Abstract
Background
No clear agreement exists on the degree of bone formation required to remove a metal plate without correction loss after medial opening-wedge high tibial osteotomy (MOWHTO). We aimed to investigate the mechanical stability of the proximal tibia with different bone formations after plate removal in MOWHTO using finite element models and determine the extent of bone formation when the plate can be removed without correction loss.
Methods
The MOWHTO models with 5, 10, and 15 mm opening gaps were generated. The mechanical stability of proximal tibial models with different extents of bone formation (from the lateral cortex of the osteotomy wedge to 20% (zone 1), 40% (zone 2), 50% (zone 2.5), 60% (zone 3), 70% (zone 3.5), 80% (zone 4), and 100% bone formation medially) after plate removal was analyzed using finite element analysis. Bone stress and strain and micromotion were evaluated to investigate fracture risk and bone stability, respectively, in various types of tibial models.
Results
Peak von Mises stress was lower than yield strength when bone formation reached zone 3.5 (70%) or more in 5- and 10-mm osteotomy gap models, and zone 4 (80%) or more in a 15-mm gap model. Maximal principal strains were lower than 6,130 microstrain when bone formation reaches zone 3.5 (70%) or more in models with osteotomy gaps of 5, 10, and 15 mm.
Conclusions
This indicates that plate removal without correction loss after MOWHTO may be possible when bone formation reaches zone 3.5 (> 70%) or more during 5- and 10-mm osteotomy gap corrections, and zone 4 (> 80%) or more during 15-mm gap correction. The present study results suggest that it would be safer to perform plate removal after obtaining sufficient bone formation rather than performing it near the osteotomy gap center (50%) to avoid correction loss considering both coronal and sagittal plane aspects.
Keywords: High tibial osteotomy, Plate removal, Optimal timing, Finite element analysis
Background
Medial opening-wedge high tibial osteotomy (MOWHTO) is a surgical intervention used to treat varus malalignment associated with medial osteoarthritis in middle or early old age [1–3]. This procedure shifts the weight-bearing axis of the lower limb laterally in the coronal plane, increases the width of the medial joint space, and reduces the medial compartment load to postpone transition to total joint surgery [4–9]. Compared with lateral closing-wedge osteotomy, MOWHTO has the advantage of easy adjustment of correction degree and a low risk of peroneal nerve damage [10, 11]. However, lateral hinge fractures, implant breakage, and correction loss occur more frequently after MOWHTO due to the relatively unstable structure generated by the opening gap [1, 12, 13]. Therefore, long and bulky metal plates with locking screws have traditionally been preferred for fixation in MOWHTO. A rigid and bulky metal plate improves stability around the osteotomy site; however, many patients complain of discomfort at the fixation site. Niemeyer et al. [14] reported that after MOWHTO, 40.6% of patients complained of local discomfort and irritation associated with the implant. Darees et al. [15] reported that 41.6% of patients treated with MOWHTO removed the plate because of the discomfort. Most Asian or lean patients with a thin subcutaneous layer visit an outpatient clinic to remove the metal plate before achieving complete bone union because of discomfort.
No clear agreement exists on the degree of bone formation required for plate removal without correction loss after MOWHTO. Staubli et al. [16] reported that approximately 90% of patients gained full consolidation of osteotomy site on simple radiography, computed tomography (CT), and magnetic resonance imaging in 1 year. Brinkman et al. [17] did not suggest hardware removal 1.5 years after corrective osteotomy. However, Kobayashi et al. [18] reported that the extent of posterior cortex union that reached zone 3 (40–60% of healing) was sufficient for metal plate removal. Goshima et al. [19] suggested that hardware removal was possible without correction loss after MOWHTO when posterior cortex union reached the midpoint (> 50%) of osteotomy space. However, studies to determine the point where correction loss occurs after plate removal in MOWHTO with various degrees of correction are still insufficient, and finding this point using only clinical studies is challenging. Furthermore, to the best of our knowledge, no study has evaluated the mechanical stability of proximal tibial models with metal plates removed after MOWHTO.
We aimed to investigate the mechanical stability of the proximal tibia with different bone formations after plate removal in MOWHTO using finite element (FE) models, and to determine the degree of bone formation where the plate can be removed without correction loss. The present study hypothesized that a possible point for plate removal without the risk of correction loss would exist in the < 50% progression zone of bone formation on anterior-posterior (AP) radiographs.
Methods
Development of proximal tibia model with plate removal after MOWHTO
This study was approved by the institutional review board of Yonsei University Gangnam Severance Hospital (IRB No. 3-2024-0263). This study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from the patient participating the present study. Cross-sectional images of the lower extremities of a 62-year-old Asian female were obtained using 64-channel computed tomography (CT) with a slice interval of 0.1 mm. Three-dimensional geometric contour of the tibia was created by stacking the CT cross-sectional images using Mimics (version 17.021.0; Materialize Inc., Belgium). Bone surfaces were modified into more sophisticated solid models using computer-aided design (CAD) software and Unigraphics NX (version 2021; Siemens PLM Software, Torrance, CA). An FE mesh was generated using HyperMesh (version 8.0; Altair Engineering).
