Abstract
Background
Rectal cancer patients are potential beneficiaries of adaptive radiotherapy (ART) which demands considerable resources. Currently, there is no definite guidance on what kind of patients and when will benefit from ART. This study aimed to develop and validate a methodology for estimating ART requirements in rectal cancer before treatment course.
Methods and materials
This study involved 66 rectal cancer patients from center 1 and 27 patients from center 2. The ART requirements were evaluated by comparing 8 dose volume histogram (DVH) metrics of targets and organs at risk (OARs) between planning and treatment fractions. Tolerance ranges of deviation of DVH metrics were derived from 10 patients and applied to assess fractional variability. Eighteen features, encompassing diagnostic, dosimetric, and time-related information, were utilized to formulate a stepwise logistic regression model for fraction-level ART requirement estimation. The super parameters were determined through 5-fold cross-validation with 250 training fractions and the methodology was validated with 109 internal testing fractions and 134 external testing fractions.
Results
The area under the curve (AUC) of training dataset was 0.74 (95% CI: 0.61 to 0.85), while in the internal and external testing, the AUC achieved 0.76 (95% CI: 0.60–0.90) and 0.68 (95% CI: 0.56–0.81). Using a best (or clinical applicable) cut-off value of 33.4% (11%), the predictive model achieved a sensitivity of 46.2% (69.2%) and specificity of 97.9% (68.7%). During the modeling, 5 features were retained: Homogeneity index (OR = 6.06, 95% CI: 2.93–14.8), planning target volume (OR = 1.77, 95% CI: 1.17–2.69), fraction dose (OR = 45.37, 95% CI: 5.74–469), accumulated dose (OR = 2.29, 95% CI: 1.35–4.14), and whether neoadjuvant chemoradiotherapy (OR > 1000).
Conclusion
ART requirements are associated with target volume, target dose homogeneity, fraction dose, dose accumulation and whether neoadjuvant radiotherapy. The predictive model exhibited the capability to predict fraction-level ART requirements.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13014-024-02567-7.
Keywords: ART requirements, Rectal cancer, DVH metrics, Logistic regression, Multi-institutional validation
Background
Neoadjuvant radiotherapy is a standard of care for patients with intermediate or locally advanced rectal cancer, generally followed by total mesorectal excision surgery, while adjuvant radiotherapy is recommended for patients with post-surgery pathological stage II-III rectal cancer and a high risk of local recurrence [1, 2]. Reduction of internal target margins could mitigate the substantial toxicity associated with radiation. However, throughout the treatment process, anatomical variations in patients may lead to a decrease in target dose coverage or an inadvertent overdosage of organs at risk (OARs) [3–5]. Adaptive radiotherapy (ART) can be adapted to the anatomical variations and the benefits of ART for rectal cancer patients have been reported [6–8].
Clinically, the implementation of ART primarily falls into two categories: online and offline [9]. Both ART strategies demand significant resources and time, involving additionally image acquisition, human review, treatment replanning, and quality assurance. It is impracticable to offer ART to every patient in busy institutions, and extending patients’ time onboard for each fraction may negatively impact their adherence. Moreover, the benefits of ART vary among patients, and not every patient can benefit greatly from it [10, 11]. Therefore, proactively selecting patients and determining the appropriate frequency of image monitoring or ART implementation are crucial [12].
Currently, there is no definite guidance regarding the criteria for patient and fraction selection for ART [12, 13]. The assessments of ART requirement utilized in studies and in clinic vary among institutions and are usually judged subjectively based on fractional images [14]. Some studies have attempted to predict ART requirement using the similarity or features extracted from fractional images [15–17]. Fractional image acquisition may not be easy for busy institutions, and predictions before treatment course are more helpful to make positive responses in target delineation, plan design, and imaging scheduling. Studies in this area primarily employing radiomic, geometric, dosimetric, and clinical features to predict tumor shrinkage or dosimetric benefits [18]. For instance, Hu et al. investigated the correlations between clinical features and the potential dosimetric benefit of ART for nasopharyngeal cancer patients [19]. However, these studies typically focused on only a small number of indicators such as planning target volume (PTV) V95% in [17] as end point. Consequently, the ART requirements of the target volume and OARs were not comprehensively evaluated. Moreover, further investigation into predicting fraction-level ART requirements before treatment course may be required.
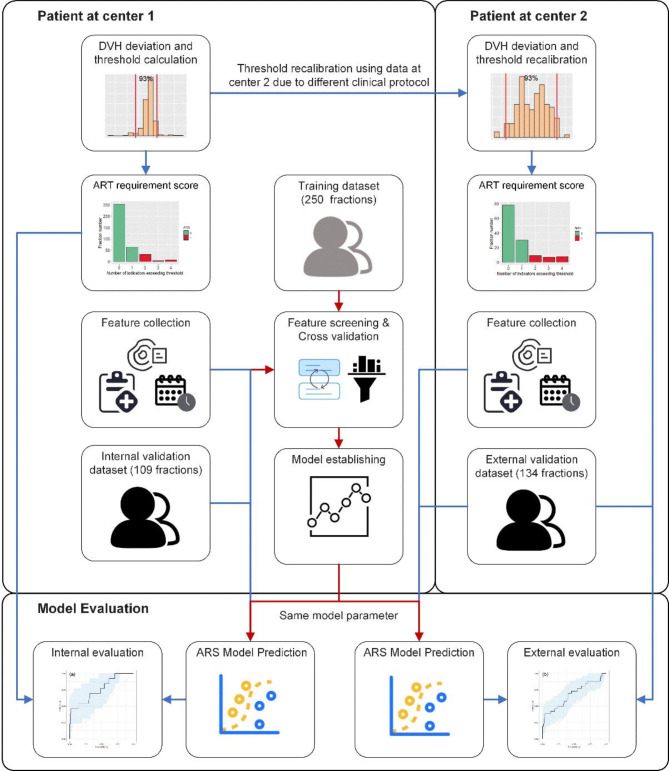
The aim of the present study was to propose a framework for objectively estimating the ART requirement and to apply the methodology for rectal cancer. As shown in Fig. 1, we introduced a dose-volume histogram (DVH)-based Adaptive radiotherapy Requirement Score (ARS) to evaluate the necessity of ART for rectal cancer patients and investigated the correlation between pre-treatment features and ARS. Furthermore, we established a quantitative model to predict ARS for untreated patients and then assessed its performance using a patient cohort from another institution.
