Abstract
Background
Patients with palliated pulmonary valve stenosis (PVS) have less cardiac remodeling and symptoms as compared to patients with repaired tetralogy of Fallot (TOF) presenting with similar severity of right ventricular outflow tract (RVOT) disease. What is not known is whether patients with PVS versus TOF presenting with similar severity of RVOT disease at baseline, would have similar (or different) pace of cardiac remodeling and disease progression over time. The study objective was to compare temporal changes in clinical and cardiac function indices between adults with palliated PVS and repaired TOF presenting with moderate/severe RVOT disease.
Methods
Cardiac function indices (based on strain imaging) and clinical indices (N-terminal pro–B-type natriuretic peptide [NT-proBNP], model for end-stage liver disease excluding international normalized ratio [MELD-XI], peak oxygen consumption [VO2]), were assessed at baseline, 3 years, and 5 years. Temporal changes were calculated as relative changes from baseline (Δ). Cardiovascular adverse event was assessed as time-to-event outcome.
Results
Compared to TOF group (n = 173), the PVS group (n = 173) had less temporal change in right atrial reservoir strain (−9±4% versus −21 ± 6%, p < 0.001), RV free wall strain (−8±4% versus −20 ± 5%, p < 0.001), NT-proBNP (8 ± 5% versus 17 ± 6 %, p < 0.001), MELD-XI (6 ± 4% versus 19 ± 4%, p = 0.008), and peak VO2 (−7±3% versus −12 ± 7%, p < 0.001) at 5 years. The 5-year freedom from cardiovascular adverse event was higher in the PVS group (76% versus 54%, p = 0.01).
Conclusions
These data suggest that a less frequent clinical and imaging follow-up may be appropriate in patients with PVS (as compared to patients with TOF).
Keywords: Valvular heart disease, Congenital heart disease, Disease progression
Highlights
-
•
PVS group had less temporal change in RA and RV function compared to TOF group.
-
•
PVS group had less temporal change in NT-proBNP, MELD-XI (, and peak VO2 compared to TOF group.
-
•
The 5-year freedom from cardiovascular adverse event was higher in the PVS group.
Abbreviations
- CMRI
Cardiac magnetic resonance imaging
- IQR
Interquartile range
- LA
Left atrium
- LV
Left ventricle
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- NYHA
New York Heart Association
- MELD-XI
Model for end-stage liver disease excluding international normalized ratio
- VO2
Oxygen consumption
- PVS
Pulmonary valve stenosis
- PVR
Pulmonary valve replacement
- RVOT
Right ventricular outflow tract
- TOF
Tetralogy of Fallot
- RV-PA
Right ventricular to pulmonary artery
- RVSP
Right ventricular systolic pressure
1. Introduction
Transcatheter and surgical pulmonary valve interventions are effective for the treatment of pulmonary valve stenosis (PVS) [[1], [2], [3], [4]]. However, most patients develop either iatrogenic pulmonary regurgitation and/or recurrent right ventricular outflow tract (RVOT) stenosis leading to RV volume and/or pressure overload [[1], [2], [3], [4]]. Chronic RV volume and/or pressure overload would, in turn, lead to RV remodeling, and cardiovascular symptoms [[1], [2], [3], [4], [5], [6]]. This pathophysiologic cascade can be halted, and potentially reversed if the patients undergo pulmonary valve replacement (PVR) prior to the onset of irreversible RV dysfunction [[7], [8], [9]]. However, the long-term clinical benefits derived from PVR are limited by the risks associated with subsequent reinterventions because of the limited longevity of bioprosthetic valves [[10], [11], [12]]. Hence, determining the optimal timing of PVR is important to avoid irreversible RV dysfunction and also minimize the risks associated with multiple RVOT reinterventions [[10], [11], [12]].
The decision to recommend PVR is based on a set of clinical and imaging criteria proposed in the guidelines for the management of adults with congenital heart disease [[13], [14], [15], [16]]. However, most of the data supporting these recommendations were derived from studies conducted in patients with repaired tetralogy of Fallot (TOF). In the absence of robust longitudinal data from patients with PVS, these guideline recommendations (designed for patients with TOF) are often applied to patients with PVS [[13], [14], [15], [16]].
