Abstract
Background
Different areas of sheep infected with different types of parasites, all will cause serious harm to the local sheep, and the widespread use and repeated use of anthelmintics have produced different degrees of anthelmintic resistance (AR) in various regions. We re-investigated the infection of common parasites and AR of Gastrointestinal Nematodes (GINs) in sheep in Horqin Right Wing Front Banner, and first investigated the common parasite types and AR of GINs in sheep at other four areas in Hinggan league (city), China.
Results
A total of 1770 fecal samples were collected from 1 prefecture-level city and 4 counties in Hinggan league, in which the infection rate of Coccidia ranged from 83.3% to 96.06%, that of Ascaris ovis ranged from 10.17% to 15.19%, that of Moniezia benedeni ranged from 0.6% to 1%, that of Moniezia expansa ranged from 0.33% to 8.15%. The infection rate of GINs was 100%, and Haemonchus contortus was still the dominant species. The AR results showed that only the closantel in Horqin Right Wing Middle Banner was low resiatant, and the other three regions had been resistant. Levamisole also occurred AR in the other four regions, the widely used ivermectin and albendazole had produced serious AR in five areas. The research shows that GINs are becoming more and more resistant to various anthelmintics, which has made the problem worse.
Conclusions
Understanding the dynamic changes of parasite infections and the development trend of AR in sheep in the region in this paper, the development trend seems to be more serious than imagined. Therefore only by deeply understanding the parasitic infections of sheep in this land can more reasonable medication guidance be carried out. It is expected to provide new ideas formore innovative, scientific and sustainable methods of preventing and controlling parasites.
Keywords: Sheep parasites, Positivity rates, Gastrointestinal nematodes, Haemonchus contortus, Anthelmintic resistance, Hinggan League, China
Introduction
Sheep parasitiosis is a common chronic wasting disease, which is mainly caused by some internal and external parasites such as tapeworms, nematodes, trematodes, coccidia, ticks, lice, fleas, mites, etc. [1]. China is an important epidemic area of Fasciola hepatica, which is mainly found in Xinjiang, Tibet, Qinghai, Gansu, Heilongjiang, Jilin, Liaoning and other areas with high cattle and sheep breeding [2]. The common parasites in nematodiasis include ascariasis, haemonchiasis, whipworm disease, Oesophagostomum, hookworm disease, etc. These parasites can cause diarrhea, anemia, loss of appetite, weight loss, bloody stools and other problems in sheep, and the mortality rate of sheep infected with these parasites is extremely high [1]. Tapeworms mainly parasitizes in the small intestine of ruminants. The disease will seriously endanger the health of lambs. After the onset of the disease, it often leads to increased drinking water, anemia, diarrhea, growth retardation, etc., and even death in severe cases, resulting in a decline in the economic benefits of the sheep farming [3].
Gastrointestinal Nematodes (GINs) disease in sheep is caused by mixed infection of various nematodes. A large number of infections can lead to gastroenteritis, slow growth, weight loss, severe anemia and malnutrition in sheep, and even death. At the same time, the anthelmintic resistance (AR) of GINs is increasing, resulting in increased breeding costs and decreased economic benefits, which seriously restricts the development of sheep industry in pastoral areas [4, 5]. GINs more common is Haemonchus contortus, which cuts the gastrointestinal mucosa through the spear teeth and feeds on blood [6]. Infected animals often suffer from loss of appetite, fur messy, wool shedding, lethargy, accompanied by obvious abdominal edema, submandibular gland edema, abdominal mucosal damage, often accompanied by diarrhea, feces were dark paste, breathing and heart rate, weight and productivity decline [7]. In severe cases, there will be high anemia, extreme weight loss, and even death [8]. GINs is widely prevalent globally to this day and remains a major problem for the livestock industry. The disease is seasonally prevalent, and the seasonal prevalence varies depending on the geographical area of the environment. Outbreaks are usually more frequent in spring, summer and autumn, when suitable temperature and humidity help the recovery and development of the worms, leading to infection of a large number of animals and, in severe cases, to mass mortality of the hosts, resulting in serious economic losses [9].
For GINs is usually treated with anthelmintic for deworming, and the commonly used drugs are albendazole, ivermectin and levamisole [10]. In recent years, a large number of studies at home and abroad have reported that the disease has developed resistance to all three drugs, and even multi-drug resistance [11]. Early in the United States, H.contortus was reported to be resistant to benzimidazoles in 1961, and H.contortus was reported to be resistant to macrolides in Australia in the 1980s [12]. So since the last century had found signs of AR and multi-anthelmintic resistance, with the development of time and technology, whether AR has been more scientific regulation, or whether there is a better solution to the prevention and control of parasites, the authors, by reviewing domestic and international reports, found that the solution to AR has not achieve the desired effect. In China, some scientific research teams have carried out AR detection of GINs in different regions. For example, in 2018, the detection of H.contortus in sheep was carried out in 16 pastures in 5 areas of Chahar Right Back Banner, Ulanqab City, Inner Mongolia, northern China. It was found that the average infection rate of H. contortus was 75%, and H. contortus in 5 pastures had strong resistance to albendazole and ivermectin [13]. In July 2019, a survey on the resistance of GINs to anthelmintics was carried out in Horqin Right Wing Front Banner, Hinggan league, Inner Mongolia. It was found that the worms had developed resistance to ivermectin and albendazole, and were relatively sensitive to closantel [14]. In 2021, the study found that the dominant nematode infecting sheep in Inner Mongolia was still H. contortus, and 85.7% of the population was resistant to ivermectin; 75% of the population was resistant to albendazole; 60% of the population was sensitive to closantel; and some of the H. contortus showed a double resistance to ivermectin and albendazole. Later in the same year, the authors found that four ivermectin-resistant isolates of H. contortus were also resistant to albendazole, while one ivermectin-sensitive isolate was also sensitive to albendazole [15, 16]. In 2022, from six provinces in southern China collected 1733 H.contortus adults, and found that the adults had serious resistance to benzimidazoles and levamisole, and the smaller the age of the sheep and more worm burden could be more resistant to BZ [17]. H. contortus in these areas of China has a tendency to develop resistance to both anthelmintics at the same time.
Due to the climatic environment and cultural characteristics of Hinggan League, the breeding industry in the region is more developed. By the end of 2023, the region livestock inventory ≥ 12 million ( only, mouth), of which: pig inventory ≥ 700,000; cow inventory ≥ 1.5 million; sheep inventory ≥ 10 million. With the development of the breeding industry, the prevention and control of animal epidemics is particularly critical for life and health and economic development. Parasitism as an animal disease prevention and control is also a key issue that cannot be ignored. Due to the lack of awareness of parasites among farmers, and the infection of parasites in sheep of different farmers is different. Most farmers in the face of the choice of anthelmintic, mostly blind and crowd psychology, so long-term development, will lead to drug abuse or misuse, so the current parasite AR will become more serious. Therefore, in order to better help farmers standardize rational anthelmintic use and reduce veterinary drug residues, the investigation of parasitic infection and analysis of AR of common anthelmintic for GINs were carried out for sheep in Hinggan League.
Sheep GINs are increasingly resistant to locally used anthelmintics, which makes the prevention and control problem more serious. In 2024, Cesar C. Bassetto et al. revisited AR in sheep flocks in S˜ao Paulo State, Brazil, and found that the average effects of ivermectin and moxidectin were the worst, 34% and 21%, respectively, and the average effective rate of albendazole was 40%. This study shows that nematodes are becoming more resistant to a variety of chemical anthelmintics than before, which makes the problem even more serious [18]. This situation highlights the need to implement sustainable and durable approaches to prevent GINs infections. In 2019, China only investigated the epidemiology and AR of GINs in Horqin Right Front Banner of Hinggan League. Five years later, it continued to investigate the infection status of common parasites in sheep in all agricultural and pastoral areas under the jurisdiction of Hinggan League, and tracked the development trend of AR of GINs. The purpose is to first grasp the relevant dynamic changes of dominant species of sheep infection in 5 years, and then summarize the trend of anthelmintic prevention and control. Only by in-depth and extensive understanding of the specific conditions of parasitic infections in sheep on this land, grazing status, medication status, AR ratio, etc., can we better formulate prevention and control measures. It is the so-called need to implement sustainable methods to prevent GINs infections according to local conditions, and provide reasonable medication guidance for the prevention and treatment of GINs diseases in relevant areas.
