Abstract
Background
Shared decision-making is an imperative in chronic pain care. However, we know little about the decision-making process, especially in primary care where most chronic pain care is provided. We sought to understand decisional needs of people living with chronic pain in Canada.
Methods
We conducted a population-based cross-sectional online survey of random samples of adults living in Canada with chronic noncancer pain and registered with the Leger Marketing panel. We followed the International Association for Study of Pain definition of chronic pain (i.e., persistent or recurrent pain lasting longer than three months). We used a stratified proportional random sampling based on the population and chronic pain prevalence of each province to achieve representativeness. Based on the Ottawa Decision Support Framework, we collected data on difficult decisions (i.e., decision with more than one option and no clear best option) related to their chronic pain condition, the level of decisional conflict associated with the most difficult decisions (i.e., Decisional Conflict Scale), the assumed and preferred role during the decision-making process (i.e., Control Preferences Scale), and respondents’ characteristics. We used descriptive quantitative analyses of survey responses.
Results
Of the 31,545 invited panellists, 2,666 met the eligibility criteria, and 1,649 respondents from the 10 Canadian provinces completed the survey. Respondents had diverse socio-demographic profiles. Mean age was 51.8 years (SD = 16.3). Half were men (51.4%), most lived in urban areas (87.8%), mean pain duration was 8.5 years (SD = 9.6), and respondents reported an average number of painful body regions of 2.3 (SD = 1.5). We observed that 96.7% of respondents faced at least one difficult decision across their care pathways. These difficult decisions were related to numerous issues from the medical consultation, diagnosis, treatment, and daily life. Almost half of respondents made their most difficult decision with a primary care physician. One third of respondents experienced a high level of clinically significant decisional conflict (Decisional Conflict Scale score ≥ 37.5). Two-thirds of respondents self-reported having a collaborative role during their decision while three-quarters wanted this role.
Conclusions
People living with chronic pain in Canada have unmet decisional needs and need support to make optimal decisions to manage their chronic pain. Our findings will guide future development of interventions to implement shared decision-making, especially to support primary care where discussions about difficult decisions often occur.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12875-024-02667-z.
Keywords: Chronic pain, Shared decision-making, Survey
Key points
1. We conducted an online survey across 10 Canadian provinces and collected responses from a wide diversity of people living with chronic pain.
2. Over 96% of respondents faced at least one difficult decision concerning pain care across their care pathways, from diagnosis to daily living modifications.
3. A third of respondents experienced a high level of clinically significant decisional conflict.
4. People living with chronic pain in Canada have unmet needs and desire to be involved in the decisions about their pain care.
5. Our national survey justifies the development of shared decision-making interventions, especially to support primary care, to address difficult decisions, reduce decisional conflict and increase involvement in chronic pain care.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12875-024-02667-z.
Introduction
Decisions made in chronic pain care often require tradeoffs that are valued differently by patients and health care providers (HCPs) [1–4]. Involving patients in the decision-making process through shared decision-making (SDM) is of great value in this context [5]. The bidirectional exchange of information allows patients and HCPs to co-construct a care plan that makes intellectual, emotional, and practical sense [6, 7]. For example, a patient and their HCP may choose a rehabilitation care plan because the patient is concerned about the side effects of pharmacological options. We have no information on current patients’ involvement in chronic pain care in Canada.
A lack of patients’ involvement can lead to unmet decisional needs resulting in decisional conflict [8, 9]. Decisional conflict is defined as “a state of uncertainty about the course of action to take” [10]. Patients experiencing decisional conflict may harbour negative emotions (e.g., self-blame) concerning the care they are receiving [9]. These emotions may adversely affect their well-being and lead to maladaptive behaviours (e.g., non-adherence to the chosen option), which could lead to poorer clinical outcomes [9].
We have no information on the magnitude of decisional conflict in the context of chronic pain. However, the prevalence of decisional conflict in primary care in Canada is estimated between 10.3% and 31.1% [11]. As chronic pain is mainly managed by primary care HCPs in Canada [12, 13], we can hypothesize that people living with chronic pain experience decisional conflict. In this context, it is essential to identify the decisional needs of people living with chronic pain to reduce decisional conflict.
According to the Ottawa Decision Support Framework, eight decisional needs core components should be assessed before developing interventions to support the decision-making process [8]. The first is identifying difficult decisions experienced by individuals [8]. A recent systematic review with chronic musculoskeletal pain (i.e., the most prevalent category of chronic pain [14, 15]) showed that studies on SDM interventions mainly focused on a single decision: the choice between treatment options (mostly surgical vs. nonsurgical treatments) [16, 17]. According to the literature [18] and patient partners, this focus does not reflect the authentic lived experience of patients, as people living with chronic pain face multiple difficult decisions across their care pathways.
Canada is currently implementing the Action Plan for Pain [19]. In this context, we collected data on current patients’ involvement, decisional conflict, and difficult decisions experienced by people living with chronic pain in Canada. The aims of this study were (1) to evaluate the magnitude of decisional conflict in chronic pain care, (2) to estimate to which extent people living with chronic pain in Canada wish to be involved in their pain management decisions, and (3) to identify difficult decisions people living with chronic pain in Canada face across their care pathways.
Methods
Study design and settings
We conducted a cross-sectional online survey of people from the 10 Canadian provinces from August 31, 2022 to September 28, 2022 (the protocol is available elsewhere [20]). We used the Checklist for Reporting Of Survey Studies (CROSS) to inform the reporting of results [21].
Respondents
We recruited community-dwelling people living with chronic non-cancer pain from the largest Canadian-owned market research and analytics company, Leger Marketing (https://leger360.com/). This company maintains a panel of 500,000 active members of Canadian society from the 10 Canadian provinces with Internet access. Respondents included a randomly selected sample of citizens, permanent residents or refugees living in Canada, aged ≥ 18 years old, with chronic primary pain (e.g., low back pain, fibromyalgia, migraine) or secondary pain (e.g., postsurgical pain, pain due to structural changes, pain associated with a disease of the nervous system). The International Association for the Study of Pain defined chronic primary pain as pain in one or more anatomical regions that persists or recurs for longer than three months and that cannot be better accounted for by another chronic pain condition [22], and chronic secondary pain as chronic pain that is linked to other diseases as the underlying cause, for which pain may initially be regarded as a symptom [23]. Respondents had to read, write, and understand French or English. We excluded respondents with chronic cancer pain or chronic post-cancer treatment pain, a type of pain which requires specific care pathways and management options [24]. We used a stratified proportional random sampling based on the population and chronic pain prevalence of each province [15, 25]. Randomization method and strategy to achieve a representative sampling are detailed in the published protocol [20].
Data collection and outcome measures
We collected data on: (1) the respondents’ characteristics, (2) the difficult decisions that Canadians living with chronic pain experienced, (3) the decisional conflict associated with the most difficult decision, (4) the assumed and preferred role during the decision-making process.
Respondents’ characteristics
We used the personal and clinical needs from the Ottawa Decision Support Framework to characterize the respondents [8]. We expanded the data collection with supplementary information such as comorbidity, sex at birth, number of people living at home and income. We collected 21 different respondents’ characteristics. These characteristics were consistent with the PROGRESS-Plus framework that is recommended by Cochrane [26].
Difficult decisions
We used two resources from Canada to describe the difficult decisions (six questions). These resources were a previously published qualitative study with people with complex care needs in Canada [27] and the report of the Canadian Pain Task Force [12]. We divided the difficult decisions into four categories: (1) medical consultation, (2) diagnosis, (3) treatment, and (4) daily life. We also collected the most difficult decision that the respondents faced and the HCP(s) with whom they made the most difficult decision.
Decisional conflict
We used the Decisional Conflict Scale (statement format) (DCS) to evaluate the decisional conflict of the most difficult decision. The DCS consists of 16 items designed to elicit information on the five dimensions of decision-making: feeling uncertain, feeling uninformed, feeling unclear about values, feeling unsupported and ineffective decision-making [28]. A subscore is available for each dimension. A continuous decisional conflict score is obtained between 0 = absence of decisional conflict and 100 = maximum decisional conflict. A score ≥ 25 is related to decisional conflict [28] that reflects “a state of uncertainty about the course of action to take” [10]. A score ≥ 37.5 is related to clinically significant decisional conflict (CSDC) [28] that is positively associated with decisional delay, departure from active treatment, decision regret and nervousness [11, 29]. Details on measurement properties are available in the protocol [20].
Assumed and preferred role
We used the Control Preferences Scale (CPS) to determine assumed and preferred role during the decision-making process of the most difficult decision (two questions). The CPS is a five-level Likert scale representing the degrees of control (roles) an individual wants to assume when making a health decision [30]. Details on measurement properties are available in the protocol [20].
Pretesting of the survey
In April 2022, we performed a clinical sensibility testing [31, 32] with our patient partners (n = 2), experts in survey methodology (n = 7), and SDM experts (n = 9) to obtain preliminary evidence of face validity of our survey. We made several modifications such as the removal of medical jargon and the addition of option responses to better discriminate HCP(s) with whom the most difficult decision was made. In August 2022, we performed a pilot test with 50 random respondents who meet the inclusion criteria to obtain information on the readability and comprehensibility of our survey. No changes were made at the end of the pilot testing.
