Abstract
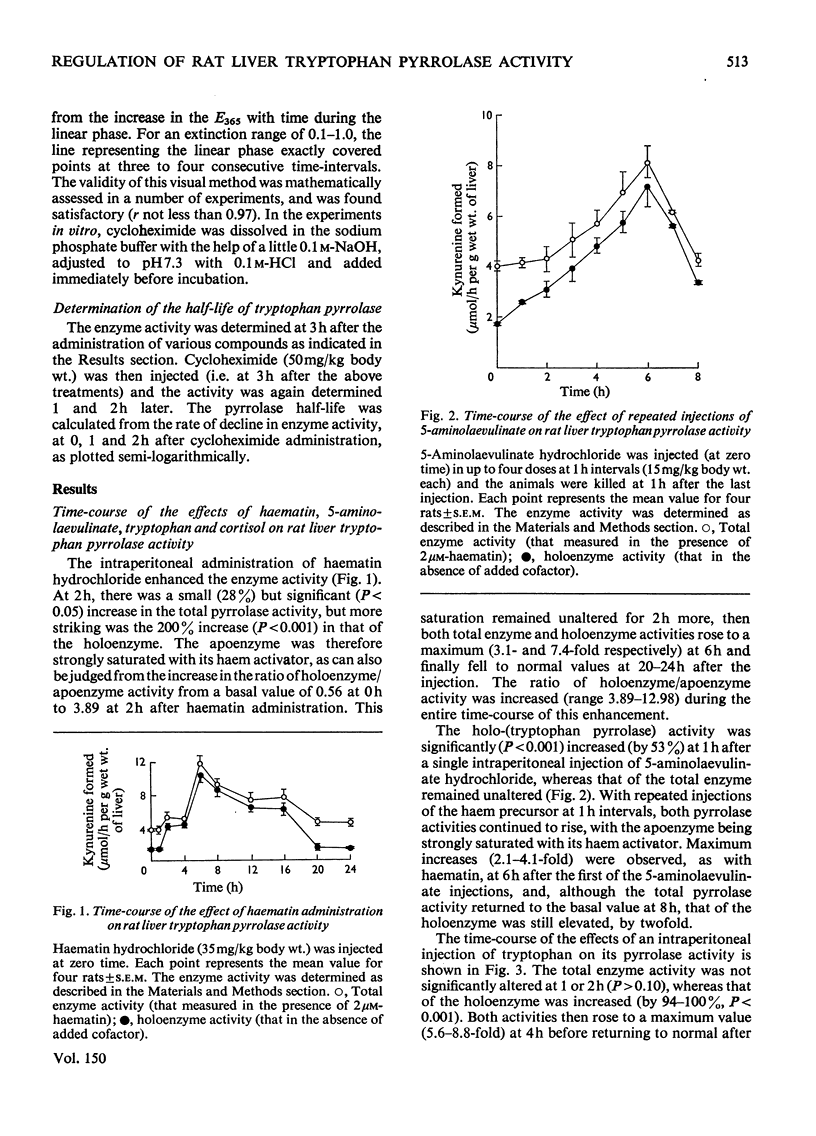
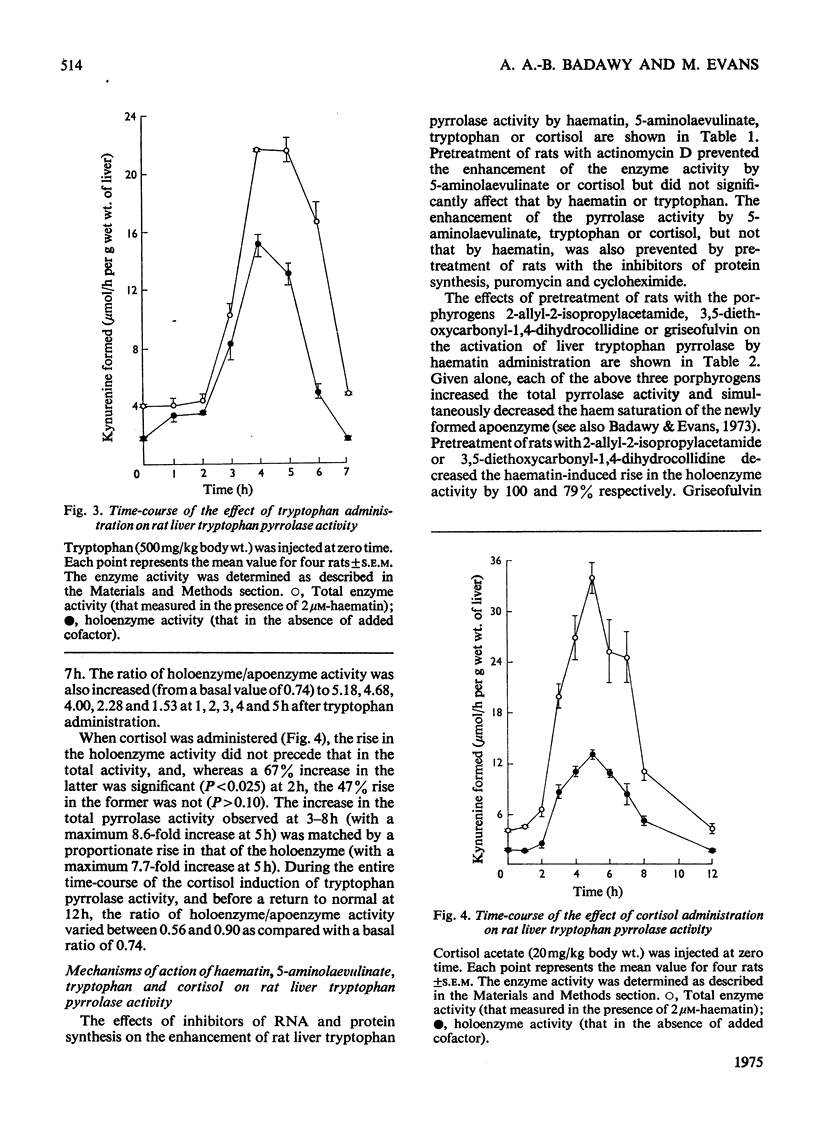
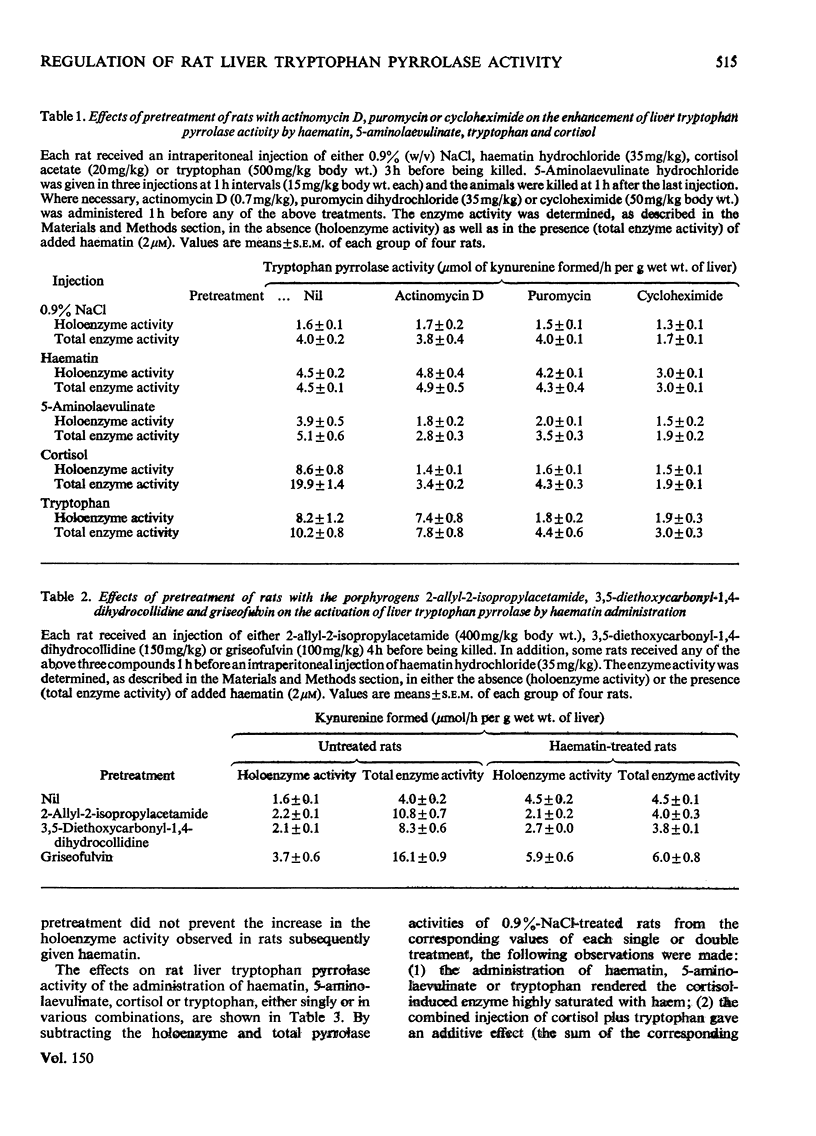
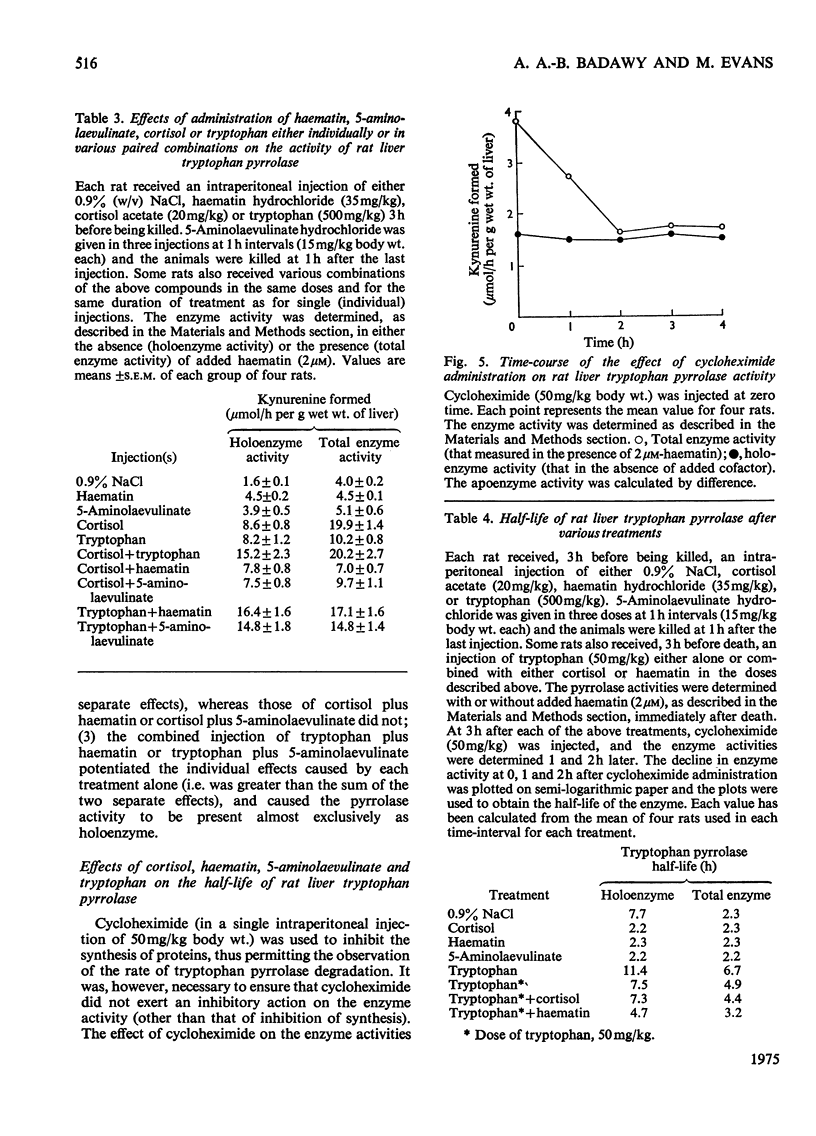
1. The administration of haematin or 5-aminolaevulinate to rat enhances the activity of liver tryptophan pyrrolase; both endogenous and newly formed apoenzymes become strongly haem-saturated. Haem activation does not stabilize tryptophan pyrrolase. 2. Actinomycin D, puromycin or cycloheximide prevent the activation of the enzyme by 5-aminolaevulinate but not that by haematin. The latter is inhibited by haem-destroying porphyrogens. 3. The combined injection of either haematin or 5-aminolaevulinate with cortisol does not produce an additive effect, whereas potentation is observed when tryptophan is jointly given with either the cofactor or the haem precursor. 4. Further experiments on the substrate (tryptophan) mechanism of pyrrolase regulation are reported, and a comparison between this and the cofactor and hormonal mechanisms is made. 5. It is suggested that the substrate mechanism may also involve increased haem synthesis. 6. The role of tryptophan pyrrolase in the utilization of liver haem, and as a possible model for the exacerbation by drugs of human hepatic porphyrias, is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman K., Greengard O. Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest. 1966 Oct;45(10):1527–1534. doi: 10.1172/JCI105459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Guinea-pig liver tryptophan pyrrolase. Absence of detectable apoenzyme activity and of hormonal induction by cortisol and possible regulation by tryptophan. Biochem J. 1974 Mar;138(3):445–451. doi: 10.1042/bj1380445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of chemical porphyrogens and drugs on the activity of rat liver tryptophan pyrrolase. Biochem J. 1973 Dec;136(4):885–892. doi: 10.1042/bj1360885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of ethanol on tryptophan pyrrolase activity and their comparison with those of phenobarbitone and morphine. Adv Exp Med Biol. 1975;59:229–251. doi: 10.1007/978-1-4757-0632-1_15. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Smith M. J. Changes in liver tryptophan and tryptophan pyrrolase activity after administration of salicylate and tryptophan to the rat. Biochem Pharmacol. 1972 Jan;21(1):97–101. doi: 10.1016/0006-2952(72)90254-7. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Smith M. J. The effects of salicylate on the activity of rat liver tryptophan pyrrolase in vitro and in vivo. Biochem J. 1971 Jun;123(2):171–174. doi: 10.1042/bj1230171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K. W., Fröhling W., Remmer H. Influence of fasting and hemin on microsomal cytochromes and enzymes. Biochem Pharmacol. 1973 Jul 1;22(13):1557–1564. doi: 10.1016/0006-2952(73)90021-x. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Drug interactions in experimental hepatic porphyria. A model for the exacerbation by drugs of human variegate porphyria. Enzyme. 