Materials and methods
Animals and experimental design
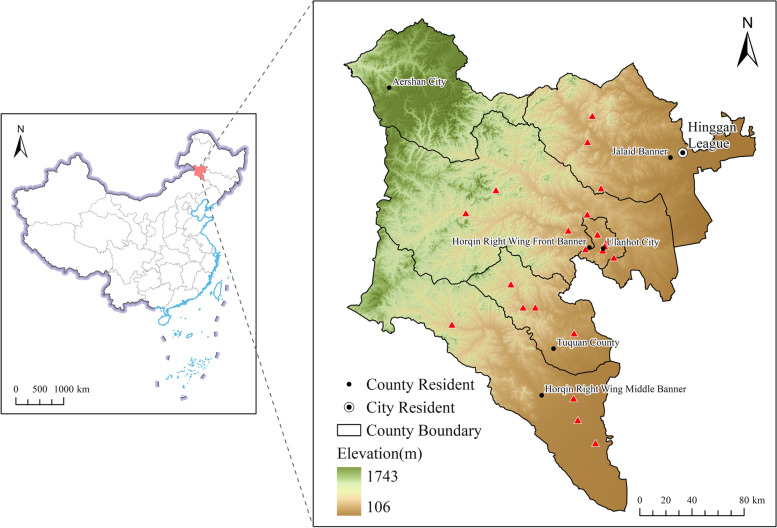
Hinggan League (city), China located 119°28′ ~ 123°39′ E, 44°15′ ~ 47°39′ N, with a total area of 55,131 square kilometers [19]. From June to July 2024, according to the administrative regions and topographic features of Hinggan League, Twenty-one sampling points were set up in five banners and counties (Fig. 1). Atotal of 1770 fecal samples of sheep were collected from five regions, among them, Ulanhot City sheep n = 300, Horqin Right Wing Middle Banner sheep n = 330, Horqin Right Wing Front Banner sheep n = 600, Jalaid Banner sheep n = 270, Tuquan County sheep n = 270, all sheep were privately owned by local farms, herdsmen and farmers, including from pastoral areas, semi-agricultural and semi-pastoral areas and different breeds, genders and ages. In each one of sampling points, animals were allocated into at five groups and treated as follows: 1) Closantel (CLO) (subcutaneous, 0.2 mL/1 kg body weight (BW)); 2) Ivermectin (IVM) (oral, 0.67 mL/10 kg BW); 3) Albendazole (ABZ) (oral, 0.3 pill/10 kg BW); 4) Levamisole (LEV) (oral, 1.5 pills/10 kg BW); 5) untreated control. The untreated control group to observe the natural decrease or increase in the eggs per gram (EPG) [20]. All the anthelmintics had passed the content determination of the Veterinary Pharmacopoeia of the People’s Republic of China. Fecal samples were collected from each flock before and 14 days after treatment, and quantitative analysis was performed using a modified McMaster counting plate.
Fig. 1.
Geographical coordinates of the sampling site Hinggan League, China
Faecal egg count reduction test (FECRT)
On the day before administration (D-1), fecal samples were collected from the rectum of each animal for faecal egg counts. In addition, these animals were weighed separately and administered at the recommended dose. The farms and their respective flocks had to: (1) at least 7 animals with EPG ≥ 200 per group [18]; (2) As recommended by Coles et al. (2006), anthelmintics were not used for at least 30 days prior to the experiment [21]. Sheep were evenly grouped by body weight and EPG, and were administered (D0) according to the specifications of each anthelmintic manufacturer mentioned above. On day 14 (D14), faeces were collected rectally and quantitatively analysed using the modified McMaster counting method for counting eggs, comparing the EPG before and after treatment and calculating the rate of faecal eggs count reduction (FECR). Meanwhile qualitatively analysed using the saturated saline floatation method while examined microscopically under a microscope, and referring to the related parasite books’ Egg Atlas for the identification of eggs [1, 22], determining the species and calculating the infection rate by the parasites in different regions.
The specific calculation formula is as follows:
Data analysis
According to the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines (2023) for determination of anthelmintic resistance [23], the resistance of sheep GINs to four anthelmintics was determined in this region. Differences in the infection of nematodes among geographical areas and sampling sites were analyzed by using the one-way ANOVA test within the software SPSS V 27 (IBM, New York, USA). When the p value < 0.05, the difference was considered significant [24]. According to the changes of EPG pre- and post-treatment measures within the same group, following the FECR calculation method by Denwood et al. (fecrt.com data analysis), to judge the results of AR test [25].
Results
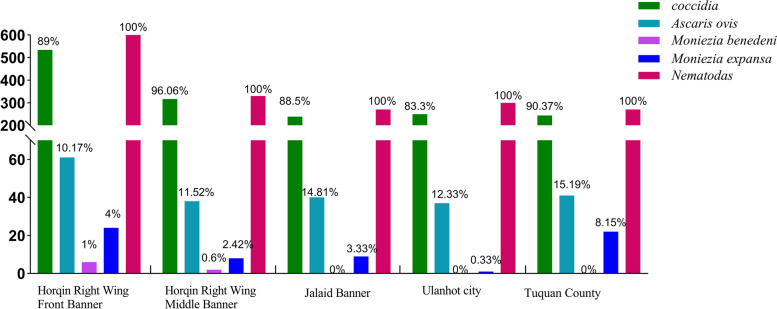
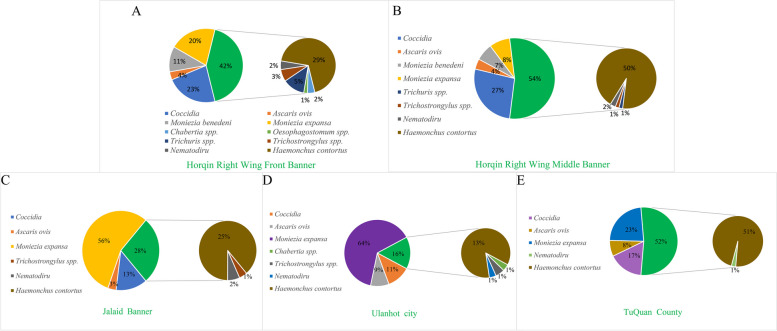
In the detection of common parasites in five regions of Hinggan league, it was found that the infection of parasites in different regions was different (Fig. 2). Among them, the infection rate of Coccidia was between 83.3% and 96.06%, the infection rate of coccidia in Horqin Right Wing Middle Banner was the highest, and a sheep was as high as EPG = 53300. The infection rate of Ascaris ovis was 10.17% -15.19%, which infection rate in Tuquan County was the highest, and a sheep was as high as EPG = 2200. Moniezia benedeni were only found in sheep in Horqin Right Wing Front Banner and Horqin Right Wing Middle Banner, and the infection rate was only 0.6% -1%. Moniezia expansa was infected in all five regions, and the infection rate ranged from 0.33% to 8.15%, the infection rate of Tuquan County was the highest, and a sheep highly reached EPG = 8100. The infection rate of GINs was 100%, and the highest infection amount of sheep was EPG = 32400. However, in order to better understand the distribution of parasites in different regions, based on the infection rate in each region, the proportion of mean EPG of different parasites was further counted (Fig. 3) to determine whether there were differences in parasite infections in different landforms in different regions.The highest percentage of EPG in sheep infections in Jalaid Banner and Ulanhot City was extended Moniezia expansa, while the highest percentage of EPG in all other regions was GINs. Figure 3 showed that the GINs infection, in addition to the large number of H. contortus in the five regions, there were Chabertia spp., Oesophagostomum spp., Trichuris spp., Trichostrongylus spp., Nematodirus spp. in Horqin Right Front Banner, but the proportion was only between 1 and 5%. Horqin Right Wing Middle Banner also had Trichuris spp., Trichostrongylus spp., Nematodirus spp.; Jalaid Banner had Trichostrongylus spp., Nematodirus spp.; three types of nematodes (Chabertia spp., Trichostrongylus spp., Nematodirus spp.) were found in Ulanhot; in Tuquan County only Nematodirus spp.was found; and these proportion of worms content were only between 1 and 2%. Therefore, H. contortus is still the dominant species of infection. Subsequently, the eggs were qualitatively analyzed by saturated saline flotation method, and the egg atlas was consulted to determine the species genus as detailed in Figs. 4 and 5.
Fig. 2.
Infection rate of different parasites by 5 regions
Fig. 3.
