Abstract
Background
Guillain-Barré syndrome (GBS) is an autoimmune disease that affects the peripheral nervous system leading to motor, sensory, and sometimes autonomic manifestations. Therapeutic plasma exchange (TPE), which involves the selective removal of pathological molecules, such as auto-antibodies, from plasma, has proven to be safe and effective in adults with GBS. However, its application in pediatric patients lacks sufficient evidence. This study aims to evaluate the efficacy and safety of TPE in pediatric patients with severe GBS, in a low-resource setting.
Methods
This is a single-center retrospective study of 36 GBS patients aged between 2 and 13 years. A total of 122 TPE sessions were administered, with a median of four sessions per patient. A human albumin solution was the exchange fluid in all the sessions. Clinical improvement was evaluated through general examination and muscle power assessment using the Medical Research Council (MRC) scale.
Results
All patients showed clinical improvement upon treatment with TPE. The grade of power in the upper extremities increased from a mean of 1.7 ± 1.1 at the peak of illness to 3.7 ± 0.9 at discharge, indicating an increase of 2.0 ± 1.1 (95% CI, 1.6 to 2.4, p< 0.001). Alternatively, in the lower extremities, it increased from 1.2 ± 1.1 to 2.5 ± 0.8, indicating a significant rise of 1.4 ± 0.8 (95% CI, 1.1 to 1.6, p< 0.001). There was a significant improvement in the cranial, autonomic, and respiratory functions among all patients. Half of the patients were available for follow-up and showed full recovery, with six of them still exhibiting minimal residual deficits. TPE-related complications were mostly mild or moderate, with tachycardia, hypotension, and mild anemia being the most common. However, serious complications occurred in three of the patients, necessitating the discontinuation of the treatment in two of them. There was no mortality related to TPE in this study.
Conclusions
TPE shows promise in treating pediatric GBS. In this study, TPE was associated with the recovery of neurological functions, yielding positive outcomes with only minimal residual deficits. However, balancing its benefits with potential risks requires careful clinical judgment and rigorous monitoring to ensure patient safety and optimize outcomes. TPE was a more cost-effective and accessible option than IVIG in this financially restricted, low-income setting.
Keywords: Guillain-Barré syndrome, Therapeutic plasma exchange, Pediatric patients, Efficacy, Safety, Plasmapheresis
Background
Guillain-Barré syndrome (GBS) is an autoimmune neurological disorder where the nervous system is attacked by the immune system. Molecular mimicry is thought to be the leading hypothesis to explain such cross interactions; however, the exact mechanism and pathophysiology are still scarce. With poliomyelitis widely eradicated, GBS is considered the most common cause of flaccid paralysis in infants and children. The incidence among children under the age of 15 ranges from 0.34 to 1.34 per 100,000. It typically occurs after a viral or bacterial infection with microorganisms, such as Campylobacter jejuni and Epstein-Barr virus [1–6].
The disorder manifests as an acute, progressive, symmetrical, usually ascending flaccid paralysis accompanied by weak or absent tendon reflexes. Additionally, diffuse pain is a common symptom among GBS patients, which can sometimes delay the accurate diagnosis [4, 5]. Other manifestations of GBS may include signs of autonomic dysfunction such as cardiac arrhythmia and blood pressure fluctuations. Cranial nerves involvement is fairly common, presenting as bulbar weakness characterized by dysphagia, difficulty handling secretions, sialorrhea, hoarseness, and dysphonia [7]. Most patients require hospitalization, and up to 30% of them may require invasive ventilation at some point [8].
The clinical progression of GBS follows three distinct phases: the acute phase, which ranges from hours to weeks and is characterized by the onset and development of symptoms; the plateau phase, with varying duration, during which symptoms cease to progress; and the recovery phase, which spans from months to years [4, 6]. GBS was long thought to be a single disorder, but later investigations showed that GBS consists of several phenotypically similar disorders depending on the target affected by the inflammatory response. These variants include acute inflammatory demyelinating polyradiculoneuropathy (AIDP), where the myelin sheath is affected; acute motor sensory axonal neuropathy (AMSAN); and acute motor axonal neuropathy (AMAN), where the axon itself is affected rather than the myelin sheath. Additionally, Miller Fisher syndrome (MFS) is another subtype of GBS. It presents itself as rapidly evolving ataxia, areflexia, and ophthalmoplegia, usually with little or no weakness [4, 7, 9].
Therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIG) are two treatment options that have been recommended by the American Academy of Neurology [10] for treating GBS patients. TPE is the process by which pathological molecules, such as antibodies and immune complexes, are selectively removed from the plasma [11–13]. It has been found to be a safe and effective treatment for various neuroimmunological diseases, including GBS, and has proven to be superior compared to supportive care alone. This was well established in adults with GBS, based on moderate quality evidence in the Cochran’s Database of Systemic Reviews [14], as well as in many previous studies [15–20]. Furthermore, the American Society for Apheresis (ASFA) strongly recommends the use of TPE as a primary treatment for GBS (category I Grade 1A) [21]. Conversely, IVIG is acknowledged as an effective first-line treatment for GBS, with some studies suggesting comparable efficacy to TPE [22–25]. Some studies even advocate for IVIG over TPE due to its enhanced convenience, ease of administration, and, at times, better outcomes, particularly seen in pediatric patients [3, 10, 18, 19, 26–28].
However, the use of TPE in pediatric cases is often based on experience extrapolated from adult clinical practice, lacking randomized controlled trials assessing its safety and efficacy in this demographic [12, 18, 19, 29–31]. Moreover, the application of TPE in pediatric patients presents distinct challenges due to their smaller size, lower blood volume, higher incidence of adverse events, lack of cooperation, and more frequent technical problems concerning vascular access [12, 18, 30, 31]. Lastly, managing GBS in low-income countries presents numerous challenges and obstacles, including limited availability of treatment options and specialized diagnostic procedures, as well as the potential for uncommon complications related to GBS or TPE.
This article presents findings regarding the safety and efficacy of TPE in pediatric patients diagnosed with GBS, in a low-income setting.
Methods
Patients and study population
This retrospective study was conducted by reviewing past records of 36 patients diagnosed with GBS at the Children’s University Hospital in Damascus, Syria, admitted between March 2015 and March 2017. During that time, Syria was classified as a low-income country due to the ongoing civil war and economic restrictions. Each patient demonstrated an acute onset of progressive, symmetric flaccid paralysis with hypo- or areflexia. Diagnosis of GBS relied on clinical observations and was subsequently validated through cerebrospinal fluid (CSF) analysis and electrophysical studies, after ensuring the absence of poliomyelitis through culture confirmation.
The choice of treatment at the hospital during the study period-whether IVIG, TPE, or supportive care-was influenced by the availability of IVIG and the financial situation of the patient’s family (i.e., whether they could afford IVIG). IVIG was the first-line treatment for pediatric GBS in our hospital. Patients who could not afford IVIG were treated with TPE. Notably, no patient diagnosed with GBS during this period received only supportive care, even those with mild symptoms. This selection process may introduce a potential socioeconomic bias in the study.
TPE procedure
A total of 122 TPE sessions were performed, with a minimum of one and a maximum of five sessions per patient, all of which were conducted on Spectra Optia apheresis machine (Manufacturer TERUMO BCT). The TPE kit was primed with 4% human albumin (H.A) solution; with normal saline solution (NS); or with screened, group specific, and cross-matched packed red blood cells diluted with saline; depending on the hematological status of the patient. Nadler’s formula [13, 30, 32] was used to calculate each patient’s whole blood volume. The exchange fluid used during each session consisted of 4% H.A solution in NS. Units of fresh frozen plasma (FFP) were infused during TPE sessions if the patient had developed coagulation factors deficiency. Additional normal saline solution was infused to patients who developed hypotension during the procedure. On average, 0.7–1.4 times of plasma volume (PV) was exchanged (Table 1).
Table 1.