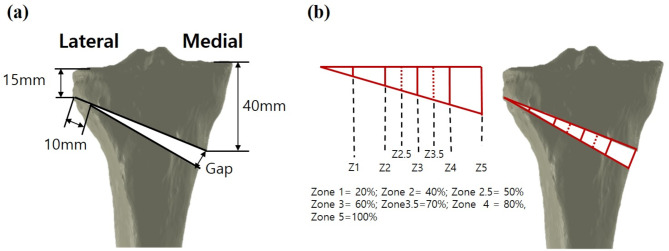
The constructed healthy tibia model was used to simulate MOWHTO with rotation of the distal part of the tibia in the coronal plane to create a valgus correction angle. The MOWHTO models with 5, 10, and 15 mm gaps were generated by removing a single-plane wedge-shaped osteotomy bone at the proximal tibia while leaving a 10 mm hinge from the lateral cortex (Fig. 1a) [20].
Fig. 1.
Specifications of the MOWHTO used in this study (a) and osteotomy filling index with seven identified zones (b)
A commercially used fixation plate, TomoFix (DePuy Synthes, Warsaw, IN, USA), was considered as the worst case in terms of bulkiness, used in MOWHTO simulation, and modeled three-dimensionally using the CAD program and Unigraphics NX (version 7.0; Siemens PLM Software, Torrance, CA, USA). The plate model was virtually implanted into the tibia to simulate MOWHTO fixation and then removed to create a proximal tibia model with bony deficiency at the opening wedge and screw holes. The plate had a length of 112 mm with 4 proximal screws and 4 distal screws, 5 mm screw diameter for proximal and distal, 65 mm proximal screw length, and 36 mm distal screw length [21] .
To create proximal tibial models with different degrees of bone formation at the time of hardware removal, we further subdivided the osteotomy filling index proposed by Brosset et al. [22]. The tibia models with different union progressions at the time of plate removal were generated by artificially forming cortical bone with trabecular bone inside formation from the lateral cortex of the osteotomy wedge to 20% (zone 1), 40% (zone 2), 50% (zone 2.5), 60% (zone 3), 70% (zone 3.5), 80% (zone 4), and 100% medially (Fig. 1b).
As for the tibia, cortical bone was considered linear elastic, isotropic, and homogeneous material with an assumed Young’s modulus (E), E = 17,000 MPa and Poisson ratio (ν), ν = 0.33 [23, 24]. Cancellous bone was simulated as a linear isotropic material with E = 910 MPa and v = 0.2 [25]. A “tie” contact condition was applied assuming full constraints between bone and bone. The distal end of the tibial bone was assumed to be fully constrained in all tests [26, 27].
Mesh convergence of the FE model was investigated to complete the modeling. Mesh convergence was defined as the maximum displacement of trabecular bone, which was within 95% of the pressure of the next two smaller mesh sizes [28]. The average FE size was 0.8 mm for the cortical and cancellous bone. To avoid singularities and achieve convergence in the FE model bone region, a graded bone model was implemented. A mesh size of 0.6 mm on at the posterolateral end of the wedge region, and 0.8 mm for other regions. Quadratic tetrahedral elements of type C3D10 was applied. The numbers of created finite elements were as follows: cortical bone, 77,576 and cancellous bone, 178,726.
Loading and boundary conditions
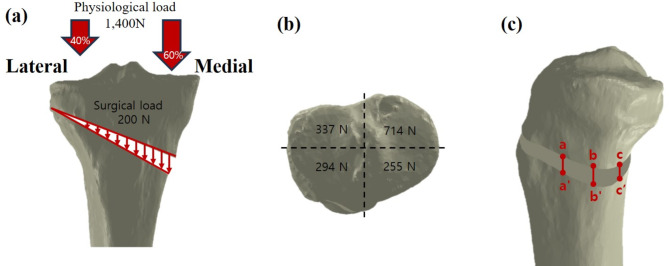
Physiological loading and intervention-induced compression were applied to the MOWHTO models. Because peak tibial stress increases with gait speed increase and increases in tibial compression during walking may not stem from concurrent increases in vertical ground reaction forces, the physiological loads were estimated to be two times of body weight (70 kgf) to simulate the compressive load on the tibia during static condition [29–31]. Additional 200 N intervention-induced compressive load was uniformly exerted on the tibial osteotomy site in a distracted cortex. The intervention-induced load induced by the stabilizing force on the graft was primarily due to the distraction of the remaining intact cortex, medial collateral ligament, and patellar ligament [23, 24, 32]. According to the results from a previous study, restoration of physiological transfer of a knee load was assumed in the present study, which led to load repartitions of 60% and 40% on the medial and lateral tibial plateaus, respectively (Fig. 2a). The components of the physiological (1,400 N) and surgical loads (200 N) on the four regions of the tibial plateau are shown in Fig. 2b [33].
