Abstract
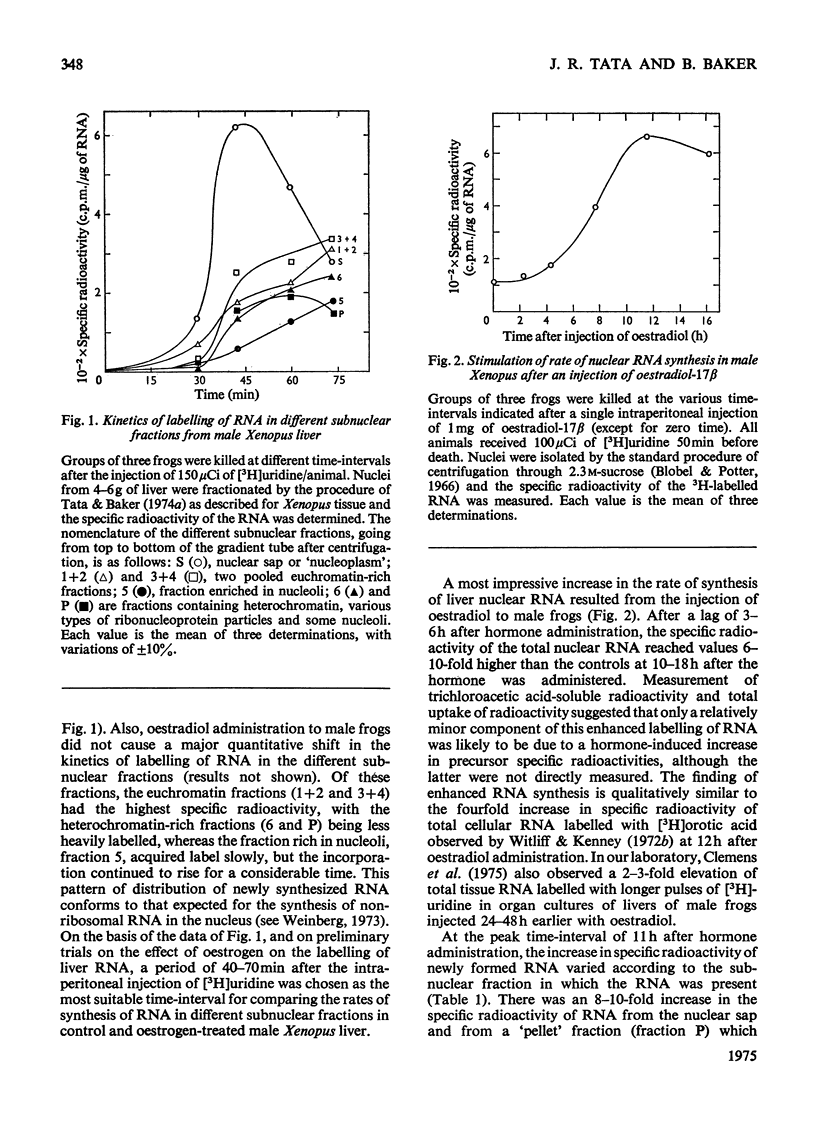
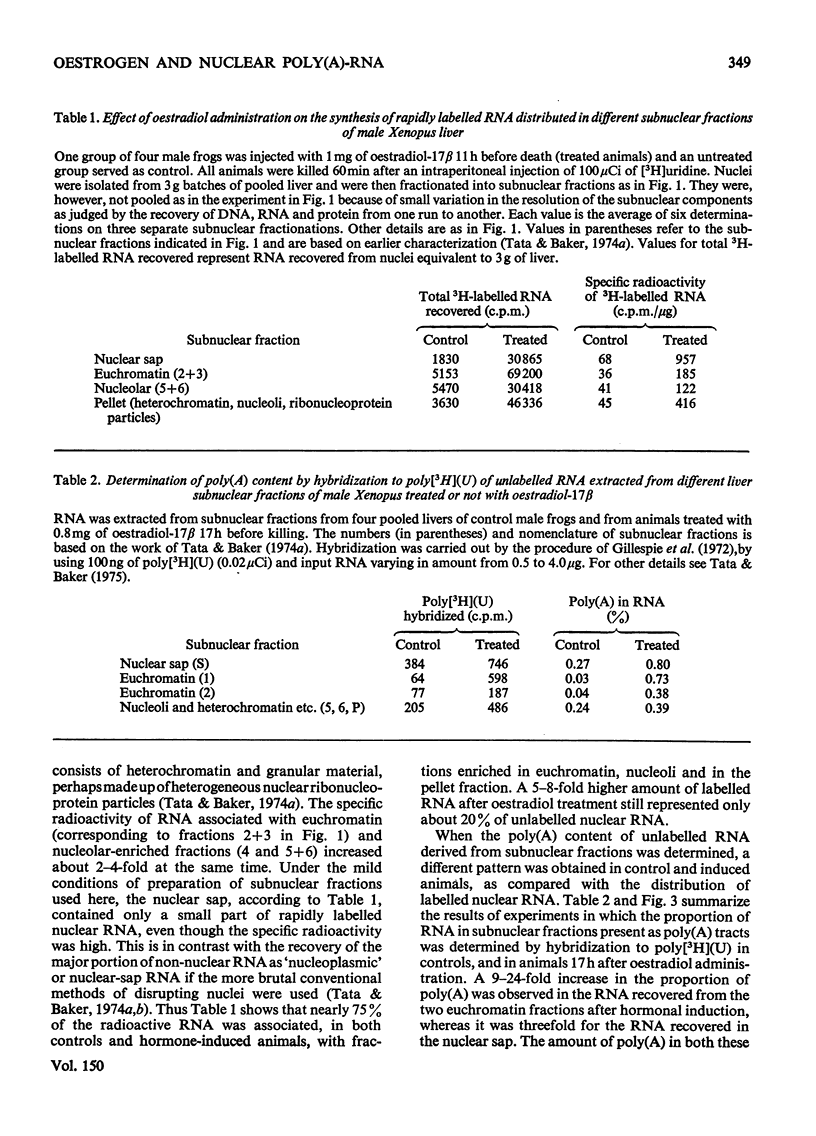
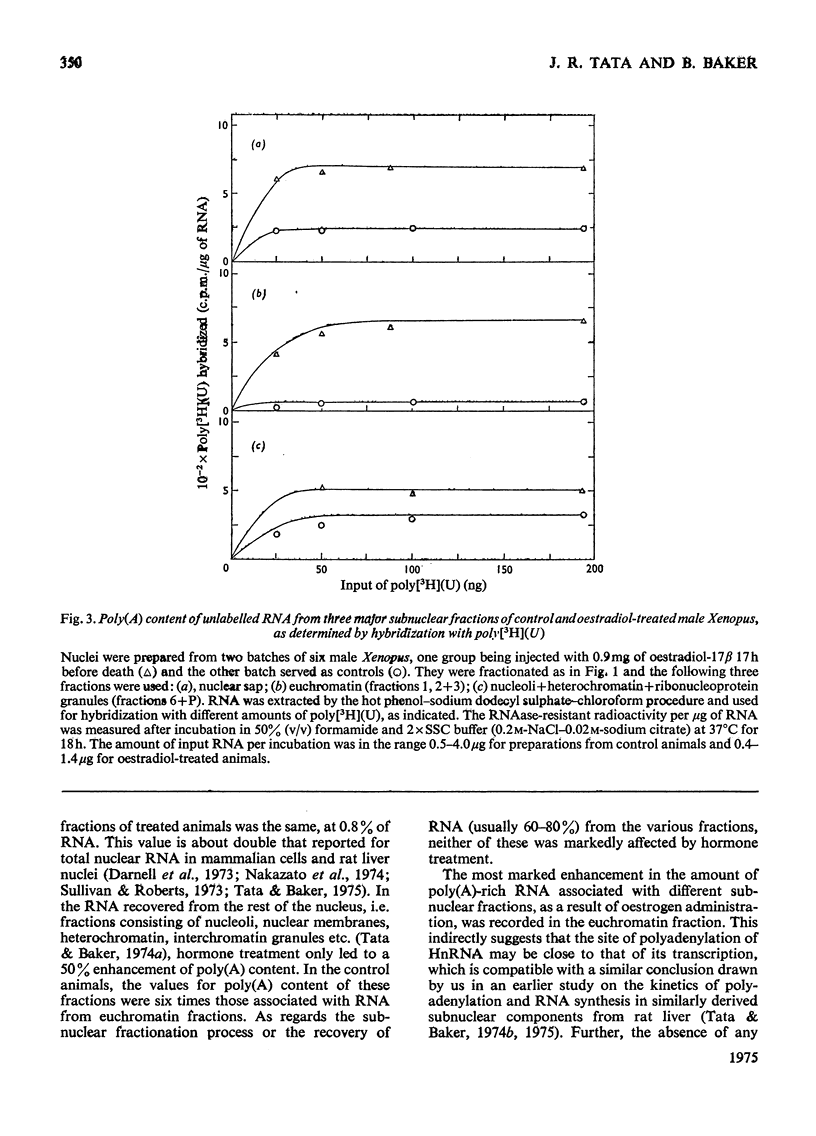
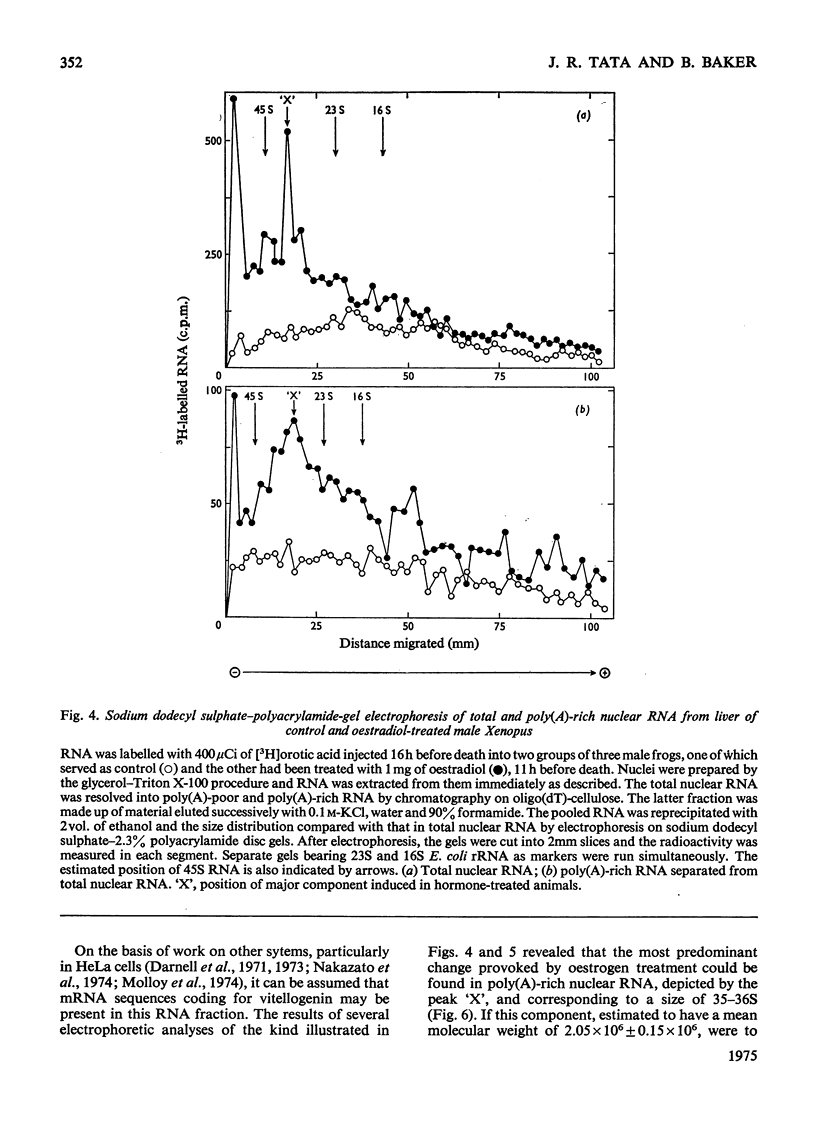
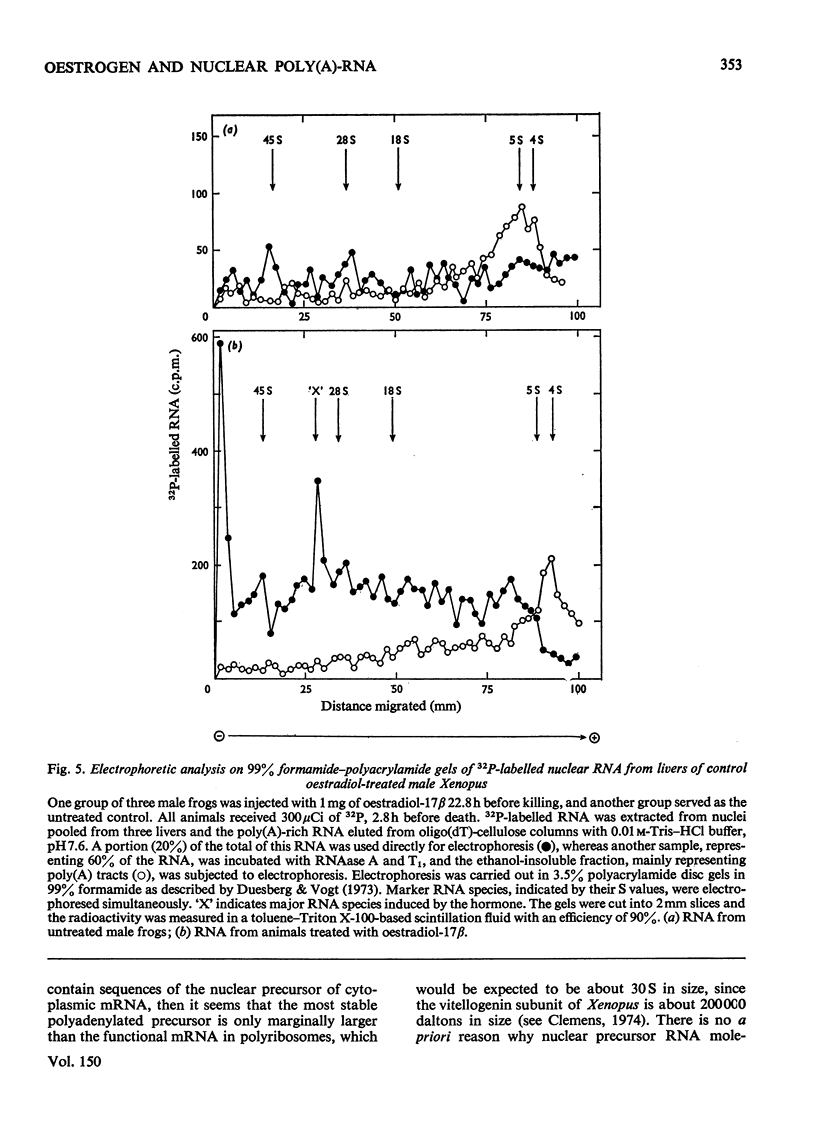
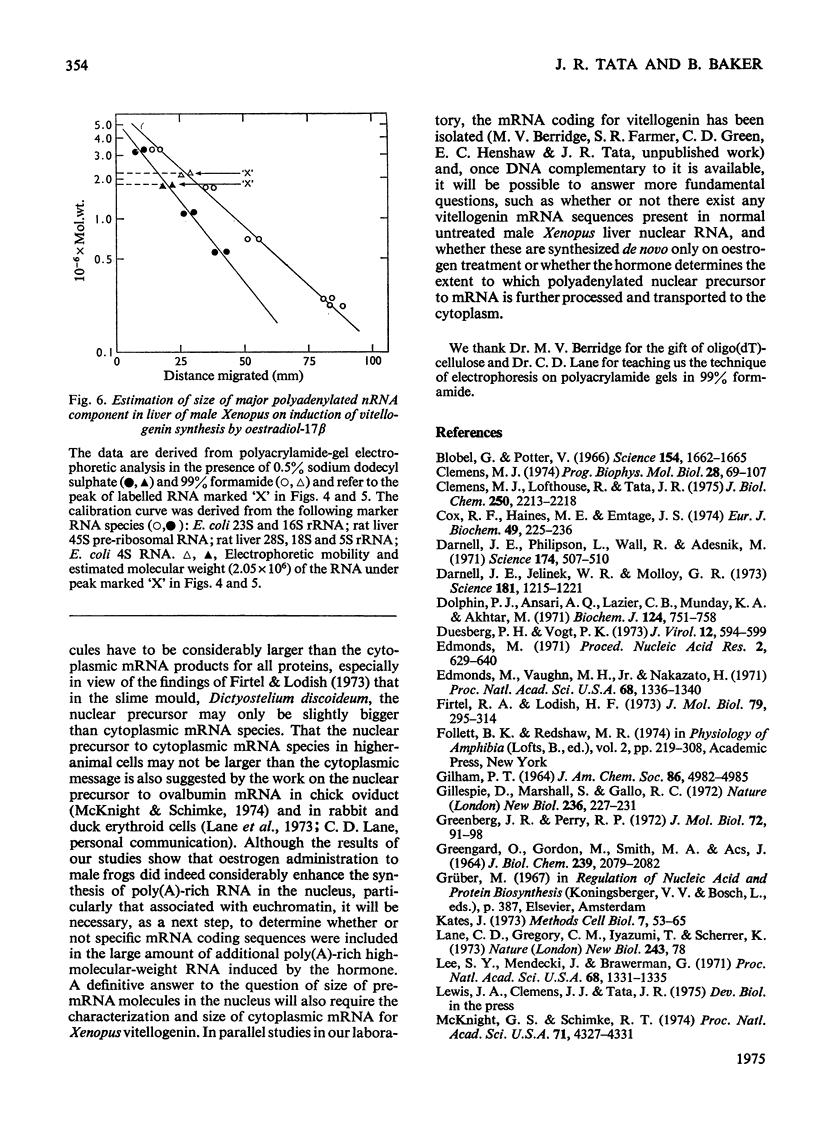
A 4-8-fold increase in the rate of hepatic nuclear RNA synthesis occurred within 11 h after a single injection of oestradiol-17 beta to male Xenopus to induce egg-yolk protein synthesis. 2. By using a gentle procedure for fractionating nuclei into their major structurally different components [J. R. Tata& B. Baker (1974) Exp. Cell Res. 83. 111-124], it was found that the hormone-induced increase in the total amount of newly made RNA was associated with a 2-10-fold increase in the poly(A) content of nuclear RNA. 3. When the poly (A) content of nuclear RNA was determined by hybridization to poly[3H](U) or specific binding to oligo(dT)-cellulose, most of the increase (10-fold) in poly (A) content of newly synthesized RNA was associated with the euchromatin fractions, whereas the increase was less marked in the other subnuclear fractions. 