The amount of plasma volume removed and exchanged, as well as the amount of ACD used for each group of patients during the plasmapheresis procedures
| Age (Years) | Number of patients | PV removed | PV exchanged | ACD used |
|---|---|---|---|---|
| 2–4 | 3 | 838.3 | 758.6 | 123.3 |
| 5–7 | 10 | 988.5 | 871.8 | 157.8 |
| 8–10 | 11 | 1316.9 | 1160.06 | 212.4 |
| 11–13 | 9 | 1660.9 | 1460.7 | 284.4 |
PV plasma volume, ACD acid-citrate dextrose
The rate of blood flow ranged from 10 to 55 ml/minute, contingent upon the patient’s weight and blood volume. The duration of each procedure varied from 26 to 136 minutes, with a mean of 89.3 ± 35.0 minutes, depending upon the amount of plasma exchange. Acid citrate dextrose (ACD) was used as the anticoagulant. ACD to whole blood ratio was maintained between 1:12 and 1:15. To prevent citrate toxicity, intravenous dextrose plus calcium glocunate were administered at a dose of one ml/kg each. Vascular access was established using either a peripheral needle or a central vain catheter, the choice depended on the patient’s clinical condition.
Throughout each session, pulse rate, respiratory rate, blood pressure, and blood oxygen saturation were closely monitored. Any adverse events occurring during or after each session were documented and subjected to analysis. Whole blood count, renal function tests, coagulation tests (including prothrombin time (PT) and activated partial thromboplastin time (aPTT)) were assessed before and after each session, along with blood potassium, sodium, and calcium concentrations.
The evaluation of clinical improvement was based on neurological, respiratory, and autonomic examination. Additionally, muscle power was evaluated using the Medical Research Council (MRC) scale (Table 2) [33]. A comprehensive comparison of the findings was conducted at three distinct time points in the disease progression: upon admission, at the peak of illness, and upon discharge. The “peak of illness” was defined as the stage characterized by the most severe status of neurological and respiratory signs and symptoms.
Table 2.
The Medical Research Council scaling system used for grading the muscle power in the extremities
| Muscle power grade | Description |
|---|---|
| 0 | No contraction |
| 1 | Flicker or trace contraction |
| 2 | Active movement, with gravity eliminated |
| 3 | Active movement against gravity |
| 4 | Active movement against gravity and resistance |
| 5 | Normal power |
However, due to substantial variations in hospitalization duration among patients, clinical findings at discharge may not accurately reflect the impact of treatment on the rate of clinical improvement. To address this, a comprehensive time-dependent evaluation of patients’ symptoms and health status was conducted. This was done by measuring the time required to restore normal functionality of different parts of the nervous system affected by GBS. The time intervals between the onset of symptoms and the return to normal functions were determined for respiratory; cranial nerves; reflexes in the upper and lower extremities; and autonomic functions.
Statistical analysis
Demographic and clinical data were subjected to descriptive statistics, incorporating measures such as (mean ± 1 standard deviation (SD)), median, range, and percentages. A two-tailed t-test for two samples, assuming unequal variances, was done to assess the mean duration of hospitalization in patients who required mechanical ventilation versus those who did not. Furthermore, a two-tailed paired t-test, with a 95% confidence interval (95% CI) was used to compare the muscle power grades before and after treatment. Statistical significance was attributed to differences with a p value less than 0.001. The entire data analysis process was carried out using Microsoft Excel for Windows, version 2016.
Results
Demographic and clinical characteristics
An overview of the demographic and clinical characteristics of the study cohort is provided in (Table 3). The study involved 36 patients, consisting of 20 boys (56%) and 16 girls (44%), with a male-to-female ratio of 1.25/1. The age range was 2 to 13 years, with a mean age of 8.3 ± 2.9 years and a median age of 8 years.
Table 3.
Demographic and clinical characteristics of the patients
| Parameter | n = 36 (%) |
|---|---|
| Age (years) | |
| Range | 2–13 |
| Mean ± SD | 8.3 ± 2.9 |
| Gender | |
| Male | 20 (56%) |
| Female | 16 (44%) |
| M/F ratio | 1.25/1 |
| GBS variant | |
| AMAN | 10 (28%) |
| AIDP | 1 (3%) |
| Mixed | 2 (6%) |
| Undefined | 23 (63%) |
| Treatment | |
| TPE only | 29 (81%) |
| IVIG and then TPE | 3 (8%) |
| TPE and then IVIG | 4 (11%) |
| Number of TPE sessions per patients | |
| 1 | 1 (3%) |
| 2 | 9 (25%) |
| 3 | 4 (11%) |
| 4 | 19 (53%) |
| 5 | 3 (8%) |
| Antecedent infection | |
| Upper respiratory infection | 19 (53%) |
| Gastrointestinal infection | 11 (31%) |
| Required invasive ventilation | |
| Yes | 15 (42%) |
| No | 21 (58%) |
Lumbar puncture was performed in all patients early after admission (within the first week of symptoms onset). CSF analysis revealed albuminocytologic dissociation in nine patients (25%), characterized by an elevated CSF protein concentration (>0.45 g/L), with a normal leukocyte count ( cells per mm). The remaining 75% exhibited normal CSF analytes. However, lumbar puncture was repeated in five of the remaining patients after the first week of symptoms onset, and CSF analysis in those patients showed albuminocytologic dissociation. Hence, in total, 14 patients (39%) exhibited this positive finding. Electromyography (EMG) was conducted in the majority of patients (31 patients). The results were consistent with GBS in all of them; however, a defined GBS variant was reported in only 13 patients. Of these, 10 patients had the AMAN variant, one patient had the AIDP variant, and two patients had a mixed variant of both AMAN and AIDP. EMG was not performed in the remaining five patients.
The time interval between the onset of neurological symptoms and hospital admission ranged from the same date of admission to 10 days, with a mean of 3.6 ± 2.2 days. Regarding antecedent infection, upper respiratory tract infections were the most common. It was reported in 19 patients (53%), preceding hospital admission by a mean of 9.5 ± 8.3 days. Gastrointestinal infections were reported in 11 patients (31%), occurring 12.2 ± 9.1 days before admission. Of the remaining six patients (16%), none exhibited any antecedent infection. However, one patient experienced fever preceding the onset of neurological symptoms, with no defined respiratory or gastrointestinal infection. Additionally, one patient had a previous diagnosis of GBS, and had undergone treatment with IVIG, resulting in complete recovery. Another patient had been diagnosed with autoimmune polyendocrine syndrome, characterized by multiple autoimmune disorders, such as type 1 diabetes mellitus, and autoimmune thyroiditis, among others.
Treatment regimens and procedures
All 36 patients received a total of 122 TPE sessions, with a median of four sessions per patient (range: 1–5). The time interval between consecutive sessions ranged from 1 to 4 days, with a median of 2 days. However, one session was performed after a 6-day gap due to the cancellation of the intervening session, attributed to hemolysis in the blood bag that was intended to be infused during that cancelled session. Plasmapheresis was initiated on an average of 6.1 ± 2.5 days after the onset of symptoms (range: 1 to 11 days, median: 6 days). TPE was the sole treatment for 29 patients; three patients received IVIG before TPE, with one patient dying from septic shock; and four patients received IVIG after TPE, with two transitioning from TPE to IVIG after two TPE sessions due to complications associated with plasmapheresis.
Clinical observations during hospitalization
The hospitalization duration varied widely among patients, ranging from 5 to 131 days, with a median of 15 days (38.5 ± 38.6 days) (Fig. 1). The time from symptoms onset to peak symptoms spanned 1 to 15 days (6.2 ± 3.2 days). During their hospital stay, 19 patients (53%) developed pneumonia, 10 patients (28%) had urinary tract infection, and seven patients (19%) had sepsis. A substantial majority, 22 patients (61%) were admitted to the pediatric intensive care unit (PICU), and their PICU stay ranged from 5 to 128 days (48.9 ± 43.1 days, median 37 days). Among the PICU admissions, 15 patients (42%) required invasive mechanical ventilation, with 12 of them (33%) undergoing tracheostomy. Ventilation-acquired pneumonia (VAP) was identified in 15 patients (42%). Throughout PICU stay, 15 patients (42%) developed lobar or lobular atelectasis, and 17 patients (47%) experienced acid-base disturbances due to respiratory failure, ventilator-driven hyperventilation, or serum electrolytes disturbances. These conditions necessitated additional medical and supportive care.