Survey administration
Leger Marketing sent an email invitation to random samples of eligible respondents including a cover letter describing the aim of the survey. Interested respondents logged to their panel membership account to consent and begin the questionnaire. They had access to the questionnaire for three weeks. A reminder was sent weekly to solicit respondents. All respondents completed the questionnaire once. The participation was free and voluntary but, respondents received standardized compensation from Leger Marketing for the time required to complete the survey. We collected data over a four-week period, from August 31, 2022 to September 28, 2022.
Ethical considerations
We obtained the ethics approval from the Research Ethics Board of the Research Centre at the Centre Hospitalier Universitaire de Sherbrooke (project #2022–4645). We conducted the survey following the Canadian Personal Information Protection and Electronic Documents Act [33] and the Marketing Research and Intelligence Association’s Charter of Respondent Rights [34]. Respondents consented to participate in the study. All respondents had to give their consent before their participation and could request to be removed from the study at any time. Respondents also had to consent to Leger Marketing’s terms of use and privacy policy [35].
Statistical analysis
We calculated the required sample size to respond to the third objective (i.e., binary logistic regression) of the DECIDE-PAIN project [20]. We used a context-specific method proposed by Riley et al. [36] using an estimated prevalence of the outcome = 0.10 (i.e., prevalence of clinically significant decisional conflict in primary care) [11], an estimated Nagelkerke’s R2 = 0.15 [36] and an estimated number of predictors in the initial model = 30 [36]. We needed to recruit 1,649 individuals living with chronic pain. This sample size yielded a margin of error of 2% [37], representing a good accuracy of our estimators [37, 38].
Two independent reviewers performed the data cleaning of the dataset. The process was supervised with an external team of biostatisticians. We managed all disagreements through discussion leading to consensus that did not require a third reviewer. We based the initial data analysis (i.e., metadata, data cleaning, and data screening) on the STRengthening Analytical Thinking for Observational Studies (STRATOS) initiative recommendations and we recorded the cleaning rules in a report [39]. We performed a complete case analysis, as there was a small amount of missing data (a maximum of five missing values, representing 0.3% of the sample).
Respondents’ characteristics
We used descriptive statistics to describe respondents’ characteristics (mean and standard deviation (SD), frequency and percentage). We performed this descriptive analysis for the entire sample, for people with a decisional conflict scale score ≥ 37.5 (i.e., presence of CSDC), and for people with no difficult decision. We investigated differences between people with CSDC and people with no difficult decision using a two-sample t-test for normally distributed continuous variables (or Mann-Whitney test for non-normally distributed continuous variables) and Chi2 test (or Fisher’s exact test) for categorical variables. We performed the same analysis with the DCS cut-off of 25 to determine whether results were different.
Difficult decisions
We used descriptive analysis (frequency and absolute number) to describe the difficult decisions faced by individuals living with chronic pain in Canada for their medical consultation, diagnosis, treatment, and daily life and report the most difficult decision experienced by our respondents. We conducted thematic analysis of the response option “Other, please specify” to identify new difficult decisions [40].
Decisional conflict
We addressed prevalence of decisional conflict by reporting the percentage of people with CSDC (DCS total score ≥ 37.5) [28] and with decisional conflict (DCS total score ≥ 25) [28]. We also described decisional conflict with mean and SD of the total score and for each dimension. To identify difficult decisions relevant for targeting with SDM interventions, we reported the mean total DCS score and the percentage of respondents with CSDC for each most difficult decision. We then compared these decisions to the one with the highest mean score and the one with the highest percentage of CSDC.
Assumed and preferred role
We determined assumed and preferred role as well as the congruence between both during the decision-making process related to the most difficult decision with descriptive analysis (percentage and absolute number). We also reported the percentage and absolute number of people whose preferred role was a collaborative role (i.e., response C of the CPS). In our statistical analysis, we also integrated responses B (i.e., “I made the decision after seriously considering my providers’ opinion.”) and D (i.e., “My providers made the decision after seriously considering my opinion.”) as a collaborative role due to the evolution of the shared decision-making concept (e.g., patient adapted paternalism as part of SDM concept) [41].
All the statistical analyses were performed on R software (version 4.3.1) and open Epi (version 3.01).
Results
Respondents’ details and respondents’ characteristics
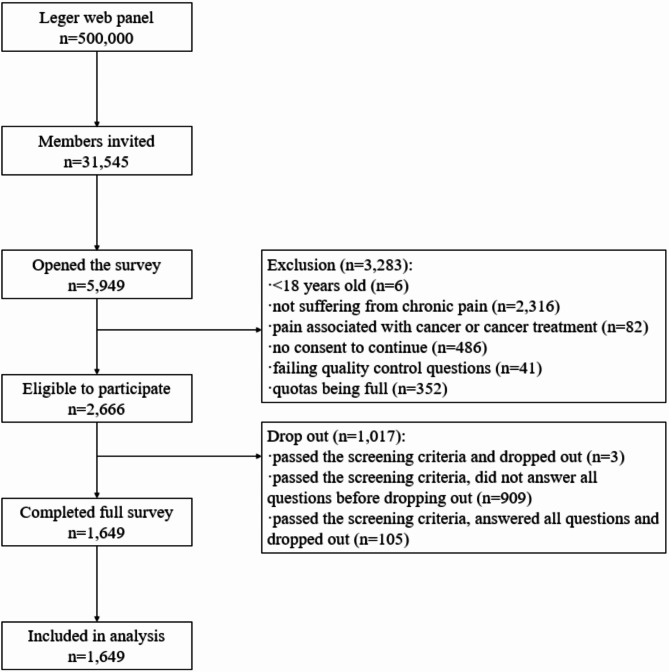
Leger Marketing randomly invited 31,545 members of their panel (Fig. 1). The invitation view rate was 18.9% (5,949/31,545). The participation rate was 44.8% (2,666/5,949) and the completion rate was 61.9% (1,649/2,666). 80% (1,323/1,649) of the respondents completed the survey in English.
Fig. 1.
Flow diagram on recruitment of people living with chronic pain in Canada
Table 1 describes the respondents’ characteristics. Mean age was 51.8 years (SD = 16.3). Half of respondents were man (51.4%), most lived in urban areas (87.8%), mean pain duration was 8.5 years (SD = 9.6) and reported an average number of painful body regions of 2.3 (SD = 1.5). Respondents with a clinically significant decisional conflict (DCS total score ≥ 37.5) had statistically significant differences from those without difficult decision regarding to age, sex, gender, number and location of painful body regions, number and type of comorbidities, perception of disability and/or emotional distress, quality of life, satisfaction with their current state of health, health literacy, education level, spirituality, and number of individuals in their household. All the data (e.g., median, first quartile) are available in Supplementary Material 1.
Table 1.