Fig. 1.
General workflow of this study, an ARS predicted model was established in training dataset of center 1 and evaluated in internal and external dataset
Methods
Patient cohort
This retrospective study involves two patient cohorts undergoing radiotherapy for rectal cancer at two centers from March 2021 to March 2023, comprising 359 fractions of 66 rectal cancer patients at center 1 and 134 fractions of 27 patients at center 2. All patients received 6 MV photon Intensity-Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT) with four different dose-fraction schemes (50 Gy/25 fx, 45 Gy/25 fx, 50.4 Gy/28 fx, 25 Gy/5 fx). Fractional CT scans (fCTs), were obtained at least once a week with diagnostic-level fan beam CT (FBCT, United Imaging Healthcare, Shanghai, China) scans at center 1 and high-quality cone beam CT (CBCT, Varian Medical Systems, Palo Alto, CA) scans at center 2. Clinical features were collected from electronic medical records (EMRs) and dosimetric features were calculated on planning CT scans (pCTs) with contours. The study was approved by the Institutional Review Boards of Fudan University Shanghai Cancer Center (2201250-16) and Chongqing University Cancer Hospital (CZLS2023164-A), with the requirement for individual informed consent waived.
ART requirement assessment
The main end point was the variation of DVH metrics between planning dose distributions and fractional dose distribution. These dosimetric variations, associated with anatomical changes, were utilized to evaluate the necessity for ART. For patients at center 1, the dose could be calculated directly on diagnostic level FBCT, while for patients at center 2, high-quality CBCT scans were utilized to generate synthetic CT scans for dose distribution calculations with ArcherQA software (Wisdom Technologies, Anhui, China). The structure of the planning target volume (PTV) on the fCT was transferred from the pCT using deformable registration, while the OARs were auto-contoured using AI model. Structures were then reviewed and approved or edited by a senior radiation oncologist. The detail of the imaging devices and algorithms can be found in Supplement.
To comprehensively evaluated the dose variations of target volume and OARs of rectal cancer, percent deviations of 8 DVH metrics of clinical concerns (PTV D95, PTV D2, bladder D15, bladder D50, left femoral head (FH-L) D25, FH-L D40, right femoral head (FH-R) D25, and FH-R D40) were calculated between dose distributions of pCT and fCT as DVH-based indicators following the methods proposed by Chen et al. [20] The empirical distribution of these percent deviations from 51 fractions of 10 patients at center 1 was used to determine the tolerance range (median 95% interval, 2.5 to 97.5 percentile) for each DVH-based indicator. To account the different clinical protocol between two institutions, a recalibration with the same method was performed to adjust the tolerance range at center 2 base on 10 patients from center 2. ART requirement estimation involved counting the number of DVH-based indicators exceeding tolerance. In this study, a fraction with two or more (≥ 2) DVH-based indicators beyond tolerance was assigned an ART requirement score (ARS) of 1. The detail of the ARS criterion can be found in [20].
Feature definition
To account for potential features related to ART, 18 features were enrolled into this study which may related to PTV volume changes and dosimetric variation base on literature or clinical experience, with their definitions outlined in Table 1.
Table 1.
The definitions of predictive features
| Features | Definitions | Categories | |||
|---|---|---|---|---|---|
| Gender | Gender (0 for female and 1 for male) | Clinical [21] | |||
| Age | Age (years) | Clinical [21, 22] | |||
| BMI | Body mass index | Clinical [21, 23] | |||
| Distance | Distance from tumor to anus (mm) | Clinical [24] | |||
| T_stage | T stage (from 0 to 4) | Clinical [21, 23] | |||
| N_stage | N stage (from 0 to 2) | Clinical [21] | |||
| MRF | Mesorectal fascia (negative 0, positive 1) | Clinical | |||
| EMVI | Extramural vascular invasion (negative 0, positive 1) | Clinical | |||
| Neo_RT | Neoadjuvant radiotherapy (0 for not and 1 for yes) | Intervention | |||
| Concurrent_chem | Concurrent chemoradiotherapy (0 for not and 1 for yes) | Intervention [23, 25] | |||
| PTV_vol | Volume of PTV in planning CT (cm3) | Planning [23] | |||
| IMRT/VMAT | Treatment technology (0 for IMRT and 1 for VMAT) | Planning | |||
| HI | Homogeneity index of PTV ((D2-D98)/DRx) | Dosimetric | |||
| CI | Conformity index of PTV ((VRx,PTV)2/(VPTV × VRx)) | Dosimetric | |||
| Frac_dose | Dose per fraction (Gy) | Dosimetric | |||
| Diff_daytime | Absolute daytime (time period) difference between pCT and fCT (hour) | Time related [26] | |||
| Passed_day | The number of days elapsed from planning CT to the current fraction (days) | Time related [25] | |||
| Accumulated_dose | Expected dose received before the selected fraction (Gy) | Time related |
Dn: minimum dose received by n% of the PTV, DRx: prescription dose, VPTV: volume of the PTV, VRx: volume received prescription dose in the whole body, VRx, PTV: volume received prescription dose in the PTV
Model develop and validation
Descriptive and statistical analyses were conducted using R software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). Spearman correlation analyses and univariate logistic analyses were utilized to investigate features associated with ARS. To enhance variable inclusion for screening in the stepwise model, all features meeting criteria of p-values < 0.2, or OR > 1000 in the univariate analysis were integrated into a multivariate logistic regression model. A backward stepwise strategy based on Akaike information criterion (AIC) was employed for feature screening.