Average percentage of EPG by parasites
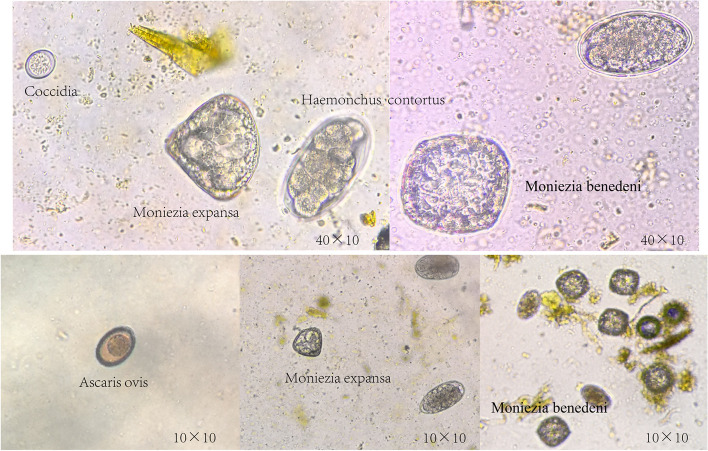
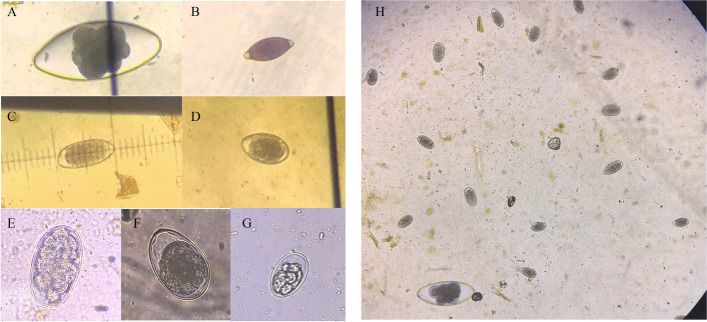
Fig. 4.
Sheep common parasite eggs
Fig. 5.
GIN eggs. A Nematodirus egg, B Trichuris spp. egg, C Chabertia spp. Egg, D/F Trichostrongylus spp.egg, E Haemonchus contortus egg, G Osertagia spp. egg, H Eggs infected by one of the sheep (10 × 40)
On the basis of previous epidemiological investigation, a total of 1770 sheep with severe infection in 5 regions of Hinggan league were screened for AR test, and the anthelmintic use in different pastures in different regions was recorded. The differences between the five regions were not statistically significant ( p > 0.05). Figure 6 showed that the most popular choice of anthelmintics for herdsmen is IVM, followed by ABZ and anthelmintic combination. The results in Table 1 showed that the widely used IVM and ABZ had produced severe resistant in the five regions of Hinggan league, and LEV also produced different degrees of resistance in the five regions. The CLO was low resistant in Horqin Right Wing Middle Banner, and in the other four regions, it was identified as having developed anthelmintic resistance.
Fig. 6.
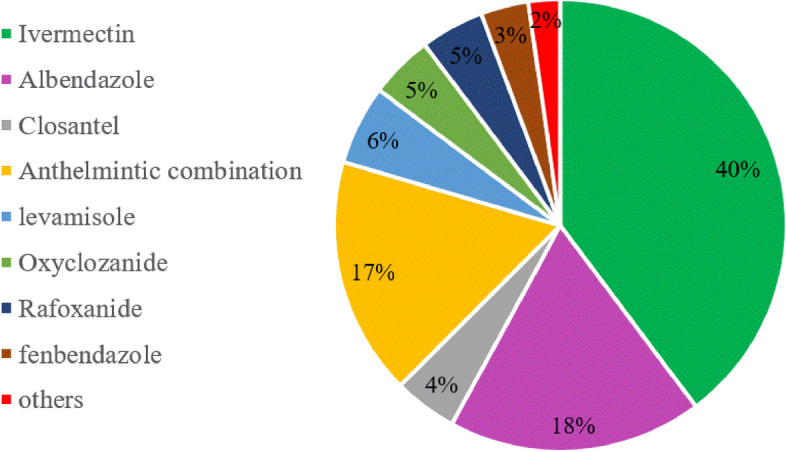
Herdsmen’s choice of anthelmintic
Table 1.
Results of anthelmintics resistance tests
| Area | Anthelmintic | n | Pre-Treatment EPG (Mean ± sd) | 14th Day Post-Treatment EPG (Mean ± sd) | FECR (%) | Guidelines: clinical | Guidelines: research |
|---|---|---|---|---|---|---|---|
| Horqin Right Wing Front Banner | Closantel | 120 | 1679 ± 2860 | 110 ± 192 | 86.2 | Low Resistant | Resistant |
| Ivermectin | 120 | 1848 ± 2424 | 817 ± 1833 | 53.99 | Resistant | Resistant | |
| Albendazole | 120 | 1460 ± 2259 | 390 ± 589 | 70.12 | Resistant | Resistant | |
| Levamisole | 120 | 1545 ± 2121 | 71 ± 149 | 91.81 | Low Resistant | Resistant | |
| Control | 120 | 1430 ± 1631 | 1410 ± 1982 | 0.53 | - | - | |
| Horqin Right Wing Middle Banner | Closantel | 66 | 2813 ± 4186 | 70 ± 158 | 96.89 | Low Resistant | Low Resistant |
| Ivermectin | 66 | 2970 ± 3801 | 1755 ± 1894 | 23.87 | Resistant | Resistant | |
| Albendazole | 66 | 2201 ± 3331 | 962 ± 1659 | 35.55 | Resistant | Resistant | |
| Levamisole | 66 | 1965 ± 2495 | 129 ± 341 | 94.73 | Resistant | Resistant | |
| Control | 66 | 1885 ± 1797 | 1684 ± 1532 | -18.84 | - | - | |
| Jalaid Banner | Closantel | 54 | 1127 ± 1256 | 145 ± 189 | 84.18 | Resistant | Resistant |
| Ivermectin | 54 | 894 ± 725 | 772 ± 1159 | 14.72 | Resistant | Resistant | |
| Albendazole | 54 | 1013 ± 703 | 670 ± 580 | 28.61 | Resistant | Resistant | |
| Levamisole | 54 | 1014 ± 1116 | 113 ± 168 | 85.01 | Resistant | Resistant | |
| Control | 54 | 932 ± 903 | 919 ± 679 | -7.12 | - | - | |
| Ulanhot city | Closantel | 60 | 1210 ± 2131 | 39 ± 62 | 94.68 | Low Resistant | Resistant |
| Ivermectin | 60 | 927 ± 914 | 338 ± 314 | 55.61 | Resistant | Resistant | |
| Albendazole | 60 | 856 ± 663 | 305 ± 319 | 58.49 | Resistant | Resistant | |
| Levamisole | 60 | 907 ± 1115 | 67 ± 367 | 91.91 | Resistant | Resistant | |
| Control | 60 | 899 ± 699 | 1058 ± 1245 | -20.18 | - | - | |
| Tuquan County | Closantel | 54 | 1506 ± 2096 | 164 ± 238 | 87.16 | Resistant | Resistant |
| Ivermectin | 54 | 1876 ± 4489 | 1188 ± 2489 | 27.85 | Resistant | Resistant | |
| Albendazole | 54 | 2346 ± 4662 | 693 ± 1060 | 57.13 | Resistant | Resistant | |
| Levamisole | 54 | 1739 ± 2186 | 181 ± 571 | 88.87 | Resistant | Resistant | |
| Control | 54 | 1435 ± 1882 | 1420 ± 1924 | -6.19 | - | - |
Citations: EPG (Egg Per Gram), FECR (Fecal Egg Count Reduction)
Guidelines (clinical): Based on an expected efficacy of 99% and a lower efficacy threshold of 90%
Guidelines (research): Based on an expected efficacy of 99% and a lower efficacy threshold of 95%
Discussion
In 2019, our laboratory tested the AR of sheep GINs in Horqin Right Front Banner of Hinggan league. In this area, sheep GINs were resistant to ABZ (FECR = 36.8%), resistant to IVM (FECR = 39.1%), and sensitive to CLO (FECR = 98.2%) [14]. In 2024, the results of AR of sheep in Horqin Right Wing Front Banner were IVM and ABZ continuous resistance, FECR was 70.12% and 53.99%, respectively. Resistant to CLO, FECR = 86.2%; and resistant to LEV, FECR = 91.81%. Although the GINs of sheep in this area were continuously resistant to IVM and ABZ, the FECR of IVM and ABZ was slightly improved after 5 years, indicating that it was necessary to regularly carry out AR investigation and provide guidance for the popularization of parasites and rational use of anthelmintics for herdsmen and farmers. There is a Chinese idiom that ' Out of the frying pan into the fire '.From the perspective of deworming effect, the degree of resistance of ABZ and IVM is still very high, and the fundamental problem of AR has not been solved. 5 years later, it was found that the GINs of sheep in this area had resistance to CLO, FECR decreased from 98.2% to 86.2%, and there was also a slight resistance to LEV. In 2024, Cesar C. Bassetto [18] revisited AR in sheep from São Paulo state, Brazil, and found that worms resistance had not improved in the past 30 years. On the contrary, the situation had deteriorated, which is consistent with the results obtained in this study. Regarding the changes in the species of GINs in the region during the five-year period, compared with the previous survey did not detect Bunosomum trigonocephalus spp. in this survey. Osteragia spp.was not detected in the McMaster technique, but three eggs were found in the qualitative test, so they were not included in the statistical results. Other nematodes were consistent with previous surveys. In summary, it is necessary to regularly carry out parasitic infection investigation and AR detection, so that new measures can be taken as soon as possible to delay the occurrence of AR and reduce the probability of AR.