Previous studies showed that patients with PVS had less RV dilation and systolic dysfunction, aerobic impairment, and cardiovascular symptoms as compared to patients with repaired TOF with similar severity of RVOT disease [2,4,7,16]. What is unknown is whether patients with PVS versus TOF presenting with similar severity of RVOT disease at baseline, would have similar (or different) pace of cardiac remodeling and disease progression over time. This is an important knowledge gap, as it will determine the frequency of clinical and imaging follow-up in patients with PVS, which would in turn, determine healthcare resource utilization. The purpose of this study was to compare the pace of cardiac remodeling and disease progression between adults with palliated PVS and those with repaired TOF presenting with similar severity of RVOT disease.
2. Methods
2.1. Study population
The Mayo Clinic Institutional Review Board approved this retrospective cohort study.
2.1.1. PVS group (case group)
We identified adults (age ≥18 years) with palliated PVS that received care at Mayo Clinic between January 1, 2003, and December 31, 2022. From this cohort, we selected consecutive patients with moderate/severe RVOT disease that had more than 2 years of clinical and imaging follow-up. Moderate/severe RVOT disease was defined as ≥ moderate RVOT stenosis (Doppler derived maximum instantaneous gradient ≥36 mmHg) and/or ≥ moderate pulmonary regurgitation.
Based on these criteria, the patients were divided into 3 RVOT disease group. (1) Isolated RVOT stenosis defined as RVOT Doppler derived maximum instantaneous gradient ≥36 mmHg with <moderate pulmonary regurgitation. (2) Isolated pulmonary regurgitation defined as ≥ moderate pulmonary regurgitation with RVOT Doppler derived maximum instantaneous gradient <36 mmHg. (3) Mixed RVOT disease defined as RVOT Doppler derived maximum instantaneous gradient ≥36 mmHg with ≥moderate pulmonary regurgitation. The severity of pulmonary regurgitation was based on multiparametric qualitative Doppler assessment. Patients with prior palliative systemic-pulmonary shunt, and those without adequate echocardiographic images for offline strain analysis were excluded.
2.1.2. TOF group (control group)
We also identified a control group comprising of adults with repaired TOF presenting with moderate/severe RVOT disease. Similarly, we defined moderate/severe RVOT disease as ≥ moderate RVOT stenosis (Doppler derived maximum instantaneous gradient ≥36 mmHg) and/or ≥ moderate pulmonary regurgitation. The patients were also divided into 3 RVOT disease groups using the same criteria as the PVS group. Patients with prior palliative systemic-pulmonary shunt, and those without adequate echocardiographic images for offline strain analysis were excluded.
We then performed 1:1 matching of patients with PVS to patients with TOF based on RVOT anatomy (native RVOT versus prosthetic pulmonary valve), and RVOT disease type (isolated RVOT stenosis versus isolated pulmonary regurgitation versus mixed RVOT disease).
2.2. Study objectives
(1) Compare temporal change in cardiac function indices between the PVS and TOF groups. (2) Compare temporal change in clinical indices (functional status, aerobic capacity, neurohormonal activation, and end-organ dysfunction) between the PVS and TOF groups. (3) Compare incidence of cardiovascular adverse events between the PVS and TOF groups.
2.3. Data collection
2.3.1. Cardiac function indices
A comprehensive assessment of cardiac structure and function was performed using 2-dimensional, Doppler and speckle tracking echocardiography according to contemporary guidelines [[17], [18], [19]]. The first echocardiogram performed within the study period showing moderate/severe RVOT disease was considered as the baseline echocardiogram. The baseline echocardiogram was reviewed to obtain the baseline cardiac function indices. Cardiac function indices at 3 years were determined based on the review of echocardiograms performed between 24 and 48 months from baseline encounter, while cardiac function indices at 5 years was determined based on a review of echocardiograms performed between 48 and 72 months.
Offline image analyses and measurements were performed, to determine the following cardiac function indices. (1) Right atrial (RA) function was assessed using RA reservoir strain. (2) Left atrial (LA) function was assessed using LA reservoir strain. (3) Right ventricular (RV) systolic function was assessed using RV free wall strain. (4) Left ventricular (LV) systolic function was assessed using LV global longitudinal strain. Temporal changes in cardiac function indices were calculated as relative changes from baseline values as follows: (indices from baseline echocardiogram minus indices from follow-up echocardiogram)/indices from baseline echocardiogram and expressed as %-change. Restrictive RV physiology was defined as pulmonary artery forward flow in atrial systole for >3 cardiac cycles, and was assessed by pulsed wave Doppler of the pulmonary valve [20]. RV to pulmonary artery (RV-PA) coupling was assessed as the ratio of RV free wall strain/RV systolic pressure (RVSP) and tricuspid annular plane systolic excursion/RVSP ratio [21].