The dataset of 359 fractions from center 1 was divided into training dataset (n = 250) and internal testing dataset (n = 109) sets. The hyperparameter including penalty parameter of AIC and the number of the maximum step was finetune through 200 times repeated 5-fold cross-validation on the training dataset. Internal testing was conducted using the testing dataset from center 1, while external testing was performed using the dataset of 134 fractions from center 2. Risk factors were assessed using the odds ratio (OR) value, and model performance was evaluated using ROC curve analysis.
Results
A total of 359 fractions with fCTs were collected from the 66 patients treated at center 1, and 134 fractions from 27 patients at center 2, the patient characters were show in Table 2.
Table 2.
Characteristics of the 66 patients at center 1 and 27 patients at center 2
| Patient characteristics | Center 1 (n = 66) |
Center 2 (n = 27) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Gender (%) | 0.699 | ||||||
| Male | 46 (69.7) | 17 (63.0) | |||||
| Female | 20 (30.3) | 10 (37.0) | |||||
| Age (mean (SD)) | 56.3 (13.2) | 62.3 (11.11) | 0.043 | ||||
| BMI (mean (SD)) | 22.9 (2.48) | 23.16 (3.21) | 0.703 | ||||
| Distance (mean (SD)) | 4.90 (2.04) | 4.95 (1.44) | 0.920 | ||||
| PTVvol (mean (SD)) | 1081 (176) | 1086 (149) | 0.896 | ||||
| HI (mean (SD)) | 0.0770 (0.0293) | 0.0662 (0.0273) | 0.108 | ||||
| CI (mean (SD)) | 0.902 (0.0247) | 0.919 (0.0152) | 0.001 | ||||
| T_stage (%) | 0.014 | ||||||
| T2 | 4 (6.1) | 3 (11.1) | |||||
| T3 | 46 (69.7) | 10 (37.0) | |||||
| T4 | 16 (24.2) | 14 (51.9) | |||||
| N_stage (%) | 0.156 | ||||||
| N0 | 16 (24.2) | 5 (18.5) | |||||
| N1 | 14 (21.2) | 11 (40.7) | |||||
| N2 | 36 (54.5) | 11 (40.7) | |||||
| MRF (%) | < 0.001 | ||||||
| Negative | 48 (72.7) | 8 (29.6) | |||||
| Positive | 18 (27.3) | 19 (70.4) | |||||
| EMVI (%) | 0.002 | ||||||
| Negative | 47 (71.2) | 9 (33.3) | |||||
| Positive | 19 (28.8) | 18 (66.7) | |||||
| Neo_RT (%) | < 0.001 | ||||||
| No | 6 (9.6) | 13 (48.1) | |||||
| Yes | 60 (90.9) | 14 (51.9) | |||||
| Chem (%) | 0.502 | ||||||
| No | 11 (16.7) | 3 (11.1) | |||||
| Yes | 55 (83.3) | 24 (88.9) | |||||
| IMRT/VMAT (%) | < 0.001 | ||||||
| IMRT | 22 (33.3) | 26 (96.3) | |||||
| VMAT | 44 (66.7) | 1 (3.7) |
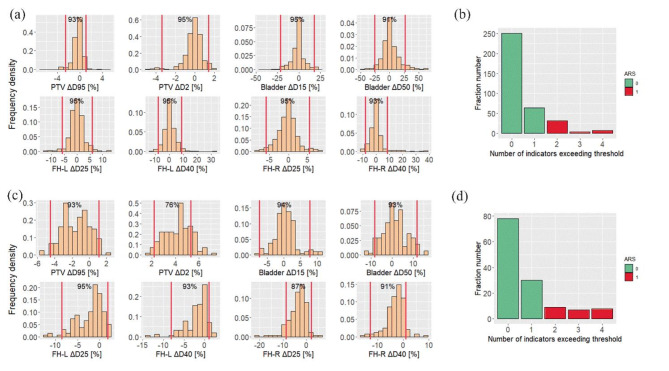
The specific tolerance thresholds of DVH-based indicators derived from 51 fractions at center 1 were (-2.54–1.05%) for PTV ΔD95 [%], (-3.39–1.37%) for PTV ΔD2 [%], (-21.30–17.57%) for Bladder ΔD15 [%], (-25.46–26.77%) for Bladder ΔD50 [%], (-5.88–5.84%) for FH-L ΔD25 [%], (-7.92–8.87%) for FH-L ΔD40 [%], (-5.86–5.52%) for FH-R ΔD25 [%], and (-7.99–8.36%) for FH-R ΔD40 [%], and the recalibrated tolerance thresholds of center 2 were shown in Supplement. The distribution of DVH-based indicators for all 359 fractions at center 1 and 134 fractions at center 2 are illustrated in Fig. 2(a) and Fig. 2(c).