In the AR survey in Horqin Right Wing Middle Banner, it was found that the region was low resistant to CLO and resistant to LEV, IVM and ABZ; the GINs of sheep in Ulanhot、Jalaid Banner and Tuquan County have developed resistance to these four anthelmintics. Throughout the deworming effect of four anthelmintics in five regions, the deworming effect of CLO was between 84.18% and 96.89%, IVM deworming effect was between 14.72% and 55.61%, ABZ was 28.61% to 70.12%, and LEV was 85.01% to 94.73%. If there is a division of the degree of AR, CLO and LEV are classified as mild drug resistance, while IVM and ABZ are moderately resistant. And even the lowest deworming rate of IVM was only 14.72%, and the lowest deworming rate of ABZ was only 28.61%. In the statistics of anthelmintics commonly used by herdsmen, we found that the frequency of use of LEV and CLO was only 6% and 4%, and IVM was highest to 40%. The herdsmen in Jalaid Banner had used LEV for a long time, so the results of this AR survey showed that the lowest deworming effect of LEV in this area was 85.01%. The CLO usually is subcutaneous injection, compared with oral administration, the subcutaneous injection is somewhat troublesome and the cost of deworming when high of a large number of deworming in sheep farms. Therefore, herdsmen still prefer IVM, ABZ and other anthelmintics for deworming. Therefore, it is further speculated that the occurrence and persistence of AR are related to the number of medications and the dosage of medication. In the process of epidemiological investigation and sampling in Hinggan League, China, Aershan City is a forest area, due to its special geomorphological environment, the sampling and detection of AR will be more difficult; secondly, its tourism industry is developed, and the relative yield of sheep is relatively small. The region is not representative, so there was no investigation of parasitic infection and AR of sheep in Aershan City. In addition, it was found that there was no significant difference in worm infection between agricultural areas, pastoral areas or semi-agricultural and semi-pastoral farming methods in this survey. We believe that it is related to the special climate influence during this survey, this year, from June to July, the rainfall in Hinggan League is larger compared to the previous years, and the rainfall lasts for a longer period of time, so most of the sheep will take the captive form of rearing under the influence of the weather. Through the literature reports and parasite life history, the breeding mode has a certain impact on the infection of sheep parasites. If you want to truly understand the influence of the breeding method on the infection of the parasite, it is necessary to design experiments for different grazing forms, find representative sites, and repeat many times. It is expected that there will be new discoveries.
This survey’s results were that five regions had developed varying degrees of resistance to common anthelmintics. By reviewing relevant reports from home and abroad, the author summarized the resistance of sheep GINs to common anthelmintics in some northern regions of China in the past 8 years [26–35] (Table 2), and summed the development of AR in international sheep GINs [36–56] (Table 3). In most parts of China, it had developed resistance to IVM and ABZ, LEV and CLO were resistant in a few areas, while Moxidectin and Nitroxynil were relatively sensitive. In the latest report ( 2024), only the Xinjiang Uygur Autonomous Region was sensitive to ABZ [35]. International studies have found that almost all ABZ were resistant, and IVM, LEV, MOX and CLO were resistant in most areas, although there had some reports of anthelmintic sensitivity, these reports were made two years ago. Monepantel, a relatively new anthelmintic, had also been reported to be resistant in Uruguay in 2014 [41]. In summary, it was found that GINs have developed more widespread and multi-drug resistance to various types of anthelmintics [57]. Due to the limited choice of available anthelmintics and the prevalence of resistance, this situation indicates that pastoral households and local governments need to implement innovative and sustainable strategies to control GINs infections.
Table 2.
Survey on anthelmintic resistance of GINs in Sheep in northern China
| Anthelmintics | Year | Area | Judge | Reference |
|---|---|---|---|---|
| Albendazole | 2017 | Eastern Inner Mongolia | Resistant | [26] |
| 2017 | Hulun Buir Inner Mongolia | Resistant | [27] | |
| 2018 | Ulanqab Inner Mongolia | Resistant | [13] | |
| 2018 | Ordos Inner Mongolia | Resistant | [28] | |
| 2019 | Ulanqab Inner Mongolia | Resistant | [29] | |
| 2020 | Xilingol League Inner Mongolia | Resistant | [30] | |
| 2021 | Hinggan League Inner Mongolia | Resistant | [14] | |
| 2021 | Tailai County, Heilongjiang Province | Resistant | [31] | |
| Xilinhot City/ Yakeshi City Inner Mongolia | ||||
| 2022 | Ordos Inner Mongolia | Resistant | [24] | |
| 2024 | Xinjiang Uygur Autonomous Region | Sensitive | [35] | |
| Ivermectin | 2017 | Eastern Inner Mongolia | Resistant | [26] |
| 2017 | Hulun Buir Inner Mongolia | Resistant | [27] | |
| 2018 | Ulanqab Inner Mongolia | Resistant | [13] | |
| 2018 | Ordos Inner Mongolia | Resistant | [28] | |
| 2019 | Ulanqab Inner Mongolia | Resistant | [29] | |
| 2020 | Xilingol League Inner Mongolia | Resistant | [30] | |
| 2021 | Hinggan League Inner Mongolia | Resistant | [14] | |
| 2021 | Tailai County, Heilongjiang Province | Resistant | [31] | |
| Xilinhot City/ Yakeshi City Inner Mongolia | ||||
| 2022 | Ordos Inner Mongolia | Resistant | [24] | |
| 2022 | Hulun Buir /Xilinhot City Inner Mongolia | Resistant | [33] | |
| Levamisole | 2017 | Eastern Inner Mongolia | Sensitive | [26] |
| 2018 | Ordos Inner Mongolia | Resistant | [28] | |
| 2019 | Ulanqab Inner Mongolia | Sensitive | [29] | |
| 2020 | Xilingol League Inner Mongolia | Sensitive | [30] | |
| 2021 | Tailai County, Heilongjiang Province | Sensitive | [31] | |
| Xilinhot City/ Yakeshi City Inner Mongolia | ||||
| 2022 | Ordos Inner Mongolia | Suspected | [24] | |
| Closantel | 2017 | Hulun Buir Inner Mongolia | Sensitive | [27] |
| 2019 | Ulanqab Inner Mongolia | Resistant | [29] | |
| 2020 | Xilingol League Inner Mongolia | Sensitive | [30] | |
| 2021 | Hinggan League Inner Mongolia | Sensitive | [14] | |
| 2022 | Ordos Inner Mongolia | Suspected | [24] | |
| Moxidectin | 2021 | Altay Prefecture in Xinjiang | Sensitive | [32] |
| 2023 | Heilongjiang Province | Sensitive | [34] | |
| Nitroxynil | 2018 | Ordos Inner Mongolia | Sensitive | [28] |
| 2020 | Xilingol League Inner Mongolia | Sensitive | [30] | |
| 2022 | Ordos Inner Mongolia | Suspected | [24] |
Table 3.