Characteristics of the respondents
| Characteristics | Descriptives analyses | All sample (n = 1649) | People with DCS score ≥ 37,5 (n = 462) | People with no difficult decision (n = 276) | Comparison between DCS ≥ 37.5 and no difficult decision |
|---|---|---|---|---|---|
| Socio-demographic characteristics | |||||
| Age (years) | Mean, [95%CI] | 51.84, [51.05 ; 52.63] | 48.24, [46.78 ; 49.70] | 54.36, [52.45 ; 56.25] | p < 0.001 |
| SD | 16.33 | 15.92 | 16.00 | ||
| Min | 18.00 | 18.00 | 18.00 | ||
| Max | 90.00 | 90.00 | 89.00 | ||
| Sex | Male: n, (%), [95%CI] |
854, (51.79%), [49.38 ; 54.19] |
218, (47.19%), [42.68 ; 51.74] |
168, (60.87%), [55.00 ; 66.44] |
p < 0.001 |
| Female: n, (%), [95%CI] |
789, (47.85%), [45.44 ; 50.26] |
241, (52.16%), [47.61 ; 56.68] |
107, (38.77%), [33.21 ; 44.63] |
||
| Intersex: n, (%), [95%CI] |
4, (0.24%), [0.09 ; 0.62] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Prefer not to say: n, (%), [95%IC] |
2, (0.12%), [0.03 ; 0.44] |
1, (0.22%), [0.04 ; 1.22] |
1, (0.36%), [0.06 ; 2.02] |
||
| Gender | Man: n, (%), [95%CI] |
847, (51.36%), [48.95 ; 53.77] |
218, (47.19%), [42.68 ; 51.74%] |
167, (60.51%), [54.63 ; 66.09] |
p < 0.01 |
| Woman: n, (%), [95%CI] |
777, (47.12%), [44.72 ; 49.53] |
234, (50.65%), [46.10 ; 55.18] |
106, (38.41%), [32.86 ; 44.27] |
||
| Non-binary: n, (%), [95%CI] |
10, (0.61%), [0.33 ; 1.11] |
3, (0.65%), [0.22 ; 1.89] |
2, (0.72%), [0.20 ; 2.60] |
||
| Transgender woman: n, (%), [95%CI] |
1, (0.06%), [0.01 ; 0.34] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Transgender man: n, (%), [95%CI] |
2, (0.12%), [0.03 ; 0.44] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Two-spirit: n, (%), [95%CI] |
3, (0.18%), [0.06 ; 0.53] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Gender fluid: n, (%), [95%CI] |
4, (0.24%), [0.09 ; 0.62] |
2, (0.43%), [0.12 ; 1.56] |
1, (0.36%), [0.06 ; 2.02] |
||
| Other: n, (%), [95%CI] |
0, (0.00%), [0.00 ; 0.23] |
0, (0,00%), [0.00 ; 0.82] |
0, (0.00%), [0.00 ; 1.37] |
||
| Prefer not to say: n, (%), [95%CI] |
0, (0.00%), [0.00 ; 0.23] |
0, (0,00%), [0.00 ; 0.82] |
0, (0.00%), [0.00 ; 1.37] |
||
| Missing data: n, (%) | 5, (0.30%) | 1, (0.22%) | 0, (0.00%) | ||
| Provinces | British Columbia: n, (%), [95%CI] |
268, (16.25%), [14.55 ; 18.11] |
80, (17.32%), [14.14 ; 21.03] |
48, (17.39%), [13.38 ; 22.30] |
p = 0.46 |
| Alberta: n, (%), [95%CI] |
217, (13.16%), [11.61 ; 14.88] |
53, (11.47%), [8.88 ; 14.70] |
29, (10.51%), [7.42 ; 14.68] |
||
| Prairies: n, (%), [95%CI] |
121, (7.34%), [6.18 ; 8.70] |
36, (7.79%), [5.68 ; 10.60] |
18, (6.52%), [4.17 ; 10.07] |
||
| Ontario: n, (%), [95%CI] |
579, (35.11%), [32.85 ; 37.45] |
175, (37.88%), [33.57 ; 42.38] |
92, (33.33%), [28.03 ; 39.09] |
||
| Quebec: n, (%), [95%CI] |
332, (20.13%), [18.27 ; 22.14] |
82, (17.75%), [14.54 ; 21.49] |
59, (21.38%), [16.95 ; 26.59] |
||
| Atlantic Canada: n, (%), [95%CI] |
132, (8.00%), [6.79 ; 9.41] |
36, (7.79%), [5.68 ; 10.60] |
30, (10.87%), [7.72 ; 15.09] |
||
| Geographical area | Rural: n, (%), [95%CI] |
202, (12.25%), [10.75 ; 13.92] |
55, (11.90%), [9.26 ; 15.18] |
40, (14.49%), [10.83 ; 19.13] |
p = 0.37 |
| Urban: n, (%), [95%CI] |
1447, (87.75%), [86.08 ; 89.25] |
407, (88.10%), [84.82 ; 90.74] |
236, (85.51%), [80.87 ; 89.17] |
||
| First learned language | French: n, (%), [95%CI] |
378, (22.92%), [20.96 ; 25.01] |
98, (21.21%), [17.73 ; 25.17] |
63, (22.83%), [18.27 ; 28.13] |
p = 0.57 |
| English: n, (%), [95%CI] |
1122, (68.04%), [65.75 ; 70.25] |
318, (68.83%), [64.47 ; 72.88] |
191, (69.20%), [63.52 ; 74.35] |
||
| An aboriginal language: n, (%), [95%CI] |
2, (0.12%), [0.03 ; 0.44] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Spanish: n, (%), [95%CI] |
10, (0.61%), [0.33 ; 1.11] |
5, (1.08%), [0.46 ; 2.51] |
1, (0.36%), [0.06 ; 2.02] |
||
| Mandarin: n, (%), [95%CI] |
15, (0.91%), [0.55 ; 1.50] |
4, (0.87%), [0.34 ; 2.21] |
0, (0.00%), [0.00 ; 1.37] |
||
| Arabic: n, (%), [95%CI] |
9, (0.55%), [0.29 ; 1.03] |
2, (0.43%), [0.12 ; 1.56] |
0, (0.00%), [0.00 ; 1.37] |
||
| Other: n, (%), [95%CI] |
113, (6.85%), [5.73 ; 8.18] |
33, (7.14%), [5.13 ; 9.86] |
21, (7.61%), [5.03 ; 11.35] |
||
| Education level | Less than a high school diploma: n, (%), [95%CI] |
46, (2.79%), [2.10 ; 3.70] |
10, (2.16%), [1.18 ; 3.94] |
16, (5.80%), [3.60 ; 9.21] |
p < 0.01 |
| High School diploma: n, (%), [95%CI] |
338, (20.50%), [18.62 ; 22.51] |
89, (19.26%), [15.93 ; 23.11] |
74, (26.81%), [21.93 ; 32.33] |
||
| College, CEGEP or other non-university certificate or diploma: n, (%), [95%CI] |
479, (29.05%), [26.91 ; 31.29] |
141, (30.52%), [26.50 ; 34.86] |
74, (26.81%), [21.93 ; 32.33] |
||
| University certificate: n, (%), [95%CI] |
163, (9.88%), [8.54 ; 11.42] |
47, (10.17%), [7.74 ; 13.27] |
28, (10.14%), [7.11 ; 14.27] |
||
| Bachelor’s degree: n, (%), [95%CI] |
398, (24.14%), [22.13 ; 26.26] |
105, (22.73%), [19.14 ; 26.76] |
47, (17.03%), [13.05 ; 21.91] |
||
| Above the bachelor’s level: n, (%), [95%CI] |
213, (12.92%), [11.38 ; 14.62] |
67, (14.50%), [11.58 ; 18.01] |
32, (11.59%), [8.33 ; 15.91] |
||
| Prefer not to say: n, (%), [95%CI] |
12, (0.73%), [0.42 ; 1.27] |
3, (0.65%), [0.22 ; 1.89] |
5, (1.81%), [0.78 ; 4.17] |
||
| Health literacy (problem understanding what healthcare professionals said) | Always: n, (%), [95%CI] |
32, (1.94%), [1.38 ; 2.73] |
9, (1.95%), [1.03 ; 3.66] |
3, (1.09%), [0.37 ; 3.15] |
p < 0.001 |
| Often: n, (%), [95%CI] |
172, (10.43%), [9.05 ; 12.00] |
73, (15.80%), [12.76 ; 19.41] |
16, (5.80%), [3.60 ; 9.21] |
||
| Sometimes: n, (%), [95%CI] |
482, (29.23%), [27.09 ; 31.47] |
200, (43.29%), [38.85 ; 47.84] |
63, (22.83%), [18.27 ; 28.13] |
||
| Occasionally: n, (%), [95%CI] |
456, (27.65%), [25.55 ; 29.86] |
101, (21.86%), [18.33 ; 25.85] |
84, (30.43%), [25.31 ; 36.10] |
||
| Never: n, (%), [95%CI] |
507, (30.75%), [28.57 ; 33.02] |
79, (17.10%), [13.94 ; 20.80] |
110, (39.86%), [34.26 ; 45.73] |
||
| Cultural and ethnical backgrounds | Aboriginal: n, (%), [95%CI] |
84, (5.09%), [4.13 ; 6.26] |
21, (4.55%), [2.99 ; 6.85] |
10, (3.62%), [1.98 ; 6.54] |
p = 0.69 |
| North American: n, (%), [95%CI] |
770, (46.69%), [44.30 ; 49.11] |
217, (46.97%), [42.46 ; 51.53] |
145, (52.54%), [46.65 ; 58.35] |
||
| European: n, (%), [95%CI] |
662, (40.15%), [37.81 ; 42.53] |
171, (37.01%), [32.73 ; 41.51] |
111, (40.22%), [34.61 ; 46.10] |
||
| Caribbean: n, (%), [95%CI] |
24, (1.46%), [0.98 ; 2.16] |
10, (2.16%), [1.18 ; 3.94] |
4, (1.45%), [0.57 ; 3.67] |
||
| Latin, south American: n, (%), [95%CI] |
16, (0.97%), [0.60 ; 1.57] |
5, (1.08%), [0.46 ; 2.51] |
1, (0.36%), [0.06 ; 2.02] |
||
| African: n, (%), [95%CI] |
22, (1.33%), [0.88 ; 2.01] |
7, (1.52%), [0.74 ; 3.09] |
2, (0.72%), [0.20 ; 2.60] |
||
| Asian: n, (%), [95%CI] |
169, (10.25%), [8.88 ; 11.81] |
55, (11.90%), [9.26 ; 15.18] |
25, (9.06%), [6.21 ; 13.03] |
||
| Oceanian: n, (%), [95%CI] |
3, (0.18%), [0.06 ; 0.53] |
0, (0,00%), [0.00 ; 0.82] |
0, (0.00%), [0.00 ; 1.37] |
||
| Other: n, (%), [95%CI] |
4, (0.24%), [0.09 ; 0.62] |
