit and energy loss, dependent on the diameter of conduit stenosis, was significantly correlated with hepatic fibrosis in recent studies [7,36,37]. Therefore, an increased resistance of the stenotic conduit can increase hepatic congestion and cause liver fibrosis progression.
The relationship of conduit stenosis with exercise intolerance was not clarified in this study. The parameters of the conduit were not correlated with peak VO2, in contrast to findings in previous studies [21,29,38]. Patel and colleagues demonstrated a correlation between minimum Fontan cross-sectional area, indexed to body surface area, and %predicted VO2 [21]. This disparity might be attributed to the present study not accounting for anatomical factors such as pulmonary artery morphology and blood flow distribution, nor considering the impact of overweight/obesity and physical activity [36,[39], [40], [41]]. Only maximal percent stenosis of the conduit was weakly correlated with the oxygen pulse and peak heart rate. Oxygen pulse is a surrogate of stroke volume; it is well-known that stroke volume and heart rate at peak exercise are lower in patients with Fontan circulation than in the normal population [42,43]. Since there is no subpulmonic ventricle present after ECFP, cardiac output is elevated mainly by muscle pump during exercise, and an increase in heart rate causes a proportional decrease in stroke volume in Fontan circulation [42,[44], [45], [46]]. Resistance of the Fontan pathway, dependent on conduit stenosis, has a negative impact on the increase in cardiac output during exercise in patients with single ventricular physiology [7,29,36,47]. Additionally, narrowing of the Fontan pathway is also associated with exercise-related energy loss [8,27]. Thus, lesser stenosis of the conduit might help patients with Fontan circulation to maintain a greater stroke volume at a relatively appropriate heart rate during exercise.
There are some limitations with this study. First, a retrospective design was adopted and did not include patients without cardiac CT findings. Since cardiac CT was not a routine part of surveillance; thus, not all patients underwent regular cardiac CT assessments, those who did undergo cardiac CT were more likely to have issues related to the Fontan pathway, introducing a selection bias. Second, although cardiac CT could provide details of anatomical variables such as calcification or thrombus, unlike cardiac MR, it did not provide information regarding blood flow and conduit resistance. Third, the determination of Fontan-associated liver disease relied solely on radiologic data, without including liver pathology via biopsy, various laboratory data, or advanced imaging, such as elastography. Fourth, only a limited number of CPETs with maximal effort and liver imaging studies, conducted within 2 years of the cardiac CT, had insufficient power to demonstrate a strong correlation between conduit narrowing and exercise intolerance or chronic liver disease. Lastly, since the mechanisms of Fontan-related adverse outcomes, such as Fontan-associated liver disease and exercise intolerance, are multifactorial, the effects of conduit stenosis should be determined after consideration of other important contributors.
In conclusion, employing a larger conduit at ECFP results in a larger minimum conduit area at follow-up. However, oversizing requires careful monitoring for stenosis near anastomotic sites and for occurrence of pseudointimal hyperplasia or thrombus. Cardiac anatomic variables and the thromboprophylaxis method are not associated with conduit narrowing. There are some associations between conduit stenosis and the clinical status of Fontan circulation, including hepatic complications and exercise intolerance, but further studies are needed to investigate this.
Funding
None declared.