In addition to the above prespecified cardiac function indices, cardiac magnetic resonance imaging (CMRI) performed within 6 months from the baseline echocardiogram were reviewed and used to define the baseline characteristics.
2.3.2. Clinical indices
The clinic notes, cardiopulmonary exercise test, and laboratory tests were reviewed, and the data obtained within 6 months from the baseline and follow-up echocardiograms were used to define the clinical indices at the different time points. The following clinical indices were assessed. (1) Functional status was assessed using the New York Heart Association (NYHA) functional classification. (2) Aerobic capacity was assessed using predicted peak oxygen consumption (VO2). (3) Neurohormonal activation was assessed using N-terminal pro–B-type natriuretic peptide (NT-proBNP). (4) End-organ function was assessed using the model for end-stage liver disease excluding international normalized ratio (MELD-XI). Similarly, temporal change in clinical indices was assessed as a relative change from baseline and calculated as: (indices from baseline assessment minus indices from follow-up assessment)/indices from baseline assessment and expressed as %-change.
2.3.3. Cardiovascular adverse event
Cardiovascular adverse event was defined as a composite outcome of sustained atrial arrhythmias requiring medical intervention, heart failure hospitalization, or progressive functional impairment (progression in NYHA functional class). Cardiovascular adverse event was assessed time-to-event outcome, from baseline echocardiogram to the occurrence of cardiovascular adverse event or December 31, 2022. The patients without cardiovascular adverse events were censored at last clinical encounter, time of PVR or December 31, 2022.
2.4. Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range [IQR]), and count (%). Between-group comparisons were performed using chi-square test, unpaired t-test, or Wilcoxon rank sum test, as appropriate. Pearson correlation was used to assess the correlations between continuous variables. Temporal change in cardiac function and clinical indices were assessed using paired t-test. RV and LV strain values were treated as absolute values in the assessment of temporal change in cardiac function indices. NYHA functional class was treated as continuous variable in the assessment of temporal change in clinical indices. The freedom from cardiovascular adverse event was assessed using the Kaplan Meier method and compared using log-rank test. All statistical analyses were performed with BlueSky Statistics software (version. 7.10; BlueSky Statistics LLC, Chicago, IL, USA), and JMP statistical software (version 17.1.0, JMP Statistical Discovery LLC, NC). P value < 0.05 was considered to be statistically significant for all analyses.
3. Results
3.1. Baseline clinical and cardiac function indices
They were 173 patients with PVS and 173 patients with TOF that met the study inclusion criteria. Both groups were adequately matched based on RVOT anatomy and RVOT disease type (Table 1). Compared to the TOF group, the patients in the PVS group were older at the time initial RVOT intervention (6.3 [IQR 1.2–17.9] versus 1.6 [IQR 0.9–3.1] years, p < 0.001), and at the beginning of the study period (35 [IQR 25–48] versus 28 [IQR 20–39] years, p < 0.001). The PVS group also had a lower prevalence of ventricular arrhythmia (1% versus 8 [5%], p = 0.03), higher aerobic capacity (predicted peak VO2 73 ± 22% versus 62 ± 16%, p < 0.001), less neurohormonal activation (NT-proBNP 175 [IQR 85–337] versus 234 (IQR 107–561) pg/ml, p = 0.02), and less end-organ dysfunction (MLED-XI score 9.8 [IQR 9.4–10.6] versus 10.7 [IQR 9.8–11.4], p = 0.01) (Table 1).
Table 1.
Comparison of baseline clinical indices.
