Abstract
Background
Two phenotypes of insomnia disorder (ID) have been identified based on objective total sleep duration (TST): one with short sleep duration (ISSD) and another with normal sleep duration (INSD). Recent proposals suggested that insomnia with objective short-sleep duration (TST < 7 h) is associated with impaired inhibitory function, leading to a dysregulation of cortical inhibition, which may underlie its prevalence. This study investigated the status of impaired response inhibition in these two phenotypes and examined the potential different effect of response inhibition training on these two phenotypes.
Methods
Twenty-two healthy controls (HC) and eighty-one patients with ID were recruited, with IDs further categorized into ISSD and INSD (with TST ≥ 7 h). Clinical behavior measures, including the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Pre-sleep Arousal Scale (PSAS), objective sleep characteristics assessed by all-night sleep electroencephalography, and the accuracy of NoGo trials in the Go/NoGo task were compared among the three groups. Subsequently, within each ID phenotype, participants were divided into training and blank control sub-groups. The two training sub-groups completed Adaptive Go/NoGo training task (Through adaptive difficulty adjustment, the task trains participants' inhibitory control) 15 times over 3 weeks, and all IDs were assessed using sleep-related subjective and objective measures and Go/NoGo task before and after the intervention.
Results
ISSD patients exhibited significantly longer sleep latency (p = 0.003) compared to HC, while wakefulness duration (p = 0.004) and light sleep duration (p < 0.001) were shorter than INSD. No significant differences in objective sleep characteristics were observed between INSD and HC. Following adaptive training, the ISSD training sub-group showed decreased scores in PSQI (p = 0.039) and ISI (p = 0.053) compared to their blank control sub-group. In the INSD groups, both training and blank control sub-groups demonstrated reductions in PSQI (p < 0.001), ISI (p < 0.001), and the cognitive arousal sub-dimension of the PSAS scores (p = 0.003) in the post-session test.
Conclusions
Impaired response inhibition is a characteristic of ISSD, potentially indicating dysfunctional cortical inhibition, whereas INSD pathogenesis may be related to cognitive-emotional arousal. Response inhibition training effectively alleviates sleep problems in ISSD. These findings provide new insights for developing precise intervention strategies in ID.
Trial registration
The study was prospectively registered on May 30, 2024, in Chinese Clinical Trials registry (ChiCTR2400085063).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03813-1.
Keywords: Insomnia disorder, Short sleep duration phenotype, Inhibitory control, Cognitive training
Background
Insomnia disorder (ID) is a highly prevalent sleep disorder, affecting approximately 30% ~ 48% of the global population [1–3]. ID significantly impairs patients' sleep quality, emotional well-being, daytime functioning, and cognitive abilities [4–7]. Response inhibition is a crucial component of cognitive function, allowing individuals to rapidly cancel inappropriate actions or suppress prepotent responses [8, 9]. Additionally, previous research has indicated that impaired inhibitory control may be critical to developing and maintaining ID [10, 11]. However, there is still no consensus on whether response inhibition is impaired in ID patients. Some studies indicate that ID patients exhibit poorer response inhibition compared to healthy controls [10–13], while others do not support this [14–16]. These discrepancies may result from small sample sizes (most studies had sample sizes of 8–30) and the different phenotypes of ID.
ID is a highly heterogeneous disorder, and the influence of its different phenotypes on outcome variables should be emphasized [17–19]. According to the International Classification of Sleep Disorders-2 (ICSD-2), ID can be categorized as psychophysiological insomnia (characterized by complaints of sleep difficulties and objective short sleep duration, ISSD) and paradoxical insomnia (characterized by complaints of sleep difficulties but normal objective sleep duration, INSD). However, the International Classification of Sleep Disorders-3 (ICSD-3) no longer distinguishes between these phenotypes, referring to them collectively as chronic insomnia [20, 21], which is debatable. Some research, including several meta-analyses [5, 6, 22] and empirical studies [23, 24] suggest that objective sleep duration is essential in determining whether cognitive functioning is impaired in ID. Generally, ISSD showed extensive cognitive impairment compared to INSD, whereas there was no difference between INSD and healthy individuals [22]. Unfortunately, previous studies examining the effect of objective sleep duration on cognitive performance in ID have not directly addressed response inhibition. Therefore, whether ISSD and INSD exhibit different response inhibition profiles requires further empirical evidence.
A complex yet valuable question is whether impaired inhibitory control is a consequence of ID or a contributing factor. Hyperarousal perspective, a key concept in ID, suggests that excessive pre-sleep arousal perpetuates the disorder, primarily involving cognitive, somatic, and cortical arousal. Cognitive arousal refers to persistent cognitive activation caused by thoughts, perceptions, attention, beliefs, attributions, and expectations, while pre-sleep cognitive arousal specifically refers to activation before sleep onset, often manifesting as worry and rumination about sleep [25]. Existing research hints at a relationship between inhibitory function and cognitive arousal. A meta-analysis found a significant positive correlation between rumination and inhibitory control [26], and another study showed that poorer inhibitory control in ID patients was associated with higher rumination levels and more severe insomnia symptoms [27]. Physiological and cortical arousal are closely related, with cortical arousal reflecting brain-level activation. One study suggested that ID patients may require more energy in the prefrontal cortex during wakefulness to maintain normal cognitive functions, including inhibitory control, and that dysfunction in this region may lead to compensatory mechanisms in other cortical areas, resulting in reduced pre-sleep cortical inhibition and subsequent over-arousal [28]. Thus, improving inhibitory control may help alleviate excessive arousal in insomnia patients. Recent studies have focused on using inhibitory control-based cognitive training to enhance prefrontal cortex function and alleviate clinical symptoms in populations with anxiety [29] and eating disorders [30]. Therefore, computerized cognitive training (CCT) targeting inhibitory control may offer a potential method for mitigating insomnia symptoms.
It is essential to explore the effects of CCT on ID. Cognitive–behavioral therapy for insomnia (CBT-I) is a widely used intervention for ID. However, research indicates that CBT-I is less effective for ISSD when comparing with INSD [31, 32]. The reason CBT-I may be ineffective for ISSD could be that CBT-I focus on addressing unhealthy sleep-related behaviors and beliefs, often characteristic of INSD [33] rather than improving cognitive function. ISSD is regarded as a biologically severe subtype of ID [34], emphasizing the need for specific interventions. Considering the growing body of research evidence indicating that impaired inhibitory control may be a significant feature of the ISSD phenotype compared to INSD [6, 22, 23], it is reasonable to believe that the targeted training focusing on inhibitory control could improve the sleep issues in ISSD. In contrast, such training may not have the same effective for INSD, as the inhibitory control deficits characteristic of the INSD phenotype do not appear to be as pronounced.
Several studies have introduced CCT in ID patients [35, 36]; however, these studies did not evaluate the patients' objective sleep duration. Consequently, the validity of these findings might be influenced by sample characteristics, potentially comprising mainly ISSD patients. When comparing with INSD, we propose that CCT might be more effective in ISSD patients who exhibit significantly impaired inhibitory control. Previous research utilized the CogniFit® cognitive training program, which encompasses various cognitive tasks. We posit that concentrating exclusively on inhibitory control training could be sufficient and less burdensome for patients. This is supported by a recent study employing Transcranial Magnetic Stimulation (TMS) to enhance inhibitory control and improve sleep in ID patients [37]. Additionally, previous interventions with multiple task types had excessively long durations (8 weeks), leading to high dropout rates (42%), which is a criticism of CBT-I [38]. In contrast, CCTs focusing on inhibitory control generally have shorter intervention periods (3–4 weeks) [39–41].
Based on the above background, the present study explored two questions: Q1. Does the inhibitory control function differ among the two insomnia phenotypes (ISSD and INSD) and healthy controls (HC)? We hypothesized that ISSD would have worse inhibitory control than HC, but INSD would not. Q2. Does the CCT intervention based on response inhibition function bring beneficial effects (improvements in response inhibition function and sleep-related measures) only to ISSD but not INSD? We hypothesized that the CCT interventions could significantly improve response inhibition function and sleep-related measures in ISSD but not INSD.
