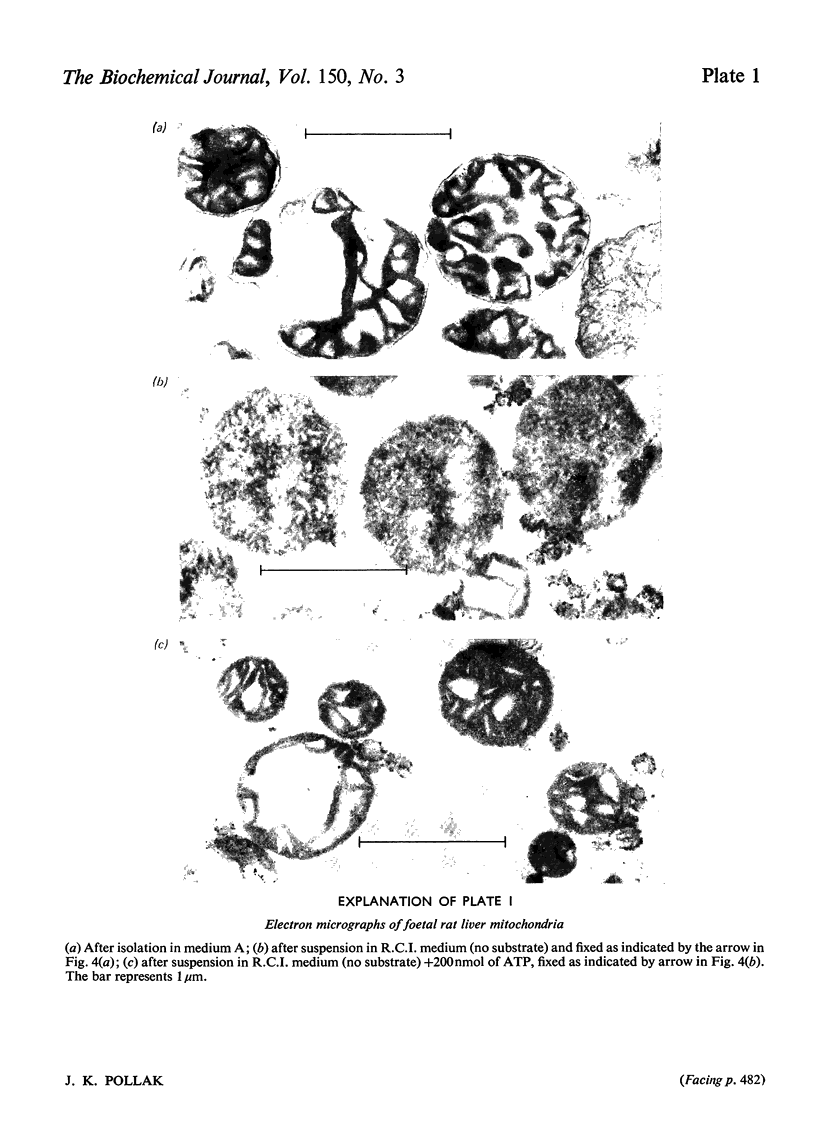
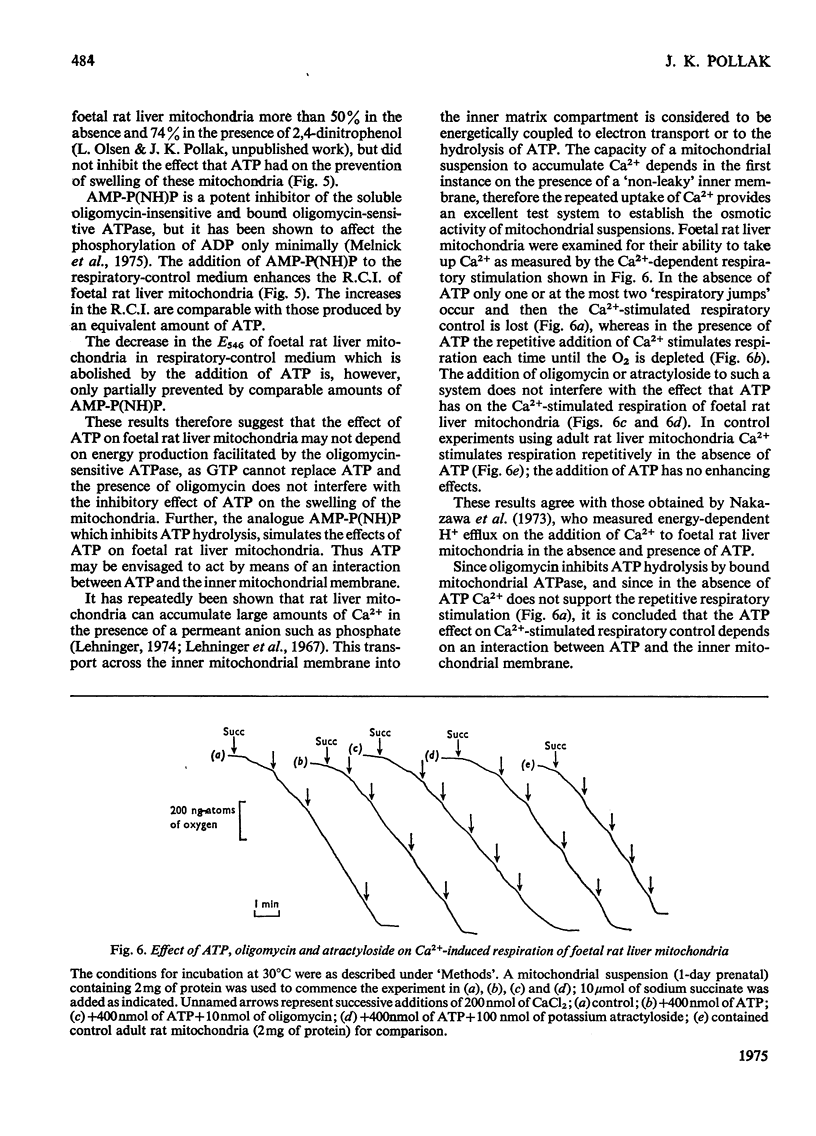
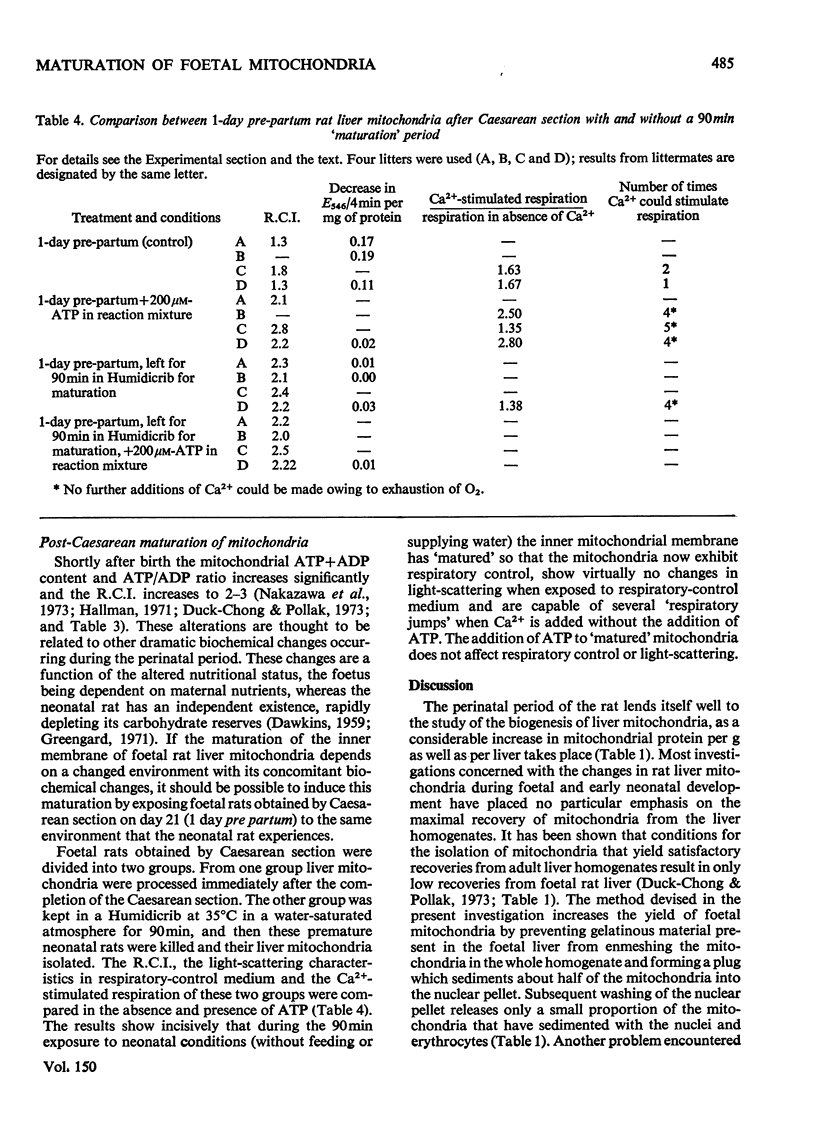
Abstract
A new method was devised for the isolation of foetal and neonatal rat lvier mitochondria, giving higher yields than conventional methods. 2. During development from the perinatal period to the mature adult, the ratio of cytochrome oxidase/succinate-cytochrome c reductase changes. 3. The inner mitochondrial membrane of foetal liver mitochondria possesses virtually no osmotic activity; the permeability to sucrose decreases with increasing developmental age. 4. Foetal rat liver mitochondria possess only marginal respiratory control and do not maintain Ca2+-induced respiration; they also swell in respiratory-control medium in the absence of substrate. ATP enhances respiratory control and prevents swelling, adenylyl imidodiphosphate, ATP+atractyloside enhance the R.C.I. (respiratory control index), Ca2+-induced respiratory control and prevent swelling, whereas GTP and low concentrations of ADP have none of these actions. It is concluded that the effect of ATP depends on steric interaction with the inner mitochondrial membrane. 5. When 1-day pre-partum foetuses are obtained by Caesarean section and maintained in a Humidicrib for 90 min, mitochondrial maturation is "triggered", so that their R.C.I. is enhanced and no ATP is required to support Ca2+-dependent respiratory control or to inhibit mitochondrial swelling. 6. It is concluded that foetal rat liver mitochondria in utero do not respire, although they are capable of oxidative phosphorylation in spite of their low R.C.I. The different environmental conditions which the neonatal rat encounters ex utero enable the hepatic mitochondria to produce ATP, which interacts with the inner mitochondrial membrane to enhance oxidative phosphorylation by an autocatalytic mechanism.