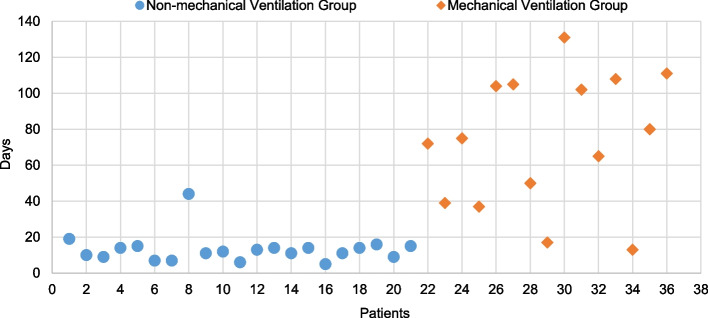
Fig. 1.
Duration of hospitalization (in days) for all 36 patients with GBS, categorized into two groups: those who required mechanical ventilation (n =15) and those who did not (n = 21). Patients in the mechanical ventilation group had significantly longer hospital stays with high variances, reflecting the increased severity of their condition
We compared the patients who required mechanical ventilation, to those who did not, in terms of the duration of hospitalization. The mean duration of hospitalization in the non-mechanical ventilation group was found to be significantly shorter than that for the mechanical ventilation group (13.1 ± 7.9 versus 73 ± 36.5 days, p< 0.001).
Disease course
Peripheral nerves examination
The detailed comparative analysis is presented in (Table 4). Generalized muscle pain emerged as a prominent symptom, affecting 20 patients (56%) upon admission, and escalating to 33 patients (92%) at the peak of illness. By the time of discharge, 19 patients (54%) continued to experience pain, but it was manageable with the aid of medications. Apart from pain, sensory involvement, manifested as numbness, was observed in only one patient (3%) on admission and at peak of illness, but resolved by the time of discharge.
Table 4.
A detailed comparison of the peripheral nerves examination findings between admission, peak and discharge
| Peripheral nerves examination | At admission no. total n = 36 | At peak no. total n = 36 | At discharge no. total n = 35a | |||
|---|---|---|---|---|---|---|
| Pain | 20 | 33 | 19 | |||
| Ability to walk | ||||||
| Unable to walk | 33 | 35 | 25 | |||
| Able to walk with assistance | 3 | 1 | 7 | |||
| Able to walk without assistance | 0 | 0 | 3 | |||
| Sensory involvement | 1 | 1 | 0 | |||
| Sphincters dysfunction | 4 | 5 | 0 | |||
| Power grading | U. limbs | L. limbs | U. limbs | L. limbs | U. limbs | L. limbs |
| 0/5 | 2 | 2 | 4 | 5 | 0 | 0 |
| 1/5 | 7 | 21 | 13 | 24 | 0 | 5 |
| 2/5 | 8 | 5 | 10 | 3 | 3 | 14 |
| 3/5 | 13 | 7 | 8 | 4 | 11 | 10 |
| 4/5 | 6 | 1 | 1 | 0 | 14 | 4 |
| 5/5 | 0 | 0 | 0 | 0 | 7 | 2 |
aExcluding the deceased patient
Upon admission, 33 patients (92%) were non-ambulatory, with three patients (8%) capable of walking only with assistance. At the peak of illness, the number of patients losing the ability to walk increased to 35 (97%), leaving one patient still able to walk with assistance. Following treatment and at discharge, three patients (9%) were able to walk independently, and seven patients (20%) regained the ability to walk with assistance; the remaining patients were still unable to walk.
Sphincters dysfunction was identified in four patients (11%) upon admission, leading to either incontinence or retention. At the peak of illness, an additional patient developed sphincters dysfunction, but all five patients regained normal sphincters function by the time of discharge after receiving treatment.
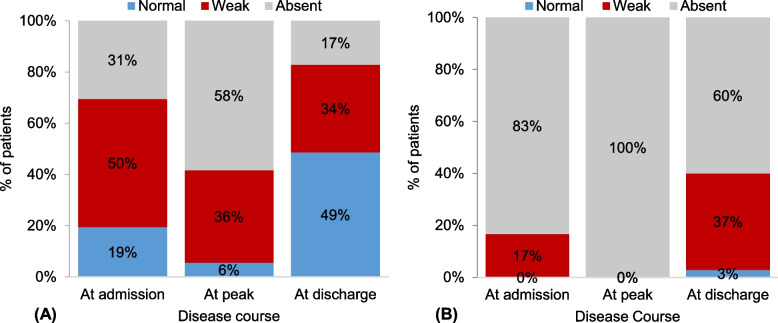
Neurological examination of the upper limbs revealed a significant improvement in upper limb reflexes upon discharge comparing to the peak. Specifically, at the peak of illness, 58% and 36% of patients demonstrated absent and weak reflexes, respectively (Fig. 2A). In contrast, at discharge, 49% of patients regained normal reflexes, with only 17% still experiencing absent reflexes. Conversely, the neurological manifestations of the disease exhibited more pronounced effects in the lower limbs, resulting in absent reflexes across all 36 patients at the peak of illness. At discharge, however, there was a notable shift, with reflexes returning to normal in only one patient (3%), and registering as weak in 13 patients (37%) (Fig. 2B).
Fig. 2.
Comparison in the reflexes of the extremities between admission, peak of illness, and discharge; A upper limbs; B lower limbs
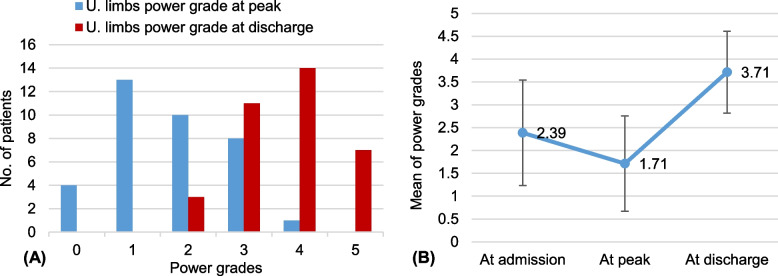
Furthermore, there was also a significant improvement in the grade of power in the upper extremities upon discharge. The grade of power increased from a mean of 1.7 ± 1.1 at the peak of illness to 3.7 ± 0.9 at discharge, indicating an increase of 2.0 ± 1.1 (95% CI, 1.6 to 2.4, p< 0.001) (Fig. 3).
Fig. 3.
The improvement in muscle power of the upper limbs. A Describes the power grades in all patients at discharge compared to peak; B Describes the improvement in the mean of power grades from admission to discharge. The error bars indicate one standard deviation
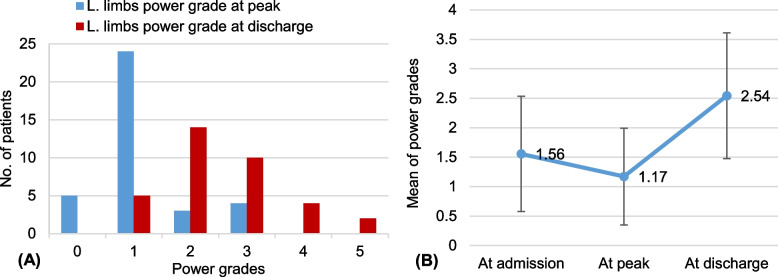
Simultaneously, the grade of power increased significantly, ascending from a mean of 1.2 ± 1.1 at the peak of illness to 2.5 ± 0.8 at discharge. This increase amounted to a statistically significant rise of 1.4 ± 0.8 (95% CI, 1.1 to 1.6, p< 0.001) (Fig. 4).
Fig. 4.