Fig. 2.
Loading conditions used in the present study; physiological and surgical loads (a), and loads on the four regions of the tibial plateau (b). Three edges aa’, bb’, and cc’ along the medial cortex were defined to evaluate the height changes in loading condition (c)
Three parameters were used to compare the differences in stress, strain, and micromotion with variations in bone formation. First, peak stress (peak von Mises stress, PVMS) was investigated in the cortical bone and lateral hinge area in the FE models, and the stress values were compared to the yield strength to verify stability. The yield strength of the tibial cortical bone (177.2 MPa for axial compression) was obtained from a previous publication [34]. Next, the maximum principal strain in the bones was evaluated. Third, change in gap (△= gap distance before applying load – gap distance after applying load) at the medial edges of the opening gap was evaluated to compare bony stability (Fig. 2c). The present study used 1st principal stress values and the FE analysis was conducted using ABAQUS (version 6.14; Dassault Systems, France).
Intact model validation
The FE model of normal tibia was validated by comparing it with data from a previous study that experimentally validated an FE model of a human cadaveric tibia [35]. The FE model is validated under axial loading conditions. The minimum and maximum principal strains were investigated. The largest values of the minimum principal strain on the tibia bone and reference model were − 542 and − 569 microstrain, respectively. The largest values of the maximum principal strain on the tibia and reference model were 403 and 426 microstrain, respectively. We considered that the constructed FE model was reliable because the differences were less than 10%. Therefore, the FE model constructed in this study was appropriate for testing by comparing the differences in the largest values of the minimum and maximum principal strains on the tibia between the FE model and previous literature data.