1, (0.22%), [0.04 ; 1.22] |
0, (0.00%), [0.00 ; 1.37] |
||
| Prefer not to say: n, (%), [95%CI] |
49, (2.97%), [2.26 ; 3.94] |
16, (3.46%), [2.14 ; 5.55] |
11, (3.99%), [2.24 ; 6.99] |
||
| Missing data: n, (%) | 4, (0.24%) | 2, (0.43%) | 1, (0.36%) | ||
| Spirituality | Buddhist: n, (%), [95%CI] |
18, (1.09%), [0.69 ; 1.72] |
6, (1.30%), [0.60 ; 2.80] |
1, (0.36%), [0.06 ; 2.02] |
p < 0.05 |
| Christian: n, (%), [95%CI] |
694, (42.09%), [39.72 ; 44.48] |
162, (35.06%), [30.85 ; 39.52] |
116, (42.03%), [36.35 ; 47.92] |
||
| Hindu: n, (%), [95%CI] |
14, (0.85%), [0.51 ; 1.42] |
8, (1.73%), [0.88 ; 3.38] |
2, (0.72%), [0.20 ; 2.60] |
||
| Jewish: n, (%), [95%CI] |
22, (1.33%), [0.88 ; 2.01] |
10, (2.16%), [1.18 ; 3.94] |
3, (1.09%), [0.37 ; 3.15] |
||
| Muslim: n, (%), [95%CI] |
32, (1.94%), [1.38 ; 2.73] |
10, (2.16%), [1.18 ; 3.94] |
1, (0.36%), [0.06 ; 2.02] |
||
| Sikh: n, (%), [95%CI] |
15, (0.91%), [0.55 ; 1.50] |
6, (1.30%), [0.60 ; 2.80] |
2, (0.72%), [0.20 ; 2.60] |
||
| Traditional (North American Indigenous) spirituality: n, (%), [95%CI] |
21, (1.27%), [0.83 ; 1.94] |
8, (1.73%), [0.88 ; 3.38] |
0, (0.00%), [0.00 ; 1.37] |
||
| Other: n, (%), [95%CI] |
33, (2.00%), [1.43 ; 2.80] |
10, (2.16%), [1.18 ; 3.94] |
7, (2.54%), [1.23 ; 5.14] |
||
| No religious or spiritual affiliations: n, (%), [95%CI] |
735, (44.57%), [42.19 ; 46.98] |
218, (47.19%), [42.68 ; 51.74] |
134, (48.55%), [42.72 ; 54.43] |
||
| Prefer not to say: n, (%), [95%CI] |
64, (3.88%), [3.05 ; 4.93] |
24, (5.19%), [3.52 ; 7.61] |
10, (3.62%), [1.98 ; 6.54] |
||
| Missing data: n, (%) | 1, (0.06%) | 0, (0.00%) | 0, (0.00%) | ||
| Marital status | Never legally married: n, (%), [95%CI] |
402, (24.38%), [22.37 ; 26.51] |
133, (28.79%), [24.85 ; 33.08] |
73, (26.45%), [21.60 ; 31.95] |
p = 0.91 |
| Legally married: n, (%), [95%CI] |
759, (46.03%), [43.63 ; 48.44] |
185, (40.04%), [35.68 ; 44.58] |
112, (40.58%), [34.95 ; 46.46] |
||
| Separated, but still legally married: n, (%), [95%CI] |
49, (2.97%), [2.26 ; 3.91] |
20, (4.33%), [2.82 ; 6.59] |
9, (3.26%), [1.73 ; 6.08] |
||
| Divorced: n, (%), [95%CI] |
144, (8.73%), [7.46 ; 10.19] |
42, (9.09%), [6.80 ; 12.06] |
28, (10.14%), [7.11 ; 14.27] |
||
| Widowed: n, (%), [95%CI] |
60, (3.64%), [2.84 ; 4.66] |
15, (3.25%), [1.98 ; 5.29] |
10, (3.62%), [1.98 ; 6.54] |
||
| Living common law: n, (%), [95%CI] |
210, (12.73%), [11.21 ; 14.43] |
57, (12.34%), [9.65 ; 15.65] |
40, (14.49%), [10.83 ; 19.13] |
||
| Prefer not to say: n, (%), [95%CI] |
25, (1.52%), [1.03 ; 2.23] |
10, (2.16%), [1.18 ; 3.94] |
4, (1.45%), [0.57 ; 3.67] |
||
| Number of people in the household | Mean, [95%CI] | 2.45, [2.38 ; 2.51] | 2.53, [2.40 ; 2.65] | 2.20, [2.05 ; 2.34] | p < 0.001 |
| SD | 1.28 | 1.34 | 1.22 | ||
| Min | 1.00 | 1.00 | 1.00 | ||
| Max | 10.00 | 10.00 | 9.00 | ||
| Household income (CAD) | Less than $50,000: n, (%), [95%CI] |
481, (29.17%), [27.03 ; 31.41] |
139, (30.09%), [26.08 ; 34.42] |
93, (33.69%), [28.38 ; 39.46] |
p = 0.47 |
| $50,000 to less than $60,000: n, (%), [95%CI] |
199, (12.07%), [10.58 ; 13.73] |
62, (13.42%), [10.61 ; 16.83] |
27, (9.78%), [6.81 ; 13.86] |
||
| $60,000 to less than $80,000: n, (%), [95%CI] |
215, (13.04%), [11.50 ; 14.75] |
58, (12.55%), [9.84 ; 15.89] |
30, (10.87%), [7.72 ; 15.09] |
||
| $80,000 to less than $100,000: n, (%), [95%CI] |
233, (14.13%), [12.53 ; 15.89] |
59, (12.77%), [10.03 ; 16.12] |
41, (14.86%), [11.14 ; 19.53] |
||
| $100,000 or more: n, (%), [95%CI] |
406, (24.62%), [22.60 ; 26.76] |
104, (22.51%), [18.94 ; 26.54] |
56, (20.29%), [15.97 ; 25.43] |
||
| Prefer not to say: n, (%), [95%CI] |
115, (6.97%), [5.84 ; 8.31] |
40, (8.66%), [6.42 ; 11.58] |
29, (10.51%), [7.42 ; 14.68] |
||
| Work status | Currently working full-time: n, (%), [95%CI] |
649, (39.36%), [37.03 ; 41.74] |
188, (40.69%), [36.31 ; 45.23] |
98, (35.51%), [30.10 ; 41.32] |
p = 0.06 |
| Currently working part-time: n, (%), [95%CI] |
153, (9.28%), [7.97 ; 10.77] |
38, (8.23%), [6.05 ; 11.09] |
30, (10.87%), [7.72 ; 15.09] |
||
| Homemaker, no outside employment: n, (%), [95%CI] |
59, (3.58%), [2.78 ; 4.59] |
18, (3.90%), [2.48 ; 6.07] |
5, (1.81%), [0.78 ; 4.17] |
||
| Student: n, (%), [95%CI] |
65, (3.94%), [3.11 ; 4.99] |
19, (4.11%), [2.65 ; 6.33] |
14, (5.07%), [3.05 ; 8.33] |
||
| Unemployed: n, (%), [95%CI] |
64, (3.88%), [3.05 ; 4.93] |
25, (5.41%), [3.69 ; 7.87] |
12, (4.35%), [2.50 ; 7.44] |
||
| Retired: n, (%), [95%CI] |
505, (30.62%), [28.45 ; 32.89] |
111, (24.03%), [20.35 ; 28.13] |
90, (32.61%), [27.35 ; 38.34] |
||
| Long term or permanent disability: n, (%), [95%CI] |
106, (6.43%), [5.34 ; 7.72] |
38, (8.23%), [6.05 ; 11.09] |
21, (7.61%), [5.03 ; 11.35] |
||
| Total sick leave with financial assistance: n, (%), [95%CI] |
16, (0.97%), [0.60 ; 1.57] |
9, (1.95%), [1.03 ; 3.66] |
1, (0.36%), [0.06 ; 2.02] |
||
| Total sick leave with no financial assistance: n, (%), [95%CI] |
11, (0.67%), [0.37 ; 1.19] |
4, (0.87%), [0.34 ; 2.21] |
0, (0.00%), [0.00 ; 1.37] |
||
| Partial sick leave: n, (%), [95%CI] |
3, (0.18%), [0.06 ; 0.53] |
3, (0.65%), [0.22 ; 1.89] |
0, (0.00%), [0.00 ; 1.37] |
||
| Prefer not to say: n, (%), [95%CI] |
18, (1.09%), [0.69 ; 1.72] |
9, (1.95%), [1.03 ; 3.66] |
5, (1.81%), [0.78 ; 4.17] |
||
| Pain characteristics | |||||
| Pain location | Head: n, (%), [95%CI] |
246, (14.92%), [13.28 ; 16.72] |
94, (20.35%), [16.93 ; 24.25] |
33, 11.96%, [8.64 ; 16.32] |
p < 0.01 |
| Face: n, (%), [95%CI] |
59, (3.58%), [2.78 ; 4.59] |
25, (5.41%), [3.69 ; 7.87] |
13, (4.71%), [2.77 ; 7.89] |
||
| Neck: n, (%), [95%CI] |
555, (33.66%), [31.42 ; 35.97] |
187, (40.48%), [36.10 ; 45.01] |
77, (27.90%), [22.94 ; 33.47] |
||
| Middle back: n, (%), [95%CI] |
465, (28.20%), [26.08 ; 30.42] |
132, (28.57%), [24.64 ; 32.85] |
56, (20.29%), [15.97 ; 25.43] |
||
| Low back: n, (%), [95%CI] |
944, (57.25%), [54.85 ; 59.61] |
284, (61.47%), [56.96 ; 65.80] |
150, (54.35%), [48.45 ; 60.12] |
||
| Upper limb: n, (%), [95%CI] |
414, (25.11%), [23.07 ; 27.26] |
110, (23.81%), [20.15 ; 27.90] |
77, (27.90%), [22.94 ; 33.47] |
||
| Lower limb: n, (%), [95%CI] |
666, (40.39%), [38.04 ; 42.78] |
188, (40.69%), [36.31 ; 45.23] |
122, (44.20%), [38.46 ; 50.10] |
||
| Abdominal: n, (%), [95%CI] |
149, (9.04%), [7.75 ; 10.52] |
58, (12.55%), [9.84 ; 15.89] |
14, (5.07%), [3.05 ; 8.33] |
||
| Pelvic: n, (%), [95%CI] |
191, (11.58%), [10.13 ; 13.22] |
76, (16.45%), [13.35 ; 20.10] |
28, (10.14%), [7.11 ; 14.27] |
||
| Chest: n, (%), [95%CI] |
86, (5.22%), [4.24 ; 6.40] |
32, (6.93%), [4.95 ; 9.61] |
13, (4.71%), [2.77 ; 7.89] |
||
| Other: n, (%), [95%CI] |
12, (0.73%), [0.42 ; 1.27] |
3, (0.65%), [0.22 ; 1.89] |
3, (1.09%), [0.37 ; 3.15] |
||
| Missing data: n, (%) | 2, (0.12%) | 1, (0.22%) | 1, (0.36%) | ||
| Mean, [95%CI] | 2.30, [2.23 ; 2.37] | 2.58, [2.42 ; 2.74] | 2.13, [1.96 ; 2.31] | p < 0.001 | |
| SD | 1.52 | 1.78 | 1.47 | ||
| Min | 1.00 | 1.00 | 1.00 | ||
| Max | 10.00 | 10.00 | 10.00 | ||
| Pain duration (months) | Mean, [95%CI] | 102.12, [96.17 ; 108.07] | 103.3, [92.76 ; 113.88] | 101.7, [84.86 ; 118.59] | p = 0.87 |