The improvement in muscle power of the lower limbs. A Describes the power grades in all patients at discharge compared to peak; B Describes the improvement in the mean of power grades from admission to discharge. The error bars indicate one standard deviation
Cranial nerve examination
Hoarseness and dysphagia emerged as the predominant cranial nerve dysfunctions on admission, affecting 42% and 36% of patients, respectively (Table 5). At the peak of illness, these percentages increased to 61% for hoarseness and 42% for dysphagia. Additionally, sialorrhea became a common symptom, observed in 36% of patients. While nystagmus, dysphonia, and facial palsy were also present, their occurrence was less frequent. Upon discharge, there was a significant improvement in cranial nerves function for the majority of patients. Only six patients (17%) did not achieve full recovery, with four patients (11%) still exhibiting hoarseness. Furthermore, gag reflex was absent in only four patients (11%) at the peak of illness, but returned to normal by the time of discharge.
Table 5.
Cranial nerve examination findings at admission, peak and discharge
| Cranial nerve examination | At admission no. (n = 36) | At peak no. (n = 36) | At discharge no. (n = 35a) |
|---|---|---|---|
| Dysphagia | 13 | 15 | 1 |
| Dysphonia | 3 | 6 | 1 |
| Nystagmus | 2 | 5 | 1 |
| Hoarseness | 15 | 22 | 4 |
| Sialorrhea | 6 | 13 | 0 |
| Facial Palsy | 0 | 1 | 1 |
| Gag reflex | |||
| Normal | 35 | 32 | 35 |
| Absent | 1 | 4 | 0 |
aExcluding the deceased patient
Autonomic findings
Upon admission to the hospital, autonomic dysfunction was observed in ten patients (28%), and this number increased to 17 patients (47%) at the peak of illness. Cardiac arrhythmias were the most common, affecting 15 patients (42%) at the peak of illness (Table 6). Additionally, hypertension (22%), urinary retention (19%), and hypotension (11%) were present at the peak of illness. Upon discharge, nearly all patients had regained normal autonomic functions, with only two individuals exhibiting autonomic dysfunction. One patient had hypertension, while the other experienced urinary retention.
Table 6.
Autonomic findings at admission, peak and discharge
| Autonomic dysfunction | At admission no. (n = 36) | At peak no. (n = 36) | At discharge no. (n = 35a) |
|---|---|---|---|
| Cardiac arrhythmias | 5 | 15 | 0 |
| Hypertension | 3 | 8 | 1 |
| Urinary retention | 4 | 7 | 1 |
| Hypotension | 1 | 4 | 0 |
aExcluding the deceased patient
Respiratory findings
Upon admission, respiratory muscles were affected in eight patients (22%) (Table 7). Five patients (14%) had dyspnea, and three patients (8%) had entirely lost the ability to breathe.
Table 7.
Respiratory findings at admission, peak and discharge
| Respiratory findings | At admission no. (n = 36) | At peak no. (n = 36) | At discharge no. (n = 35a) |
|---|---|---|---|
| Ability to breathe | |||
| Normal | 28 | 17 | 35b |
| Weak (dyspnea) | 5 | 7 | 0 |
| Inability to breathe | 3 | 13 | 0 |
| Cyanosis | 0 | 4 | 0 |
a Excluding the deceased patient
b Five of these patients were still experiencing mild dyspnea
Subsequently, during the peak of illness, more than half of the patients (56%) developed respiratory compromise. Among them, 13 patients (36%) entirely lost the ability to breathe, and seven patients (19%) experienced dyspnea. Consequently, 15 patients required invasive mechanical ventilation. At discharge, all patients (n = 35, 100%) successfully restored normal respiratory function, achieving optimal oxygen saturation in ambient air without the need for respiratory support. Notably, five patients (14%) reported lingering mild dyspnea.
Time required to regain normal functions
The duration to regain normal respiratory function proved to be the most prolonged (Fig. 5). Among the 20 patients with respiratory compromise, 19 achieved normal respiratory function within a mean of 56.0 ± 37.4 days (range: 5 to 132 days, median: 47 days), with the exception of the patient who unfortunately died before regaining the ability to breathe normally.
Fig. 5.
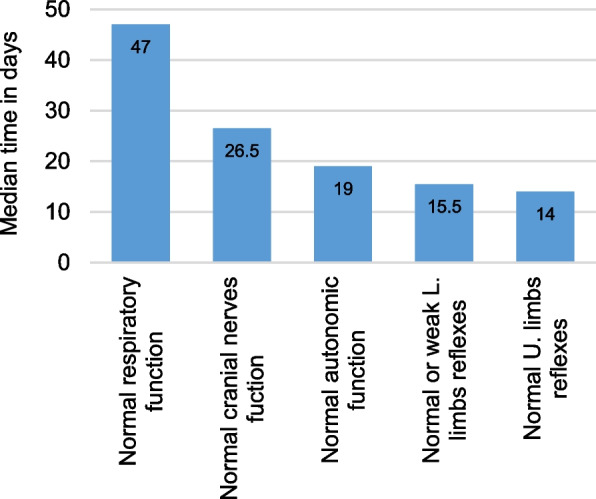
The median time required to achieve normal functionality of the different parts of the nervous system affected by GBS. The function of the extremities is described by the time required to regain only normal reflexes in the upper limbs, and either normal or weak reflexes in the lower limbs
This is followed by the time required to regain normal cranial nerves function. Of the 24 patients experiencing cranial nerves dysfunction at the peak of their illness, 18 fully regained normal cranial nerves function within a mean of 38.4 ± 34.3 days (range: 8 to 129 days, median: 26.5 days). However, six patients were discharged with some degree of cranial nerves dysfunction.
For the 17 patients with autonomic dysfunction, 14 patients fully regained normal autonomic function within a mean of 20.4 ± 14.1 days (range: 1 to 48 days, median: 19 days), while two patients did not fully recover, and one patient passed away before regaining normal autonomic function.
For the upper limbs, only the time required to regain normal reflexes was considered in calculations, whereas for the lower limbs, both weak and normal reflexes were taken into account. This decision was made because upper limb reflexes was restored to normal in 13 patients, but the lower limb reflexes was restored to normal in only one patient. Hence, the time required for the return of normal upper limb reflexes ranged from 7 to 77 days, with a mean of 26.1 ± 23.6 days, and a median of 14 days. Conversely, 14 patients regained weak or normal lower limb reflexes in a mean of 35.7 ± 36.9 days (range: 7 to 105 days, median: 15.5 days), highlighting the variability in recovery times between upper and lower limb reflexes.
Plasmapheresis complications
TPE was found to be generally safe when applied in this cohort of pediatric patients, and it is routinely performed in our hospital, despite the challenges associated with its application. However, some adverse events occurred during or after plasmapheresis sessions, including a few that were serious and life-threatening. We will describe the details of these complications next.
Complications during plasmapheresis procedures
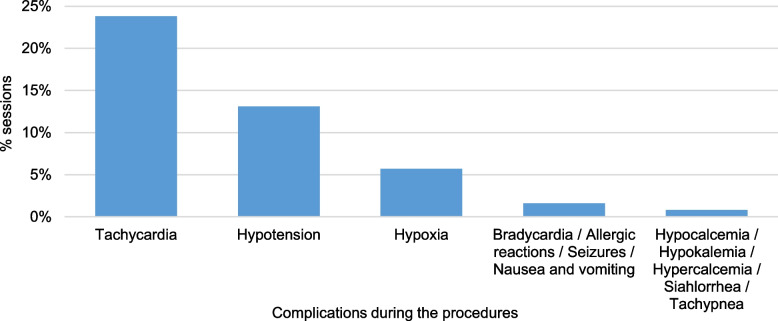
Throughout the course of 122 TPE sessions, complications were observed in 47 sessions (39%) involving 29 patients (81%). Tachycardia emerged as the most prevalent complication, occurring in 24% of sessions, followed by hypotension at 13%. Other observed complications included hypoxia (6%), and other less frequent complications, which are illustrated in (Fig. 6).