1973;16(1):266–275. doi: 10.1159/000459390. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Drug-induced destruction of cytochrome P-450. Drug Metab Dispos. 1973 Jan-Feb;1(1):267–274. [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. Stimulation of liver 5-aminolaevulinate synthetase by drugs and its relevance to drug-induced accumulation of cytochrome P-450. Studies with phenylbutazone and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Biochem J. 1972 Mar;126(5):1149–1160. doi: 10.1042/bj1261149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druyan R., Kelly A. The effect of exogenous -aminolaevulinate on rat liver haem and cytochromes. Biochem J. 1972 Oct;129(5):1095–1099. doi: 10.1042/bj1291095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEIGELSON P., GREENGARD O. A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase. J Biol Chem. 1961 Jan;236:153–157. [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD O., FEIGELSON P. The activation and induction of rat liver tryptophan pyrrolase in vivo by its substrate. J Biol Chem. 1961 Jan;236:158–161. [PubMed] [Google Scholar]
- GREENGARD O., SMITH M. A., ACS G. Relation of cortisone and synthesis of ribonucleic acid to induced and developmental enzyme formation. J Biol Chem. 1963 Apr;238:1548–1551. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- KNOX W. E. Two mechanisms which increase in vivo the liver tryptophan peroxidase activity: specific enzyme adaptation and stimulation of the pituitary adrenal system. Br J Exp Pathol. 1951 Oct;32(5):462–469. [PMC free article] [PubMed] [Google Scholar]