| PVS |
TOF |
|||
|---|---|---|---|---|
| (N = 173) | (N = 173) | p | ||
| Demographic indices | ||||
| Age, years | 35 (25–48) | 28 (20–39) | <0.001 | |
| Male sex | 81 (47%) | 95 (53%) | 0.2 | |
| Body mass index, kg/m2 | 26 ± 5 | 27 ± 4 | 0.2 | |
| Body surface area, m2 | 1.91 ± 0.26 | 1.87 ± 0.29 | 0.1 | |
| Initial RVOT intervention | ||||
| Age of initial RVOT intervention, years | 6.3 (1.2–17.9) | 1.6 (0.9–3.1) | <0.001 | |
| Type of initial RVOT intervention | <0.001 | |||
| Balloon valvuloplasty | 47 (27%) | 0 | ||
| Transannular patch repair | 29 (17%) | 36 (21%) | ||
| Non-transannular patch repair | 97 (56%) | 137 (79%) | ||
| RVOT anatomy | 0.9 | |||
| Pulmonary valve bioprosthesis | 42 (24%) | 42 (36%) | ||
| Native RVOT | 131 (76%) | 131 (76%) | ||
| RVOT disease type | 0.9 | |||
| Isolated RVOT stenosis | 34 (20%) | 34 (20%) | ||
| Isolated pulmonary regurgitation | 97 (56%) | 97 (56%) | ||
| Mixed RVOT disease | 42 (24%) | 42 (24%) | ||
| Comorbidities | ||||
| Atrial flutter/tachycardia | 12 (7%) | 21 (12%) | 0.07 | |
| Atrial fibrillation | 2 (1%) | 3 (2%) | 0.6 | |
| All atrial arrhythmias | 12 (7%) | 22 (13%) | 0.05 | |
| Non-sustained ventricular tachycardia | 2 (1%) | 8 (5%) | 0.03 | |
| Sustained ventricular tachycardia | 0 | 0 | – | |
| Any ventricular arrhythmia | 2 (1%) | 8 (5%) | 0.03 | |
| Hypertension | 39 (23%) | 32 (19%) | 0.4 | |
| Coronary artery disease | 6 (4%) | 5 (3%) | 0.6 | |
| NYHA functional class | 0.3 | |||
| I | 146 (84%) | 138 (80%) | ||
| II | 27 (16%) | 35 (20%) | ||
| Aerobic capacity | ||||
| Peak VO2 (ml/kg/min) | 26 ± 5 | 22 ± 7 | <0.001 | |
| Peak VO2 (%-predicted) | 73 ± 22 | 62 ± 16 | <0.001 | |
| Medications | ||||
| Diuretics | 29 (17%) | 40 (23%) | 0.03 | |
| Beta blockers | 33 (19%) | 42 (24%) | 0.2 | |
| ACEI/ARB | 36 (21%) | 39 (23%) | 0.6 | |
| Mineralocorticoid receptor antagonist | 5 (3%) | 8 (5%) | 0.3 | |
| Laboratory data | ||||
| NT-proBNP, pg/ml | 175 (85–337) | 234 (107–561) | 0.02 | |
| MELD-XI | 9.8 (9.4–10.6) | 10.7 (9.8–11.4) | 0.01 | |
| Estimated GFR, ml/min/1.73 m2 | 93 (79–109) | 89 (76–102) | 0.4 | |
Abbreviations: ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin-II receptor blockers; GFR: glomerular filtration rate; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NYHA: New York Heart Association; NT-proBNP: N-terminal pro–B-type natriuretic peptide; PVS: Pulmonary valve stenosis; RVOT: Right ventricular outflow tract; TOF: Tetralogy of Fallot; VO2: Oxygen consumption.
Footnote: p values were derived from pairwise comparisons using chi-squared test, unpaired t-test, and Wilcoxon rank sum test.
Table 2 shows the baseline echocardiographic indices. Compared to the TOF group, the PVS group had better RA function (RA reservoir strain 36% [IQR 28–49] versus 25% [IQR 20–32], p < 0.001), RV systolic function (RV free wall strain −25 ± 4% versus −22 ± 5%, p = 0.007), LA function (LA reservoir strain 38 ± 9% versus 31 ± 10%, p = 0.008), and LV systolic function (LV global longitudinal strain −23 ± 5% versus −19 ± 4%, p < 0.001). However, there was no significant between-group difference in RV-PA coupling between the PVS group versus the TOF group as measured by RV free wall strain/RVSP ratio (0.54 ± 0.93 versus 0.52 ± 1.06 %/mmHg, p = 0.1) or as measured by tricuspid annular plane systolic excursion/RVSP ratio (0.42 ± 0.39 versus 0.41 ± 0.32 mm/mmHg, p = 0.7) (Table 2). Similalry, there was no significant between-group difference in the prevalence of restrictive RV physiology (8% [n = 14] versus 9% [n = 16], p = 0.5).
Table 2.