Fig. 2.
(a), (c) Distributions of the DVH-based indicators of the 359 fractions at center 1 and 134 fractions at center 2. The vertical red line represents the tolerance range based on 10 patients. The notation on the top of each subgraph shows the proportion of fractions within tolerance. (b), (d) Distribution of fractions with the number of DVH-based indicators exceeding the threshold at center 1 and center 2. PTV: planning target volume, FH-L: left femoral head, FH-R: right femoral head, Dn: The dose received by n% of the volume in the structure
As shown in Fig. 2(b) and Fig. 2(d), the number of DVH-based indicators exceeding the threshold for each fraction of center 1 and center 2 was calculated. Out of the 359 fractions at center 1, 108 (30.1%) had at least 1 metric above the threshold, 44 (12.3%) had at least 2, and 12 (3.3%) had at least 3. Based on the distribution, we chose to label fractions with at least 2 indicators beyond the thresholds with ARS = 1, which allow us to obtain a certain proportion of positive samples for subsequent analysis, without causing excessive demand for ART.
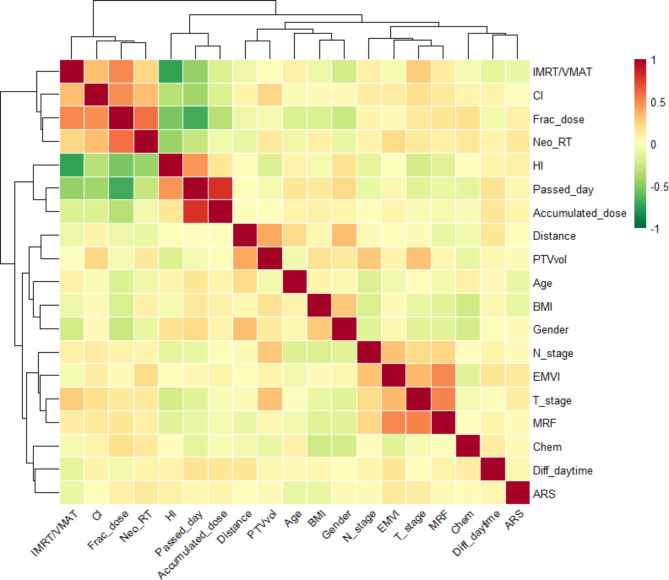
The Spearman correlation coefficients between features and ARS are depicted in Fig. 3. The correlation coefficient between a single feature and ARS is relatively small (∣ρ∣<0.2). The features with the strongest correlation were T_stage, Neo_RT, and Age, each with ∣ρ∣=0.12. Among the time-related or daytime-related features (Passed_day, Accumulated_dose, and Diff-daytime), Accumulated _dose showed the highest correlation, with ∣ρ∣=0.07.
Fig. 3.
Correlation heat map between features and ARS. The value of the grid indicates the magnitude of the correlation coefficient
The results of univariate and multivariate analyses are shown in Table 3. No single feature showed significant difference between the ARS = 0 and ARS = 1 groups (p-values < 0.05), and there were 4 features (Age, PTVvol, EMVI, Diff_daytime) with p-values between 0.05 and 0.1, and 3 features (HI, Accumulated_dose, Frac_dose) with p-values between 0.1 and 0.2. Due to the extremely small sample size, or even the absence of samples in a particular subgroup, T_stage and Neo_RT showed exceptionally large OR values (> 1000) with a p-value close to 1, for example, all samples with Neo_RT = 0 have ARS = 0. We believe these factors have significant impact on clinical outcomes in reality, and thus, it was included in subsequent multivariate analysis. Therefore, in order to include the right amount of features for the subsequent multivariate analysis, p-values < 0.2 or OR > 1000 in the univariate analysis were selected (a total of 9 features: Accumulated_dose, Age, HI, Frac_dose, IMRT/VMAT, Neo_RT, T_stage, EMVI, Diff_daytime). These features were included in the backward stepwise logistic regression analysis, with all continuous features normalized using Z-score before analysis. Through 200 times 5-fold cross validation, penalty parameter of AIC = 3 and the number of the maximum step = 100 were set, and the average AUC of cross-validation was 0.74 (95% CI: 0.61–0.85). Five features remained after stepwise logistic regression: HI (OR = 6.06, 95CI: 2.93–14.8), PTVvol (OR = 1.77, 95%CI: 1.17–2.69), Neo_RT (OR > 1000), Frac_dose (OR = 45.37, 95CI: 5.74–469), and Accumulated_dose (OR = 2.29, 95%CI: 1.35–4.14).
Table 3.