Fig. 6.
Complications that occurred during the plasmapheresis procedures, presented as the percentages of the sessions out of the total sessions. In some sessions, there was more than one complication. The total number of sessions is 122 sessions
Notably, these complications were severe in two patients during their second session, prompting the discontinuation of plasmapheresis and a transition to IVIG. The first patient experienced complications such as hypertension, tachycardia, hypoxia, acidosis, and hypercalcemia, while the second patient encountered hypotension with hemodynamic instability, along with tachycardia, seizures, hypoxia, acidosis, and hypercalcemia.
Complications following plasmapheresis procedures
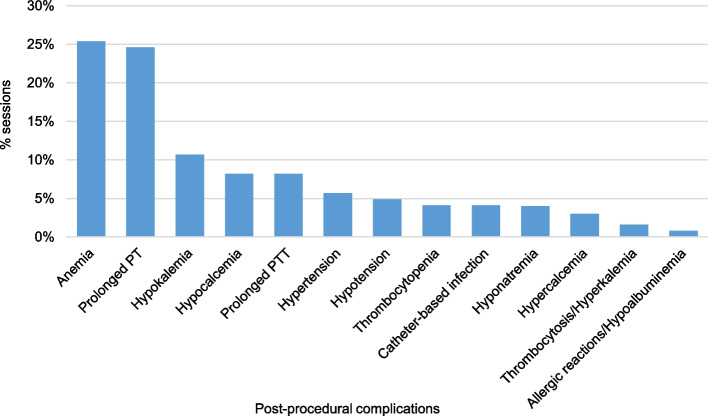
An examination of patients’ medical records yielded valuable insights into clinical observations and laboratory test outcomes following each plasmapheresis session, albeit with some sessions lacking documentation of certain laboratory tests. Complications were evident after a total of 70 TPE sessions (57%), impacting 31 patients. Anemia was the most common complication, observed in 31 sessions (25%). This was managed by the transfusion of packed red blood cell units as deemed necessary.
Moreover, prolonged PT and aPTT were observed following 30 sessions (25%) and 10 sessions (8%), respectively. Anomalous coagulation test results prompted the transfusion of FFP units. A total of 16 FFP units were transfused after 11 different TPE sessions, including one FFP unit administered during a patient’s final plasmapheresis session. Furthermore, hypokalemia occurred after 13 sessions (11%), hypocalcemia after 10 sessions (8%), and hypotension after six sessions (5%). Hypocalcemia was managed through oral calcium administration. Additional, albeit milder, complications are detailed in (Fig. 7).
Fig. 7.
Complications that occurred after the plasmapheresis procedures, presented as the percentages of the sessions out of the total sessions. In some sessions, there was more than one complication. The total number of sessions is 122 sessions
In a specific case, a patient developed pulmonary edema accompanied by hypoxia and periorbital edema shortly after their second plasmapheresis session. Arterial blood oxygen saturation dropped to 60%, classifying the incident as an anaphylactic shock, necessitating manual ventilation using an AMBO bag. Subcutaneous adrenaline administration and intravenous furosemide were used to treat the patient, along with the transfusion of one unit of FFP. In a separate incident, a central venous catheter was erroneously inserted into the right femoral artery instead of the intended vein, resulting in subsequent cellulitis in the affected area.
One patient passed away 65 days after admission to the hospital, and after receiving four TPE sessions. There was a 48-day time lapse between the patient’s last plasmapheresis session and date of death; thus, the cause of death could not be attributed to plasmapheresis. The determined cause of death was septic shock arising from severe VAP.
Follow-up evaluation
Upon discharge, the patients were recommended to undergo continued physical therapy, as they did not achieve a full recovery at the hospital. Communication was established with the parents of 18 of the patients (50%). We could not reach out to the rest of the patients because of contacting problems. Since the patients were admitted to this referral hospital from different regions, it was not feasible to coordinate with the multiple medical centers that handled the follow-up. The follow-up data was obtained by asking parents about changes that occurred four to six years before the conduct of this study. Hence, all the information is just estimations from the parents, and is not based on accurate medical approach.
Following consultations with the parents, it was revealed that the duration of physical therapy varied widely, spanning from 2 to 64 months post-discharge, with a median duration of 12 months for each patient. Meanwhile, the timeline for regaining the ability to walk with assistance ranged from 0.3 to 12.6 months after the onset of symptoms (mean duration of 5.8 months), while achieving independent walking took between 0.5 and 18.6 months (mean duration of 9.2 months). One of the patients included in the above statistics, faced a car accident 20 days post-discharge, resulting in hip and lower extremity fractures that delayed their physical therapy and overall recovery. Moreover, six patients (17%) did not achieve full recovery and exhibited a mild residual disability, including abnormal gait, general muscle weakness, or foot drop.
Discussion
The availability of advanced plasmapheresis machines and various types of central venous catheters has encouraged the adoption of TPE in pediatric neuroimmunological diseases. There is ongoing debate about whether TPE or IVIG is superior in terms of efficacy and safety in treating pediatric GBS. Notably, TPE has demonstrated effectiveness comparable to, or even surpassing, IVIG, while also proving to be more cost effective. This assertion aligns with findings reported from numerous previous case series and cohort studies [2, 11, 29, 30, 34, 35]. A recent systematic review by Nandeesha et al stated that TPE could have a slight advantage over IVIG, particularly in the pediatric group, although it carries a marginally higher risk of adverse reactions [36]. This retrospective study investigates and delves into the efficacy and safety of TPE in the management of severe GBS in pediatric patients, with the objective of providing valuable insights for potential inclusion in future larger-scale trials.
Our study encompassed 36 patients aged between 2 and 13 years (median 8 years), undergoing a total of 122 TPE sessions as a treatment for GBS. The number of TPE sessions per patient was influenced by several factors, including the initial response to treatment, the financial ability of the patients’ families to afford albumin and TPE kits, the occurrence of adverse events, and concerns related to the vulnerability of the central venous line. TPE was administered as the sole therapeutic intervention in 81% of the cases, while in the remaining 19%, it was used in conjunction with IVIG. Males were affected more than females (M/F = 1.25/1), proving to be consistent with prior studies that demonstrate a slight predominance of GBS in males [1, 37–40].
While CSF analysis was primarily conducted within the initial week of symptoms onset, albuminocytologic dissociation was evident in only 25% of patients. This contrasts with some studies reporting higher percentages (above 75%) in pediatric patients early in the disease course [1, 34, 37, 41]. Other studies suggest that normal CSF results may be observed within one week after symptoms onset [2, 4, 7, 9], which correlates with our findings in the six patients who had lumbar puncture repeated and may explain the results in our study. This warrants further investigations for future work to determine the optimal timing for diagnostically informative CSF analysis, at which it would serve as a good diagnostic test for GBS.
The AMAN variant (28%) prevailed over AIDP (3%) among our patients, aligning with findings reported by previous studies in China, Japan, Thailand, Central and South America [1, 37, 42]. However, AIDP was more prevalent among children in North America, Europe, Egypt, Taiwan, and Iran [7, 34, 39, 40, 43]; and also among adults [19, 44]. This variation in the incidence of different GBS variants among pediatric patients based on geographical aspects has not been fully explained, and requires further investigation [34, 42]. Given that only about one-third of the patients included in this study had defined GBS variants, the results obtained from this study may not accurately represent the prevalence of each variant within our region; thus, additional studies are required to establish and determine regional trends.
The lack of resources and financial restrictions at the time of the study limited the availability of further diagnostic investigations, including bacterial and viral panels for preceding infections, brain and spinal magnetic resonance imaging, nerve and muscle biopsies, and auto-antibody titers.