Comparison of baseline cardiac indices.
| VPS |
TOF |
||
|---|---|---|---|
| Echocardiographic indices | (N = 173) | (N = 173) | p |
| RA indices | |||
| RA volume index (ml/m2) | 35 (24–45) | 37 (29–47) | 0.2 |
| RA reservoir strain (%) | 36 (28–49) | 25 (20–32) | <0.001 |
| RA pressure (mmHg) | 6 ± 3 | 8 ± 3 | 0.002 |
| RV indices | |||
| RV end-diastolic area index (cm2/m2) | 15.7 ± 3.8 | 18.3 ± 4.2 | 0.008 |
| RV end-systolic area index (cm2/m2) | 9.8 ± 2.2 | 12.6 ± 3.1 | 0.01 |
| RV fractional area change (%) | 39 ± 8 | 36 ± 10 | 0.1 |
| RVFWS (%) | −25 ± 4 | −22 ± 5 | 0.007 |
| TAPSE (mm) | 19 ± 7 | 17 ± 6 | 0.1 |
| ≥Moderate tricuspid regurgitation | 22 (13%) | 28 (16%) | 0.3 |
| Pulmonary mean gradient (mmHg) | 24 (13–41) | 22 (9–38) | 0.3 |
| RVSP (mmHg) | 46 (31–57) | 41 (32–53) | 0.6 |
| Restrictive RV physiology | 13 (8%) | 16 (9%) | 0.5 |
| RVFWS/RVSP (%/mmHg) | 0.54 ± 0.93 | 0.52 ± 1.06 | 0.1 |
| TAPSE/RVSP (mm/mmHg) | 0.42 ± 0.39 | 0.41 ± 0.32 | 0.7 |
| LA indices | |||
| LA volume index (ml/m2) | 25 ± 7 | 26 ± 8 | 0.7 |
| LA reservoir strain (%) | 38 ± 9 | 31 ± 10 | 0.008 |
| Lateral E/e’ | 7.1 ± 2.8 | 8.4 ± 3.5 | 0.03 |
| LV indices | |||
| LV end-diastolic volume index (ml/m2) | 52 ± 15 | 55 ± 16 | 0.4 |
| LV end-systolic volume index (ml/m2) | 21 ± 10 | 24 ± 11 | 0.1 |
| LV ejection fraction (%) | 61 ± 9 | 56 ± 8 | 0.06 |
| LV global longitudinal strain (%) | −23 ± 5 | −19 ± 4 | <0.001 |
| LV stroke volume index (ml/m2) | 43 ± 11 | 37 ± 8 | 0.03 |
| Cardiac index (l/min/m2) | 2.98 ± 0.74 | 2.69 ± 0.71 | 0.01 |
| CMRI indices | (N = 148) | (N = 159) | |
| RV end-diastolic volume (ml/m2) | 126 ± 15 | 139 ± 32 | 0.02 |
| RV end-systolic volume (ml/m2) | 49 ± 14 | 63 ± 21 | 0.008 |
| RV ejection fraction (%) | 54 ± 10 | 47 ± 13 | 0.01 |
| RV stroke volume index (ml/m2) | 78 ± 18 | 76 ± 21 | 0.8 |
| Pulm regurgitant volume index (ml/m2) | 36 ± 12 | 38 ± 10 | 0.6 |
| LV end-diastolic volume (ml/m2) | 79 ± 18 | 84 ± 26 | 0.1 |
| LV end-systolic volume (ml/m2) | 38 ± 16 | 45 ± 14 | 0.06 |
| LV ejection fraction (%) | 62 ± 15 | 58 ± 16 | 0.3 |
| LV stroke volume index (ml/m2) | 41 ± 12 | 39 ± 12 | 0.2 |
Abbreviations: CMRI: Cardiac magnetic resonance imaging; E/e’: ratio of mitral inflow pulsed wave Doppler early velocity to tissue Doppler early velocity; LA: Left atrium; LV: Left ventricle; PVS: Pulmonary valve stenosis; RA: Right atrium; RV: Right ventricle; RVFWS: Right ventricular free wall strain; RVSP: Right ventricular systolic pressure; TOF: Tetralogy of Fallot; TAPSE: Tricuspid anular plane systolic excursion.
Footnote: p values were derived from pairwise comparisons using chi-squared test, unpaired t-test, and Wilcoxon rank sum test.