Aggregation of international reports on anthelmintic resistance about GINs
| Anthelmintics | Year | Area | Judge | Reference |
|---|---|---|---|---|
| Albendazole | 2011 | Trinidad | Resistant | [37] |
| 2012 | São Paulo state, Brazil | Resistant | [39] | |
| 2017 | Antioquia,Colombia | Resistant | [43] | |
| 2020 | Bangladesh | Resistant | [50] | |
| 2020 | Costa Rica | Resistant | [49] | |
| 2021 | Beni-Suef province, Egypt | Resistant | [51] | |
| 2022 | Southwest England | Resistant | [56] | |
| 2022 | Sweden | Resistant | [55] | |
| 2023 | Minas Gerais, Brazil | Resistant | [54] | |
| 2024 | S˜ao Paulo State, Brazil | Resistant | [18] | |
| Ivermectin | 2010 | Netherlands | Sensitive | [36] |
| 2011 | Trinidad | Resistant | [37] | |
| 2012 | São Paulo state, Brazil | Resistant | [39] | |
| 2014 | Inland southern Queensland | Resistant | [40] | |
| 2017 | Kashmir valley | Sensitive | [45] | |
| 2017 | Antioquia,Colombia | Resistant | [43] | |
| 2018 | Netherlands | Resistant | [46] | |
| 2020 | Bangladesh | Resistant | [50] | |
| 2020 | Costa Rica | Resistant | [49] | |
| 2021 | Beni-Suef province, Egypt | Sensitive | [51] | |
| 2022 | Southwest England | Resistan | [56] | |
| 2022 | Sweden | Resistant | [55] | |
| 2023 | Minas Gerais, Brazil | Resistant | [54] | |
| 2024 | S˜ao Paulo State, Brazil | Resistant | [18] | |
| Levamisole | 2011 | Trinidad | Resistant | [37] |
| 2012 | São Paulo state, Brazil | Resistant | [39] | |
| 2014 | Inland southern Queensland | Resistant | [40] | |
| 2017 | Antioquia,Colombia | Resistant | [43] | |
| 2020 | Bangladesh | Resistant | [50] | |
| 2020 | Costa Rica | Low Resistant | [49] | |
| 2021 | Beni-Suef province, Egypt | Sensitive | [51] | |
| 2022 | Southwest England | Resistant | [56] | |
| 2022 | Sweden | Sensitive | [55] | |
| 2023 | Minas Gerais, Brazil | Resistant | [54] | |
| 2024 | S˜ao Paulo State, Brazil | Suspected | [18] | |
| Closantel | 2012 | São Paulo state, Brazil | Resistant | [39] |
| 2014 | Inland southern Queensland | Resistant | [40] | |
| 2016 | Ontario,Canada | Sensitive | [42] | |
| 2017 | Kashmir valley | Sensitive | [45] | |
| 2018 | Netherlands | Resistant | [46] | |
| 2021 | Northeastern Brazil | Suspected | [53] | |
| 2024 | S˜ao Paulo State, Brazil | Resistant | [18] | |
| Monepantel | 2011 | Australia | Sensitive | [38] |
| 2014 | Uruguay | Resistant | [41] | |
| 2017 | Botucatu, SP, Brazil | Resistant | [44] | |
| 2019 | UK | Resistant | [47] | |
| 2020 | Sweden | Resistant | [48] | |
| 2021 | Espírito Santo, Brazil | Resistant | [52] | |
| 2024 | S˜ao Paulo State, Brazil | Sensitive | [18] | |
| Moxidectin | 2010 | Netherlands | Sensitive | [36] |
| 2012 | São Paulo state, Brazil | Resistant | [39] | |
| 2014 | Inland southern Queensland | Resistant | [40] | |
| 2017 | Antioquia,Colombia | Resistant | [43] | |
| 2018 | Netherlands | Resistant | [46] | |
| 2022 | Southwest England | Resistant | [56] | |
| 2022 | Sweden | Sensitive | [55] | |
| 2024 | S˜ao Paulo State, Brazil | Resistant | [18] |
Studies have shown that anthelmintic combination may be able to solve worms that have developed AR. S. Luque used the combination of MOX and LEV, and no obvious adverse pharmacokinetic changes were observed compared with the single anthelmintic. In 2014, FECR was 99% ( MOX), 85% ( LEV) and 100% ( MOX + LEV), respectively. After 4 years, the anthelmintic effect of MOX + LEV combination ( 87%) was significantly higher than that of MOX ( 42%) or LEV (69%) alone [58]. In 2023, Gonzalo Su 'arez reported that CLO + MXD administered simultaneously by sc and oral administration will not lead to any related drug-drug pharmacokinetic interaction. After combined administration, the efficacy of CLO and MOX resistant nematodes was the highest [59]. At present, the combination of anthelmintic seems to be an effective strategy to control AR GINs. If it is overused, there will be a risk of producing multi-drug resistant parasite populations, in this case, the anthelmintic combination under specific management conditions to use, only when the animals are parasitized by a large number of nematodes and difficult to control, it is meaningful to combine policy use.
Of course, researchers have also been engaged in the study of AR in recent years, hoping to overcome the problem of AR as soon as possible. In the research progress of H. contortus resistance to IVM, three possible resistance mechanisms have been reported, which are targeting GABA-gated Cl-channels, reducing glutamate-gated Cl-opening frequency, and binding efflux transporter proteins [60–63]. The production of ABZ resistance is closely related to β-tubulin. The single nucleotide polymorphisms (snp) at the codons 167,198 and 200 of the isotype-1β-tubulin gene are related to the occurrence of parasite resistance [64, 65], and also related to the p-glycoprotein coding gene (P-gps) of nematodes, which help to excrete the drug into the extracellular compartment, thus leading to drug resistance [66]. LEV acts as an agonist on nicotinic acetylcholine receptors (nAChRs) at the neuromuscular junction of nematodes, leading to persistent neuromuscular depolarization and spastic paralysis. The resistance to LEV may also be due to gene mutations that do not encode the structural components of nAChR itself [67, 68]. In view of the pharmacological complexity of anthelmintics and the potential non-specific resistance mechanisms (such as P-gps), AR may be polygenic and may vary between different helminth species and even between isolates within a species. In addition to understanding the mechanism of resistance to anthelmintics, researchers also used advanced scientific methods such as whole genome, proteomics, whole transcriptomics (the role of non-coding RNA in AR), AR genetic analysis and other methods to compare the differences between the sensitive group and the drug-resistant group, combined with q-PCR, western-blot and other methods to narrow the range of AR genes, so as to screen differential genes. Subsequently, some teams tried to use RNAi, gene knockout, overexpression and other methods [69–74] to detect whether the selected AR genes play a key role, or to reverse the AR problem by changing the AR genes through manual intervention, but this still seems to be some difficulty and challenge.
In addition to the above common GINs infections, the survey also found that some sheep were infected with Coccidia, Ascaris ovis and Moniezia. These parasites also cause irreversible damage to the host, and the anthelmintics of different species are also different. In addition, in the survey of sheep in Hinggan league, only one sheep farm was infected with cerebral echinococcosis, and the fetal sheep died in the abdomen. Because the owner of the farm did not raise separately in time, other sheep had been infected. Parasitic diseases are easy to be ignored in both animal and human healthy life, people are not enough awareness of parasites, and their development and parasitism lead to a long time for the host to produce diseases. Compared with other viral and bacterial epidemic diseases, the outbreak process is slow. In fact, most of parasitosis bring extremely serious damage to the host, even death. With the development of breeding industry and the influence of grazing environment, the probability of animals infected with parasites to spread parasites is also increasing. A large number of parasites will lead to host emaciation, anemia, abortion and death, so farmers will take corresponding measures to solve parasites. As we all know, the fundamental method to solve infectious diseases is divided into three steps: controlling the source of infection, cutting off the route of transmission, and protecting susceptible animals. So the prevention and control measures for parasites mainly include: 1.Strengthen feeding management: pay attention to the hygiene of feeding environment, avoid drinking polluted water, reasonable feeding, and improve the immunity of livestock. 2. Innocent treatment of feces: regular cleaning of feces, harmless treatment, composting and fermentation of feces, and high temperature can effectively kill parasite eggs and larvae in feces. 3. Elimination of intermediate hosts: such as mice, mosquitoes, etc., can be medicated bath, spray, etc., to eliminate intermediate hosts. 4. Scientific deworming: First, regularly check and monitor the parasite infection status of livestock, select the appropriate drugs, do not follow the trend and do not blindly use anthelmintics. 5. Environmental disinfestation and diseased animals for isolation rearing, etc. [75]. These relatively effective prevention and control measures need to increase publicity, so that more herdsmen reasonable scientific breeding.