| SD | 115.36 | 109.57 | 123.66 | ||
| Min | 3.00 | 3.00 | 3.00 | ||
| Max | 708.00 | 600.00 | 600.00 | ||
| Don’t remember precisely: n, (%), [95%IC] |
202, (12.25%), [10.75 ; 13.92] |
46, (9.96%), [7.55 ; 13.03] |
67, (24.28%), [19.59 ; 29.66] |
||
| Comorbidity | Diabetes: n, (%), [95%CI] |
243, (14.74%), [13.11 ; 16.53] |
63, (13.64%), [10.81 ; 17.07] |
39, (14.13%), [10.51 ; 18.73] |
p < 0.001 |
| Mental health disorders: n, (%), [95%CI] |
501, (30.38), [28.21 ; 32.65] |
188, (40.69%), [36.31 ; 45.23] |
63, (22.83%), [18.27 ; 28.13] |
||
| Alcohol use disorders: n, (%), [95%CI] |
53, (3.21%), [2.47 ; 4.18] |
15, (3.25%), [1.98 ; 5.29] |
4, (1.45%), [0.57 ; 3.67] |
||
| Substance use disorders: n, (%), [95%CI] |
58, (3.52%), [2.73 ; 4.52] |
22, 4.76%, [3.17 ; 7.10] |
4, (1.45%), [0.57 ; 3.67] |
||
| Sleep disorders: n, (%), [95%CI] |
489, (29.65%), [27.50 ; 31.90] |
147, (31.82%), [27.74 ; 36.20] |
72, (26.09%), [21.26 ; 31.57] |
||
| Hypertension: n, (%), [95%CI] |
436, (26.44%), [24.37 ; 28.62] |
104, (22.51%), [18.94 ; 26.54] |
63, (22.83%), [18.27 ; 28.13] |
||
| Respiratory disorders: n, (%), [95%CI] |
227, (13.77%), [12.19 ; 15.51] |
61, (13.20%), [10.42 ; 16.60] |
32, (11.59%), [8.33 ; 15.91] |
||
| Other: n, (%), [95%CI] |
195, (11.83%), [10.35 ; 13.47] |
57, (12.34%), [9.65 ; 15.65] |
23, (8.33%), [5.62 ; 12.19] |
||
| No comorbidity: n, (%), [95%CI] |
465, (28.20%), [26.08 ; 30.42] |
106, (22.94%), [19.34 ; 26.99] |
104, (37.68%), [32.17 ; 43.53] |
||
| Mean, [95%CI] | 1.34, [1.28 ; 1.39] | 1.42, [1.32 ; 1.53] | 1.09, [0.95 ; 1.23] | p < 0.001 | |
| SD | 1.20 | 1.15 | 1.17 | ||
| Min | 0.00 | 0.00 | 0.00 | ||
| Max | 6.00 | 5.00 | 6.00 | ||
| Perceived disability and/or emotional distress | Yes: n, (%), [95%CI] |
794, (48.15%), [45.75 ; 50.56] |
252, (54,55%), [49.99 ; 59.03] |
105, (38.04%), [32.52 ; 43.90] |
p < 0.001 |
| No: n, (%), [95%CI] |
855, (51.85%), [49.44 ; 54.25] |
210, (45,45%), [40.97 ; 50.01] |
171, (61.96%), [56.10 ; 67.48] |
||
| Quality of life (seven-point Likert scale) | Mean, [95%CI] | 4.59, [4.53 ; 4.65] | 4.08, [3.96 ; 4.19] | 4.70, [4.55 ; 4.84] | p < 0.001 |
| SD | 1.25 | 1.27 | 1.19 | ||
| Min | 1.00 | 1.00 | 1.00 | ||
| Max | 7.00 | 7.00 | 7.00 | ||
| Health state satisfaction | Yes: n, (%), [95%CI] |
1080, (65.49%), [63.17 ; 67.75] |
214, (46.32%), [41.82 ; 50.88] |
206, (74.64%), [69.19 ; 79.41] |
p < 0.001 |
| No: n, (%), [95%CI] |
569, (34.51%), [32.25 ; 36.83] |
248, (53.68%), [49.12 ; 58.18] |
70, (25.36%), [20.59 ; 30.81] |
||
CAD: Canadian Dollars, DCS: Decisional Conflict Scale, 95%CI: 95% Confidence Interval
Difficult decisions
Table 2 describes the difficult decisions faced by the respondents. We classify difficult decisions according to four categories: consultations, diagnosis, treatment, and daily life. Responses rate of the 26 predefined difficult decisions ranged from 4.6% (n = 76) to 51.7% (n = 852). Of the 1,649 respondents, 1,595 (96.7%) reported having faced at least one difficult decision among the four categories.
Table 2.
Difficult decisions and the most difficult decision
| Healthcare decisions | n | (%) | [95%CI] |
|---|---|---|---|
| Difficult decisions regarding medical consultation (n = 1649, no missing data) | |||
| Do I need to consult a health care provider or a health service for my current need or not? | 727 | (44.09%) | [41.71 ; 46.49] |
| Which health care provider or health service is the best suited for my problem or condition? | 713 | (43.24%) | [40.87 ; 45.64] |
| Do I need to change the health care provider or to obtain a second opinion or not? | 423 | (25.65%) | [23.6 ; 27.81] |
| Do I need to go to the emergency department or not? | 397 | (24.08%) | [22.07 ; 26.20] |
| Should my health problem be handled by the health system or not? | 369 | (22.38%) | [20.43 ; 24.45] |
| Will my health care provider still want to follow me if I ask for a second opinion? | 317 | (19.22%) | [17.39 ; 21.20] |
| Do I have to ask for emergency assistance or not? | 243 | (14.74%) | [13.11 ; 16.53] |
| Other(s) | 5 | (0.30%) | [0.13 ; 0.71] |
| I have not made a difficult decision in medical consultation | 397 | (24.08%) | [22.07 ; 26.2] |
| Difficult decisions regarding diagnosis (n = 1649, no missing data) | |||
| Do I need more tests to find the cause of my pain? | 805 | (48.82%) | [46.41 ; 51.23] |
| Do I need medical imaging (e.g., X-Ray, MRI) for my condition or not? | 728 | (44.15%) | [41.77 ; 46.56] |
| Which therapist(s) should I trust for my diagnosis? | 621 | (37.66%) | [35.35 ; 40.02] |
| Other(s) | 18 | (1.09%) | [0.69 ; 1.72] |
| I have not made difficult decision | 422 | (25.59%) | [23.54 ; 27.75] |
| Difficult decisions regarding treatment (n = 1649, no missing data) | |||
| Which treatment or approach is the best suited for my problem or condition? | 816 | (49.48%) | [47.08 ; 51.90] |
| Should I adopt new lifestyle habits and behaviours or maintain the status quo? | 647 | (39.24%) | [36.91 ; 41.61] |
| Should I need to consult rehabilitation professionals (e.g., occupational therapist, physiotherapist)? | 525 | (31.84%) | [29.63 ; 34.13] |
| What elements of my condition should I prioritize? | 423 | (25.65%) | [23.60 ; 27.81] |
| Should I permanently end my treatment or pursue it? | 340 | (20.62%) | [18.74 ; 22.64] |
| Should I need to consult mental-health professionals (e.g., psychiatrist, psychologist, mental health counsellor, social workers)? | 320 | (19.41%) | [17.57 ; 21.38] |
| Should I choose the terms of an intervention plan/treatment or let health care professionals do it? | 301 | (18.25%) | [16.46 ; 20.19] |
| Do I accept to be involved in my chronic pain management? | 295 | (17.89%) | [16.12 ; 19.81] |
| Other(s) | 37 | (2.24%) | [1.63 ; 3.08] |
| I have not made difficult decision | 275 | (16.68%) | [14.96 ; 18.55] |
| Difficult decisions regarding daily life (n = 1649, no missing data) | |||
| Am I able to maintain daily activities or not? | 852 | (51.67%) | [49.25 ; 54.07] |
| Should I stop/reduce working or continue to work as usual? | 544 | (32.99%) | [30.76 ; 35.30] |
| Should I choose or maintain a social activity or not? | 410 | (24.86%) | [22.84 ; 27.01] |
| Should I tell my family and friends about my condition? | 310 | (18.80%) | [16.99 ; 20.76] |
| Should I organize my lifestyle to reduce the burden on my family? | 301 | (18.25%) | [16.46 ; 20.19] |
| Should I keep driving my car or not? | 170 | (10.31%) | [8.93 ; 11.87] |
| Should I accept a proposed service (other than health services) from a community organization or not? | 166 | (10.07%) | [8.71 ; 11.61] |
| Should I return to my country/ city/region of origin or stay here? | 76 | (4.61%) | [3.70 ; 5.73] |
| Other(s) | 24 | (1.46%) | [0.98 ; 2.16] |
| I have not made difficult decision | 397 | (24.08%) | [22.07 ; 26.20] |
| Most difficult decisions (n = 1649, no missing data) | |||
| Should I take medication or not? | 278 | (16.86%) | [15.13 ; 18.74] |