4. Resolution of nuclear RNA into poly (A)-poor and poly(A)-rich RNA species by chromatography on oligo(dT)-cellulose, followed by polyacrylamide-gel electrophoresis with sodium dodecyl sulphate or in the pressence of 99% formamide, revealed that the hormone caused a preferential enhancement of high-molecular-weight (25S-60S) poly (A)-rich HnRNA (heterogeneous nuclear RNA,) much of which was associated with euchromatin and not with the nuclear sap. 5. Induction of vitellogenin in male frogs was in particular characterized by the appearance of a high-molecular-weight polyadenylated component exhibiting a peak at 35-36S, i.e. a molecular weight of approx. 2.05x10(6)+/-0.15x10(6). Although there is no evidence as yet that such a polyadenylated high-molecular-weight nuclear RNA species contains sequences corresponding to vitellogenin mRNA, it is possible that a high proportion of the most stable form of the putative nuclear precursor to vitellogenin mRNA induced by oestrogen in male Xenopus liver may be only marginally bigger than the cytoplasmic mRNA, and may at any one time be predominantly associated with the euchromatin fraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Lofthouse R., Tata J. R. Sequential changes in the protein synthetic activity of male Xenopus laevis liver following induction of egg-yolk proteins by Estradiol-17 beta. J Biol Chem. 1975 Mar 25;250(6):2213–2218. [PubMed] [Google Scholar]
- Clemens M. J. The regulation of egg yolk protein synthesis by steroid hormones. Prog Biophys Mol Biol. 1974;28:69–108. doi: 10.1016/0079-6107(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Dolphin P. J., Ansari A. Q., Lazier C. B., Munday K. A., Akhtar M. Studies on the induction and biosynthesis of vitellogenin, an oestrogen-induced glycolipophosphoprotein. Biochem J. 1971 Oct;124(4):751–758. doi: 