Methods
Study design
The study is a randomized double-blind trial. Patients with insomnia disorder (ID) and sex- age-matched healthy controls (HCs) were recruited through electronic advertisements and posters in Chongqing, China. From a total of 136 participants who were initially contacted, 103 met the inclusion criteria and agreed to participate in the study (20 not meet the inclusion criteria and 13 declined to participate). The inclusion criteria for ID were as follows: (1) Criteria for ID meeting the DSM-V (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) were determined to be met after a semi-structured interview conducted by a professional psychological practitioner; (2) Pittsburgh Sleep Quality Index (PSQI) ≥ 7 and Insomnia Severity Index (ISI) ≥ 11; (3) experiencing sleep disturbances for at least one month; (4) no other psychiatric or physiological diseases or other sleep disorders. The inclusion criteria for HCs were: (1) PSQI < 7 and ISI < 11; (2) no history of shift work or sleep complaints; (3) no other psychiatric history or brain injury.
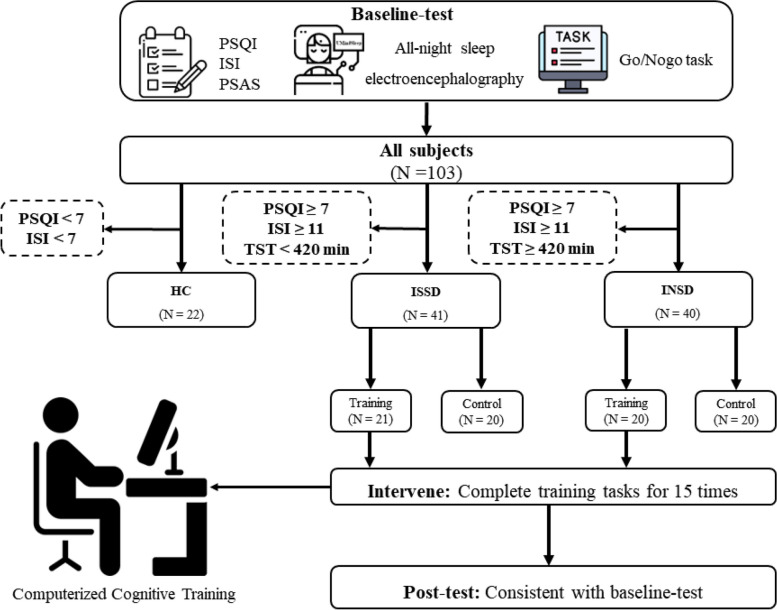
All participants signed informed consent forms confirming their participation and full knowledge of the study protocol, and they were compensated in cash. The study was approved by the ethical review committee of the Faculty of Psychology, Southwest University (Ethics approval number: H24061) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2400085063). Data collection took place between May 2024 and July 2024. This study adheres to CONSORT guidelines on reporting [42]. The procedures (Fig. 1) were performed following the Declaration of Helsinki.
Fig. 1.
Flow chart of the study. The sample was first divided into healthy control (HC) and patients with insomnia disorder (IDs) by the Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI). Then, IDs were further categorized into objective short sleep duration (ISSD) and objective normal sleep duration (INSD) by objective total sleep time measured by All-night sleep electroencephalography. This was followed sequentially by the intervention period (completing 15 computerized training tasks during 3 weeks) and post-testing. PSAS, Pre-sleep arousal scale. TST; Total Sleep Time measured by all night sleep electroencephalography
Outcome measures
The outcome measures in this study align with the preregistration, and no outcomes have been omitted. All outcome measures were collected both before and immediately after the end of the intervention phase. The primary outcomes include the PSQI and ISI, while the secondary outcomes include the Pre-sleep Arousal Scale, objective sleep parameters measured by all-night sleep EEG, and response inhibition assessed by the Go/No-Go task. Further details are provided below.
Primary outcomes
Pittsburgh sleep quality index
The PSQI was used to assess subjective sleep quality, including 19 items covering 7 clinical components (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medicines, and daytime functioning) [43]. The total PSQI score (range 0 to 21) is the sum of the 7 clinical components (range 0 to 3), with higher scores reflecting poorer sleep. Although Buysse and colleagues suggested using 5 as the cut-off for poor sleep [43], Chinese researchers found that using 7 produced better results in the Chinese adult population [44]. Therefore, we used 7 as the cut-off in this study.
Insomnia severity index
The ISI assessed the presence and severity of insomnia symptoms, including 7 items along a five-point scale (0–4) [45]. The total ISI score ranges from 0 (no insomnia) to 28 (severe insomnia), with 10 generally used as the cut-off to identify individuals with clinical insomnia in the community [46]. Therefore, we used 10 as the cut-off in this study.
Secondary outcomes
Pre-sleep arousal scale
The Pre-sleep arousal scale (PSAS) assessed arousal right before sleep (somatic and cognitive arousal, PSAS-SA and PSAS-CA), including 16 items along a five-point scale (1–5). Somatic arousal (1–8) and cognitive arousal (9–16) each consisted of 8 questions, with higher total scores indicating higher levels of arousal [47].
All-night sleep electroencephalography
UMindSleep is a wearable forehead sleep recorder designed for sleep monitoring (UMindSleep, EEGSmart Co., Ltd.). Although it uses a single-channel electroencephalogram (EEG), it has been shown to be highly consistent (kappa agreements range = 0.69–0.79) with polysomnography (PSG) in recording sleep-related parameters [48]. We used the device's built-in algorithm to extract the total sleep time (TST) as the primary measure, with other measures including sleep latency (SL) and the time of each sleep stage (wakefulness, WT; light sleep, LST; deep sleep, DST).
Go/NoGo task
The Go/NoGgo task (GNT) was used to evaluate participants’ inhibitory control before and after the intervention. GNT was compiled using the jsPsych framework (version 6.0.4) in JavaScript, an open-source library for creating psychology experiments that offers a wealth of features and flexibility [49]. The task contained 210 trials, with the first 10 as practice trials, and the remaining 200 as testing trials. Participants were required to press the space key when a blue box was displayed (Go trial) and not press the key when an orange box was displayed (NoGo trial), with a ratio of 9 Go trials to 1 NoGo trial. In each trial, the stimuli (blue or orange boxes) were presented for 750 ms, and participants had to choose whether to respond based on the color. The subsequent trial began once the participant pressed the space key or the presentation duration exceeded 750 ms. Feedback was given on practice trials but not on test trials. The dependent measure was the accuracy of NoGo trials (NoGo-ACC).
Training task
The training task, called the Adaptive GNT, is adapted from the GNT. The Adaptive GNT uses images of two animals (cat and dog), two fruits (banana and apple), and two celestial bodies (sun and moon). The task comprises six blocks, each containing 100 trials. Each pair of pictures is used in two blocks of 100 trials each. The order of blocks concerning the image pairs is random, but each picture pair is used in two consecutive blocks. In each set of 100 trials, participants respond to a specific stimulus (e.g., the sun) but withhold response to another stimulus (e.g., the moon). In the following 100 trials, participants respond to the previous NoGo stimulus but not to the previous Go stimulus. The ratio of Go to NoGo stimuli is 7:3. Adaptive refers to the dynamic adjustment of task difficulty based on the participant's performance. This dynamic adjustment ensures that the task difficulty is tailored to everyone, allowing participants to face an appropriate level of challenge to enhance their abilities, while avoiding excessive difficulty that could lead to a loss of motivation. Task difficulty is adjusted by varying the inter-stimulus interval time (ISIT), starting at 1600 ms during the initial training phase, depending on the participants' average percentage of correct answers in the preceding stage. If the accuracy rate for NoGo trials falls below 60%, the response time (RT) is increased by 200 ms to maintain motivation. If the accuracy rate for NoGo trials exceeds 90%, the RT is decreased by 200 ms. If the accuracy rate for NoGo trials ranges between 60 and 90%, the RT remains unchanged. Participants are instructed to respond as quickly and accurately as possible. After each block, participants receive feedback regarding their accuracy. Training spans 15 sessions during 3 weeks, each involving 20 min of task completion.