In this epidemiological survey of parasites, we found that most farmers rely on anthelmintics for deworming. Especially in recent years, the resistance of commonly used anthelmintics had become increasingly serious, so farmers believed that increasing the dose of drugs can be effective, and administered multiple times, and the interval between deworming was short. In addition, we also counted their faeces disposal, environmental disinfection and isolation of diseased animals, and most of them did not take reasonable precautions. In fact, it can be understood, these works need to consume human, material and financial resources, large-scale farms can do it, but some other herdsmen cannot do it. After all, the conditions and environment are limited, and it is difficult to achieve health standards and environmental standards at all times. So they will choose chemical drugs to deworm quickly. The irrational use of drugs over time has also become the key to accelerating the development of AR, of course, with the impact of geomorphological environment and climate, the degree will change slightly. The diagnosis of AR and the lack of awareness of AR issues are still the key to increasing the development of AR in more farms. It is clear that herders need to reduce their dependence on traditional anthelmintics and cannot use them as the only way to control GINs [76]. Therefore, must be according to the actual situation of herdsmen to selectively use anthelmintic treatment and combine with one or more alternative methods for the control of GINs. In this implementation process, sheep farmers, researchers and local governments need to cooperate. Otherwise, the sustainability of existing and upcoming anthelmintic molecules will remain unfeasible.
Through this epidemiological investigation of Hinggan League, we know the real farming environment and parasite infection in agricultural and pastoral areas, so we will think about a question, how to really improve the preventive measures of parasites, and how to use anthelmintic conveniently, quickly and standardly. Under the premise of protecting the fundamental interests of farmers and herdsmen, more efficient and rapid prevention and control of parasitic diseases, which has to be thought-provoking. In summary, it was found that sheep in Hinggan League of China were infected with a variety of parasites, and GINs have developed more extensive and multi-drug resistance to various types of anthelmintics. Due to the limited choice of available anthelmintics and the prevalence of AR, this situation indicates that herders need to implement innovative and sustainable strategies to control gastrointestinal nematode infections.
Conclusion
This study mainly reported the common parasites infected by sheep in Hinggan league, China, including Coccidia, Ascaris ovis, Moniezia and Haemonchus contortus, and tested the anthelmintic resistance of gastrointestinal nematodes, and found that only Horqin Right Wing Middle Banner was low resistant to CLO in the five regions, and the rest had different degrees of anthelmintic resistance to IVM, ABZ, CLO and LEV. Due to the limited selectivity of available anthelmintics and the prevalence of anthelmintic resistance, it is necessary to standardize breeding and rationally use anthelmintics for deworming. According to the different anthelmintic resistance situation in each region, according to local conditions to provide medication guidance, combine with local conditions, put forward sustainable strategies to control gastrointestinal nematode infection is the primary prevention and control task at present.
Acknowledgements
The authors appreciate Hinggan league Agricultural and Animal Husbandry Technology Extension Centre for their strong support, gratitude Animal Disease Prevention and Control Center of Horqin Right Front Banner, Horqin Right Middle Banner, Jalaid Banner, Ulanhot City and Tuquan County for their strong supports and help during the sampling period.
Abbreviations
- GINs
Gastrointestinal Nematodes
- AR
Anthelmintic resistance
- H. contortus
Haemonchus contortus
- BZ
Benzimidazoles
- IVM
Ivermectin
- ABZ
Albendazole
- LEV
Levamisole
- CLO
Closantel
- EPG
Eggs per gram
- FECRT
Faecal egg count reduction test
- FECR
Faecal eggs count reduction
- MOX
Moxidectin
- nAChRs
Nicotinic acetylcholine receptors
- P-gps
P-glycoprotein coding genes
- snp
Single nucleotide polymorphisms
Authors’ contributions
WL and CX designed the research; YM conducted the data analysis and wrote the manuscript. YM,HZ,HL and LL collected samples and detected eggs. WJ,ZM,CG and ML were responsible for sampling and coordination. JL and WG instructed the writing and provided critical comments. All authors read and approved the final manuscript.
Funding
This work was funded by the Monitoring and Deworming of Major Parasitic Diseases in Sheep in Hinggan League (MB202306) and the “Science and Technology for the Development of Mongolia” initiative priority program (NMKJXM202203).
Data availability
The datasets supporting the results of this document are contained within the article. Any additional data may be requested to the corresponding author.
Declarations
Ethics approval and consent to participate
The experimental animals used in this project were treated in strict accordance with the Guidelines for Animal Ethics and Experimentation of the People’s Republic of China. The experimental animals were used in accordance with the protocols approved by The Scientific Ethics Committee of Inner Mongolia Agricultural University (Approval code: NND2023119). In the course of this investigation, all of our experimental animals obtained the informed consent of the animal owners, and all animal owners agreed to use these animals in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenlong Wang, Email: wwl.imau@163.com.
Chunxia Liu, Email: lcx.imau@163.com.
References
- 1.Kong FY. Livestock parasitology (second edition). Beijing: China Agricultural University Press; 2016. p. 164–94 (in Chinese). [Google Scholar]
- 2.Duan JH. Screening of Antigens for Detection of Fasciola hepaticaInfection in Sheep and Establishment and Application ofImmunological Detection Methods. Jilin University. 2023. (in Chinese)
- 3.Lu SL. Epidemiological study of Intestinal Parasites and RiskAnalysis of Zoonotic in sheep and goats of differentgeographical environments in China. Henan Agricultural University. 2024. (in Chinese)
- 4.Wilmsen MO, Silva BF, Bassetto CC, Amarante AF. Gastrointestinal nematode infections in sheep raised in Botucatu, state of São Paulo. Brazil Rev Bras Parasitol Vet. 2014;23(3):348–54. [DOI] [PubMed] [Google Scholar]
- 5.Besier RB, Kahn LP, Sargison ND, Van Wyk JA. The pathophysiology, ecology and epidemiology of haemonchus contortus infection in small ruminants. Adv Parasitol. 2016;93:95–143. [DOI] [PubMed] [Google Scholar]
- 6.Feng K L. Establishment of early diagnostic method. Nanjing University. 2020. (in Chinese)
- 7.Huang Y. Preliminary Research on the Function and Molecular Mechanism of Hc-nas-33 Involved in the Molting Process of Haemonchus contortus. Zhejiang University. 2021. (in Chinese)
- 8.Wang C, Li F, Zhang Z. Recent Research Progress in China on Haemonchus contortus. Front Microbiol. 2017;8:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao XC. Preliminary Study on Immune Responses Induced by Exosome sfrom Haemonchus contortus in Goat. Chinese Academy of Agricultural Sciences. 2018. (in Chinese)
- 10.Xv SS. Epidemiology, clinical signs and control measures of Haemonchus contortus disease in sheep. Modern Animal Husbandry Sci Technol. 2021;No.77(05):133–4. [Google Scholar]
- 11.Muchiut SM, Fernandez AS, Steffan PE. Anthelmintic resistance: Management of parasite refugia for Haemonchus contortus through the replacement of resistant with susceptible populations. Vet Parasitol. 2018;254:43–8. [DOI] [PubMed] [Google Scholar]
- 12.Kotze AC, Prichard RK. Anthelmintic resistance in haemonchus contortus: history mechanisms and diagnosis. Adv Parasitol. 2016;93:397–428. [DOI] [PubMed] [Google Scholar]
- 13.Zhao XL, Liu XL, Su Q. Epidemiological Investigation on Haemonchus contortus in Sheep in Chayouhouqi of Inner Mongolia and Detection of Its Drug Resistance. China Animal Health Inspection. 2016;36(05):6–10 (in Chinese). [Google Scholar]
- 14.Wang TY, Zhai S, Wang Q. Investigation on infection and drug resistance of digestive tract nematodes of sheep in Keyouyiqianqi area. Chinese J Veter Med. 2021;57(07):29–32+37 (in Chinese). [Google Scholar]
- 15.Luo XP, Li JY, Geng WG. Investigation on anthelmintic resistance of Haemonchus contortus in sheep in Inner Mongolia [C]//Veterinary Parasitology Branch of China Association of Animal Husbandry and Veterinary Medicine.2021 Thesis Collection of the Second Young Scientists Academic Forum of Veterinary Parasitology Branch of China Association of Animal Husbandry and Veterinary Medicine. 2021. (in Chinese)
- 16.Luo XP, Wang PL, Li JY. Characteristics of albendazole resistance of different ivermectin-resistant Haemonchus contortus isolates in China. Chinese J Veter Sci. 2021;41(07):1301–9+1347 (in Chinese). [Google Scholar]
- 17.Li ZH. Detection of benzimidazoles and levamisole resistance in Haemonchus contortus in some areas of China. Nanjing Agricultural University. 2022. (in Chinese)
- 18.Bassetto CC, Albuquerque ACA, Lins JGG, Marinho-Silva NM, Chocobar MLE, Bello HJS, Mena MO, Niciura SCM, Amarante AFT, Chagas ACS. Revisiting anthelmintic resistance in sheep flocks from São Paulo State, Brazil. Int J Parasitol Drugs Drug Resist. 2024;24:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Q, Fu HL, Wang TM. Simulation of potential suitable growth areas of shrubs in hinggan league steppe. Acta Agrestia Sinica. 2024;32(02):579–87 (in Chinese). [Google Scholar]
- 20.Neves JH, Carvalho N, Rinaldi L, Cringoli G, Amarante AFT. Diagnosis of anthelmintic resistance in cattle in Brazil: a comparison of different methodologies. Vet Parasitol. 2014;206:216–26. [DOI] [PubMed] [Google Scholar]
- 21.Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136:167–85. [DOI] [PubMed] [Google Scholar]
- 22.Taylor MA, Coop RL, Wall RL. Veterinary parasitology. Third edition. Black well. 2007.