Characteristics of the 250 training fractions and their results of univariate and multivariate analyses
| Characteristics | ARS = 0 (n = 219) |
ARS = 1 (n = 31) |
Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Gender (%) | ||||||||
| Male | 156 (71.2) | 23 (74.2) | 1.16 | (0.51 ~ 2.89) | 0.73 | |||
| Female | 63 (28.8) | 8 (25.8) | ||||||
| Age (mean (SD)) | 57.1 (13.6) | 22.6 (2.69) | 0.70 | (0.47 ~ 1.01) | 0.06 | |||
| BMI (mean (SD)) | 23.1 (2.57) | 21.24 (2.05) | 0.85 | (0.57 ~ 1.23) | 0.39 | |||
| Distance (mean (SD)) | 4.81 (2.03) | 5.09 (2.01) | 1.14 | (0.80 ~ 1.61) | 0.47 | |||
| PTVvol (mean (SD)) | 1060 (175) | 1120 (171) | 1.36 | (0.96 ~ 1.90) | 0.075 | 1.77 | (1.17 ~ 2.69) | 6.8e-3 |
| HI (mean (SD)) | 0.0764 (0.0291) | 0.0839 (0.0311) | 1.22 | (0.88 ~ 1.61) | 0.19 | 6.06 | (2.93 ~ 14.8) | 1.0e-5 |
| CI (mean (SD)) | 0.901 (0.0252) | 0.899 (0.0247) | 0.95 | (0.68 ~ 1.34) | 0.70 | |||
| T_stage (%) | ||||||||
| T2 | 14 (6.4) | 0 (0.0) | Ref | Ref | Ref | |||
| T3 | 161 (73.5) | 19 (61.3) | > 1000 | (< 0.001 ~ > 1000) | 0.99 | |||
| T4 | 44 (20.1) | 12 (38.7) | > 1000 | (< 0.001 ~ > 1000) | 0.99 | |||
| N_stage (%) | ||||||||
| N0 | 54 (24.7) | 5 (16.1) | Ref | Ref | Ref | |||
| N1 | 56 (25.6) | 8 (25.8) | 1.54 | (0.48 ~ 5.38) | 0.47 | |||
| N2 | 109 (49.8) | 18 (58.1) | 1.78 | (0.67 ~ 5.63) | 0.28 | |||
| MRF (%) | ||||||||
| Negative | 166 (75.8) | 21 (67.7) | Ref | Ref | Ref | |||
| Positive | 53 (24.2) | 10 (32.3) | 1.49 | (0.64 ~ 3.30) | 0.34 | |||
| EMVI (%) | ||||||||
| Negative | 159 (72.6) | 17 (54.8) | Ref | Ref | Ref | |||
| Positive | 60 (27.4) | 14 (45.2) | 2.18 | (0.98 ~ 4.75) | 0.06 | |||
| Neo_RT (%) | > 1000 | (< 0.001 ~ > 1000) | 0.98 | |||||
| No | 21 (9.6) | 0 (0.0) | Ref | Ref | Ref | |||
| Yes | 198 (90.4) | 31 (100) | > 1000 | (< 0.001 ~ > 1000) | 0.99 | |||
| Chem (%) | - | |||||||
| No | 32 (14.6) | 3 (9.7) | Ref | Ref | Ref | |||
| Yes | 187 (85.4) | 28 (90.3) | 1.60 | (0.53 ~ 6.95) | 0.46 | |||
| IMRT/VMAT (%) | ||||||||
| IMRT | 83 (37.9) | 13 (41.9) | Ref | Ref | Ref | |||
| VMAT | 136 (62.1) | 18 (58.1) | 0.85 | (0.40 ~ 1.85) | 0.67 | |||
| Frac_dose (mean (SD)) | 2.91 (1.41) | 3.16 (1.49) | 1.82 | (0.88 ~ 1.91) | 0.18 | 45.37 | (5.74 ~ 469) | 6.1e-4 |
| Diff_daytime (mean (SD)) | 4.90 (3.14) | 5.97 (3.14) | 3.88 | (0.88 ~ 19) | 0.08 | |||
| Passed_day (mean (SD)) | 16.6 (14.7) | 17.6 (13.0) | 1.07 | (0.74 ~ 1.52) | 0.72 | |||
| Accumulated_dose (mean (SD)) | 16.8 (13.8) | 20.9 (12.8) | 1.34 | (0.92 ~ 1.96) | 0.12 | 2.29 | (1.35 ~ 4.14) | 3.5e-3 |
ARS: The ART requirement score, OR: Odd ratio, CI: Confidence interval, SD: standard deviation
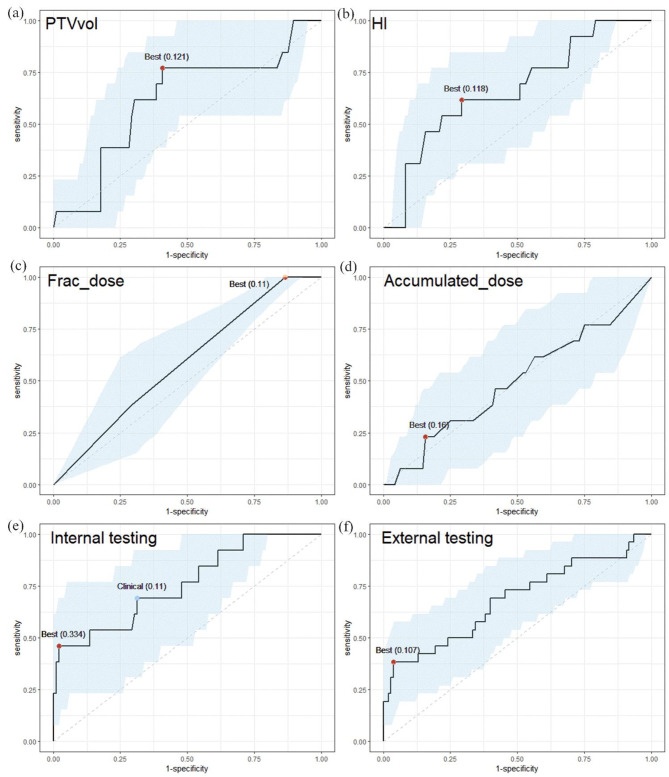
Figure 4(a)-(d) show the ROC curves of some univariant model in 109 internal validation datasets. The classification performance of individual factors was suboptimal, with the highest AUC observed for HI, reaching 0.665 (95% CI: 0.50 to 0.81). Figure 4(e) displays the ROC curve of the model in 109 internal validation datasets, achieving an AUC of 0.76 (95% CI: 0.60 to 0.90). Using a predicted probability of ART requirement equal to the best cut-off value of 33.4%, the sensitivity was 46.2%, and specificity was 97.9%. In clinical practice, this threshold can be adjusted according to the resources and needs of the institution. For instance, a threshold of 11% may be more appropriate if identifying patients who require ART is prioritized, resulting in a sensitivity of 69.2% and a specificity of 68.7%. Figure 4(f) illustrates the ROC curve of the model using 134 external validation datasets, with an AUC of 0.68 (95% CI: 0.56 to 0.81).