- Knox W. E. The regulation of tryptophan pyrrolase activity by tryptophan. Adv Enzyme Regul. 1966;4:287–297. doi: 10.1016/0065-2571(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Schmid R., Schützel H. Heme and methemoglobin: naturally occurring repressors of microsomal cytochrome. Biochem Biophys Res Commun. 1968 Dec 30;33(6):969–974. doi: 10.1016/0006-291x(68)90408-7. [DOI] [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Coordinate synthesis of heme and apoenzyme in the formation of tryptophan pyrrolase. Science. 1966 Oct 28;154(3748):501–503. doi: 10.1126/science.154.3748.501. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Shore M. L., Felten R. P., Lamanna A., Sheppard E. P. Relative rates of tryptophan pyrrolase degradation following the administration of hydrocortisone and tryptophan to intact and adrenalectomized rat. Biochim Biophys Acta. 1971 Oct;252(1):117–124. doi: 10.1016/0304-4165(71)90099-7. [DOI] [PubMed] [Google Scholar]
- Sourkes T. L. Alpha-methyltryptophan and its actions on tryptophan metabolism. Fed Proc. 1971 May-Jun;30(3):897–903. [PubMed] [Google Scholar]
- Tyrrell D. L., Marks G. S. Drug-induced porphyrin biosynthesis. V. Effect of protohemin on the transcriptional and post-transcriptional phases of -aminolevulinic acid synthetase induction. Biochem Pharmacol. 1972 Aug 1;21(15):2077–2093. doi: 10.1016/0006-2952(72)90161-x. [DOI] [PubMed] [Google Scholar]