Sample size
The sensitivity power analysis was conducted to determine study power (G*Power 3.1.9.4). For the cross-sectional group comparison, power calculations (α = 0.05, power = 0.80) found that the current sample size had the capacity to detect a moderate amount of effect (f = 0.31). For the analysis of the subsequent intervention phase, power calculations (α = 0.05, power = 0.80) also found that the current sample size had the capacity to detect a moderate amount of effect (fISSD = 0.23, fINSD = 0.23).
Procedure
The study consisted of three phases: the baseline test, the training/control period, and the post-test phase. HCs were only involved in the baseline test. The baseline test was completed over two days, and tasks included the PSQI, ISI, PSAS, all-night sleep EEG recordings (Wear the device at home to complete all-night sleep EEG recordings), and GNT. IDs were then categorized into ISSD (TST < 7 h) and INSD (TST ≥ 7 h) based on objective sleep duration and further randomly divided into training and control groups within ISSD and INSD. The choice of 7 h rather than other values like 6 h or 6.5 h as cut-off is based on our laboratory's most recent work (unpublished data), which suggests that 7 h may be more appropriate as the cut-off value. The training process was conducted in a standard computerized behavioral experiment, supervised by an experimental assistant. Participants in the training group completed adaptive GNT training 15 times, one (lasting approximately 20 min) per day, on every weekday (between 9 a.m. and 8 p.m., at least 1 h before go to bed) over a three-week period, while the blank control group did not perform any tasks. After the training period, all IDs (both ISSD and INSD) completed the post-test, which included the same tasks as the baseline test and following the same setting.
Randomisation and blinding
The participants in the ISSD and INSD were randomly assigned to training or control group, based on simple randomization (computer-generated random numbers for the randomization process). Research staff who were not aware of the training allocation conveyed computer-generated random numbers to the non-blinded staff, who then communicated the randomization assignment and training plans to the participants. Participants were not informed of the study hypothesis. All outcome assessments and data analyses were conducted by blinded research staff who were unaware of the treatment allocations. No interim analyses were conducted.
Statistical analysis
Statistical analyses were conducted after all data collection was completed. The Shapiro–Wilk test was used to assess the normality of the data distribution. First, we tested the differences in task measurements among the three groups (HC, ISSD, INSD) at baseline. If the data met the normal distribution assumption, we used one-way analysis of variance (ANOVA); otherwise, we used the Kruskal–Wallis H test for non-parametric analysis. When a significant main effect is found, pairwise comparisons are further conducted, with p-values adjusted using the Bonferroni correction. Second, the associations between measures were analyzed using partial correlation analysis, in which age and gender were set as covariates. We then compared the baseline characteristics between the training and control groups within both the ISSD and INSD cohorts to identify any pre-intervention differences. Independent samples t-test was performed for normally distributed variables, while Mann–Whitney U test was applied to non-normally distributed data. Finally, for the intervention effect, we performed a 2 (condition: training, control) × 2 (session: pre-test, post-test) repeated measures ANOVA within each of the two groups (ISSD, INSD) for the data that follows the normal distribution. When the data violated the assumptions of normality or homogeneity of variance, we used the rank-based Scheirer-Ray-Hare test.
Results
Sample characteristics
For the cross-sectional group comparison, no participants were excluded from the data analysis. The demographic characteristics and sleep parameters of the participants are shown in Table 1. There were 22 participants in the HC group, 41 in the ISSD, and 40 in the INSD, with mean age of 35.55 years (SD = 1.47), 20.44 years (SD = 1.67), and 20.80 years (SD = 1.84), respectively.
Table 1.
Demographic and clinical characteristics of participants
| HC (N = 22) | ISSD (N = 41) | INSD (N = 40) | p | |
|---|---|---|---|---|
| Male, no. (%) | 6 (27%) | 10 (24%) | 7 (18%) | .48a |
| Age, mean (SD) | 35.55 (12.47) | 20.44 (1.67) | 20.80 (1.84) | < .001b |
| PSQI, mean (SD) | 3.09 (0.75) | 10.34 (1.94) | 9.93 (2.38) | < .001b |
| PSQI-TST, mean (SD) | 502.64 (63.01) | 390.00 (60.37) | 396.00 (59.69) | < .001b |
| ISI, mean (SD) | 5.32 (1.17) | 14.59 (3.00) | 15.58 (3.66) | < .001b |
| PSAS, mean (SD) | 29.59 (8.09) | 47.37 (10.41) | 47.98 (10.50) | < .001b |
| PSAS-SA, mean (SD) | 12.14 (2.70) | 19.88 (5.68) | 18.98 (6.44) | < .001b |
| PSAS-CA, mean (SD) | 17.45 (6.04) | 27.49 (6.27) | 29.00 (5.59) | < .001b |
| TST, mean (SD) | 456.61 (78.72) | 360.54 (45.57) | 507.34 (60.29) | < .001b |
| SL, mean (SD) | 19.32 (14.41) | 57.40 (50.31) | 49.34 (46.01) | .003b |
| WT, mean (SD) | 59.16 (56.51) | 44.76 (46.90) | 94.29 (79.36) | .006b |
| LST, mean (SD) | 246.11 (87.60) | 208.07 (49.35) | 252.98 (64.67) | .009c |
| DST, mean (SD) | 43.57 (32.93) | 53.65 (30.31) | 65.43 (32.09) | .008c |
HC healthy control, ISSD insomnia with objective short sleep duration, INSD insomnia with objective normal sleep duration, PSQI Pittsburg Sleep Quality Index, PSQI-TST Subjective Total Sleep Duration reported by PSQI’s item 4, ISI Insomnia Severity Index, PSAS Pre-sleep arousal scale, SA Somatic arousal, CA Cognitive arousal, TST Total Sleep Time, SL Sleep Latency, WT Wakefulness Time, LST Light Sleep Time, DST Deep Sleep Time
athe p-value of χ2 t-test
bthe p-value of Kruskal–Wallis H test
cthe p-value of one-way analysis of variance
For the analysis of the subsequent intervention phase, one participant from the ISSD control group and one participant from the INSD training group were excluded from data analysis due to dropping out for personal reasons unrelated to the study. The final sample sizes were: ISSD-Training: 21 (Male: 5, Mage = 20.57 years, SDage = 1.54), ISSD-Control: 19 (Male: 4, Mage = 20.32 years, SDage = 1.89), INSD-Training: 19 (Male: 3, Mage = 20.42 years, SDage = 1.50), INSD-Control: 20 (Male: 4, Mage = 21.05 years, SDage = 2.09). Baseline characteristics of the training and control groups within ISSD and INSD are provided in Table 2, showing no significant differences between groups before the intervention.
Table 2.