- 23.Kaplan RM, Denwood MJ, Nielsen MK, Thamsborg SM, Torgerson PR, Gilleard JS, Dobson RJ, Vercruysse J, Levecke B. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol. 2023;318:109936. [DOI] [PubMed] [Google Scholar]
- 24.Hou B, Yong R, Wuen J, Zhang Y, Buyin B, Subu D, Zha H, Li H, Hasi S. Positivity rate investigation and anthelmintic resistance analysis of gastrointestinal nematodes in Sheep and Cattle in Ordos, China. Animals (Basel). 2022;12(7):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denwood MJ, Kaplan RM, McKendrick IJ, Thamsborg SM, Nielsen MK, Levecke B. A statistical framework for calculating prospective sample sizes and classifying efficacy results for faecal egg count reduction tests in ruminants, horses and swine. Vet Parasitol. 2023;314:109867. [DOI] [PubMed] [Google Scholar]
- 26.Han T, Wang M, Zhang G, Han D, Li X, Liu G, Li X, Wang Z. Gastrointestinal nematodes infections and anthelmintic resistance in grazing sheep in the Eastern Inner Mongolia in China. Acta Parasitol. 2017;62(4):815–22. [DOI] [PubMed] [Google Scholar]
- 27.Luo XP, Ao DGW, Zhan SB. The study of anthelminthic effect on gastrointestinal nematodes of sheep by three current anthelmintics in Inner Mongolia. Chinese Journal of Veterinary Medicine. 2017;53(10):35–7 (in Chinese). [Google Scholar]
- 28.E Ye LDG, Cao ZF, Hai Y. Studies on infections and drug resistance of gastrointestinal nematodes in sheep in Uxin Banner. Chinese Veter Sci. 2018;48(06):735–42 (in Chinese). [Google Scholar]
- 29.Liu XL. The Investigation of Conventional anthelmintics drug resistance of sheep GINs in Chayouhouqi Area. Inner Mongolia Agricultural University. 2019.(in Chinese)
- 30.Qi LMG. Epidemiological investigation of cattle and sheep helminth infections and comparative anthelmintic studies in Bayanhua of west Uzhemchin. Inner Mongolia Agricultural University. 2020. (in Chinese)
- 31.Wen J. Investigation of Gastrointestinal Parasitesand Comparison of Insect Repellent among Tailai. Xilinhot and Yakeshi Sheep: Northeast Agricultural University; 2021. (in Chinese). [Google Scholar]
- 32.Li X, Chen Z, Zhong C. Evaluation on the Pharmacodynamics of a Pour-on Agent of Moxidectin Against Melophagus ovinus and the Gastrointestinal Tract Nematodes in Sheep. China Animal Husbandry & Veterinary Medicine. 2021;48(05):1784–93 (in Chinese). [Google Scholar]
- 33.Liu Y, Wang P, Wang R, Li J, Zhai B, Luo X, Yang X. An epidemiological investigation and drug-resistant strain isolation of nematodirus oiratianus in sheep in inner Mongolia, China. Animals (Basel). 2022;13(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang GH, Jin ZH, Wang LK. Therapeutic effect of moxidectin microemulsion preparation on ovine digestive tract nematodes. Modern J An Husban Veter Med. 2023;02:23–6 (in Chinese). [Google Scholar]
- 35.Ding J, Ma Z, Zhang D. Detection of the resistance of major gastrointestinal nematodes in sheep in Xinjiang to Albendazole. Modern An Husban Sci Technol. 2024;06:31–3 (in Chinese). [Google Scholar]
- 36.Borgsteede F, Verkaik J, Moll L, Dercksen D, Vellema P, Bavinck G. How widespread is resistance to invermectin among gas-trointestinal nematodes in sheep in The Netherlands? Tijdschr Diergeneeskd. 2010;135:782–5. [PubMed] [Google Scholar]
- 37.George N, Persad K, Sagam R, Offiah VN, Adesiyun AA, Harewood W, Lambie N, Basu AK. Efficacy of commonly used anthelmintics: first report of multiple drug resistance in gastrointestinal nematodes of sheep in Trinidad. Vet Parasitol. 2011;183(1–2):194–7. [DOI] [PubMed] [Google Scholar]
- 38.Kaminsky R, Bapst B, Stein PA, Strehlau GA, Allan BA, Hosking BC, Rolfe PF, Sager H. Differences in efficacy of monepantel, derquantel and abamectin against multi-resistant nematodes of sheep. Parasitol Res. 2011;109(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veríssimo CJ, Niciura SC, Alberti AL, Rodrigues CF, Barbosa CM, Chiebao DP, Cardoso D, da Silva GS, Pereira JR, Margatho LF, da Costa RL, Nardon RF, Ueno TE, Curci VC, Molento MB. Multidrug and multispecies resistance in sheep flocks from São Paulo state. Brazil Vet Parasitol. 2012;187(1–2):209–16. [DOI] [PubMed] [Google Scholar]
- 40.Lyndal-Murphy M, Ehrlich WK, Mayer DG. Anthelmintic resistance in ovine gastrointestinal nematodes in inland southern Queensland. Aust Vet J. 2014;92(11):415–20. [DOI] [PubMed] [Google Scholar]
- 41.Mederos AE, Ramos Z, Banchero GE. First report of monepantel Haemonchus contortus resistance on sheep farms in Uruguay. Parasit Vectors. 2014;7:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westers T, Jones-Bitton A, Menzies P, Van Leeuwen J, Poljak Z, Peregrine AS. Efficacy of closantel against ivermectin- and fenbendazole-resistant Haemonchus sp. in sheep in Ontario. Canada. Vet Parasitol. 2016;228:30–41. [DOI] [PubMed] [Google Scholar]
- 43.Chaparro JJ, Villar D, Zapata JD, López S, Howell SB, López A, Storey BE. Multi-drug resistant Haemonchus contortus in a sheep flock in Antioquia Colombia. Vet Parasitol Reg Stud Reports. 2017;10:29–34. [DOI] [PubMed] [Google Scholar]
- 44.de Albuquerque ACA, Bassetto CC, Almeida FA, Amarante AFT. Development of Haemonchus contortus resistance in sheep under suppressive or target selective treatment with monepantel. Vet Parasitol. 2017;246:112–7. [DOI] [PubMed] [Google Scholar]
- 45.Tramboo SR, Shahardar RA, Allaie IM, Wani ZA, Abbas M. Efficacy of ivermectin, closantel and fenbendazole against gastrointestinal nematodes of sheep in Kashmir valley. J Parasit Dis. 2017;41(2):380–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ploeger HW, Everts RR. Alarming levels of anthelmintic resistance against gastrointestinal nematodes in sheep in the Netherlands. Vet Parasitol. 2018;262:11–5. [DOI] [PubMed] [Google Scholar]
- 47.Bartley DJ, Hamer K, Andrews L, Sargison ND, Morrison AA. Multigeneric resistance to monepantel on a UK sheep farm. Vet Parasitol. 2019;X 1:100003. [DOI] [PubMed] [Google Scholar]
- 48.Höglund J, Enweji N, Gustafsson K. First case of monepantel resistant nematodes of sheep in Sweden. Vet Parasitol Reg Stud Reports. 2020;22:100479. [DOI] [PubMed] [Google Scholar]
- 49.Castro-Arnáez IC, Montenegro VM, Vargas-Leitón B, Álvarez-Calderón V, Soto-Barrientos N. Anthelmintic resistance in commercial sheep farms in Costa Rica. Vet Parasitol Reg Stud Reports. 2021;23:100506. [DOI] [PubMed] [Google Scholar]