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Ballard F. J. Adenine nucleotides and the adenylate kinase equilibrium in livers of foetal and newborn rats. Biochem J. 1970 Apr;117(2):231–235. doi: 10.1042/bj1170231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. The development of gluconeogenesis in rat liver. Controlling factors in the newborn. Biochem J. 1971 Sep;124(2):265–274. doi: 10.1042/bj1240265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- DAWKINS M. J. Respiratory enzymes in the liver of the newborn rat. Proc R Soc Lond B Biol Sci. 1959 Mar 17;150(939):284–298. doi: 10.1098/rspb.1959.0022. [DOI] [PubMed] [Google Scholar]
- Filler R., Criss W. E. Development of adenylate kinase isoenzymes in rat liver. Biochem J. 1971 May;122(4):553–555. doi: 10.1042/bj1220553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian tissues. Essays Biochem. 1971;7:159–205. [PubMed] [Google Scholar]
- Hallman M. Changes in mitochondrial respiratory chain proteins during perinatal development. Evidence of the importance of environmental oxygen tension. Biochim Biophys Acta. 1971 Dec 7;253(2):360–372. doi: 10.1016/0005-2728(71)90040-5. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Federman M., Greengard O. Subcellular morphometric and biochemical analysis of developing rat hepatocytes. J Cell Biol. 1973 May;57(2):475–483. doi: 10.1083/jcb.57.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. Aspartate aminotransferase in fat tissues: changes with growth and hormones. Biochim Biophys Acta. 1971 Apr 20;237(1):88–98. doi: 10.1016/0304-4165(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Pyruvate dehydrogenase activity in rat liver during development. Biol Neonate. 1974;24(1):41–48. doi: 10.1159/000240630. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Role of phosphate and other proton-donating anions in respiration-coupled transport of Ca2+ by mitochondria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1520–1524. doi: 10.1073/pnas.71.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz H. A., Yawn D. H., Safer B., Bresnick E., Liebelt A. G., Blailock Z. R., Rabin E. R., Schwartz A. Morphological and biochemical studies of isolated mitochondria from fetal, neonatal, and adult liver and from neoplastic tissues. J Cell Biol. 1967 Aug;34(2):513–523. doi: 10.1083/jcb.34.