| Should I get surgery or not? | 252 | (15.28%) | [13.63 ; 17.10] |
| Should I change my lifestyle habits and behaviours? | 191 | (11.58%) | [10.13 ; 13.22] |
| Should I consult a complementary and alternative medicine professional (e.g., chiropractor, osteopath, naturopath, acupuncture) | 146 | (8.85%) | [7.58 ; 10.32] |
| Should I undergo more diagnostic tests? | 139 | (8.43%) | [7.18 ; 9.87] |
| Should I change my treatment? | 102 | (6.19%) | [5.12 ; 7.45] |
| Should I change the health care provider to manage my condition? | 93 | (5.64%) | [4.63 ; 6.86] |
| Should I stop my treatment? | 60 | (3.64%) | [2.84 ; 4.65] |
| Should I consult a rehabilitation professional? | 59 | (3.58%) | [2.78 ; 4.59] |
| Should I consult a mental-health professional? | 50 | (3.03%) | [2.31 ; 3.98] |
| Other(s) | 3 | (0.18%) | [0.06 ; 0.53] |
| I have not made difficult decision with a health care provider | 276 | (16.74%) | [15.01 ; 18.62] |
95%CI: 95% Confidence Interval
Medical consultation
From the 1,649 respondents, 79% (n = 1,302) had faced at least one difficult decision regarding medical consultation. Two difficult decisions showed higher 95% confidence interval (CI) than others: (1) “Do I need to consult a health care provider or a health service for my current need or not?” (44.1%, n = 727, 95%CI [41.7 ; 46.5]), and (2) “Which health care provider or health service is the best suited for my problem or condition?” (43.2%, n = 713, 95%CI [40.9 ; 45.6]).
Diagnosis
From the 1,649 respondents, 74.4% (n = 1,227) had faced at least one difficult decision regarding diagnosis. Two difficult decisions showed higher 95%CI than others: (1) “Do I need more tests to find the cause of my pain?” (48.8%, n = 805, 95%CI [46.4 ; 51.2]), and (2) “Do I need medical imaging (e.g., X-Ray, MRI) for my condition or not?” (44.2%, n = 728, 95%CI [41.8 ; 46.6]).
Treatment
From the 1,649 respondents, 83.3% (n = 1,374) had faced at least one difficult decision regarding treatment. One difficult decision showed higher 95%CI than others: 1) “Which treatment or approach is the best suited for my problem or condition?” (49.5%, n = 816, 95%CI [47.1 ; 51.9]).
Daily life
From the 1,649 respondents, 75.9% (n = 1,252) had faced at least one difficult decision regarding daily life. One difficult decision showed higher 95%CI than others: 1) “Am I able to maintain daily activities or not?” (51.7%, n = 852, 95%CI [49.3 ; 54.1]).
New difficult decisions
From the open-ended response option, 10 new themes were identified (Supplementary Material 2): (1) access to HCPs and diagnostic tests, (2) trust regarding HCPs, diagnostic test results and treatment options, (3) knowledge issues and implicit bias from patients and HCPs, (4) financial issues, (5) options consequences, (6) resignation and suicidal ideation, (7) pacing, (8) returning or reorganizing work, (9) adaptation of responders’ living environment, and (10) integrative care.
Most difficult decisions
Of the 1,649 respondents, 16.7% (n = 276) reported they had no most difficult decision with a HCP. Two most difficult decisions showed higher 95%CI than others: (1) “Should I take medication or not?” (16.9%, n = 278, 95%CI [15.1 ; 18.7]), and (2) “Should I get surgery or not?” (15.3%, n = 252, 95%IC [13.6 ; 17.1]).
HCP(s) with whom the most difficult decision was made
From the 1,373 respondents who were able to identify a single most difficult decision, 48.8% (n = 670) had made the most difficult decision with a primary care physician, 16.1% (n = 221) with a medical specialist (e.g., rheumatologist, pain specialist), 10.3% (n = 142) with a rehabilitation professional, 9.8% (n = 135) with a surgeon, 6.9% (n = 95) with a complementary and alternative medicine professional, and 6.2% (n = 85) with a primary care professional (e.g., nurse, pharmacist). From the open-ended response option (1.5%, n = 21), respondents reported not having consulted a HCP for this decision, or having made this most difficult decision by themselves, or having made this most difficult decision with multiple healthcare providers.
Decisional conflict
Table 3 provides details on the DCS total score and subscales. Respondents who made difficult decisions (n = 1,373, 83.3%) had an average decisional conflict score of 30.8 (SD = 17.0). The highest subscale score was for the “uncertainty” subscale with an average score of 36.3 (SD = 21.3). Of the 1,373 respondents with a most difficult decision, 69.3% (n = 952) displayed decisional conflict (i.e., DCS total score ≥ 25) and 33.7% (n = 462) had a CSDC (i.e., DCS total score ≥ 37.5).
Table 3.
Decisional Conflict Scale scores among respondents
| Decisional Conflict Scale | People with a most difficult decision (n = 1373) | People with DCS score ≥ 37.5 (n = 462) |
|
|---|---|---|---|
| Total score | Mean [95%CI] | 30.76 [29.86 ; 31.66] | |
| SD | 17.02 | ||
| Min | 0.00 | ||
| Max | 93.75 | ||
| Nb ≥ 25 | 952 | ||
| % ≥25 | 69.34% | ||
| Nb ≥ 37.5 | 462 | ||
| % ≥ 37.5 | 33.65% | ||
| Informed subscale score | Mean [95%CI] | 30.38 [29.37 ; 31.40] | 49.12 [47.68 ; 50.55] |
| SD | 19.24 | 15.69 | |
| Min | 0.00 | 0.00 | |
| Max | 100.00 | 100.00 | |
| Values clarity subscale score | Mean [95%CI] | 28.51 [27.54 ; 29.49] | 46.16 [44.76 ; 47.55] |
| SD | 18.42 | 15.27 | |
| Min | 0 | 0.00 | |
| Max | 100 | 100.00 | |
| Support subscale score | Mean [95%CI] | 30.92 [29.88 ; 31.96] | 50.29 [48.90 ; 51.68] |
| SD | 19.64 | 15.20 | |
| Min | 0.00 | 16.67 | |
| Max | 100.00 | 100.00 | |
| Uncertainty subscale score | Mean [95%CI] | 36.28 [35.15 ; 37.40] | 56.67 [55.32 ; 58.02] |
| SD | 21.28 | 14.76 | |
| Min | 0.00 | 16.67 | |
| Max | 100.00 | 100.00 | |
| Effective decision subscale score | Mean [95%CI] | 28.48 [27.52 ; 29.44] | 46.55 [45.36 ; 47.74] |
| SD | 18.11 | 12.97 | |
| Min | 0.00 | 6.25 | |
| Max | 100.00 | 100.00 | |
| Comparison between | |||
| Informed and Values clarity subscales | p < 0.001 | p < 0.001 | |
| Informed ans Support subscales | p = 0.18 | p = 0.06 | |
| Informed and Uncertainty subscales | p < 0.001 | p < 0.001 | |
| Values clarity and Support subscales | p < 0.001 | p < 0.001 | |
| Values clarity and Uncertainty subscales | p < 0.001 | p < 0.001 | |
| Uncertainty and Support subscales | p < 0.001 | p < 0.001 | |
%: percentage, DCS: Decisional Conflict Scale, Nb: number of respondents with a DCS total score, SD: Standard Deviation, 95%CI: 95% Confidence Interval
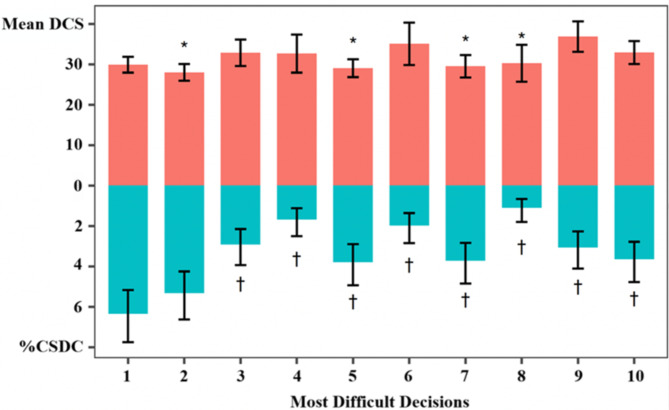
Figure 2 presents mean DCS total score (upper part of the figure) and percentage of CSDC (lower part of the figure) for each most difficult decision. When compared to the most difficult decision with the highest total DCS score (i.e., “Should I change the health care provider to manage my condition?”), four of the most difficult decisions had a statistically significant lower DCS score. When compared to the most difficult decision with the higher percentage of CSDC (i.e., “Should I take medication or not?”), eight of the most difficult decisions had a statistically significant lower percentage of CSDC.