CRediT authorship contribution statement
Joowon Lee: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Mi Kyoung Song: Writing – review & editing, Supervision. Sang-Yun Lee: Writing – review & editing, Supervision. Gi Beom Kim: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization. Eun Jung Bae: Writing – review & editing, Supervision, Conceptualization. Hye Won Kwon: Writing – review & editing, Supervision. Sungkyu Cho: Writing – review & editing, Supervision. Jae Gun Kwak: Writing – review & editing, Supervision. Woong-Han Kim: Writing – review & editing, Supervision. Whal Lee: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for their consultation on statistical analyses.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcchd.2024.100505.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.d'Udekem Y., Iyengar A.J., Galati J.C., Forsdick V., Weintraub R.G., Wheaton G.R., et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–S38. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 2.Kverneland L.S., Kramer P., Ovroutski S. Five decades of the Fontan operation: a systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. 2018;13:181–193. doi: 10.1111/chd.12570. [DOI] [PubMed] [Google Scholar]
- 3.Deshaies C., Hamilton R.M., Shohoudi A., Trottier H., Poirier N., Aboulhosn J., et al. Thromboembolic risk after atriopulmonary, lateral tunnel, and extracardiac conduit fontan surgery. J Am Coll Cardiol. 2019;74:1071–1081. doi: 10.1016/j.jacc.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 4.Lardo A.C., Webber S.A., Friehs I., del Nido P.J., Cape E.G. Fluid dynamic comparison of intra-atrial and extracardiac total cavopulmonary connections. J Thorac Cardiovasc Surg. 1999;117:697–704. doi: 10.1016/S0022-5223(99)70289-8. [DOI] [PubMed] [Google Scholar]
- 5.Zheng J., Li Z., Li Q., Li X. Meta-analysis of Fontan procedure : extracardiac conduit vs. intracardiac lateral tunnel. Herz. 2018;43:238–245. doi: 10.1007/s00059-017-4553-6. [DOI] [PubMed] [Google Scholar]
- 6.Ovroutski S., Ewert P., Alexi-Meskishvili V., Peters B., Hetzer R., Berger F. Dilatation and stenting of the fontan pathway: impact of the stenosis treatment on chronic ascites. J Interv Cardiol. 2008;21:38–43. doi: 10.1111/j.1540-8183.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- 7.Tang E., McElhinney D.B., Restrepo M., Valente A.M., Yoganathan A.P. Haemodynamic impact of stent implantation for lateral tunnel Fontan stenosis: a patient-specific computational assessment. Cardiol Young. 2016;26:116–126. doi: 10.1017/S1047951114002765. [DOI] [PubMed] [Google Scholar]
- 8.Tang E., Wei Z.A., Whitehead K.K., Khiabani R.H., Restrepo M., Mirabella L., et al. Effect of Fontan geometry on exercise haemodynamics and its potential implications. Heart. 2017;103:1806–1812. doi: 10.1136/heartjnl-2016-310855. [DOI] [PubMed] [Google Scholar]
- 9.Udink Ten Cate F.E.A., Trieschmann U., Germund I., Hannes T., Emmel M., Bennink G., Sreeram N. Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart. 2017;103:1111–1116. doi: 10.1136/heartjnl-2016-310511. [DOI] [PubMed] [Google Scholar]
- 10.Hagler D.J., Miranda W.R., Haggerty B.J., Anderson J.H., Johnson J.N., Cetta F., et al. Fate of the Fontan connection: mechanisms of stenosis and management. Congenit Heart Dis. 2019;14:571–581. doi: 10.1111/chd.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreeram N., Emmel M., Bennink G. Stent therapy for acute and chronic obstructions in extracardiac Fontan conduits. Cardiol Young. 2013;23:766–768. doi: 10.1017/S1047951112001886. [DOI] [PubMed] [Google Scholar]
- 12.Babu-Narayan S.V., Giannakoulas G., Valente A.M., Li W., Gatzoulis M.A. Imaging of congenital heart disease in adults. Eur Heart J. 2016;37:1182–1195. doi: 10.1093/eurheartj/ehv519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han B.K., Huntley M., Overman D., Witt D., Dassenko D., Garberich R.F., Lesser J.R. Cardiovascular CT for evaluation of single-ventricle heart disease: risks and accuracy compared with interventional findings. Cardiol Young. 2018;28:9–20. doi: 10.1017/S1047951117001135. [DOI] [PubMed] [Google Scholar]
- 14.Hauser J.A., Taylor A.M., Pandya B. vol. 10. Circ Cardiovasc Imaging; 2017. (How to image the adult patient with fontan circulation). [DOI] [PubMed] [Google Scholar]