Fig. 4.
(a)-(d) The ROC curves of the univariant model for PTVvol, HI, Frac_dose, Accmulated_dose in 109 internal validation datasets. (e) The ROC curves of the multivariant models in 109 internal validation datasets with AUC = 0.76. (f) The ROC curves of the multivariant models in 134 external validation datasets with AUC = 0.68. Light blue regions were the 95% CI. The red points indicate the best threshold by making sum of sensitivity and specificity maximum, and blue point indicates a threshold that may be clinically applicable. In parentheses is their corresponding cut-off value
Discussion
ART presents challenge due to resource, time, and knowledge requirements. This study aimed to identify features associated with ART requirements prior to treatment for better early clinical strategy. Our results identified 5 features—HI, PTVvol, Neo_RT, Frac_dose, and Accumulated_dose—as risk factors. Not neoadjuvant radiotherapy suggests smaller target volume variations after surgery. Interestingly, all non-neoadjuvant radiotherapy fractions in center 1 had ARS = 0, while in center 2, only 8% (5/62) achieved ARS = 1. HI, PTVvol, and Frac_dose imply that larger target volume, higher fractional dose, and lower homogeneity (in our definition, the higher HI, the lower homogeneity) can affect ARS, likely due to anatomical changes induced by single fraction treatment. This aligns with previous studies by Zhong et al. [27] and Corvo et al. [28], which also demonstrated correlations between PTV volume or fractional dose with ART requirements, and HI has an impact on tumor control probability, which may also influence tumor deformation [29]. Additionally, Sanguineti et al. demonstrated that the efficacy of radiotherapy may not be directly influenced by concurrent chemotherapy [30]. Among time-related features, only Accumulated_dose remained, as patient deformation may increase over treatment course, with less impact from different daytime conditions.
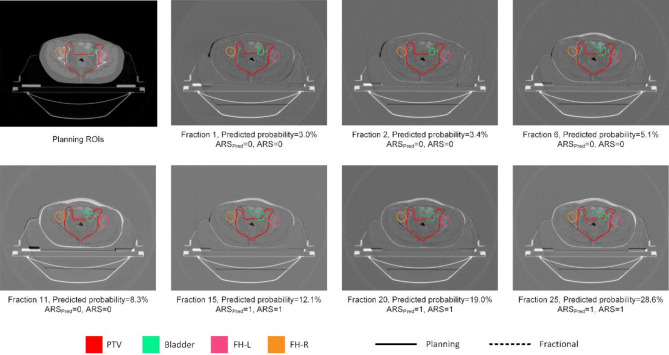
As shown in Fig. 4, although a single feature may exhibit a large OR value, using only one feature to assess ART demand is not reliable. Therefore, the application of this method must be based on the establishment of a multifactorial model using a patient cohort. However, our results demonstrate that the model follows consistent patterns across different centers. When features of patients from one center are collected and input into a model developed by another center, the results of ART requirements still provide meaningful guidance. An example of using the model from center 1 to predict the time-dependent ARS at center 2 is shown in Fig. 5. For this patient, there were relatively small deformation during the initial fractions. However, as the dose accumulated to a certain extent, significant deformation began to occur, leading to substantial dose changes. This indicates an increased requirement for ART, and an imaging examination or ART implementation can be scheduled for the patient.
Fig. 5.
Planning CT scan and differences between planning CT scans and fractional CT scans during the whole treatment course of one patient at center 2. The solid lines present the ROIs of planning and dash lines present the ROIs of fractional image. Target volume and OARs varies along with treatment, and the predicted probability of ARS increased. Using the best cut-off value of ROC curve, ARS was precisely classified. ARSpred: predicted ARS of the model. FH-L: left femoral head, FH-R: right femoral head
The distribution of DVH deviations in OARs was similar between the two centers. However, the PTV ΔD95 [%] showed significant differences, attributed to the substantial variations in clinical features of patient cohorts such as age and T stage. While the vendor-provided algorithm demonstrated a high gamma passing rate for dose calculation on synthetic CT, indicating that this is likely not the primary reason for the observed discrepancies. For OARs, we also set a lower threshold. This is because some OARs, such as the bladder, may overlap with the target volume and require adequate dosing to minimize recurrence rates.