The patients involved in this study reached the peak of illness in 6.2 ± 3.2 days, akin to previous studies [7, 43, 45]. However, our cohort of patients exhibited more severe GBS symptoms, in terms of respiratory manifestations, compared to other previous reports. That is, 61% of patients were admitted to PICU and 42% required invasive mechanical ventilation due to pulmonary failure. This is higher than reported by many previous studies [1, 26, 34, 37, 39, 46], yet aligns with two others [12, 45], and is lower than results obtained from one [40]. Mechanical ventilation was almost always accompanied by VAP among our patients, which further worsened the respiratory function, necessitating extended PICU and hospital stays; additional supportive care, including multiple antibiotic treatment regimens; and also delaying full recovery in numerous cases. This high incidence of pneumonia can be traced to lack of logistical assets and infrastructure of the hospital due to the economic manifestations of the civil war in Syria. This study occurred in a time of low supplies and capabilities which made the hospital more vulnerable to spreading of bacteria and other pathogens. In addition, the high number of patients arriving every day to the hospital was too high as it was one of very few hospitals still in business at that time, which made sticking with standard dis-infection procedures and guidelines so hard.
Furthermore, 97% of patients in this study were unable to walk when they reached the maximal deficit at the peak of illness, signifying a high disability score (4 or higher on the GBS disability scale established by Hughes et al. [8]). This contrasts with lower figures reported in many previous pediatric studies [1, 7, 26, 40, 43].
Other clinical features of GBS in our cohort of patients were similar to most previous studies [1, 7, 26, 34, 40, 47].
Factors such as the need for mechanical ventilation, cranial nerve involvement, the axonal subtype of GBS, and a disability score exceeding 3 were consistently linked to poorer outcomes and/or slower recovery in children with GBS across numerous reports [1, 7, 19, 20, 23, 40, 48].
Despite the severity of the disease, clinical improvement in all neurological functions was well established in our patients after plasma exchange. This includes the one deceased patient, who had regained normal cranial nerves function and showed some improvement in motor functions prior to their death. This contrasts with studies where some patients failed to respond at all to TPE treatment [12, 15, 30].
All mechanically-ventilated patients regained normal respiratory function at discharge. This excludes one patient who succumbed to septic shock in the PICU. Studies have highlighted the superiority of TPE over IVIG or conservative therapy, particularly in mechanically ventilated patients, by reducing the length of mechanical ventilation [35, 41, 45]. Furthermore, Saad et al. reported that the need for mechanical ventilation was lower in patients treated with TPE compared to IVIG [34]. Although the median duration of hospitalization in our study aligned with a study by Jansen et al. [45], mean hospital and PICU stays (38.5 and 48.9 days, respectively) exceeded those in pediatric patients treated with IVIG or TPE [3, 26, 34, 35, 40, 45]. This can be attributed to the higher severity of the disease, the occurrence of other pathologies, and the higher incidence of mechanical ventilation-related complications among our patients. Hence, if we compared the mean duration of hospitalization in the patients who did not require mechanical ventilation (13.1 days), we would find similar results in the same previous studies. Furthermore, the critical condition of patients admitted to the PICU may have contributed to the development of critical illness polyneuropathy, potentially worsening neurological function, delaying weaning, and extending rehabilitation. However, the confirmation of such diagnosis was not established by further investigations. This suggests that higher caution should be prompted in children who require intubation to enhance prognosis and shorten hospital stays.
The GBS disability score established by Hughes et al. [8], while widely employed, may lack sensitivity to detect clinically meaningful differences, as it mainly assesses the ability to walk, which reflects the function of the lower extremities. It does not evaluate upper limb motor functions, nor cranial, autonomic, or sensory functions, and has large steps between the grades [26, 49]. Thus, in this study, the MRC scoring system was used to evaluate motor functions in both upper and lower extremities. All the patients showed an improvement of at least one grade of power. However, the improvement was more profound in the upper limbs compared to the lower limbs. The mean increase in grade of power in the upper limbs among the patients in this study was slightly higher than that reported by Vikrant et al. among adult patients treated with TPE [44], but it was slightly lower in the lower limbs compared to that in the same study. Sixty percent of our patients exhibited a grade-4 or grade-5 improvement in the upper limbs which is higher than reported by Gajjar et al. [30]. Further studies are needed to assess the efficacy of TPE using the MRC scale for a more precise measurement of power improvement.
Furthermore, careful analysis was conducted to determine the duration necessary for the restoration of individual normalcy in cranial, autonomic, and respiratory functions. This additional investigation was undertaken to enhance our evaluation of TPE efficacy in expediting and speeding the recovery process, marking a pioneering effort as this study is the first to do so.
Long-term outcomes revealed full recovery in most patients available for follow-up, with only 17% exhibiting minimal residual deficits that may impact daily life functions. This indicates a better outcome among our patients compared to other reports in which IVIG was the treatment [26, 40, 46], and also compared to what was observed in other studies in which TPE or other therapies were applied [19, 29]. Overall, 92% of the patients who were available for follow-up achieved independent ambulation within 1 year after disease onset, with a median time of 8.7 months. Comparable findings were reported by Chaweekulrat and Sanmaneechai in IVIG-treated patients [1] though the same study along with some others reported shorter duration for achieving independent walking [7, 41]. Chaweekulrat and Sanmaneechai found that the axonal subtype of GBS and a disability score higher than 3 were associated with longer duration for independent walking, which may explain the results in this study.
In this investigation, complications associated with TPE primarily manifested as mild or moderate adverse events, indicating that the procedure is generally safe for pediatric patients. The two most common complications during the procedure were tachycardia (24%) and hypotension (13%), with mild anemia (25%) emerging as the most frequent post-procedural complication. Hypotension was the most common complication seen during TPE sessions in one recent study [50]. Nevertheless, electrolyte disturbances, such as hypocalcemia and hypokalemia, were the predominant complications observed in two studies [29, 51], with incidence rates reported similar to ours. Comparatively, our study revealed a higher overall incidence of complications in our patient cohort when contrasted with studies in children [12, 29, 30, 34]. However, our findings align closely with those reported by Michon et al. and Eyre et al. [31, 51]. Despite certain studies showing no major or life-threatening complications among pediatric cases subjected to TPE treatment [12, 29, 30, 34, 45], our study identified serious complications in three patients (8% of the patients, and 2.5% of the sessions). Two incidents necessitated termination of plasmapheresis, while the third involved an anaphylactic shock occurring two hours post-session. The latter was effectively managed without discontinuation of the treatment.
The French Cooperative Group has advocates for the use of albumin as the replacement fluid in plasmapheresis, which is preferred over FFP due to a higher incidence of adverse and allergic reactions associated with the latter [52]. This is also recommended by some studies [18, 45, 53]. While our study supported this recommendation by demonstrating fewer allergic reactions with albumin, it unveiled a notable drawback. The use of albumin was linked to a depletion of clotting factors, resulting in abnormal coagulation test results (prolonged PT and aPTT), constituting the second most common post-procedural complication (25% for the prolongation of PT) observed in our study. Despite this, no clinical signs of bleeding disorders were observed. This required replenishment of the clotting factors by transfusing FFP units. Close monitoring of coagulopathies should be considered when using albumin as a replacement fluid, particularly in pediatric cases.
Notably, no mortality attributable to TPE was recorded within our patient cohort, which is a trend consistent with several previous studies involving pediatric cases [12, 18, 29, 30, 45]. A comprehensive retrospective study involving 186 pediatric patients undergoing apheresis for various indications, reported a mortality rate of 1%, but with no clear attribution to the procedure [31].
Limitations
This study had several limitations, primarily the absence of a comparison group (i.e., patients with GBS who were treated with IVIG or supportive care alone). Furthermore, we acknowledge the presence of a potential socioeconomic bias in the selection of treatment option, as the treatment selection was influenced by the patients’ financial ability to afford IVIG. This could have affected the comparability of outcomes between groups and may limit the generalization of our findings. Additionally, only 36% of the patients had defined GBS variants, limiting our ability to establish a relationship between specific variants and the severity of the disease in our cohort. Limited resources restricted the availability of additional diagnostic tests, potentially complicating the exclusion of other conditions in the differential diagnosis of GBS. The follow-up process also had significant limitations. Only 50% of the patients were available for follow-up, and the follow-up was not based on a standardized medical approach. Instead, it relied on reports from parents whose children continued physical therapy at various centers across the country. Moreover, the follow-up data was not derived from saved medical records but rather from the parents’ recollections, which may have introduced substantial bias into the results.