- 50.Dey AR, Begum N, Anisuzzaman, Alim MA, Alam MZ. Multiple anthelmintic resistance in gastrointestinal nematodes of small ruminants in Bangladesh. Parasitol Int. 2020;77:102105. [DOI] [PubMed] [Google Scholar]
- 51.Aboelhadid SM, Arafa WM, El-Ashram S, Noaman AF, Shokier KA, Darwish AB, Mahmoud MM, Gadelhaq SM. Haemonchus contortus Susceptibility and Resistance to Anthelmintics in Naturally Infected Egyptian Sheep. Acta Parasitol. 2021;66(2):329–35. [DOI] [PubMed] [Google Scholar]
- 52.Viana MVG, Silva YHD, Martins IVF, Scott FB. Resistance of Haemonchus contortus to monepantel in sheep: first report in Espírito Santo, Brazil. Rev Bras Parasitol Vet. 2021;30(4):e013121. [DOI] [PubMed] [Google Scholar]
- 53.Nascimento LS, Evaristo AMCF, Oliveira GMB, Ferreira MS, Silva DLR, Azevedo SS, Yamamoto SM, Araújo MM, Horta MC. Anthelmintic resistance of gastrointestinal nematodes in sheep grazing in irrigated and dry areas in the semiarid region of northeastern Brazil. Trop Anim Health Prod. 2021;53(2):267. [DOI] [PubMed] [Google Scholar]
- 54.Batista LF, Oliveira LLDS, Silva FVE, Lima WDS, Pereira CAJ, Rocha RHF, Santos IS, Dias Júnior JA, Alves CA. Anthelmintic resistance in sheep in the semiarid region of Minas Gerais, Brazil. Vet Parasitol Reg Stud Reports. 2023;37:100821. [DOI] [PubMed] [Google Scholar]
- 55.Höglund J, Baltrušis P, Enweji N, Gustafsson K. Signs of multiple anthelmintic resistance in sheep gastrointestinal nematodes in Sweden. Vet Parasitol Reg Stud Reports. 2022;36:100789. [DOI] [PubMed] [Google Scholar]
- 56.Bull K, Glover MJ, Rose Vineer H, Morgan ER. Increasing resistance to multiple anthelmintic classes in gastrointestinal nematodes on sheep farms in southwest England. Vet Rec. 2022;190(11):e1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papadopoulos E, Gallidis E, Ptochos S. Anthelmintic resistance in sheep in Europe: a selected review. Vet Parasitol. 2012;189(1):85–8. [DOI] [PubMed] [Google Scholar]
- 58.Luque S, Lloberas M, Cardozo P, Virkel G, Farias C, Viviani P, Lanusse C, Alvarez L, Lifschitz A. Combined moxidectin-levamisole treatment against multidrug-resistant gastrointestinal nematodes: a four-year efficacy monitoring in lambs. Vet Parasitol. 2021;290:109362. [DOI] [PubMed] [Google Scholar]
- 59.Suárez G, Castells D, Imperiale F, Fagiolino P, Canton C, Lanusse C, Alvarez L. Therapeutic advantages of the combined use of closantel and moxidectin in lambs parasitized with resistant gastrointestinal nematodes. Int J Parasitol Drugs Drug Resist. 2023;23:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang YM, Zhao DX, Wang WL. Mechanism of Resistance to Ivermectin in the Haemonchus contortus. Acta Veterinaria et Zootechnica Sinica. 2024;55(04):1511–20 (in Chinese). [Google Scholar]
- 61.Blackhaii WJ, Prichard RK, Beech RN. Selection at a gamma-aminobutyric acid receptor gene in Haemonchus contortus resistant to avermectins/milbemycins. Mol Biochem Parasitol. 2003;131(2):137–45. [DOI] [PubMed] [Google Scholar]
- 62.Martin RJ, Murray I, Robertson AP. Anthelmintics and ion-channels: after a puncture, use a patch. Int J Parasitol. 1998;28(6):849–62. [DOI] [PubMed] [Google Scholar]
- 63.Mate L, Ballent M, Cantón C. ABC-transporter gene expression in ivermectin-susceptible and resistant Haemonchus contortus isolates. Vet Parasitol. 2022;302:109647. [DOI] [PubMed] [Google Scholar]
- 64.dos Santos JML, Monteiro JP, Ribeiro WLC, Macedo ITF, Camurça Vasconcelos ALF, da Silva VL. Identification and quantification of benzimidazole resistance polymorphisms in Haemonchus contortus isolated in Northeastern Brazil. Vet Parasitol. 2014;199:160–4. [DOI] [PubMed] [Google Scholar]
- 65.Atanásio-Nhacumbe A, Lambert SM, da Silva Souza BMP, Ayres MCC. Molecular detection of benzimidazole resistance levels associated with F167Y and F200Y polymorphisms in Haemonchus contortus of goats from Mozambique. Parasitol Res. 2019;118:245–53. [DOI] [PubMed] [Google Scholar]
- 66.Blackhall WJ, Prichard RK, Beech RN. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Vet Parasitol. 2008;152:101–7. [DOI] [PubMed] [Google Scholar]
- 67.Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134(Pt 8):1093–104. [DOI] [PubMed] [Google Scholar]
- 68.Sarai RS, Kopp SR, Coleman GT, Kotze AC. Acetylcholine receptor subunit and P-glycoprotein transcription patterns in levamisole-susceptible and -resistant Haemonchus contortus. Int J Parasitol Drugs Drug Resist. 2013;3:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan S, Nisar A, Yuan J, Luo X, Dou X, Liu F, Zhao X, Li J, Ahmad H, Mehmood SA, Feng X. A Whole Genome Re-Sequencing Based GWA analysis reveals candidate genes associated with ivermectin resistance in haemonchus contortus. Genes (Basel). 2020;11(4):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hart EH, Brophy PM, Prescott M, Bartley DJ, Wolf BT, Hamilton JV. A new enabling proteomics methodology to investigate membrane associated proteins from parasitic nematodes: case study using ivermectin resistant and ivermectin susceptible isolates of Caenorhabditis elegans and Haemonchus contortus. Vet Parasitol. 2015;207(3–4):266–75. [DOI] [PubMed] [Google Scholar]
- 71.Evans KS, Wit J, Stevens L. Two novel loci underlie natural differences in Caenorhabditis elegans abamectin responses. PLoS Pathog. 2021;17(3):e1009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Wang T, Guo W, Yan X, Kou H, Yu Y, Liu C, Gao W, Wang W, Wang R. Transcriptome reveals the roles and potential mechanisms of lncRNAs in the regulation of albendazole resistance in Haemonchus contortus. BMC Genomics. 2024;25(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang YM, Guo WR, Wen HF, Shi YQ, Gao W, Chen XD, Wang TY, Wang WL, Wu WJ. Analysis of lncRNA-related studies of ivermectin-sensitive and -resistant strains of Haemonchus contortus. Parasitol Res. 2024;123(5):226. [DOI] [PubMed] [Google Scholar]
- 74.Yu L, Yin Y, Wang Q, Zhao P, Han Q, Liao C. Impact of Ae-GRD on Ivermectin Resistance and Its Regulation by miR-71-5p in Aedes aegypti. Insects. 2024;15(6):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang JY. Prevention and control technology of parasitic diseases in large-scale sheep farms. Today Animal Husban Veter Med. 2024;40(05):110–2 (in Chinese). [Google Scholar]
- 76.Torres-Acosta JF, Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet Parasitol. 2012;189(1):89–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the results of this document are contained within the article. Any additional data may be requested to the corresponding author.