To avoid data reuse and potential overfitting, we used a sample of 10 patients to set the tolerance threshold instead of the entire dataset. As shown in Fig. 2, approximately 95% of the eight indicators for center 1 were within the threshold, validating the stability of results. For center 2, similar trends were observed, although only PTV ΔD95 [%] showed an abnormity with only 78% within the threshold. This discrepancy is likely due to the smaller number of datasets in center 2 and a slightly skewed distribution of observed, which will be addressed by incorporating more patient data.
To our knowledge, this is the first study to establish fraction-level ART requirements predictive model for rectal cancer. Other groups may achieve higher AUCs ranging from 0.75 to 0.93 [16, 28–30]. However, these models often incorporate more complex features for patient-level prediction or rely on daily images, and their criteria are relatively subjective, restricting applicability across multiple institutions.
This study also has several limitations. Firstly, in defining ARS, we only counted the number of dosimetric indicators out of tolerance, which means the PTV and OARs were treated equally in our method. To choose comprehensive DVH metrics and assign appropriate weights considering different clinical protocols will be necessary for more accurate assessments in the future. Secondly, the tolerance ranges were based on data from only 10 patients, indicating the need for a larger patient population to establish more reliable tolerance ranges for clinical application. Thirdly, the relatively small sample size may have resulted in a lack of specific sample types (for example, simultaneous Neo_RT = 0 and ARS = 1), warranting further observation.
In summary, we propose a novel strategy to identify patients likely to benefit from ART and determine the timing of adaptive schedule based on the patient population. With this strategy, the PTV margin for patients with fewer ART requirements could potentially be reduced, along with decreasing onboard imaging or ART schedule frequency in clinical practice.
Conclusions
Rectal cancer patients undergoing neoadjuvant radiotherapy with large PTV, large fractional dose and low target dose homogeneity, would theoretically benefit the most from ART. Moreover, attention to ART should be heightened in the later period of treatment course. The binary logistic predictive model based on pre-treatment features exhibits robust predictive ability for estimating ART requirements in rectal cancer. The objective nature of the DVH-based indicators reduces variation among different institutions, and the model’s efficacy was validated at external institutions as well.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ART
Adaptive radiation therapy
- DVH
Dose volume histogram
- OAR
Organ at risk
- AUC
Area under the curve
- PTV
Planning target volume
- ARS
Adaptive radiotherapy Requirement Score
- IMRT
Intensity modulated radiation therapy
- VMAT
Volumetric modulated arc therapy
- ROC
Receiver operating characteristic curve
- FBCT
Fan beam computed tomography
- CBCT
Cone beam computed tomography
- EMRs
Electronic medical records
- FH-L
Left femoral head
- FH-R
Right femoral head
- BMI
Body mass index
- MRF
Mesorectal fascia
- EMVI
Extramural vascular invasion
- HI
Homogeneity index
- CI
Conformity index
- AIC
Akaike information criterion
- OR
odds ratio
Author contributions
LC, LY and JW proposed the study idea and methodology, LC wrote the main manuscript text, HL and YY acquired and cleaned data. WH acquired the fundings. FJ and ZZ administrated the project. LC and LY established the regression model. LC write the original draft. All authors reviewed the manuscript.
Funding
This work is supported by the National Key Research and Development Program of China (2022YFC2404603, 2022YFC2404600), National Natural Science Foundation of China (12375339, 11675042).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This retrospective study was reviewed and approved by the Institutional Review Boards of Fudan University Shanghai Cancer Center (2201250-16) and Chongqing University Cancer Hospital (CZLS2023164-A), and the requirement for individual informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhen Zhang, Email: zhen_zhang@fudan.edu.cn.
Fu Jin, Email: jfazj@126.com.
Weigang Hu, Email: jackhuwg@gmail.com.
Jiazhou Wang, Email: wjiazhou@gmail.com.
References
- 1.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv263. [DOI] [PubMed] [Google Scholar]
- 2.2020 exceptional surveillance of colorectal cancer (NICE guideline NG151). London2020. [PubMed]
- 3.Alickikus ZA, Kuru A, Aydin B, Akcay D, Gorken IB. The importance of mesorectum motion in determining PTV margins in rectal cancer patients treated with neoadjuvant radiotherapy. J Radiat Res. 2020;61(2):335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijkamp J, de Haas-Kock DF, Beukema JC, Neelis KJ, Woutersen D, Ceha H, et al. Target volume delineation variation in radiotherapy for early stage rectal cancer in the Netherlands. Radiother Oncol. 2012;102(1):14–21. [DOI] [PubMed] [Google Scholar]
- 5.Kleijnen JP, van Asselen B, Burbach JP, Intven M, Philippens ME, Reerink O, et al. Evolution of motion uncertainty in rectal cancer: implications for adaptive radiotherapy. Phys Med Biol. 2016;61(1):1–11. [DOI] [PubMed] [Google Scholar]
- 6.Lutkenhaus LJ, de Jong R, Geijsen ED, Visser J, van Wieringen N, Bel A. Potential dosimetric benefit of an adaptive plan selection strategy for short-course radiotherapy in rectal cancer patients. Radiother Oncol. 2016;119(3):525–30. [DOI] [PubMed] [Google Scholar]