Conclusion
Therapeutic plasma exchange emerges as a potentially promising treatment for GBS in pediatric patients. It may accelerate the restoration of normal neurological functions, yielding favorable outcomes, often with only mild residual deficits. In terms of its safety in children, TPE is generally safe and can be applied as a first-line treatment for GBS. This means that it can substitute IVIG in that concern. However, this procedure can occasionally be associated with life-threatening complications, such as anaphylaxis or sever hemodynamic instability, alongside several milder adverse events. Consequently, extra care and diligent attention to safety considerations is essential when implementing TPE in pediatric patients. In terms of cost, TPE was more cost-effective and accessible than IVIG, considering that this study took place in a low-income country facing financial restrictions.
Acknowledgements
The authors wish to thank Dr. Ragheed Alturkmani and Ms. Zainab Merza for their contribution in proofreading and reviewing the manuscript.
Abbreviations
- GBS
Guillain-Barré syndrome
- TPE
Therapeutic plasma exchange
- MRC
Medical Research Council
- AIDP
Acute inflammatory demyelinating polyradiculoneuropathy
- AMSAN
Acute motor sensory axonal neuropathy
- AMAN
Acute motor axonal neuropathy
- MSF
Miller Fisher syndrome
- IVIG
Intravenous immunoglobulin
- ASFA
American Society for Apheresis
- CSF
Cerebrospinal fluid
- H.A
Human albumin
- NS
Normal saline
- FFP
Fresh frozen plasma
- PV
Plasma volume
- ACD
Acid citrate dextrose
- PT
Prothrombin time
- aPTT
Activated partial thromboplastin time
- SD
Standard deviation
- CI
Confidence interval
- EMG
Electromyography
- PICU
Pediatric intensive care unit
- VAP
Ventilation-acquired pneumonia
Authors' contributions
T.A. contributed to the conception of the study, acquisition and interpretation of the data, and revision of the work. F.H.A.F. contributed to the conception of the study, acquisition and interpretation of the data, and revision of the work. M.A. participated in the conceptualization of the study, in acquisition, analysis, and interpretation of the data, and in drafting and revising the manuscript. A.A. participated in the conceptualization of the study, in acquisition, analysis, and interpretation of the data, and in drafting and revising the manuscript. A.S.H. participated in data collection.
Funding
Not applicable.
Data availability
The datasets generated and analyzed are not publicly available but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was waived by the local Ethics Committee of Children’s Hospital of Damascus University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Informed consent to participate in this study was obtained from the parents or the legal guardians of all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chaweekulrat P, Sanmaneechai O. Prognostic model for time to achieve independent walking in children with Guillain-Barré syndrome. Pediatr Res. 2022;92(5):1417–22. 10.1038/s41390-021-01919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Hamdani S, Aljanabi F, Abdulrasool M, Salman A. Child with Guillain-Barré Syndrome Responding to Plasmapheresis: A Case Report. Case Rep Acute Med. 2020;3:4–11. 10.1159/000505964. [Google Scholar]
- 3.Elahi E, Ashfaq M, Nisa BU, Chachar S. Plasma Exchange Versus Intravenous Immunoglobulin in Children with Guillain Barré Syndrome. J Dow Univ Health Sci. 2019;13:133–7. 10.36570/JDUHS.2019.3.693.
- 4.Rosen BA. Guillain-Barré Syndrome. Pediatr Rev. 2012;33:164–71. 10.1542/PIR.33-4-164. [DOI] [PubMed] [Google Scholar]
- 5.Jones RH Jr. Guillain-Barre syndrome in children. Curr Opin Pediatr. 1995;7(6):663–8. 10.1097/00008480-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–27. 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 7.Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Guillain-Barré syndrome: a prospective multicentre study. Neuropediatrics. 2007;38:10–7. 10.1055/S-2007-981686. [DOI] [PubMed] [Google Scholar]
- 8.Hughes RAC, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet (London, England). 1978;2:750–3. 10.1016/S0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- 9.Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31:491–510. 10.1016/J.NCL.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beydoun HA, Beydoun MA, Hossain S, Zonderman AB, Eid SM. Nationwide study of therapeutic plasma exchange vs intravenous immunoglobulin in Guillain-Barré syndrome. Muscle Nerve. 2020;61:608–15. 10.1002/MUS.26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigi S, Banwell B, Yeh EA. Outcomes After Early Administration of Plasma Exchange in Pediatric Central Nervous System Inflammatory Demyelination. J Child Neurol. 2015;30(7):874–80. PMID: 25246301. 10.1177/0883073814545883. [DOI] [PubMed]
- 12.Özkale M, Erol I, Özkale Y, İlknur Kozanoğlu. Overview of therapeutic plasma exchange in pediatric neurology: a single-center experience. Acta Neurol Belg. 2018;118:451–8. 10.1007/S13760-018-0961-5. [DOI] [PubMed]
- 13.Bobati SS, Naik KR. Therapeutic Plasma Exchange - An Emerging Treatment Modality in Patients with Neurologic and Non-Neurologic Diseases. J Clin Diagn Res. 2017;11:EC35–7. 10.7860/JCDR/2017/27073.10480. [DOI] [PMC free article] [PubMed]
- 14.Chevret S, Hughes RAC, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017;2017. 10.1002/14651858.CD001798.PUB3/EPDF/ABSTRACT. [DOI] [PMC free article] [PubMed]
- 15.Kaya E, Keklik M, Şencan M, Yilmaz M, Keskin A, Kiki I, et al. Therapeutic plasma exchange in patients with neurological diseases: multicenter retrospective analysis. Transf Apheres Sci: Off J World Apher Assoc: Off J Eur Soc Haemapheresis. 2013;48:349–52. 10.1016/J.TRANSCI.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Yücesan C, Önder Arslan, Arat M, Yücemen N, Ayyildiz E, Ilhan O, et al. Therapeutic plasma exchange in the treatment of neuroimmunologic disorders: review of 50 cases. Transf Apheres Sci: Off J World Apher Assoc: Off J Eur Soc Haemapheresis. 2007;36:103–7. 10.1016/J.TRANSCI.2006.06.008. [DOI] [PubMed]
- 17.Korach JM, Guillevin L, Petitpas D, Berger P, Chillet P. Apheresis registry in France: indications, techniques, and complications. French Registry Study Group. Ther Apher: Off J Int Soc Apher Jpn Soc Apher. 2000;4:207–10. 10.1046/J.1526-0968.2000.00201.X. [DOI] [PubMed]
- 18.Gafoor V, Jose J, Saifudheen K, Musthafa M. Plasmapheresis in neurological disorders: experience from a tertiary care hospital in South India. Ann Indian Acad Neurol. 2015;18:15. 10.4103/0972-2327.144301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes RAC, Cornblath DR. Guillain-Barré syndrome. Lancet (London, England). 2005;366:1653–66. 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 20.Verboon C, Doets AY, Galassi G, Davidson A, Waheed W, Péréon Y, et al. Current treatment practice of Guillain-Barré syndrome. Neurology. 2019;93:e59–76. 10.1212/WNL.0000000000007719. [DOI] [PubMed] [Google Scholar]
- 21.Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apher. 2023;38:77–278. 10.1002/JCA.22043. [DOI] [PubMed]