- 15.Kardoš M., Mikuláš J., Vulev I., Mašura J. CT angiography in Fontans with implanted stents. Cor Vasa. 2013;55:e434–e438. [Google Scholar]
- 16.Goo H.W. Contrast-enhanced CT protocol for the fontan pathway: comparison between 1- and 3-minute scan delays. Pediatr Cardiol. 2022;43:1104–1113. doi: 10.1007/s00246-022-02830-2. [DOI] [PubMed] [Google Scholar]
- 17.Hong S.H., Kim Y.M., Lee C.-H., Park S.-J., Kim S.H. CT and MRI evaluation of the fontan pathway: pearls and pitfalls. Cardiovasc Imaging Asia. 2017;1:133–145. [Google Scholar]
- 18.Steinberg C., Weinstock D.J., Gold J.P., Notterman D.A. Measurements of central blood vessels in infants and children: normal values. Cathet Cardiovasc Diagn. 1992;27:197–201. doi: 10.1002/ccd.1810270308. [DOI] [PubMed] [Google Scholar]
- 19.Nandwana S.B., Olaiya B., Cox K., Sahu A., Mittal P. Abdominal imaging surveillance in adult patients after fontan procedure: risk of chronic liver disease and hepatocellular carcinoma. Curr Probl Diagn Radiol. 2018;47:19–22. doi: 10.1067/j.cpradiol.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Lee C., Lee C.H., Hwang S.W., Lim H.G., Kim S.J., Lee J.Y., et al. Midterm follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur J Cardio Thorac Surg. 2007;31:1008–1012. doi: 10.1016/j.ejcts.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Patel N.D., Friedman C., Herrington C., Wood J.C., Cheng A.L. Progression in Fontan conduit stenosis and hemodynamic impact during childhood and adolescence. J Thorac Cardiovasc Surg. 2021;162:372–380 e2. doi: 10.1016/j.jtcvs.2020.09.140. [DOI] [PubMed] [Google Scholar]
- 22.Amodeo A., Galletti L., Marianeschi S., Picardo S., Giannico S., Di Renzi P., Marcelletti C. Extracardiac Fontan operation for complex cardiac anomalies: seven years' experience. J Thorac Cardiovasc Surg. 1997;114:1020–1030. doi: 10.1016/S0022-5223(97)70016-3. ; discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 23.Grewal J., Al Hussein M., Feldstein J., Kiess M., Ellis J., Human D., Leipsic J. Evaluation of silent thrombus after the Fontan operation. Congenit Heart Dis. 2013;8:40–47. doi: 10.1111/j.1747-0803.2012.00699.x. [DOI] [PubMed] [Google Scholar]
- 24.Alsaied T., Alsidawi S., Allen C.C., Faircloth J., Palumbo J.S., Veldtman G.R. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101:1731–1737. doi: 10.1136/heartjnl-2015-307930. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar A.J., Winlaw D.S., Galati J.C., Wheaton G.R., Gentles T.L., Grigg L.E., et al. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardio Thorac Surg. 2016;50:980–987. doi: 10.1093/ejcts/ezw159. [DOI] [PubMed] [Google Scholar]
- 26.Monagle P., Cochrane A., Roberts R., Manlhiot C., Weintraub R., Szechtman B., et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58:645–651. doi: 10.1016/j.jacc.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 27.Itatani K., Miyaji K., Tomoyasu T., Nakahata Y., Ohara K., Takamoto S., Ishii M. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann Thorac Surg. 2009;88:565–572. doi: 10.1016/j.athoracsur.2009.04.109. ; discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 28.Hut T., Roest A.A.W., Gaillard D., Hazekamp M., van den Boogaard P., Lamb H., et al. Interdiscip Cardiovasc Thorac Surg; 2023. Virtual surgery to predict optimized conduit size for adult fontan patients with 16mm conduits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijnberg F.M., van 't Hul L.C., Hazekamp M.G., van den Boogaard P.J., Juffermans J.F., Lamb H.J., et al. Haemodynamic performance of 16-20-mm extracardiac Goretex conduits in adolescent Fontan patients at rest and during simulated exercise. Eur J Cardio Thorac Surg. 2022;63 doi: 10.1093/ejcts/ezac522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rijnberg F.M., van der Woude S.F.S., Hazekamp M.G., van den Boogaard P.J., Lamb H.J., Terol Espinosa de Los Monteros C., et al. Extracardiac conduit adequacy along the respiratory cycle in adolescent Fontan patients. Eur J Cardio Thorac Surg. 2022;62 doi: 10.1093/ejcts/ezab478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogel M.A., Trusty P.M., Nicolson S., Spray T., Gaynor J.W., Whitehead K.K., Yoganathan A.P. Cross-sectional magnetic resonance and modeling comparison from just after fontan to the teen years. Ann Thorac Surg. 2020;109:574–582. doi: 10.1016/j.athoracsur.2019.07.066. [DOI] [PubMed] [Google Scholar]