Baseline characteristics of participants in training and control group within two insomnia phenotypes
| ISSD | p | INSD | p | |||
|---|---|---|---|---|---|---|
| Training (N = 21) | Control (N = 19) | Training (N = 19) | Control (N = 20) | |||
| Male, no. (%) | 5(23.8%) | 4(21.1%) | 0.855a | 3(15.8%) | 4(20.0%) | 0.732a |
| Age, years | 20.57(1.54) | 20.32(1.89) | 0.491b | 20.42(1.50) | 21.05(2.09) | 0.677b |
| NoGo-ACC | 0.88(0.07) | 0.86(0.07) | 0.476b | 0.92(0.07) | 0.90(0.06) | 0.338b |
| PSQI | 10.33(2.18) | 10.32(1.77) | 0.848b | 9.95(1.96) | 9.90(2.83) | 0.541b |
| PSQI-TST, min | 384.29(64.08) | 397.89(58.17) | 0.488c | 394.75(58.53) | 399.00(63.15) | 0.729b |
| ISI | 14.14(3.35) | 15.00(2.62) | 0.137b | 15.47(2.67) | 15.45(4.43) | 0.552b |
| PSAS | 45.86(10.22) | 49.26(10.83) | 0.162b | 45.32(9.83) | 49.70(10.53) | 0.188c |
| PSAS-SA | 19.38(5.30) | 20.74(6.12) | 0.457c | 16.89(6.57) | 20.45(5.69) | 0.079c |
| PSAS-CA | 26.48(6.27) | 28.53(6.42) | 0.232b | 28.42(5.17) | 29.25(6.05) | 0.649c |
| TST, min | 371.55(43.98) | 345.79(58.44) | 0.083b | 508.03(60.56) | 513.70(94.43) | 0.833b |
| SL, min | 68.02(56.30) | 45.82(42.77) | 0.180b | 47.42(38.2) | 50.05(54.16) | 0.933b |
| WT, min | 49.07(45.75) | 42.05(49.47) | 0.542b | 89.5(68.79) | 103.73(99.12) | 0.922b |
| LST, min | 210.40(45.84) | 200.34(65.08) | 0.572c | 273.53(49.03) | 239.38(87.69) | 0.060b |
| DST, min | 52.86(33.76) | 53.32(23.8) | 0.961c | 58.45(30.12) | 70.93(33.87) | 0.233c |
| PSQISQ | 1.95(0.38) | 1.95(0.4) | 0.965b | 2.00(0.47) | 2.10(0.55) | 0.532b |
| PSQILATEN | 2.19(0.75) | 2.47(0.61) | 0.229b | 2.26(0.65) | 2.15(0.88) | 0.774b |
| PSQIDURAT | 1.05(1.02) | 0.63(0.68) | 0.219b | 0.63(0.76) | 0.55(1.00) | 0.409b |
| PSQISE | 0.95(1.02) | 0.63(0.9) | 0.258b | 0.95(0.78) | 0.70(0.98) | 0.205b |
| PSQIDISTB | 1.33(0.48) | 1.79(0.71) | 0.057b | 1.63(0.68) | 1.75(0.72) | 0.601b |
| PSQIUOSN | 0.24(0.77) | 0.05(0.23) | 0.573b | 0(0) | 0.05(0.22) | 0.330b |
| PSQIDF | 2.62(0.5) | 2.79(0.42) | 0.361b | 2.47(0.77) | 2.60(0.60) | 0.728b |
ISSD Insomnia with objective short sleep duration, INSD Insomnia with objective normal sleep duration, NoGo-ACC accuracy of correct NoGo trials in the Go/NoGo task, PSQSI Pittsburg Sleep Quality Index, PSQI-TST Subjective Total Sleep Duration reported by PSQI’s item 4, ISI Insomnia Severity Index, PSAS Pre-sleep arousal scale, SA Somatic arousal, CA Cognitive arousal, TST Total Sleep Time, SL Sleep Latency, WT Wakefulness Time, LST Light Sleep Time, DST Deep Sleep Time, PSQISQ PSQI Sleep Quality, PSQILATEN PSQI Sleep Latency, PSQIDURAT PSQI Sleep Duration, PSQISE PSQI Sleep Efficiency, PSQIDISTB PSQI Sleep Disturbance, PSQIUOSM PSQI Use of Sleep Medicines, PSQIDF PSQI Daytime Functioning
athe p-value of χ2 t-test
bthe p-value of Mann–Whitney U test
cthe p-value of two sample t test
Comparison of demographic and clinical characteristics between three groups
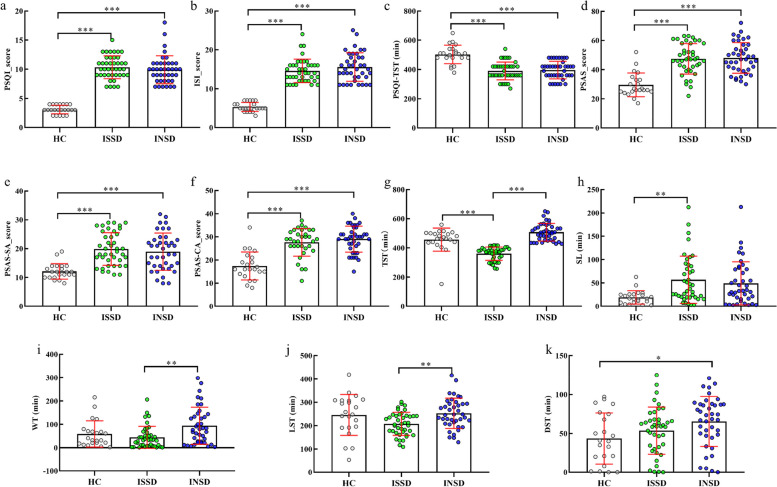
The Age, PSQI, PSQI-TST (Subjective Total Sleep Duration reported by PSQI’s item 4), ISI, PSAS, SA, CA, TST, SL, and WT were analyzed by non-parametric analysis, as those measures did not follow a normal distribution The results showed that there are no significant gender differences among the groups, but significant age differences were observed. The HC group was significantly older than both the ISSD and INSD groups (p < 0.001). The comparison results between groups show that (Table 1) significant between-group differences in PSQI (H = 53.180, p < 0.001), ISI (H = 52.645, p < 0.001), PSAS (H = 34.926, p < 0.001), TST (H = 69.659, p < 0.001), SL (H = 11.793, p = 0.003), WT (H = 10.238, p = 0.006), LST (F = 4.925, ηp2 = 0.90, p = 0.009), and DST (F = 5.022, ηp2 = 0.92, p = 0.008). Further paired comparisons (Fig. 2) showed that PSQI (p < 0.001, Cliff's Delta = 1.00, 95% CI: 0.99, 1.00), ISI (p < 0.001, Cliff's Delta = 1.00, 95% CI: 0.99, 1.00), PSAS (p < 0.001, Cliff's Delta = 0.80, 95% CI: 0.58, 0.92), Somatic arousal (PSAS-SA) (p < 0.001, Cliff's Delta = 0.79, 95% CI: 0.58, 0.90), and Cognitive arousal (PSAS-CA) scores (p < 0.001, Cliff's Delta = 0.74, 95% CI: 0.48, 0.88) were significantly lower in the HC group compared to the ISSD. The PSQI (p < 0.001, Cliff's Delta = 1.00, 95% CI: 0.99, 1.00), ISI (p < 0.001, Cliff's Delta = 1.00, 95% CI: 0.99, 1.00), PSAS (p < 0.001, Cliff's Delta = 0.84, 95% CI: 0.63, 0.94), Somatic arousal (PSAS-SA) (p < 0.001, Cliff's Delta = 0.66, 95% CI: 0.40, 0.81), and Cognitive arousal (PSAS-CA) scores (p < 0.001, Cliff's Delta = 0.82, 95% CI: 0.59, 0.93) were also significantly lower in the HC group compared to the INSD. However, there were no significant differences between ISSD and INSD. Additionally, the ISSD group exhibited significantly shorter TST than HC (p < 0.001, Cliff's Delta = −0.87, 95% CI: −0.99, −0.09) and INSD (p < 0.001, Cliff's Delta = 1.00, 95% CI: −1.00, −0.99), with no significant difference observed between HC and INSD (p = 0.229, Cliff's Delta = 0.16, 95% CI: −0.08, 0.29). SL was shorter in the HC group than ISSD (p = 0.003, Cliff's Delta = 0.51, 95% CI: 0.24, 0.70), but not different from INSD (p = 0.105, Cliff's Delta = 0.08, 95% CI: −0.17, 0.32). WT (p = 0.004, Cliff's Delta = −0.41, 95% CI: −0.60, −0.17) and LST (p < 0.001, Cohen’s d = 0.78, 95% CI: −80.04, −9.76) were shorter in ISSD compared to INSD. Finally, INSD had longer DST than HC (p = 0.031, Cohen’s d = 0.67, 95% CI: −42.26, −1.45), with no significant difference observed between ISSD and HC (p = 0.690, Cohen’s d = 0.32, 95% CI: −30.39, 10.24).
Fig. 2.