Results
Stress distribution on the bone model with plate removal after MOWHTO
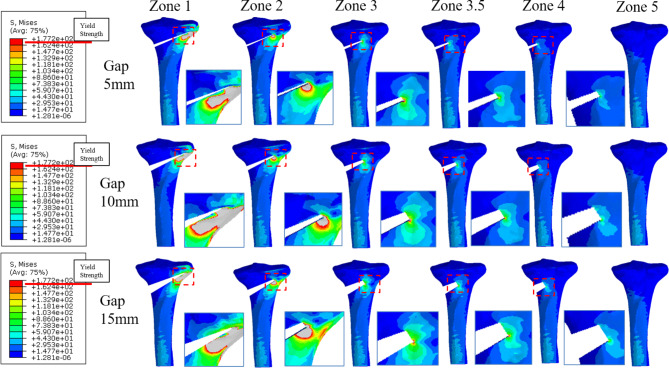
Peak von Mises stress was greatest around the posterolateral end of the osteotomy wedge in all the models, and tended to decrease with progressive gap filling (Fig. 3).
Fig. 3.
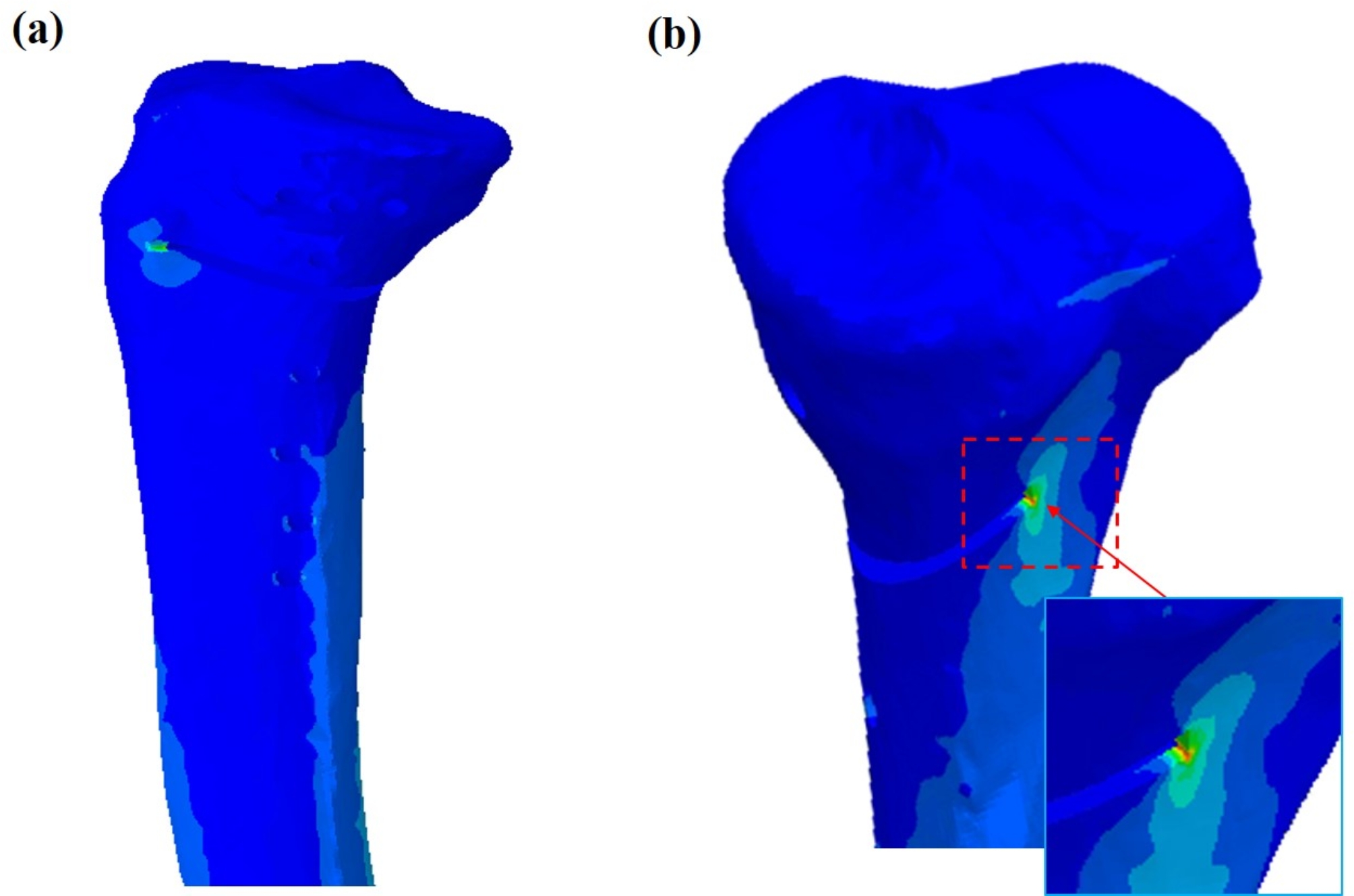
Stress distribution around the proximal tibia with opening gap of 5 mm filled with zone 3 (60%) bone formation after plate removal; medial view (a) and posterior view (b). The arrow indicates that the peak stress was observed at the posterolateral osteotomy wedge site
In models with osteotomy gaps of 5 and 10 mm, PVMS was greater than yield strength (177.2 MPa) when bone formation reached zone 3 (60%) or less; however, PVMS was lower than yield strength when bone formation reached zone 3.5 (70%) or more (Fig. 4a and b).
Fig. 4.
Stress distribution around the proximal tibia with opening gaps of 5 mm (top), 10 mm (middle), and 15 mm (bottom) after plate removal (posterior view). The enlarged image portion represents the point at which the peak stress was observed
In models with 15 mm gaps, PVMS was greater than yield strength when bone formation reached zone 3.5 (70%) or less (Fig. 4c); however, PVMS was lower than yield strength when bone formation reached zone 4 (80%) or more (Table 1).
Table 1.
Peak von Mises stress (PVMS) at bone models
| Opening Gap (mm) | Peak Von Mises Stress (MPa) | ||||||
|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 2.5 | Zone 3 | Zone 3.5 | Zone 4 | Zone 5 | |
| Gap 5 | 4230 | 1009 | 769 | 286 | 159* | 101* | 39* |
| Gap 10 | 2707 | 794 | 455 | 285 | 171* | 128* | 39* |
| Gap 15 | 1954 | 855 | 589 | 336 | 231 | 127* | 39* |
* Lower than the yield strength: axial compression 177.2 MPa
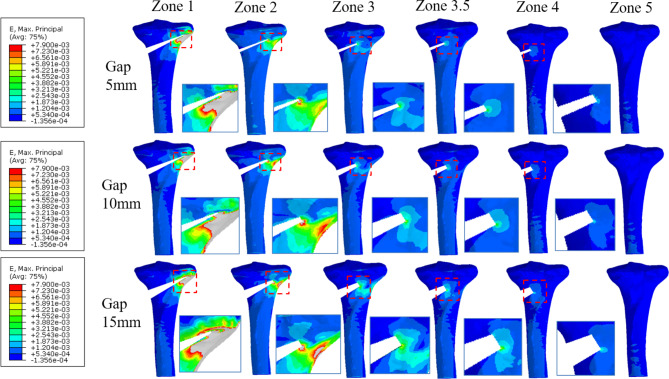
Strain distribution on the bone model with plate removal after MOWHTO
Strain was also greatest around the posterolateral end of the osteotomy wedge in all the models and tended to decrease with gap-filling progression.