CMRI data were available in 148 (86%) and 159 (92%) patients in the PVS and TOF groups, respectively. Compared to the TOF group, the PVS group had smaller RV end-diastolic volume index (126 ± 15 versus 139 ± 32 ml/m2, p = 0.02), and RV end-systolic volume index (49 ± 14 versus 63 ± 21 ml/m2, p = 0.008), as well as a higher RV ejection fraction (54 ± 10 versus 47 ± 13%, p = 0.01) (Table 2). There was a modest correlation between CMRI derived RV ejection fraction and echocardiography derived RV free wall strain in the PVS group (r = −0.56, p = 0.004) and in the TOF group (r = −0.52, p = 0.008). Similarly, there was a modest correlation between CMRI derived LV ejection fraction and echocardiography derived LV global longitudinal strain in the PVS group (r = −0.63, p < 0.001) and in the TOF group (r = −0.61, p < 0.001).
3.2. Temporal change in clinical and cardiac function indices
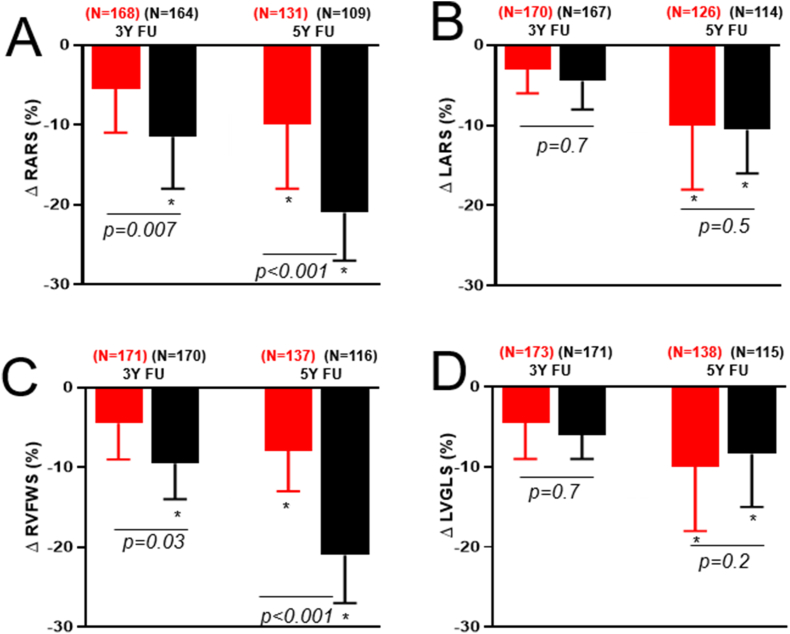
Compared to the TOF group, the PVS group had less temporal decline in RA function at 3 years (Δ RA reservoir strain −6±3% versus −11 ± 4%, p = 0.007) and 5 years (Δ RA reservoir strain −9±4% versus −21 ± 6%, p < 0.001). Similarly, the PVS group had less temporal decline in RV systolic function at 3 years (Δ RV free wall strain −5±2% versus −9±4%, p = 0.03) and 5 years (Δ RV free wall strain −8±4% versus −20 ± 5%, p < 0.001) (Fig. 1). The temporal change in left-sided cardiac indices, compared to right-sided indices were less pronounced in both groups. There were no significant temporal changes in LA reservoir strain and LV global longitudinal strain in either group at 3 years. However, both groups showed statistically significant decline in LA reservoir strain and LV global longitudinal strain at 5 years, but the magnitude of change was similar in both groups (Fig. 1).
Fig. 1.
Box and whisker plots comparing relative change in cardiac function indices between patients with pulmonary valve stenosis (PVS, red) versus tetralogy of Fallot (TOF, black). Data were presented as relative changes from baseline values. Abbreviations: FU: Follow-up; LARS: Left atrial reservoir strain; LVGLS: Left ventricular global longitudinal strain; RVFWS: Right ventricular free wall strain; RARS: Right atrial reservoir strain. Footnote: * statistically significant change from baseline value based on paired t-test. P values were derived from between-group comparisons using unpaired t-tests. N: Number of patients with available data at each time point.