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Asami K., Suzuki H., Yukawa O. Appearance of energy conservation system in rat liver mitochondria during development. The role of adenine nucleotide translocation. J Biochem. 1973 Feb;73(2):397–406. [PubMed] [Google Scholar]
- PACKER L. Metabolic and structural states of mitochondria. I. Regulation by adenosine diphosphate. J Biol Chem. 1960 Jan;235:242–249. [PubMed] [Google Scholar]
- Packer L., Pollak J. K., Munn E. A., Greville G. D. Effect of high sucrose concentrations on mitochondria: analysis of mitochondrial populations by density-gradient centrifugation after fixation with glutaraldehyde. J Bioenerg. 1971 Dec;2(5):305–316. doi: 10.1007/BF01963827. [DOI] [PubMed] [Google Scholar]
- Philippidis H., Ballard F. J. The development of gluconeogenesis in rat liver: experiments in vivo. Biochem J. 1969 Jul;113(4):651–657. doi: 10.1042/bj1130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J. K., Duck-Chong C. G. Changes in rat liver mitochondria and endoplasmic reticulum during development and differentiation. Enzyme. 1973;15(1):139–160. [PubMed] [Google Scholar]
- Pollak J. K., Munn E. A. The isolation by isopycnic density-gradient centrifugation of two mitochondrial populations from livers of embryonic and fed and starved adult rats. Biochem J. 1970 May;117(5):913–919. doi: 10.1042/bj1170913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergonet G., Hommes F. A., Molenaar I. A morphometric and biochemical study of fetal and adult rat liver cells, with special reference to energy metabolism. Biol Neonate. 1970;16(5):297–305. doi: 10.1159/000240287. [DOI] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Development of gluconeogenesis in neonatal rat liver. Effect of premature delivery. Biochem J. 1967 Dec;105(3):1229–1233. doi: 10.1042/bj1051229. [DOI] [PMC free article] [PubMed] [Google Scholar]