Maximal principal strain was greater than 10,000 microstrain when bone formation reached zone 3 (60%) or less in models with osteotomy gaps of 5, 10, and 15 mm; however, it was lower than 6,130 microstrain when bone union reached zone 3.5 (70%) or more in all the models (Fig. 5) (Table 2).
Fig. 5.
Strain distribution arournd the proximal tibia with opening gaps of 5 mm (top), 10 mm (middle), and 15 mm (bottom) after plate removal (posterior view). The enlarged image portion represents the point at which the maximal strain was observed
Table 2.
The maximum principal strain
| Opening Gap (mm) | Maximum principal strain (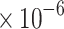 ) ) |
||||||
|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 2.5 | Zone 3 | Zone 3.5 | Zone 4 | Zone 5 | |
| Gap 5 | 170,000 | 90,700 | 44,900 | 10,100 | 5920 | 3470 | 1580 |
| Gap 10 | 107,300 | 76,400 | 31,400 | 11,100 | 5470 | 3850 | 1580 |
| Gap 15 | 96,500 | 88,000 | 34,800 | 10,500 | 6130 | 3030 | 1580 |
Micromotion at the edges of the opening gap
As the osteotomy correction gap increased, change in gap at the edges of the opening gap tended to increase. Change in gap at the edges of the osteotomy gap tended to increase from anterior to posterior (aa’< bb’< cc’) (Table 3).
Table 3.
Change in height at the medial edges of the opening gap
| Gap (mm) |
Change in the height at medial edges (mm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone1 | Zone 2 | Zone 2.5 | Zone 3 | Zone 3.5 | Zone 4 | |||||||||||
| aa’, bb’, cc’ | aa’ | bb’ | cc’ | aa’ | bb’ | cc’ | aa’ | bb’ | cc’ | aa’ | bb’ | cc’ | aa’ | bb’ | cc’ | |
| Gap 5 | > 5 | 1.61 | 1.83 | 1.86 | 1.02 | 1.12 | 1.14 | 0.34 | 0.37 | 0.37 | 0.18 | 0.19 | 0.19 | 0.10 | 0.11 | 0.11 |
| Gap10 | > 10 | 2.12 | 2.31 | 2.38 | 0.98 | 1.00 | 1.01 | 0.43 | 0.46 | 0.47 | 0.24 | 0.25 | 0.26 | 0.15 | 0.16 | 0.17 |
| Gap15 | > 15 | 2.78 | 2.99 | 3.05 | 1.53 | 1.65 | 1.69 | 0.56 | 0.60 | 0.61 | 0.32 | 0.34 | 0.35 | 0.21 | 0.22 | 0.22 |
Discussion
The most critical finding of the present study is that PVMS was lower than yield strength when bone formation reached zone 3.5 (70%) or more in the 5- and 10-mm gap models, and zone 4 (80%) or more in the 15-mm gap model. However, PVMS was greater than yield strength when bone formation reached zone 3 (60%) or less in the 5- and 10-mm gap models, and zone 3.5 (70%) or less in the 15-mm gap model, which could lead to bone fracture or correction loss after metal plate removal.
The maximum stress and strain in zones 1–4 were measured at the posterolateral end of the wedge, which is thought to be due to the loading, was more concentrated in the posterior direction than the anterior. The maximal principal strain was lower than 6,130 microstrain when bone union reached zone 3.5 (70%) or more in all the models; these were lower than 7,900 microstrain, which was previously reported to cause low cycle (< 10,000 cycles) fatigue microcracking [36]. Micromotion at the medial edge of the opening gap tended to increase from anterior to posterior, which was thought to be due to the higher load on the posterior part than the anterior part. As the osteotomy correction gap increased, change in gap at the edges of the opening gap tended to increase; greater micromotion may lead to potentially unstable proximal tibial structure, but the risk of correction loss was assessed based on the maximum stress and strain results.
When considering metal plate removal to relieve discomfort and obtain clinical benefits, no clear agreement exists on the degree of bone formation that allows hardware removal without correction loss after MOWHTO. Brinkman et al. [17] reported that complete union was achieved in 90% 1 year after the surgery and recommended that the metal plate should not be removed before 1.5 years after MOWHTO. However, there is no consensus that plate removal is possible only when complete union is achieved, and many surgeons attempt to remove the plate before complete union. Goshima et al. [37] reported that metal plate removal without correction loss is possible if gap filling arrives at zone 2 (25–50%) on simple AP X-ray radiographs; however, in another follow-up study, they experienced loss of correction in 6 (5.9%) of 101 patients who underwent plate removal according to these criteria [19].