Fig. 2.
Mean Decisional Conflict Scale total score (upper part of the figure, in salmon) and percentage of Clinically Significant Decisional Conflict (lower part of the figure, in blue) for each most difficult decision with 95% confidence interval. 1: Should I take medication or not?; 2: Should I get surgery or not?; 3: Should I change my treatment?; 4: Should I stop my treatment?; 5: Should I change my lifestyle habits and behaviours?; 6: Should I consult a rehabilitation professional?; 7: Should I consult a complementary and alternative medicine professional?; 8: Should I consult a mental-health professional?; 9: Should I change the health care provider to manage my condition?; 10: Should I undergo more diagnostic tests?. *statistically significantly lower than reference (i.e., most difficult decision #9). †statistically significantly lower than reference (i.e., most difficult decision #1). %CSDC: Percentage of Clinically Significant Decisional Conflict, DCS: Decisional Conflict Scale
Congruence between assumed and preferred role in the decision-making process
From the 1,373 respondents with a difficult decision, 27.0% (n = 371) reported having an active role in the decision-making process (i.e., response A of the CPS), 68.0% (n = 934) reported having a collaborative role (i.e., responses B, C, and D of the CPS), and 5.0% (n = 68) reported having a passive role (i.e., response E of the CPS). If they would make the same difficult decision in the future, 20.8% (n = 285) would prefer an active role, 75.7% (n = 1,040) would prefer a collaborative role, and 3.5% (n = 48) would prefer a passive role in the decision-making process. Of the 1,373 respondents with a difficult decision, 685 (49.9%) had an assumed role that was congruent with their preferred role (n = 192 for active role, n = 241 for collaborative role, and n = 10 for passive role).
Discussion
In this national survey, we identified multiple difficult decisions that people living with chronic pain in Canada experience in their care and in their life. The level of decisional conflict associated with these difficult decisions was high with a third of respondents experiencing CSDC. Our respondents expressed a preference for a collaborative role, which could highlight SDM as a potential solution to reduce decisional conflict.
During the decision-making process for the most difficult decision, half of the respondents assumed a role that was not congruent with their preferred role. This finding suggests that HCPs are not discussing with patients about the role and the information needs in the decision-making process. A patient with unmet information needs may report dissatisfaction [42], while a patient receiving unwanted information may experience emotional distress [42], both of which can negatively affect the quality of care. Interestingly, most people who prefer a passive (e.g., paternalistic) role want to be informed about their condition [42]. This central role of information exchange may partly explain why 75.7% of the respondents wanted a collaborative role. Determining role preference and information needs is part of several SDM models [43]. The implementation of SDM in chronic pain care can meet decisional role and information needs. This is in line with current health policy in Canada to implement person-centered pain care [44, 45]. Although HCPs have positive attitudes towards SDM [46–48], SDM remains poorly implemented [49], with several barriers directly reported by HCPs (time constraints, lack of training) [50, 51]. Involving HCPs in the development phase of SDM interventions is crucial to consider their experiential knowledge and ultimately to co-identify strategies that can facilitate its implementation.
People living with chronic pain in Canada experience a high level of decisional conflict. Thompson-Leduc et al. found a prevalence of decisional conflict ranging from 10.3 to 31.1% using a cutoff score of 25 in general consultation in primary care [11]. Using this threshold, we found that 69.3% of our sample experienced decisional conflict. Half of the respondents made their most difficult decision with a primary care physician. Implementing SDM in primary care could improve the patient-HCP relationship [52], leading to a potential reduction in decisional conflict [53]. From the DCS, we found that uncertainty may be a relevant target for SDM interventions, as this subscale appears to drive higher decisional conflict scores. We need a better understanding of what people want to know before making a decision (i.e., decisional needs) and which decisional needs contribute to decisional conflict. We could target these factors through the development of SDM interventions to support primary care.
Our decisional needs assessment found that over 96% of respondents faced very diverse difficult decisions throughout their care pathways, from diagnosis to daily life modifications. All the 26 pre-defined difficult decisions in the four categories (i.e., medical consultation, diagnosis, treatment, and daily living) were endorsed by respondents. This diversity of difficult decisions is consistent with two studies conducted in Canada on difficult decisions in the general population and in people with complex care needs [27, 54]. Our findings and those of these two studies highlight that decision-making process requires a larger support than current SDM interventions which focus in most cases on treatment choices. Unmet decisional needs on diagnostic decisions could affect the entire care pathway [18]. If HCPs and patients disagree about the nature and meaning of chronic pain, decision-making process on treatment options could be conflictual due to diverging preferences [18]. Therefore, SDM interventions need to evolve [7]. Patient decision aids are relevant to matching preferences within SDM [7] (e.g., comparing and matching the characteristics of available treatment options with the patient’s values and preferences), but may be less helpful to conflict reconciliation [7] (e.g., understanding the patient’s reasons for wanting an MRI scan and reconciling them with the different options). We believe it is important to propose innovative interventions that can adapt to the wide diversity and sheer number of possible decisions made throughout the care pathway.
Individual characteristics may also contribute to decisional conflict. We found several characteristics that were significantly different in people who experienced CSDC. These people were younger, more likely to be female and women, and had more painful body regions. Some of these individual characteristics are not consistent with research in other contexts [11, 55–57]. Thompson-Leduc et al. found that being older was associated with greater decisional conflict, whereas sex was not, among Canadians from the province of Quebec who consulted primary care physicians [11]. We need to explore individual characteristics associated with decisional conflict to develop personalized SDM interventions that can adapt to the patient’s own situation.
Strengths and limitations
In this national survey, we were able to recruit a diverse sample of people living with chronic pain in Canada which improves the representativeness of our results. The diversity of our steering committee (researchers, HCPs and patient partners) reduced interpretation bias. These strengths allow generalizability of our findings to adults living with chronic pain in the 10 Canadian provinces and who have Internet access.
A limitation of our study is that people living in Canadian territories are underrepresented in our survey as they could not be reached in this study. Regarding Internet access, 94% of the Canadian population reported having an Internet access at home in a national census [58]. These limitations reduce the generalizability of our findings for hard-to-reach individuals in Canada. Overall, we would value future studies using qualitative designs (e.g., interviews in person or over phone) to specifically target and collect data from underserved and hard-to-reach populations that most likely experience significant barriers to making decisions about their pain care. A second limitation is that our results highlight the prevalence of difficult decisions in daily life. However, the response options for the most difficult decision were mainly focused on medical consultation, diagnosis, and treatment. Despite the open-ended response options, we may have unintentionally biased our respondents’ choice. This limitation could have underestimated the prevalence of the most difficult decisions and could have impacted our findings on the decisional conflict. Future data collection should include more response options for difficult decisions in daily life.
Conclusion
This national cross-sectional survey found that 96% of people living with chronic pain in Canada faced at least one difficult decision about pain care across their care pathways from diagnosis to daily living modifications. Most of these difficult decisions were made in primary care and resulted in a high level of CSDC. Our results highlight that people living with chronic pain in Canada have unmet decisional needs and need support to make optimal decisions to manage their chronic pain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Samuel Lemaire-Paquette and Catherine Allard, biostatisticians, for their support in this article. We also thank the Canadians Institutes of Health Research for their financial support.
Abbreviations
- CI
Confidence Interval
- CPS
Control Preferences Scale
- CSDC
Clinically Significant Decisional Conflict
- DCS
Decisional Conflict Scale
- HCPs
Health care providers
- SD
Standard deviation
- SDM
Shared Decision-Making
Author contributions
Concept/idea/research design: FN, YTL, SD, FL.Writing: FN, YTL, SD.Data analysis: FN, CC, YTL, SD.Project management: FN, SD.Fund procurement: SD, FL, YTL, AL, JSP, KTA.Consultation (including review of manuscript before submitting): FN, FL, CC, TG, KTA, MS, JSP, AL, IG, MEP, LCL, AMH, MDP, YTL, SD.