10.1042/bj1240751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., GORDON M., SMITH M. A., ACS G. STUDIES ON THE MECHANISM OF DIETHYLSTILBESTROL-INDUCED FORMATION OF PHOSPHOPROTEIN IN MALE CHICKENS. J Biol Chem. 1964 Jun;239:2079–2082. [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Kates J. Detection and utilization of poly(A) sequences in messenger RNA. Methods Cell Biol. 1973;7:53–65. doi: 10.1016/s0091-679x(08)61771-9. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Gregory C. M., Iyazumi T., Scherrer K. The use of the Xenopus oocyte to prove the existence of messenger sequences in high molecular weight RNA. Nat New Biol. 1973 May 16;243(124):78–78. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Schibler U., Weber R. A new method for the isolation of undegraded nuclear and cytoplasmic RNA from liver of Xenopus larvae. Anal Biochem. 1974 Mar;58(1):225–230. doi: 10.1016/0003-2697(74)90461-8. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Rhoads R. E., Palacios R., Sullivan D. Ovalbumin mRNA, complementary DNA and hormone regulation in chick oviduct. Acta Endocrinol Suppl (Copenh) 1973;180:357–379. doi: 10.1530/acta.0.074s357. [DOI] [PubMed] [Google Scholar]
- Sullivan N., Roberts W. K. Characterization and poly(adenylic acid) content of Ehrlich ascites cell ribonucleic acids fractionated on unmodified cellulose columns. Biochemistry. 1973 Jun 19;12(13):2395–2403. doi: 10.1021/bi00737a005. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. Sub-nuclear fractionation. I. Procedure and characterization of fractions. Exp Cell Res. 1974 Jan;83(1):111–124. doi: 10.1016/0014-4827(74)90694-6. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. Sub-nuclear fractionation. II. Intranuclear compartmentation of transcription in vivo and in vitro. Exp Cell Res. 1974 Jan;83(1):125–138. doi: 10.1016/0014-4827(74)90695-8. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. Sub-nuclear fractionation. III. Sub-nuclear distribution of poly(A)-rich RNA. Exp Cell Res. 1975 Jun;93(1):191–201. doi: 10.1016/0014-4827(75)90439-5. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Nickol J. M., Ho T., Jared D. W. Studies on amphibian yolk. X. The relative roles of autosynthetic and heterosynthetic processes during yolk protein assembly by isolated oocytes. Dev Biol. 1972 Nov;29(3):255–272. doi: 10.1016/0012-1606(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Kenney F. T. Regulation of yolk protein synthesis in amphibian liver. I. Induction of lipovitellin synthesis by estrogen. Biochim Biophys Acta. 1972 May 29;269(3):485–492. doi: 10.1016/0005-2787(72)90136-0. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Lee K. L., Kenney F. T. Regulation of yolk protein synthesis in amphibian liver. II. Elevation of ribonucleic acid synthesis by estrogen. Biochim Biophys Acta. 1972 May 29;269(3):493–504. doi: 10.1016/0005-2787(72)90137-2. [DOI] [PubMed] [Google Scholar]