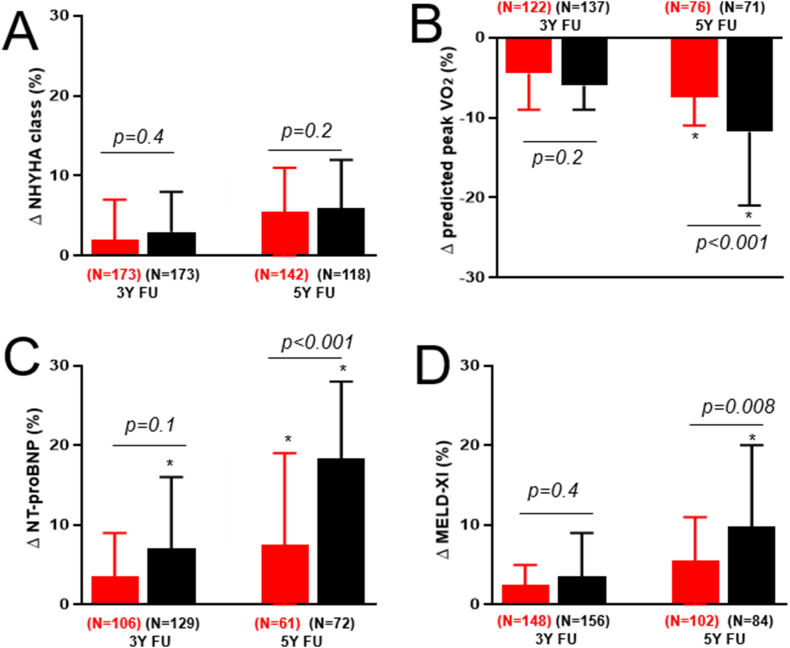
Fig. 2 shows temporal change in clinical indices. Compared to the TOF group, the PVS group had less temporal change in NT-proBNP (8 ± 5% versus 17 ± 6 %, p < 0.001), MELD-XI (6 ± 4% versus 19 ± 4%, p = 0.008), and aerobic capacity (Δ predicted peak VO2 -7±3% versus −12 ± 7%, p < 0.001) at 5 years follow-up (Fig. 2). However, there was no significant temporal change in NYHA functional class in either group (Fig. 2).
Fig. 2.
Box and whisker plots comparing relative change in clinical indices between patients with PVS (red) versus TOF (black). Data were presented as relative changes from baseline values. Abbreviations: NYHA: New York Heart Association; MELD-XI: Model for end-stage liver disease excluding international normalized ratio; NT-proBNP: N-terminal pro–B-type natriuretic peptide; VO2: Oxygen consumption. Footnote: * statistically significant change from baseline value based on paired t-test. P values were derived from between-group comparisons using unpaired t-tests. N: Number of patients with available data at each time point.
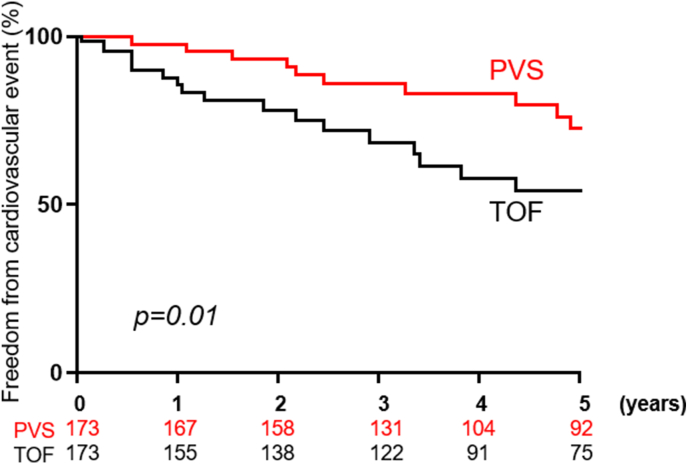
The median follow-up was 6.9 (4.3–8.5) years and 5.8 (3.9–7.2) years in the PVS and TOF groups, respectively. During this period 31 (18%) patients experienced at least one cardiovascular adverse event, while 16 (9%) patients underwent PVR in the PVS. Similarly, 43 (25%) patients experienced at least one cardiovascular adverse event, while 29 (17%) patients underwent PVR in the TOF. The 5-year freedom from cardiovascular adverse event was higher in the PVS group as compared to the TOF group (76% versus 54%, p = 0.01), (Fig. 3).
Fig. 3.
Kaplan Meier curves comparing freedom from cardiovascular adverse events between patients with PVS (red) versus TOF (black). P value was derived from log-rank test.