- 22.Diener HC, Haupt WF, Kloss TM, Rosenow F, Philipp T, Koeppen S, et al. A preliminary, randomized, multicenter study comparing intravenous immunoglobulin, plasma exchange, and immune adsorption in Guillain-Barré syndrome. Eur Neurol. 2001;46:107–9. 10.1159/000050777. [DOI] [PubMed] [Google Scholar]
- 23.Hughes RAC. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barre syndrome. Lancet. 1997;349:225–30. 10.1016/S0140-6736(96)09095-2. [PubMed] [Google Scholar]
- 24.van der Meché FGA, Schmitz PIM. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. 1992;326:1123–9. 10.1056/NEJM199204233261705. [DOI] [PubMed]
- 25.Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78:1009–15. 10.1212/WNL.0B013E31824DE293. [DOI] [PubMed] [Google Scholar]
- 26.Ma YM, Liu TKT, Wong V. Guillain-Barre syndrome in southern Chinese children: 32 year experience in Hong Kong. Pediatr Int: Off J Jpn Pediatr Soc. 2010;52:13–9. 10.1111/J.1442-200X.2009.02951.X. [DOI] [PubMed] [Google Scholar]
- 27.Hughes RAC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014;2014. 10.1002/14651858.CD002063.PUB6. [DOI] [PubMed]
- 28.Kleyweg RP, van der Meché FGA, Meulstee J. Treatment of Guillain-Barré syndrome with high-dose gammaglobulin. Neurology. 1988;38:1639–41. 10.1212/WNL.38.10.1639. [DOI] [PubMed] [Google Scholar]
- 29.Yıldırım M, Ömer Bektaş, Botan E, Şahin S, Gurbanov A, Teber S, et al. Therapeutic plasma exchange in clinical pediatric neurology practice: Experience from a tertiary referral hospital. Clin Neurol Neurosurg. 2021;207. 10.1016/J.CLINEURO.2021.106823. [DOI] [PubMed]
- 30.Gajjar M, Patel T, Bhatnagar N, Solanki M, Patel V, Soni S. Therapeutic plasma exchange in pediatric patients of Guillain-Barre syndrome: Experience from a Tertiary Care Centre. Asian J Transfus Sci. 2016;10:98–100. 10.4103/0973-6247.165834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michon B, Moghrabi A, Winikoff R, Barrette S, Bernstein ML, Champagne J, et al. Complications of apheresis in children. Transfusion. 2007;47:1837–42. 10.1111/J.1537-2995.2007.01405.X. [DOI] [PubMed] [Google Scholar]
- 32.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32. [PubMed] [Google Scholar]
- 33.Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40(8):665–71. 10.2340/16501977-0235. [DOI] [PubMed] [Google Scholar]
- 34.Saad K, Mohamad IL, Hamed MAA, Tawfeek MSK, Ahmed AE, Baseer KAA, et al. A comparison between plasmapheresis and intravenous immunoglobulin in children with Guillain-Barre syndrome in Upper Egypt. Ther Adv Neurol Disord. 2016;9. 10.1177/1756285615610471. [DOI] [PMC free article] [PubMed]
- 35.El-Bayoumi MA, El-Refaey AM, Abdelkader AM, El-Assmy MMA, Alwakeel AA, El-Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: A randomized study. Crit Care. 2011;15:1–6. 10.1186/CC10305/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nandeesha SS, Kasagga A, Hawrami C, Ricci E, Hailu KT, Salib K, et al. Treatment Efficacy of Plasmapheresis Versus Intravenous Immunoglobulin in Guillain-Barré Syndrome Management: A Systematic Review. Cureus. 2024;16. 10.7759/CUREUS.57066. [DOI] [PMC free article] [PubMed]
- 37.Tang J, Dai Y, Li M, Cheng M, Hong S, Jiang L, et al. Guillain-Barré syndrome in Chinese children: a retrospective analysis. Pediatr Neurol. 2011;45:233–7. 10.1016/J.PEDIATRNEUROL.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS). Pharmacol Rep. 2010;62:220–32. 10.1016/S1734-1140(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 39.Barzegar M, Dastgiri S, Karegarmaher MH, Varshochiani A. Epidemiology of childhood Guillan-Barre syndrome in the north west of Iran. BMC Neurol. 2007;7:1–5. 10.1186/1471-2377-7-22/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halawa EF, Ahmed D, Nada MAF. Guillain-Barré syndrome as a prominent cause of childhood acute flaccid paralysis in post polio eradication era in Egypt. Eur J Paediatr Neurol: Off J Eur Paediatr Neurol Soc. 2011;15:241–6. 10.1016/J.EJPN.2010.11.008. [DOI] [PubMed]
- 41.Epstein MA, Sladky JT. The role of plasmapheresis in childhood Guillain-Barré syndrome. Ann Neurol. 1990;28:65–9. 10.1002/ANA.410280112. [DOI] [PubMed] [Google Scholar]
- 42.McGrogan A, Madle GC, Seaman HE, Vries CSD. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review Neuroepidemiology. 2009;32:150–63. 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 43.Hung PL, Chang WN, Huang LT, Huang SC, Chang YC, Chang CJ, et al. A clinical and electrophysiologic survey of childhood Guillain- Barré syndrome. Pediatr Neurol. 2004;30:86–91. 10.1016/S0887-8994(03)00403-X. [DOI] [PubMed] [Google Scholar]
- 44.Vikrant S, Thakur S, Sharma A, Gupta D, Sharma S. Safety and efficacy of therapeutic membrane plasmapheresis in the treatment of Guillain-Barré syndrome: A study from a tertiary care hospital from India. Neurol India. 2017;65:527. 10.4103/NEUROINDIA.NI_907_15. [DOI] [PubMed] [Google Scholar]
- 45.Jansen PW, Perkin RM, Ashwal S. Guillain-Barré syndrome in childhood: natural course and efficacy of plasmapheresis. Pediatr Neurol. 1993;9:16–20. 10.1016/0887-8994(93)90004-V. [DOI] [PubMed] [Google Scholar]
- 46.Rees JH, Thompson RD, Smeeton NC, Hughes RAC. Epidemiological study of Guillain-Barré syndrome in south east England. J Neurol Neurosurg Pychiatry. 1998;64:74–7. 10.1136/JNNP.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradiso G, Tripoli J, Galicchio S, Fejerman N. Epidemiological, clinical, and electrodiagnostic findings in childhood Guillain-Barré syndrome: a reappraisal. Ann Neurol: Off J Am Neurol Assoc Child Neurol Soc. 1999;46(5):701–7. 10.1002/1531-8249(199911)46:5<701::AID-ANA4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Barzegar M, Toopchizadeh V, Maher MHK, Sadeghi P, Jahanjoo F, Pishgahi A. Predictive factors for achieving independent walking in children with Guillain-Barre syndrome. Pediatr Res. 2017;82:333–9. 10.1038/PR.2017.67. [DOI] [PubMed] [Google Scholar]
- 49.Kleyweg RP, Meché FGAVD, Schmitz PIM. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–9. 10.1002/MUS.880141111. [DOI] [PubMed]
- 50.Fateen T, Sultana N, Sarwar M, Saqlain N. Complications of Therapeutic Plasma Exchange in pediatric patients: an experience at a tertiary care hospital. Pak J Med Sci. 2023;39:994. 10.12669/PJMS.39.4.7002. [DOI] [PMC free article] [PubMed]
- 51.Eyre M, Hacohen Y, Lamb K, Absoud M, Agrawal S, Gadian J, et al. Utility and safety of plasma exchange in paediatric neuroimmune disorders. Dev Med Child Neurol. 2019;61:540–6. 10.1111/DMCN.14150. [DOI] [PubMed] [Google Scholar]
- 52.Korach JM, Berger P, Giraud C, Perff-Desman CL, Chillet P. Role of replacement fluids in the immediate complications of plasma exchange. French Registry Cooperative Group. Intensive Care Med. 1998;24:452–8. 10.1007/S001340050595. [DOI] [PubMed]
- 53.Basic-Jukic N, Kes P, Glavas-Boras S, Brunetta B, Bubic-Filipi L, Puretic Z. Complications of therapeutic plasma exchange: experience with 4857 treatments. Ther Apher Dial: Off Peer-Rev J Int Soc Apher Jpn Soc Apher Jpn Soc Dial Ther. 2005;9:391–5. 10.1111/J.1744-9987.2005.00319.X. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed are not publicly available but are available from the corresponding author upon reasonable request.