The difference between three groups. a ~ i were analyzed by non-parametric analysis and j ~ k by parametric test. HC, healthy control; ISSD, insomnia with objective short sleep duration; INSD, insomnia with objective normal sleep duration; PSQI, Pittsburg Sleep Quality Index; PSQI-TST, Subjective Total Sleep Duration reported by PSQI’s item 4; ISI, Insomnia Severity Index; PSAS, Pre-sleep arousal scale; SA, Somatic arousal; CA, Cognitive arousal; TST, Total Sleep Duration; SL, Sleep Latency; WT, Wakefulness Time, LST, Light Sleep Time; DST, Deep Sleep Time. *p < 0.05; **p < 0.01; ***p < 0.001. All p-values are Kruskal–Wallis H test with Dunn post-test
Comparison of inhibitory control between three groups
The results of the Kruskal–Wallis H test showed a significant difference in NoGo-ACC among the groups, H = 13.967, p < 0.001. The ACC of ISSD (M = 0.87, SD = 0.07) was lower than these of INSD (M = 0.91, SD = 0.07, p = 0.006, Cliff's Delta = −0.37, 95% CI: −0.58, −0.12) and HC (M = 0.92, SD = 0.04, p = 0.004, Cliff's Delta = −0.51, 95% CI: −0.70, −0.25). No significant difference was found between the NoGo-ACC of INSD and that of HC (p = 1.00), as shown in Fig. 3.
Fig. 3.
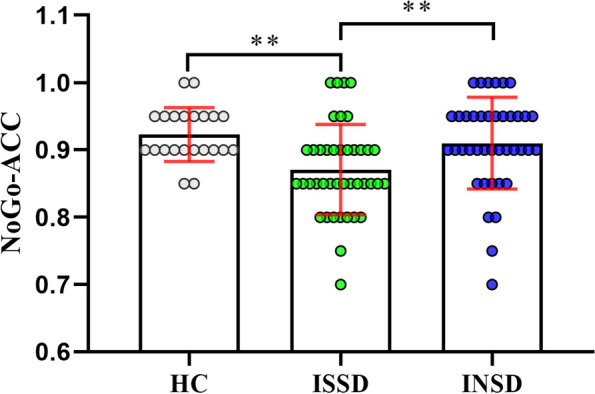
The difference of response inhibition between three groups. HC, healthy control; ISSD, insomnia with objective short sleep duration; INSD, insomnia with objective normal sleep duration; NoGo-ACC, accuracy of correct NoGo trials in the Go/NoGo task. **p < 0.01 (Kruskal–Wallis test with Dunn post-test)
Potential association between measures
Partial correlation analyses with gender and age as covariates within each group revealed a significant positive correlation between TST and NoGo-ACC (r = 0.44, p = 0.004) and between TST and LST (r = 0.57, p < 0.001) in the ISSD group (Additional file 1: Table S1). In the INSD group, no significant correlation was found between TST and NoGo-ACC, but there were significant positive correlations between TST and cognitive arousal (r = 0.34, p = 0.034) and between cognitive arousal and PSQI (r = 0.34, p = 0.033) (Additional file 1: Table S2).
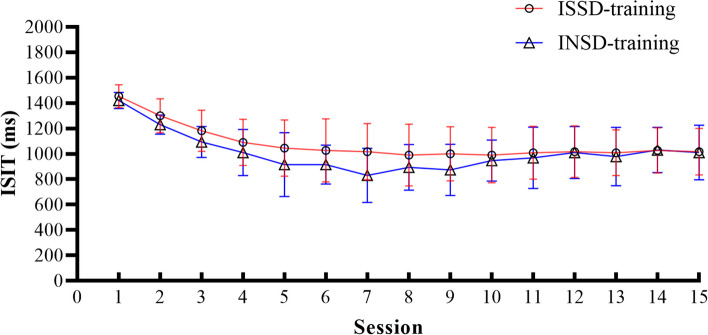
Training effects of ISSD and INSD
The performance of the two training groups with different ID phenotypes in each training session was shown in Fig. 4. In the ISSD-training group, the mean ISIT decreased from 1457.14 ms to 1019.05 ms with training sessions, F (1,20) = 22.27, p < 0.001, η2 = 0.58, indicating that the participants in training condition completed the training as required and made progress. For INSD-training group, it was also found that with the increase of training sessions, the mean ISIT decreased from 1421.05 ms to 1010.53 ms, F (1,18) = 30.62, p < 0.001, η2 = 0.63.
Fig. 4.
Mean (± SD) inter-stimulus interval time (ISIT) of participants in training conditions on the 15 training sessions for two groups. ISSD, insomnia with objective short sleep duration; INSD, insomnia with objective normal sleep duration
The transfer effect of training on inhibitory control ability
The results showed that in the ISSD group, the training group had a significant improvement in inhibitory control ability after training, while the control group did not. The non-parametric test revealed a significant main effect of the condition, H = 10.927, p < 0.001, no significant main effect of the session, H = 0.488, p = 0.485, and a significant interaction effect, H = 6.023, p = 0.014. Post hoc simple effects analysis indicated that the NoGo-ACC was significantly higher in the training group (M = 0.92, SD = 0.12) compared to the control group (M = 0.80, SD = 0.13) in the post-session test (p = 0.002, Cliff's Delta = −0.44, 95% CI: −0.70, −0.07). However, there was no significant difference between the training (M = 0.88, SD = 0.07) and control (M = 0.86, SD = 0.07) groups in the pre-session test (p = 0.476, Cliff's Delta = 0.30, 95% CI: −0.09, 0.61).
In the INSD group, no significant differences were found in inhibitory control ability between the training and control groups in the post-session test. The non-parametric test showed there were no significant effects (effect of the condition: H = 3.211, p = 0.073; effect of the session: H = 2.651, p = 0.104; interact effect: H = 0.374, p = 0.541).
The Table 3 shows the descriptive statistics of the training and control conditions measures pre- and post-session tests within two groups (ISSD and INSD).
Table 3.
Descriptive statistics of dependent measures in four conditions
| ISSD-Training (N = 21) | ISSD-Control (N = 19) | INSD-Training (N = 19) | INSD-Control (N = 20) | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| NoGo-ACC | 0.88 (0.07) | 0.92 (0.12) | 0.86 (0.07) | 0.80 (0.13) | 0.92 (0.07) | 0.89 (0.09) | 0.90 (0.06) | 0.85 (0.10) |
| PSQI | 10.33 (2.18) | 8.48 (2.69) | 10.32 (1.77) | 10.26 (1.52) | 9.95 (1.96) | 7.79 (1.58) | 9.90 (2.83) | 7.95 (2.70) |
| PSQI-TST, min | 384.29 (64.08) | 427.14 (68.35) | 397.89 (58.17) | 367.89 (47.79) | 394.75 (58.53) | 457.89 (50.84) | 399.00 (63.15) | 426.00 (64.27) |
| ISI | 14.14 (3.35) | 11.81 (3.52) | 15.00 (2.62) | 14.47 (2.46) | 15.47 (2.67) | 11.58 (3.66) | 15.45 (4.43) | 11.70 (4.55) |
| PSAS | 45.86 (10.22) | 41.52 (8.52) | 49.26 (10.83) | 45.74 (12.51) | 45.32 (9.83) | 41.42 (6.91) | 49.70 (10.53) | 44.60 (9.87) |
| PSAS-SA | 19.38 (5.30) | 16.19 (4.19) | 20.74 (6.12) | 19.37 (6.75) | 16.89 (6.57) | 15.26 (4.91) | 20.45 (5.69) | 18.70 (5.00) |
| PSAS-CA | 26.48 (6.27) | 25.33 (6.18) | 28.53 (6.42) | 26.37 (7.20) | 28.42 (5.17) | 26.16 (4.83) | 29.25 (6.05) | 25.90 (6.20) |
| TST, min | 371.55 (43.98) | 416.90 (90.15) | 345.79 (58.44) | 456.74 (100.91) | 508.03 (60.56) | 430.50 (95.12) | 513.70 (94.43) | 389.15 (94.60) |
| SL, min | 68.02 (56.30) | 54.36 (57.58) | 45.82 (42.77) | 52.63 (51.72) | 47.42 (38.20) | 59.32 (53.40) | 50.05 (54.16) | 68.65 (61.90) |
| WT, min | 49.07 (45.75) | 40.38 (49.87) | 42.05 (49.47) | 75.26 (89.63) | 89.50 (68.79) | 66.89 (62.91) | 103.73 (99.12) | 55.93 (59.69) |
| LST, min | 210.40 (45.84) | 235.38 (61.69) | 200.34 (65.08) | 235.47 (92.28) | 273.53 (49.03) | 225.89 (66.87) | 239.38 (87.69) | 210.83 (66.41) |
| DST, min | 52.86 (33.76) | 69.90 (21.77) | 53.32 (23.80) | 58.76 (22.99) | 58.45 (30.12) | 70.79 (25.39) | 70.93 (33.87) | 57.95 (28.64) |
ISSD insomnia with objective short sleep duration, INSD insomnia with objective normal sleep duration, PSQI Pittsburg Sleep Quality Index, PSQI-TST Subjective Total Sleep Duration reported by PSQI’s item 4, ISI Insomnia Severity Index, PSAS Pre-sleep arousal scale, SA Somatic arousal, CA Cognitive arousal, TST Total Sleep Time, SL Sleep Latency, WT Wakefulness Time, LST Light Sleep Time, DST Deep Sleep Time
The transfer effect of training on sleep
In this section, we focused on significant interaction effects, and the full results have been presented in Additional file 1 (Table S3 for ISSD, and Table S4 for INSD).