In a follow-up study, Goshima et al. [19] reported in their follow-up study that posterior cortical-bone union rate was the only predictor of correction loss. They suggested using receiver operating characteristic curve analysis cut-off value that posterior cortex bone union rate of < 43.3% caused an increase in posterior tibial slope and decrease in medial proximal tibial angle [19]. They finally concluded that when the posterior cortical bone union reached the gap center (50%), the plate could be removed without loss of correction. However, statistical analysis using data from the 6(5.9%) of 101 correction loss cases seems to have limitations in representativeness. In addition, they considered the correction loss only from a coronal plane point of view, not including a sagittal plane aspect such as posterior tibial slope change; therefore, biomechanical analysis using an FE model to determine the degree of bone formation extent where the plate can be removed without correction loss considering both coronal and sagittal plane aspects seems to be meaningful. According to the present study, PVMS was lower than yield strength when bone formation reached zone 3.5 (70%) or more in 5- and 10-mm osteotomy gap models, and zone 4 (80%) or more in a 15-mm gap model. Our study results suggest that it would be safer to perform plate removal after obtaining sufficient bone formation rather than performing it near the osteotomy gap center (50%) to avoid correction loss despite continued patient discomfort.
The hypothesis of this study was to question the previous clinical study report that > 50% bone formation was safe from correction loss, and to establish that there may be a safe zone even in < 50% bone formation on anterior-posterior (AP) radiographs. However, the results of our study did not support the null hypothesis of the present study, suggesting that more bone formation than the degree of bone formation suggested in the hypothesis was necessary to achieve mechanical stability of the proximal tibia after metal plate removal. The difference between the null hypothesis and the present study results may be due to the different perspective regarding the term “correction loss”. The previous clinical report by Goshima et al. considered correction loss as a varus change of 2 degrees or more in the coronal plane lower extremity mechanical axis over 1 year. In our study, when < 70% bone formation was progressed, peak stress measured at the posterolateral part of the osteotomy wedge was higher than yield stress, which suggested a possible risk of bony fracture and potential correction loss not only in coronal plane aspect, but also in sagittal plane perspective. Clinical research should be followed for matching the present FE model results with clinical situations.
The present study has several limitations. First, simple assumptions in material properties (linear homogenous isotropic material properties), uniform bone healing, and the simplified geometry of the gap were applied in this study. Second, this study was conducted under static conditions because dynamic knee joint motion is too complicated in terms of computing resources and time. In future studies, a more suitable representation of the mobile joint and study of the models under cyclic loading should be conducted. Third, only the tibia was simulated in this study. Expanding the scope of the cartilage may be required to investigate the pressing biomechanics of MOWHTO. Forth, our results cannot be generalized to patients with osteoporosis. Fifth, the present study used a single anatomical model (a 62-year-old Asian woman), which limits generalizability. Future studies should include multiple anatomical models to enhance applicability to diverse patient populations. Sixth, because the model constructed in this research was based on living subject, our research model could not be validated using mechanical test of full cadaver model.
However, to the best of our knowledge, this is the first FE analysis to demonstrate the appropriate timing of metal plate removal without correction loss after MOWHTO. The results of the biomechanical mechanics based on this study will contribute to the body of decision-making regarding the timing of plate removal after MOWHTO.
Conclusions
The present study demonstrated that plate removal seems to be possible without correction loss after MOWHTO when bone formation reached zone 3.5 (> 70%) or more during 5- and 10-mm osteotomy gap corrections, and zone 4 (> 80%) or more during 15-mm gap correction. Our study results suggest that it would be safer to perform plate removal after obtaining sufficient bone formation rather than performing it near the osteotomy gap center (50%) to avoid correction loss considering both coronal and sagittal plane aspects despite continued patient discomfort.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Abbreviations
- MOWHTO
Medial opening-wedge high tibial osteotomy
- FE
Finite element
- AP
Anterior-posterior
- CT
Computed tomography
- CAD
Computer-aided design
- PVMS
peak von Mises stress
Author contributions
C-Y. Jang (ORCID ID:0000-0002-1150-2968): Conceptualization, Writing – original draft. K-T. Kang: Data curation, Investigation. Hyongtaek Hong: Data analysis, Investigation, Manuscript revision. Min Jung (ORCID ID: 0000-0002-7527-4802): Methodology, Investigation. Sungjun Kim: Conceptualization, Investigation. Je-Hyun Yoo (ORCID ID: 0000-0002-0777-1575): Conceptualization, Methodology. S-H. Kim (ORCID ID: 0000-0001-5743-6241): Conceptualization, Supervision, Writing – review & editing. Chul-Young Jang and Kyoung-Tak Kang contributed equally to this work and are the first authors of this work. All authors have read and approved the final submitted manuscript.
Funding
There is no funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Yonsei University Gangnam Severance Hospital (IRB No. 3-2024-0263). This study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from the patient participating the present study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chul-Young Jang and Kyoung-Tak Kang contributed equally to this work and are the first authors of this work.