- 7.de Jong R, Visser J, Crama KF, van Wieringen N, Wiersma J, Geijsen ED et al. Dosimetric benefit of an adaptive treatment by means of plan selection for rectal cancer patients in both short and long course radiation therapy. Radiat Oncol. 2020;15(1). [DOI] [PMC free article] [PubMed]
- 8.de Jong R, Crama F, Visser J, van Wieringen N, Wiersma J, Geijsen ED et al. Online adaptive radiotherapy compared to plan selection for rectal cancer: quantifying the benefit. Radiat Oncol. 2020;15(1). [DOI] [PMC free article] [PubMed]
- 9.Thornqvist S, Hysing LB, Tuomikoski L, Vestergaard A, Tanderup K, Muren LP, et al. Adaptive radiotherapy strategies for pelvic tumors - a systematic review of clinical implementations. Acta Oncol. 2016;55(8):943–58. [DOI] [PubMed] [Google Scholar]
- 10.Castelluccia A, Marchesano D, Grimaldi G, Annessi I, Bianciardi F, Borrazzo C, et al. Stereotactic MR-guided adaptive radiotherapy (SMART) for primary rectal cancer: evaluation of early toxicity and pathological response. Rep Pract Oncol Radi. 2023;28(4):437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong R, Visser J, van Wieringen N, Wiersma J, Geijsen D, Bel A. Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer. Radiat Oncol. 2021;16(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green OL, Henke LE, Hugo GD. Practical clinical workflows for Online and Offline Adaptive Radiation Therapy. Semin Radiat Oncol. 2019;29(3):219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passoni P, Fiorino C, Slim N, Ronzoni M, Ricci V, Di Palo S, et al. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. 2013;87(1):67–72. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Ahunbay E, Paulson ES, Chen G, Li XA. A daily end-to-end quality assurance workflow for MR-guided online adaptive radiation therapy on MR-Linac. J Appl Clin Med Phys. 2020;21(1):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasief HG, Parchur AK, Omari E, Zhang Y, Chen X, Paulson E, et al. Predicting necessity of daily online adaptive replanning based on wavelet image features for MRI guided adaptive radiation therapy. Radiother Oncol. 2022;176:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kavanaugh J, Roach M, Ji Z, Fontenot J, Hugo GD. A method for predictive modeling of tumor regression for lung adaptive radiotherapy. Med Phys. 2021;48(5):2083–94. [DOI] [PubMed] [Google Scholar]
- 17.Iezzi M, Cusumano D, Piccari D, Menna S, Catucci F, D’Aviero A et al. Dosimetric impact of Inter-fraction Variability in the treatment of breast Cancer: towards New Criteria to evaluate the appropriateness of Online Adaptive Radiotherapy. Front Oncol. 2022;12. [DOI] [PMC free article] [PubMed]
- 18.Chen H, Li X, Pan XY, Qiang YQ, Qi XS. Feature selection based on unsupervised clustering evaluation for predicting neoadjuvant chemoradiation response for patients with locally advanced rectal cancer. Phys Med Biol. 2023;68(23). [DOI] [PubMed]
- 19.Hu YC, Tsai KW, Lee CC, Peng NJ, Chien JC, Tseng HH, et al. Which nasopharyngeal cancer patients need adaptive radiotherapy? BMC Cancer. 2018;18(1):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LY, Zhang ZY, Yu L, Peng JY, Feng B, Zhao J et al. A clinically relevant online patient QA solution with daily CT scans and EPID-based dosimetry: a feasibility study on rectal cancer. Phys Med Biol. 2022;67(22). [DOI] [PubMed]
- 21.Zeng WG, Liang JW, Wang Z, Zhang XM, Hu JJ, Hou HR, et al. Clinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Chin J Cancer. 2015;34(10):468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surucu M, Shah KK, Mescioglu I, Roeske JC, Small W Jr., Choi M, et al. Decision Trees Predicting Tumor Shrinkage for Head and Neck Cancer: implications for adaptive Radiotherapy. Technol Cancer Res Treat. 2016;15(1):139–45. [DOI] [PubMed] [Google Scholar]
- 23.Hu YC, Tsai KW, Lee CC, Peng NJ, Chien JC, Tseng HH et al. Which nasopharyngeal cancer patients need adaptive radiotherapy? BMC Cancer. 2018;18. [DOI] [PMC free article] [PubMed]
- 24.Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer-Am Cancer Soc. 2007;109(9):1750–5. [DOI] [PubMed] [Google Scholar]
- 25.Tan WY, Li YP, Han G, Xu JZ, Wang XH, Li Y, et al. Target volume and position variations during intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Oncotargets Ther. 2013;6:1719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brolese EK, Cihoric N, Bojaxhiu B, Sermaxhaj B, Schanne DH, Mathier E, et al. The impact of delivery daytime and seasonality of radiotherapy for head and neck cancer on toxicity burden. Radiother Oncol. 2021;158:162–6. [DOI] [PubMed] [Google Scholar]
- 27.Zhong XL, Zeng GH, Zhang LX, You SY, Fu YX, He W et al. Prediction of pathologic complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. Front Oncol. 2024;14. [DOI] [PMC free article] [PubMed]
- 28.Corvo R, Giaretti W, Geido E, Sanguineti G, Orecchia R, Scala M, et al. Cell kinetics and tumor regression during radiotherapy in head and neck squamous-cell carcinomas. Int J Cancer. 1996;68(2):151–5. [DOI] [PubMed] [Google Scholar]
- 29.Tomé WA, Fowler JF. On cold spots in tumor subvolumes. Med Phys. 2002;29(7):1590–8. [DOI] [PubMed] [Google Scholar]
- 30.Sanguineti G, Del Mastro L, Guenzi M, Ricci P, Cavallari M, Canavese G, et al. Impact of chemotherapy dose-density on radiotherapy dose-intensity after breast conserving surgery. Ann Oncol. 2001;12(3):373–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.