Funding
This work is supported by the Canadian Institutes of Health Research. The funder did not have any specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
We obtained the ethics approval from the Research Ethics Board of the Research Centre at the Centre Hospitalier Universitaire de Sherbrooke (project #2022–4645). We conducted the survey following the Canadian Personal Information Protection and Electronic Documents Act and the Marketing Research and Intelligence Association’s Charter of Respondent Rights. Respondents consented to participate in the study. All respondents had to give their consent before their participation and could request to be removed from the study at any time. Respondents also had to consent to Leger Marketing’s terms of use and privacy policy.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–97. [DOI] [PubMed] [Google Scholar]
- 2.Heen AF, Vandvik PO, Brandt L, Montori VM, Lytvyn L, Guyatt G, et al. A framework for practical issues was developed to inform shared decision-making tools and clinical guidelines. J Clin Epidemiol. 2021;129:104–13. [DOI] [PubMed] [Google Scholar]
- 3.Matthias MS, Talib TL, Huffman MA. Managing Chronic Pain in an Opioid Crisis: what is the Role of Shared Decision-Making? Health Commun. 2020;35(10):1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, et al. Opioids for chronic Noncancer Pain: a systematic review and Meta-analysis. JAMA. 2018;320(23):2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Horst DEM, Garvelink MM, Bos WJW, Stiggelbout AM, Pieterse AH. For which decisions is Shared decision making considered appropriate? - a systematic review. Patient Educ Couns. 2022. [DOI] [PubMed]
- 6.Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ. 1999;319(7212):780–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montori VM, Ruissen MM, Hargraves IG, Brito JP, Kunneman M. Shared decision-making as a method of care. BMJ Evid Based Med. 2023;28(4):213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacey D, Légaré F, Boland L, Lewis KB, Loiselle MC, Hoefel L, et al. 20th anniversary Ottawa decision support Framework: part 3 overview of systematic reviews and updated Framework. Med Decis Mak. 2020;40(3):379–98. [DOI] [PubMed] [Google Scholar]
- 9.Hoefel L, O’Connor AM, Lewis KB, Boland L, Sikora L, Hu J, et al. 20th anniversary update of the Ottawa decision support Framework Part 1: a systematic review of the Decisional needs of people making Health or Social decisions. Med Decis Mak. 2020;40(5):555–81. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 11.Thompson-Leduc P, Turcotte S, Labrecque M, Légaré F. Prevalence of clinically significant decisional conflict: an analysis of five studies on decision-making in primary care. BMJ Open. 2016;6(6):e011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Canada. Working together to better understand, prevent, and manage chronic pain: what we heard2020 2020.
- 13.Finley CR, Chan DS, Garrison S, Korownyk C, Kolber MR, Campbell S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018;64(11):832–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. [DOI] [PubMed] [Google Scholar]
- 15.Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag. 2011;16(6):445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen E, Nayfe R, Milburn N, Mayo H, Reid MC, Fraenkel L, et al. Do decision Aids Benefit patients with Chronic Musculoskeletal Pain? A systematic review. Pain Med. 2020;21(5):951–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korownyk CS, Montgomery L, Young J, Moore S, Singer AG, MacDougall P, et al. PEER simplified chronic pain guideline: management of chronic low back, osteoarthritic, and neuropathic pain in primary care. Can Fam Physician. 2022;68(3):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthias MS, Henry SG. Reducing frustration and improving management of Chronic Pain in Primary Care: Is Shared decision-making sufficient? J Gen Intern Med. 2022;37(1):227–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Canada. An action plan for pain in Canada2021 2021.
- 20.Naye F, Légaré F, Paquette JS, Tousignant-Laflamme Y, LeBlanc A, Gaboury I, et al. Decisional needs assessment for patient-centred pain care in Canada: the DECIDE-PAIN study protocol. BMJ Open. 2023;13(5):e066189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A Consensus-based checklist for reporting of Survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37. [DOI] [PubMed] [Google Scholar]
- 23.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of Chronic Pain for the International classification of diseases (ICD-11). Pain. 2019;160(1):19–27. [DOI] [PubMed] [Google Scholar]
- 24.Bennett MI, Kaasa S, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain. 2019;160(1):38–44. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Canada. Population and demography statistics 2022. https://www.statcan.gc.ca/en/subjects-start/population_and_demography
- 26.O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- 27.Poitras ME, Hudon C, Godbout I, Bujold M, Pluye P, Vaillancourt VT, et al. Decisional needs assessment of patients with complex care needs in primary care. J Eval Clin Pract. 2020;26(2):489–502. [DOI] [PubMed] [Google Scholar]
- 28.Garvelink MM, Boland L, Klein K, Nguyen DV, Menear M, Bekker HL, et al. Decisional Conflict Scale findings among patients and surrogates making Health decisions: part II of an anniversary review. Med Decis Mak. 2019;39(4):315–26. [DOI] [PubMed] [Google Scholar]
- 29.Knops AM, Goossens A, Ubbink DT, Legemate DA, Stalpers LJ, Bossuyt PM. Interpreting patient decisional conflict scores: behavior and emotions in decisions about treatment. Med Decis Mak. 2013;33(1):78–84. [DOI] [PubMed] [Google Scholar]
- 30.Degner LF, Sloan JA, Venkatesh P. The Control preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 31.Santesso N, Akl E, Bhandari M, Busse JW, Cook DJ, Greenhalgh T, et al. A practical guide for using a survey about attitudes and behaviors to inform health care decisions. J Clin Epidemiol. 2020;128:93–100. [DOI] [PubMed] [Google Scholar]
- 32.McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess. 2001;5(31):1–256. [DOI] [PubMed] [Google Scholar]
- 33.Personal Information Protection and Electronic Documents Act. (2000).
- 34.Charter of Respondents Rights. (2006).
- 35.Marketing L. Privacy Notice 2023. https://leo.tech/privacy-statement/
- 36.Riley RD, Ensor J, Snell KIE, Harrell FE Jr., Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. [DOI] [PubMed] [Google Scholar]
- 37.Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bethlehem J, Schouten B. Nonresponse Error: Detection and Correction. In: Sage, editor. The SAGE Handbook of Survey Methodology2016.
- 39.Baillie M, le Cessie S, Schmidt CO, Lusa L, Huebner M. Ten simple rules for initial data analysis. PLoS Comput Biol. 2022;18(2):e1009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 41.Landry JT. Current models of shared decision-making are insufficient: the professionally-driven zone of patient or surrogate discretion offers a defensible way forward. Patient Educ Couns. 2023;115:107892. [DOI] [PubMed] [Google Scholar]
- 42.Kiesler DJ, Auerbach SM. Optimal matches of patient preferences for information, decision-making and interpersonal behavior: evidence, models and interventions. Patient Educ Couns. 2006;61(3):319–41. [DOI] [PubMed] [Google Scholar]
- 43.Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9(12):e031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canadian Institutes of Health Research. Canadian Institutes of Health Research-Institute of Musculoskeletal Health and Arthritis Strategic Plan 2021–2026 2021.
- 45.Ministère de la Santé et des Services Sociaux du Québec. Continuum de soins et de services en douleur chronique - Orientations et lignes directrices 2021–2026 2021. https://publications.msss.gouv.qc.ca/msss/document-003204/
- 46.Hoffmann T, Gibson E, Barnett C, Maher C. Shared decision making in Australian physiotherapy practice: a survey of knowledge, attitudes, and self-reported use. PLoS ONE. 2021;16(5):e0251347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathijssen EGE, van den Bemt BJF, Wielsma S, van den Hoogen FHJ, Vriezekolk JE. Exploring healthcare professionals’ knowledge, attitudes and experiences of shared decision making in rheumatology. RMD Open. 2020;6(1). [DOI] [PMC free article] [PubMed]
- 48.Yen RW, Barr PJ, Cochran N, Aarts JW, Légaré F, Reed M, et al. Medical students’ knowledge and attitudes toward Shared decision making: results from a Multinational, cross-sectional survey. MDM Policy Pract. 2019;4(2):2381468319885871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couët N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18(4):542–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison T, Foster E, Dougherty J, Barton J. Shared decision making in rheumatology: a scoping review. Semin Arthritis Rheum. 2022;56:152041. [DOI] [PubMed] [Google Scholar]
- 51.Tang C, Wang A, Yan J. Exploring motivations and resistances for implementing shared decision-making in clinical practice: a systematic review based on a structure-process-outcome model. Health Expect. 2022;25(4):1254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nannenga MR, Montori VM, Weymiller AJ, Smith SA, Christianson TJ, Bryant SC, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The statin choice randomized trial. Health Expect. 2009;12(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauqeer F, Moen A, Myhr K, Wilson CA, Lupattelli A. Assessing decisional conflict and challenges in decision-making among perinatal women using or considering using antidepressants during pregnancy-a mixed-methods study. Arch Womens Ment Health. 2023;26(5):669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor AM, Drake ER, Wells GA, Tugwell P, Laupacis A, Elmslie T. A survey of the decision-making needs of canadians faced with complex health decisions. Health Expect. 2003;6(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goh ZZS, Chia JMX, Seow TY, Choo JCJ, Foo M, Seow PS, et al. Treatment-related decisional conflict in pre-dialysis chronic kidney disease patients in Singapore: prevalence and determinants. Br J Health Psychol. 2022;27(3):844–60. [DOI] [PubMed] [Google Scholar]
- 56.Lee YH, Chou XY, Lai YH, Liang YH, Hung CT, Hsaio CC, et al. Decisional conflict and its determinants among patients with cancer undergoing immunotherapy combined with chemotherapy or targeted therapy: a cross-sectional study. Sci Rep. 2023;13(1):12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wamkpah NS, Gerndt SP, Kallogjeri D, Piccirillo JF, Chi JJ. Patients’ views of Shared decision-making and Decisional conflict in otolaryngologic surgery during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2021;147(10):879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Statistics Canada. Acces to the Internet in Canada. 2020 2021. https://www150.statcan.gc.ca/n1/en/daily-quotidien/210531/dq210531d-eng.pdf?st=thuP_y8m
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.