References
- 1.Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279–89. [DOI] [PubMed] [Google Scholar]
- 2.Kang BY, Lee DK, Kim HS, Wang JH. How to achieve an optimal alignment in medial opening wedge high tibial osteotomy? Knee Surg Relat Res. 2022;34(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerciello S, Vasso M, Maffulli N, Neyret P, Corona K, Panni AS. Total knee arthroplasty after high tibial osteotomy. Orthopedics. 2014;37(3):191–8. [DOI] [PubMed] [Google Scholar]
- 4.Moon HS, Choi CH, Yoo JH, Jung M, Lee TH, Byun JW, Kim SH. An Increase in Medial Joint Space Width After Medial Open-Wedge High Tibial Osteotomy Is Associated With an Increase in the Postoperative Weight-Bearing Line Ratio Rather Than With Cartilage Regeneration: Comparative Analysis of Patients Who Underwent Second-Look Arthroscopic Assessment. Arthroscopy. 2021;37(2):657–e668654. [DOI] [PubMed] [Google Scholar]
- 5.Park JY, Kim JH, Cho JW, Kim MS, Choi W. Clinical and radiological results of high tibial of osteotomy over the age of 65 are comparable to that of under 55 at minimum 2-year follow-up: a propensity score matched analysis. Knee Surg Relat Res. 2024;36(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song SJ, Bae DK, Park SH, Park CH. Surgical accuracy of coronal and sagittal alignment in conventional closed-wedge high tibial osteotomy after computer-assisted surgery experience. Knee Surg Relat Res. 2023;35(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HU, Kim DH, Lee SW, Choi BC, Bae KC. Comparison of Lower-Limb Alignment in Patients with Advanced Knee Osteoarthritis: EOS Biplanar Stereoradiography versus Conventional Scanography. Clin Orthop Surg. 2022;14(3):370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onoi Y, Hiranaka T, Hida Y, Fujishiro T, Okamoto K, Matsumoto T, Kuroda R. Second-Look Arthroscopic Findings and Clinical Outcomes after Adipose-Derived Regenerative Cell Injection in Knee Osteoarthritis. Clin Orthop Surg. 2022;14(3):377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gougoulias N, Khanna A, Maffulli N. Sports activities after lower limb osteotomy. Br Med Bull. 2009;91:111–21. [DOI] [PubMed] [Google Scholar]
- 10.Kang KT, Koh YG, Lee JA, Lee JJ, Kwon SK. Biomechanical effect of a lateral hinge fracture for a medial opening wedge high tibial osteotomy: finite element study. J Orthop Surg Res. 2020;15(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi F, Longo UG, Ruzzini L, Marinozzi A, Maffulli N, Denaro V. Simultaneous arthroscopic implantation of autologous chondrocytes and high tibial osteotomy for tibial chondral defects in the varus knee. Knee. 2008;15(4):309–13. [DOI] [PubMed] [Google Scholar]
- 12.Stuart MJ, Beachy AM, Grabowski JJ, An KN, Kaufman KR. Biomechanical evaluation of a proximal tibial opening-wedge osteotomy plate. Am J Knee Surg. 1999;12(3):148–53. discussion 153 – 144. [PubMed] [Google Scholar]
- 13.Martinez de Albornoz P, Leyes M, Forriol F, Del Buono A, Maffulli N. Opening wedge high tibial osteotomy: plate position and biomechanics of the medial tibial plateau. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2641–7. [DOI] [PubMed] [Google Scholar]
- 14.Niemeyer P, Schmal H, Hauschild O, von Heyden J, Südkamp NP, Köstler W. Open-wedge osteotomy using an internal plate fixator in patients with medial-compartment gonarthritis and varus malalignment: 3-year results with regard to preoperative arthroscopic and radiographic findings. Arthroscopy. 2010;26(12):1607–16. [DOI] [PubMed] [Google Scholar]
- 15.Darees M, Putman S, Brosset T, Roumazeille T, Pasquier G, Migaud H. Opening-wedge high tibial osteotomy performed with locking plate fixation (TomoFix) and early weight-bearing but without filling the defect. A concise follow-up note of 48 cases at 10 years’ follow-up. Orthop Traumatol Surg Res. 2018;104(4):477–80. [DOI] [PubMed] [Google Scholar]
- 16.Sabzevari S, Ebrahimpour A, Roudi MK, Kachooei AR. High Tibial Osteotomy: A Systematic Review and Current Concept. Arch Bone Jt Surg. 2016;4(3):204–12. [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkman JM, Lobenhoffer P, Agneskirchner JD, Staubli AE, Wymenga AB, van Heerwaarden RJ. Osteotomies around the knee: patient selection, stability of fixation and bone healing in high tibial osteotomies. J Bone Joint Surg Br. 2008;90(12):1548–57. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H, Akamatsu Y, Kumagai K, Kusayama Y, Saito T. Radiographic and computed tomographic evaluation of bone union after medial opening wedge high tibial osteotomy with filling gap. Knee. 2017;24(5):1108–17. [DOI] [PubMed] [Google Scholar]