- 32.Ochiai Y., Imoto Y., Sakamoto M., Kajiwara T., Sese A., Watanabe M., et al. Mid-term follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur J Cardio Thorac Surg. 2009;36:63–67. doi: 10.1016/j.ejcts.2009.02.013. ; discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 33.Alexi-Meskishvili V., Ovroutski S., Ewert P., Dahnert I., Berger F., Lange P.E., Hetzer R. Optimal conduit size for extracardiac Fontan operation. Eur J Cardio Thorac Surg. 2000;18:690–695. doi: 10.1016/s1010-7940(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 34.Baek J.S., Bae E.J., Ko J.S., Kim G.B., Kwon B.S., Lee S.Y., et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750–1755. doi: 10.1136/hrt.2010.201772. [DOI] [PubMed] [Google Scholar]
- 35.Nii M., Inuzuka R., Inai K., Shimada E., Shinohara T., Kogiso T., et al. Incidence and expected probability of liver cirrhosis and hepatocellular carcinoma after fontan operation. Circulation. 2021;144:2043–2045. doi: 10.1161/CIRCULATIONAHA.121.056870. [DOI] [PubMed] [Google Scholar]
- 36.Rijnberg F.M., Jjm Westenberg, van Assen H.C., Juffermans J.F., Kroft L.J.M., van den Boogaard P.J., et al. 4D flow cardiovascular magnetic resonance derived energetics in the Fontan circulation correlate with exercise capacity and CMR-derived liver fibrosis/congestion. J Cardiovasc Magn Reson. 2022;24:21. doi: 10.1186/s12968-022-00854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trusty P.M., Wei Z.A., Rychik J., Graham A., Russo P.A., Surrey L.F., et al. Cardiac magnetic resonance-derived metrics are predictive of liver fibrosis in fontan patients. Ann Thorac Surg. 2020;109:1904–1911. doi: 10.1016/j.athoracsur.2019.09.070. [DOI] [PubMed] [Google Scholar]
- 38.Khiabani R.H., Whitehead K.K., Han D., Restrepo M., Tang E., Bethel J., et al. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart. 2015;101:139–143. doi: 10.1136/heartjnl-2014-306337. [DOI] [PubMed] [Google Scholar]
- 39.Alsaied T., Sleeper L.A., Masci M., Ghelani S.J., Azcue N., Geva T., et al. Maldistribution of pulmonary blood flow in patients after the Fontan operation is associated with worse exercise capacity. J Cardiovasc Magn Reson. 2018;20:85. doi: 10.1186/s12968-018-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinreb S.J., Dodds K.M., Burstein D.S., Huang J., Rand E.B., Mancilla E., et al. End-organ function and exercise performance in patients with fontan circulation: what characterizes the high performers? J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravndal M., Idorn L., Nielsen A.K.M., Kelly B., Nielsen K.G., Nielsen D.G., Hjortdal V. Exercise capacity in the Danish Fontan population remains stable after ten years of follow-up - is physical activity the key to success? Int J Cardiol. 2023;387 doi: 10.1016/j.ijcard.2023.131137. [DOI] [PubMed] [Google Scholar]
- 42.Claessen G., La Gerche A., Van De Bruaene A., Claeys M., Willems R., Dymarkowski S., et al. Heart rate reserve in fontan patients: chronotropic incompetence or hemodynamic limitation? J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hedlund E.R., Soderstrom L., Lundell B. Appropriate heart rate during exercise in Fontan patients. Cardiol Young. 2020;30:674–680. doi: 10.1017/S1047951120000761. [DOI] [PubMed] [Google Scholar]
- 44.Barber G., Di Sessa T., Child J.S., Perloff J.K., Laks H., George B.L., Williams R.G. Hemodynamic responses to isolated increments in heart rate by atrial pacing after a Fontan procedure. Am Heart J. 1988;115:837–841. doi: 10.1016/0002-8703(88)90887-3. [DOI] [PubMed] [Google Scholar]
- 45.Hebert A., Jensen A.S., Mikkelsen U.R., Idorn L., Sorensen K.E., Thilen U., et al. Hemodynamic causes of exercise intolerance in Fontan patients. Int J Cardiol. 2014;175:478–483. doi: 10.1016/j.ijcard.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Shafer K.M., Garcia J.A., Babb T.G., Fixler D.E., Ayers C.R., Levine B.D. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation) J Am Coll Cardiol. 2012;60:2115–2121. doi: 10.1016/j.jacc.2012.08.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundareswaran K.S., Pekkan K., Dasi L.P., Whitehead K., Sharma S., Kanter K.R., et al. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am J Physiol Heart Circ Physiol. 2008;295:H2427–H2435. doi: 10.1152/ajpheart.00628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.