ISSD group
Due to issues with normal distribution, the non-parametric test was used to analyze PSQI, PSQI-TST, ISI, TST, SL, and WT, while mixed ANOVA was conducted for the remaining measures. The results indicated significant interaction effects on PSQI, PSQI-TST (Subjective Total Sleep Duration reported by PSQI’s item 4), and ISI (Additional file 2: Fig. S1.A). The PSQI was significantly lower in the post-session test (M = 8.48, SD = 2.69) compared to the pre-session test (M = 10.33, SD = 2.18) in the training condition, p = 0.039, Cliff's Delta = 0.42, 95% CI: 0.09, 0.66. However, the difference between the post-session test (M = 10.26, SD = 1.52) and the pre-session test (M = 10.32, SD = 1.77) was insignificant for the control condition, p = 0.848, Cliff's Delta = −0.04, 95% CI: −0.39, 0.32. The PSQI-TST of the training group (M = 427.14, SD = 68.35) was significantly longer than the control group (M = 367.89, SD = 47.79) in the post-session test, p = 0.015, Cliff's Delta = 0.51, 95% CI: 0.15, 0.75. However, the difference between the training group (M = 384.29, SD = 64.08) and the control group (M = 397.89, SD = 58.17) was not significant in the pre-session test, p = 0.464, Cliff's Delta = 0.13, 95% CI: −0.45, 0.22. For ISI, the ISI in the training condition was marginally lower in the post-session test (M = 11.81, SD = 3.52) than in the pre-session test (M = 14.14, SD = 3.35), p = 0.053, Cliff's Delta = 0.40, 95% CI: 0.03, 0.67. However, the difference between the post-session test (M = 14.47, SD = 2.46) and the pre-session test (M = 15.00, SD = 2.62) was insignificant for the control condition, p = 0.392, Cliff's Delta = 0.16, 95% CI: −0.23, 0.50. Additionally, of the seven subdimensions of the PSQI, training also had beneficial effects on the sleep duration and daytime functioning sub-dimensions, with the training group scoring significantly lower on the post-session test than on the pre-session. In contrast, there was no difference between the pre and post-tests in the control group. See the Additional file 2 (content in Sect. 1.1 ISSD group and Fig. S2) for a detailed report.
INSD group
Due to issues with normal distribution, the non-parametric test was used to analyze PSQI, PSQI-TST, ISI, TST, SL, and WT, while mixed ANOVA was conducted for the remaining measures. In the INSD group, the results did not reveal any significant interaction effect. In contrast, the results showed significant main effects of the session on PSQI, PSQI-TST, ISI, and CA, as shown in Additional file 2 (Fig. S1.B). The PSQI and ISI were significantly lower in the post-session test (PSQI: M = 7.87, SD = 2.20; ISI: M = 11.64, SD = 4.09) than in the pre-session test (PSQI: M = 9.92, SD = 2.41; ISI: M = 15.46, SD = 3.63), pPSQI < 0.001, Cliff's Delta = 0.47, 95% CI: 0.22, 0.65 and pISI < 0.001, Cliff's Delta = 0.55, 95% CI: 0.31, 0.72. The PSQI-TST was significantly longer in the post-session test (M = 441.54, SD = 59.58) than in the pre-session test (M = 396.92, SD = 60.18), p = 0.003, Cliff's Delta = −0.39, 95% CI: −0.58, −0.15. While CA in the post-session test (M = 26.03, SD = 5.50) was significantly lower than in the pre-session test (M = 28.85, SD = 5.58), p = 0.038, Cliff's Delta = 0.27, 95% CI: 0.01, 0.50. Additionally, no significant interaction effects were found across the seven sub-dimensions of the PSQI. However, significant main effects of the session on sleep quality, sleep efficiency, sleep disturbance, and daytime function were observed. See the Additional file 2 (content in Sect. 1.2 INSD group and Fig. S3) for a detailed report.
Discussion
The present study focuses on the impaired response inhibition in two insomnia phenotypes (ISSD and INSD) classified by objective sleep duration, and explores the potential effects of inhibitory control training on different phenotypes. The study's results first showed that both phenotypes differed significantly from health control in terms of several subjective sleep perceptions and objective sleep parameters. Simultaneously, the light sleep time was significantly shorter in ISSD than in INSD. Secondly, the response inhibition was found to be significantly worse in ISSD compared to HC, but not in INSD, while NoGo-ACC was significantly and positively correlated with the total sleep time in ISSD. Within the INSD group, PSAS-CA was significantly and positively correlated with both TST and PSQI. Finally, by applying inhibition control-based cognitive training, it was found that in the ISSD group, there was a significant improvement in sleep in the training group but not in the ISSD control group. In summary, these findings illustrate the critical influence of objective sleep duration on inhibitory function in ID and suggest that inhibition-related training may serve as a potential effective intervention for specific phenotypes.
Clinical and sleep-related differences between the two insomnia phenotypes
Both ID phenotypes showed significant complaints of poor sleep compared to the HC, as evidenced by higher PSQI, ISI, and PSQIDURAT scores and significantly higher scores on PSAS. Hyperarousal has been recognized as a main feature of ID [50]. Additionally, ISSD exhibited distinct differences in objective sleep parameters derived from EEG. Specifically, ISSD showed significantly longer objective sleep latency and deep sleep duration compared to healthy individuals. Conversely, ISSD exhibited shorter duration of wakefulness and light sleep compared to INSD. Importantly, no significant differences were observed between INSD and HC across all objective sleep parameters. This suggests that the sleep structure of INSD is no different from that of HC, which is consistent with the findings of another study that classified ID subtypes based on structural brain heterogeneity [17]. Zhang et al. (2023) proposed that one subtype is similar to paradoxical ID, retaining an intact sleep structure due to overcompensation of the temporal cortex but subjectively experiencing extremely poor sleep [17]. This explains why INSD is indistinguishable from healthy individuals in terms of objective sleep parameters but still subjectively reports sleep complaints.
Differences of response inhibition between two insomnia phenotypes
Previous studies on response inhibition in ID have yielded conflicting results, which may stem from the influence of different phenotypes. This study observed that the NoGo-ACC of ISSD was significantly lower than that of HC and INSD, whereas no significant difference was found between INSD and HC. Numerous studies have explicitly indicated cognitive impairment specifically in ISSD but not in INSD [5, 6, 22–24]. Although these studies did not directly investigate response inhibition, given its pivotal role in executive functioning [8], it is reasonable to speculate that the executive function deficits observed in ISSD may primarily result from impaired response inhibition.
Association between behavioural and sleep-related measures
Many studies have linked response inhibition to cortical hyperarousal in ID [11, 51, 52], suggesting that the pathogenesis of INSD and ISSD may differ. Specifically, we speculated that ISSD is associated with cortical hyperarousal, whereas INSD may stem from cognitive-level concerns. To investigate this further, correlation analyses were conducted between behavioral and sleep-related measures.
In the ISSD group, we identified a significant positive correlation between TST and NoGo-ACC. Surprisingly, NoGo-ACC did not correlate with other subjective and objective sleep-related measures, such as PSQI and ISI. One possible explanation is that the behavioral measures may not adequately capture cortical hyperarousal. This is supported by a study conducted by Fang et al. (2022) [13], which assessed response inhibition in ID using GNT and EEG. While no significant behavioral differences were observed, higher amplitudes of N2 and P3 were recorded during NoGo trials in ID, potentially reflecting cortical hyperarousal [13].