- 19.Goshima K, Sawaguchi T, Shigemoto K, Iwai S, Fujita K, Kataoka T. Plate removal without loss of correction after open-wedge high tibial osteotomy is possible when posterior cortex bone union reaches osteotomy gap center even in incompletely filled gaps. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1827–34. [DOI] [PubMed] [Google Scholar]
- 20.Mederake M, Eleftherakis G, Schull D, Springer F, Maffulli N, Migliorini F, Konrads C. The gap height in open wedge high tibial osteotomy is not affected by the starting point of the osteotomy. BMC Musculoskelet Disord. 2023;24(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh YG, Lee JA, Lee HY, Chun HJ, Kim HJ, Kang KT. Design optimization of high tibial osteotomy plates using finite element analysis for improved biomechanical effect. J Orthop Surg Res. 2019;14(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brosset T, Pasquier G, Migaud H, Gougeon F. Opening wedge high tibial osteotomy performed without filling the defect but with locking plate fixation (TomoFix) and early weight-bearing: prospective evaluation of bone union, precision and maintenance of correction in 51 cases. Orthop Traumatol Surg Res. 2011;97(7):705–11. [DOI] [PubMed] [Google Scholar]
- 23.Luo CA, Hua SY, Lin SC, Chen CM, Tseng CS. Stress and stability comparison between different systems for high tibial osteotomies. BMC Musculoskelet Disord. 2013;14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo C-A, Lin S-C, Hwa S-Y, Chen C-M, Tseng C-S. Biomechanical effects of plate area and locking screw on medial open tibial osteotomy. Comput Methods Biomech BioMed Eng. 2015;18(12):1263–71. [DOI] [PubMed] [Google Scholar]
- 25.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36(7):897–904. [DOI] [PubMed] [Google Scholar]
- 26.Pauchard Y, Ivanov TG, McErlain DD, Milner JS, Giffin JR, Birmingham TB, Holdsworth DW. Assessing the local mechanical environment in medial opening wedge high tibial osteotomy using finite element analysis. J Biomech Eng 2015, 137(3). [DOI] [PubMed]
- 27.Golovakhsmall a CML, Orljanski W, Benedetto KP, Panchenko S, Buchler P, Henle P, Aghayev E. Comparison of theoretical fixation stability of three devices employed in medial opening wedge high tibial osteotomy: a finite element analysis. BMC Musculoskelet Disord. 2014;15:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon OR, Kang KT, Son J, Suh DS, Baek C, Koh YG. Importance of joint line preservation in unicompartmental knee arthroplasty: Finite element analysis. J Orthop Res. 2017;35(2):347–52. [DOI] [PubMed] [Google Scholar]
- 29.Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. J Orthop Res. 2004;22(3):625–32. [DOI] [PubMed] [Google Scholar]
- 30.Walker EM, Nelson M, Drew MD, Krammer SM, Brown TN. Tibial compression during sustained walking with body borne load. J Biomech. 2022;133:110969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meardon SA, Derrick TR, Willson JD, Baggaley M, Steinbaker CR, Marshall M, Willy RW. Peak and Per-Step Tibial Bone Stress During Walking and Running in Female and Male Recreational Runners. Am J Sports Med. 2021;49(8):2227–37. [DOI] [PubMed] [Google Scholar]
- 32.Luo CA, Hwa SY, Lin SC, Chen CM, Tseng CS. Placement-induced effects on high tibial osteotomized construct - biomechanical tests and finite-element analyses. BMC Musculoskelet Disord. 2015;16:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rankin KE, Dickinson AS, Briscoe A, Browne M. Does a PEEK Femoral TKA Implant Preserve Intact Femoral Surface Strains Compared With CoCr? A Preliminary Laboratory Study. Clin Orthop Relat Res. 2016;474(11):2405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemper A, McNally C, Manoogian S, Duma S. Tensile material properties of human tibia cortical bone: Effects of orientation and loading rate. Biomed Sci Instrum. 2008;44:419–27. [PubMed] [Google Scholar]
- 35.Gray HA, Taddei F, Zavatsky AB, Cristofolini L, Gill HS. Experimental validation of a finite element model of a human cadaveric tibia. J Biomech Eng. 2008;130(3):031016. [DOI] [PubMed] [Google Scholar]
- 36.Nicolella DP, Bonewald LF, Moravits DE, Lankford J. Measurement of microstructural strain in cortical bone. Eur J Morphol. 2005;42(1–2):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goshima K, Sawaguchi T, Shigemoto K, Iwai S, Nakanishi A, Inoue D, Shima Y. Large opening gaps, unstable hinge fractures, and osteotomy line below the safe zone cause delayed bone healing after open-wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1291–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.