In the INSD group, NoGo ACC did not show any significant correlation with the measured variables, whereas cognitive arousal was significantly positively correlated with both TST and PSQI. This may indicate that excessive cognitive arousal before sleep could be a potential cause of sleep issues in INSD. Pre-sleep cognitive arousal refers to the activation of cognitive processes before falling asleep and can manifest as worries about sleep, hypervigilance, or rumination [25]. According to the cognitive model of insomnia [50], both rumination and worry are linked to sleep problems, and while they represent different cognitive processes, they share the core issue of overthinking [53]. Therefore, INSD patients are likely to excessively focus on their daytime functioning or/and worry about their sleep, leading to the perception of poor sleep quality. However, this study did not assess rumination or worry, so it is unclear which plays a more critical role. Nevertheless, the finding suggests that the poor subjective sleep quality in INSD patients may be associated with maladaptive cognition.
Potential effects of inhibitory control training
We observed that training improved NoGo-ACC significantly in the ISSD-trained group compared to the ISSD control group, and also led to significant improvements in WT, ISI, PSQI, and two sub-dimensions of PSQI (Sleep Duration and Daytime Functioning). These findings suggest that inhibitory control-based training improves sleep quality in the ISSD phenotype, potentially contributing to increased sleep duration and reduced WT during sleep. The observed training effects are likely attributable to improved cortical inhibition in ISSD, which may lower cortical arousal and support sleep homeostasis.
An interesting finding emerged in INSD: both the training and control groups showed significant improvement in PSQI, PSQI-TST, ISI, and CA in the post-session test compared to the pre-session test. This suggests that sleep disturbances in INSD improved irrespective of participation in the training. This finding does not necessarily imply the effectiveness of inhibitory control training for the INSD training group. Instead, it may support the notion that sleep issues in INSD may be more closely linked to cognitive-emotional arousal [32].
Limitation
Several limitations need emphasis. First, although we conducted sensitivity power analysis and found that the current sample size had the capacity to detect a moderate amount of effect, both in a one-way ANOVA (α = 0.05, power = 0.80, f = 0.31) and in subsequent repeated-measures ANOVA done for each of the insomnia phenotypes (α = 0.05, power = 0.80, fISSD = 0.23, fINSD = 0.23). However, the current sample sizes are still small and future validation studies should further expand the sample size to 159 and 40, corresponding to the testing of differences among the three groups (HC, ISSD, INSD) and the observation of training effects, respectively, as estimated by G*Power (α = 0.05, power = 0.08). Meanwhile, the sample size lacks representativeness. Our patient sample was too age-concentrated and predominantly young, making it debatable whether the findings of this study can be generalized to other age groups. Additionally, there were gender differences between groups, and although gender was controlled as a covariate in the statistical analysis, the associated results should be carefully considered. Therefore, this study should be viewed as a pilot study. Secondly, the lack of direct measurements of cortical arousal is another limitation. The present study interprets the relevant intervention results, but it must be recognized that we lacked relevant neurophysiological indicators to support these interpretations. Meanwhile, the UMindSleep uses only one electrode, and its accuracy in sleep staging still requires further validation. Although existing study [48] suggested a high correlation with PSG, the potential issue remains whether a single night of data is sufficient for accurately measuring the duration of whole-night sleep, and the availability of a single night of data also limits our ability to observe night-to-night variability in sleep, which may indeed have a greater clinical significance than a single night. Thus, multiple nights of standard EEG recordings and even functional magnetic resonance imaging (MRI) techniques are necessary for future research. Finally, we only designed a blank control group as a contrast, so we can't clarify that the effect of training whether may be part of the placebo effect. Additionally, we did not follow up on the long-term effects of the training, so it remains unclear whether the improved effects of inhibitory control training on sleep in ISSD are temporary. This is a critical consideration for clinical practice.
Conclusions
In conclusion, this study provides evidence that impaired response inhibition is associated with ISSD but not INSD. Sleep problems in ISSD may be improved through response inhibition-based cognitive training, while INSD does not appear to benefit from this approach. These results suggest that sleep problems in ISSD might be linked to dysfunctional cortical inhibition, potentially contributing to cortical arousal. In contrast, sleep problems in INSD could be more closely related to excessive cognitive-emotional arousal. Response inhibition training may be beneficial for restoring cortical inhibition in ISSD, which could help alleviate sleep issues. Our findings contribute to the understanding of potential intervention strategies in ID, though further research is required to confirm these observations.
Supplementary Information
Additional file 1: Table S1. Results of the partial correlation analysis between measures in the insomnia with objective short sleep duration group. Table S2. Results of the partial correlation analysis between measures in the insomnia with objective normal sleep duration. Table S3. Results of transfer test data of the insomnia with objective short sleep duration group. Table S4. Results of transfer test data of the insomnia with objective normal sleep duration group. Table S5. Descriptive statistics of sub-dimensions of the Pittsburg Sleep Quality Index in four conditions. Table S6. Results of sub-dimensions of the Pittsburg Sleep Quality Index in the insomnia with objective short sleep duration group. Table S7. Results of sub-dimensions of the Pittsburg Sleep Quality Index in the insomnia with objective normal sleep duration group.
Additional file 2: The specific impact of intervention on the sub dimensions of PSQI and Figures S1-S3. Fig S1. Significant results were observed in the analysis. Fig S2. The result of the insomnia with objective short sleep duration group. Fig S3. The result of the insomnia with objective normal sleep duration group.
Acknowledgements
We express our gratitude to all those who participated in this study.
Abbreviations:
- ID
Insomnia disorder
- ISSD
Insomnia with short sleep duration
- INSD
Insomnia with normal sleep duration
- HC
Healthy controls
- GNT
Go/NoGo task
- MRI
Magnetic resonance imaging
- TMS
Transcranial magnetic stimulation
- TST
Total sleep time
- PSQI
Pittsburgh sleep quality index
- ISI
Insomnia severity index
- PSAS
Pre-sleep arousal scale
- CCT
Computerized cognitive training
- SL
Sleep latency
- WT
Wakefulness time
- LST
Light sleep time
- DST
Deep sleep time
Authors’ contributions
X.L. led the project. X.L. and H.B.Z. Involved in the study concept and the design of the study. H.B.Z., H.F.C., and Z.J.T. were responsible for experimenting. H.B.Z. was responsible for the analysis of all data. H.B.Z. wrote the draft of the manuscript. X.L. and Z.W.L. made valuable corrections to the contents of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2501500) and the Scientific Innovation Project of Postgraduates of Southwest University (SWUB23025).
Data availability
The raw data are not publicly available due to ethical issues. The other processed data and materials that support the findings of this study are available on request from the corresponding author upon reasonable request, after consideration by the local ethics committee.
Declarations
Ethics approval and consent to participate
This study received authorization from the Ethics Committee at the Faculty of Psychology, Southwest University (Ethics approval number: H24061; Registration number of the Chinese Clinical Trial Registry: ChiCTR2400085063, https://www.chictr.org.cn/showproj.html?proj=224518), and all participants provided informed consent by signing the appropriate form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y, Ren R, Lei F, Zhou J, Zhang J, Wing YK, Sanford LD, Tang X. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, Spiegelhalder K. Insomnia disorder. Nat Rev Dis Primers. 2015;1:15026. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 4.Brownlow JA, Miller KE, Gehrman PR. Insomnia and cognitive performance. Sleep Med Clin. 2020;15(1):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardle-Pinkston S, Slavish DC, Taylor DJ. Insomnia and cognitive performance: a systematic review and meta-analysis. Sleep Med Rev. 2019;48: 101205. [DOI] [PubMed] [Google Scholar]
- 6.Ballesio A, Aquino M, Kyle SD, Ferlazzo F, Lombardo C. Executive functions in insomnia disorder: a systematic review and exploratory meta-analysis. Front Psychol. 2019;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. [DOI] [PubMed] [Google Scholar]
- 8.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Gao D, Yue F, Wang Y, Mao D, Chen X, Lei X. Response inhibition deficits in insomnia disorder: an event-related potential study with the stop-signal task. Front Neurol. 2018;9: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covassin N, de Zambotti M, Sarlo M, De Min TG, Sarasso S, Stegagno L. Cognitive performance and cardiovascular markers of hyperarousal in primary insomnia. Int J Psychophysiol. 2011;80(1):79–86. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Sun H, Li J, Yang J, Fan Y, Jülich ST, Lei X. Response inhibition impairment related to altered frontal-striatal functional connectivity in insomnia disorder: a pilot and non-clinical study. J Psychiatr Res. 2024;170:138–46. [DOI] [PubMed] [Google Scholar]
- 13.Fang Z, Liu X, Wang C, Cao J, Peng Y, Lv Y. Insomnia attenuates response inhibition: evidence from Go/NoGo research. Sleep Med. 2022;100:518–33. [DOI] [PubMed] [Google Scholar]
- 14.Sivertsen B, Hysing M, Wehling E, Pallesen S, Nordhus IH, Espeseth T, Lundervold AJ. Neuropsychological performance in older insomniacs. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(1):34–48. [DOI] [PubMed] [Google Scholar]
- 15.Sagaspe P, Philip P, Schwartz S. Inhibitory motor control in apneic and insomniac patients: a stop task study. J Sleep Res. 2007;16(4):381–7. [DOI] [PubMed] [Google Scholar]
- 16.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–30. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Sun H, Li J, Fan Y, Jülich ST, Lei X. Subtypes of insomnia revealed by the heterogeneity of neuroanatomical patterns: a structural MRI study. Biol Psychol. 2023;180: 108591. [DOI] [PubMed] [Google Scholar]
- 18.Tahmasian M, Noori K, Samea F, Zarei M, Spiegelhalder K, Eickhoff SB, Van Someren E, Khazaie H, Eickhoff CR. A lack of consistent brain alterations in insomnia disorder: an activation likelihood estimation meta-analysis. Sleep Med Rev. 2018;42:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, Te Lindert BHW, Sjauw Mook J, Van Someren EJW. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med Rev. 2017;36:71–81. [DOI] [PubMed] [Google Scholar]
- 20.Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest. 2015;147(4):1179–92. [DOI] [PMC free article] [PubMed]
- 21.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–94. [DOI] [PubMed] [Google Scholar]
- 22.Ren D, Jiang B. Insomnia disorder with objective short sleep duration (ISS) phenotype and cognitive performance: a systematic review and meta-analysis. Neurol Sci. 2023;44(7):2363–8. [DOI] [PubMed] [Google Scholar]
- 23.Olaithe M, Ree M, McArdle N, Donaldson S, Pushpanathan M, Eastwood PR, Bucks RS. Cognitive dysfunction in insomnia phenotypes: further evidence for different disorders. Front Psychiatry. 2021;12: 688672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Mendoza J, He F, Puzino K, Amatrudo G, Calhoun S, Liao D, Vgontzas AN, Bixler E. Insomnia with objective short sleep duration is associated with cognitive impairment: a first look at cardiometabolic contributors to brain health. Sleep. 2021;44(1):zsaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38(7):679–93. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Cao S, Shields GS, Teng Z, Liu Y. The relationships between rumination and core executive functions: a meta-analysis. Depress Anxiety. 2017;34(1):37–50. [DOI] [PubMed] [Google Scholar]
- 27.Ballesio A, Ottaviani C, Lombardo C. Poor cognitive inhibition predicts rumination about insomnia in a clinical sample. Behav Sleep Med. 2019;17(5):672–81. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Liu X, Wang Y, Liu C, Zhang N, Lu J, Lv Y. Exploration of cortical inhibition and habituation in insomnia: based on CNV and EEG. Methods. 2022;204:73–83. [DOI] [PubMed] [Google Scholar]
- 29.Pillai V, Steenburg LA, Ciesla JA, Roth T, Drake CL. A seven day actigraphy-based study of rumination and sleep disturbance among young adults with depressive symptoms. J Psychosom Res. 2014;77(1):70–5. [DOI] [PubMed] [Google Scholar]
- 30.Chami R, Cardi V, Lawrence N, MacDonald P, Rowlands K, Hodsoll J, Treasure J. Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: a feasibility trial. Psychol Med. 2020;52(5):874–83. [DOI] [PubMed] [Google Scholar]
- 31.He D, Guo Z, McClure MA, Mu Q, Jiang B. Cognitive-behavioral therapy for insomnia with objective short sleep duration phenotype: a systematic review with meta-analysis. Sleep Med Rev. 2023;67: 101736. [DOI] [PubMed] [Google Scholar]
- 32.Bathgate CJ, Edinger JD, Krystal AD. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep. 2017;40(1):zsw012. [DOI] [PMC free article] [PubMed]
- 33.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63. [DOI] [PubMed] [Google Scholar]
- 35.Tapia JL, Puertas FJ, Duñabeitia JA. Study protocol for a randomized controlled trial assessing the effectiveness of personalized computerized cognitive training for individuals with insomnia. Front Behav Neurosci. 2022;16: 779990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One. 2013;8(4): e61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding X, He L, Kang T, Yang Y, Ji H, Zhao H, Lang X, Sun C, Zhang X. The role of the left dorsolateral prefrontal cortex in conflict control during insomnia disorder. J Psychiatr Res. 2024;171:271–6. [DOI] [PubMed] [Google Scholar]
- 38.Ong JC, Kuo TF, Manber R. Who is at risk for dropout from group cognitive-behavior therapy for insomnia? J Psychosom Res. 2008;64(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Shang Y, Zhuang W, Mai W, Cheng W, Chen Z. Effectiveness of response inhibition training and its long-term effects in healthy adults: a systematic review and meta-analysis. Front Neurosci. 2022;16: 813975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Jia L. Training and transfer effects of interference control training in children and young adults. Psychol Res. 2019;83(7):1519–30. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, Chen L, Maes JHR. Training and transfer effects of response inhibition training in children and adults. Dev Sci. 2018;21(1):e12511. [DOI] [PubMed] [Google Scholar]
- 42.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 44.Liu XC, Tang MQ. Reliability and validity study of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;29(2):5. [Google Scholar]
- 45.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 46.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–71. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Jin X, Zhang J, Ho KW, Wei Y, Cheng H. Validation of a wearable forehead sleep recorder against polysomnography in sleep staging and desaturation events in a clinical sample. J Clin Sleep Med. 2023;19(4):711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Leeuw JR. jsPsych: a JavaScript library for creating behavioral experiments in a Web browser. Behav Res Methods. 2015;47(1):1–12. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–93. [DOI] [PubMed] [Google Scholar]
- 51.Ling J, Lin X, Li X, Chan NY, Zhang J, Wing YK, Hu X, Li SX. Altered brain activity related to inhibitory processing in youth with insomnia. J Sleep Res. 2021;30(6):e13398. [DOI] [PubMed] [Google Scholar]
- 52.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31(6):887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carney CE, Harris AL, Moss TG, Edinger JD. Distinguishing rumination from worry in clinical insomnia. Behav Res Ther. 2010;48(6):540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Results of the partial correlation analysis between measures in the insomnia with objective short sleep duration group. Table S2. Results of the partial correlation analysis between measures in the insomnia with objective normal sleep duration. Table S3. Results of transfer test data of the insomnia with objective short sleep duration group. Table S4. Results of transfer test data of the insomnia with objective normal sleep duration group. Table S5. Descriptive statistics of sub-dimensions of the Pittsburg Sleep Quality Index in four conditions. Table S6. Results of sub-dimensions of the Pittsburg Sleep Quality Index in the insomnia with objective short sleep duration group. Table S7. Results of sub-dimensions of the Pittsburg Sleep Quality Index in the insomnia with objective normal sleep duration group.
Additional file 2: The specific impact of intervention on the sub dimensions of PSQI and Figures S1-S3. Fig S1. Significant results were observed in the analysis. Fig S2. The result of the insomnia with objective short sleep duration group. Fig S3. The result of the insomnia with objective normal sleep duration group.
Data Availability Statement
The raw data are not publicly available due to ethical issues. The other processed data and materials that support the findings of this study are available on request from the corresponding author upon reasonable request, after consideration by the local ethics committee.