4. Discussion
In this study, we compared temporal changes in cardiac function and clinical indices in patients with palliated PVS to patients with repaired TOF presenting with similar severity of RVOT disease. The main findings are follows: (1) The PVS group had better cardiac function and clinical indices at baseline compared to the TOF group. (2) The PVS group had less temporal decline in right-sided cardiac function indices (RA function and RV systolic function) as compared to the TOF group. (3) The patients with PVS had less temporal decline in clinical indices (neurohormonal activation, end-organ function, and aerobic capacity), and lower incidence of cardiovascular adverse events as compared to the patients with TOF.
About half of patients with palliated PVS would develop hemodynamically significant pulmonary regurgitation and/or recurrent RVOT stenosis on long-term follow-up [5,22]. While isolated recurrent RVOT stenosis typically responds to balloon pulmonary valvuloplasty, PVR is often required in patients with concomitant pulmonary regurgitation [4,7,22]. The timing of PVR is dependent on the presence of clinical indices such as functional capacity (symptoms) and aerobic capacity, as well as cardiac function indices such as RV dilation and systolic dysfunction [13,14]. In the absence of well-defined criteria for PVR in patients with palliated PVS, the decision to proceed with PVR in this population is often based on extrapolation of data derived from studies conducted in patients with repaired TOF [13,14]. Similarly, the frequency of clinical and imaging surveillance for patients that currently do not meet criteria for PVR are also based on extrapolations from data derived from the TOF population [13,14]. The current study demonstrates a slower pace of disease progression in patients with PVS as compared to patients with TOF, as evidenced by a less temporal decline in clinical indices (aerobic capacity, neural hormonal activation, and hepatorenal function), and right-sided cardiac function indices (RA function and RV systolic function).
Previous studies have shown that patients with PVS had less symptoms and cardiac remodeling at baseline as compared to patients with TOF [4,22]. Joynt et al. compared CMRI data between 24 patients with PVS and 47 patients with TOF with similar severity of pulmonary regurgitation [23]. They observed that the PVS group had a higher RV ejection fraction and less late gadolinium enhancement as compared to the TOF group. Within the TOF group, RV systolic dysfunction and late gadolinium enhancement were more pronounced in the RVOT [23]. Similarly, Mercer-Rosa et al., observed a higher CMRI derived RV ejection fraction and aerobic capacity, as well as better functional capacity in patients with palliated PVS as compared to TOF [23]. While these previous studies demonstrated better baseline clinical status and cardiac function in patients with PVS (as compared to TOF), they do not provide data about the pace of disease progression during follow-up. The current study addressed this knowledge gap.
The observed differences in the pace of disease progression may be related to differences in disease pathophysiology and impact of initial palliative interventions [24]. Patients with TOF are exposed to cyanosis within the first year of life which may have long-term adverse effects on RV myocardial structure and function, leading to RV fibrosis and diastolic dysfunction [24]. This is consistent with the higher prevalence and severity of late gadolinium enhancement (marker of myocardial fibrosis) and diastolic dysfunction described in patients with TOF [22,24,25]. This may explain the higher RA pressure observed in the TOF group in the current study. Since RA hypertension has been linked to right heart failure and hepatorenal dysfunction in adults with congenital heart disease, we postulate that RV fibrosis and diastolic dysfunction described in TOF patients in previous studies [26,27], may also explain the higher NT-proBNP and MELD-XI score observed in the TOF group in the current study. Other factors that may contribute to the rapid pace of disease progression in the TOF group include abnormal pulmonary artery compliance and worse RV to pulmonary arterial coupling that have been described in patients with TOF as compared to PVS [6,11,28].
Additionally, differences in the type of initial RVOT intervention may explain some of the observed differences in outcomes. About one-quarter of the patients in the PVS group underwent balloon pulmonary valvuloplasty, and hence were not exposed to the hypoxic-ischemic injury associated with cardiopulmonary bypass. Presumably, these patients started off with less RV myocardial injury as compared to those that underwent surgical repair.
An important negative finding from this study was the absence of temporal change in NYHA functional class in either group, even though both groups had decline in aerobic capacity and other clinical indices of disease severity. This is consistent with observation from previous studies showing a discordance between objectively measured aerobic capacity and subjective assessment of symptoms by the patient [29,30]. This highlights the limitations of relying solely on patient reported symptoms for clinical decision-making regarding timing of surgical or transcatheter interventions.
4.1. Clinical implications
Patients with PVS and residual RVOT disease not meeting the criteria for PVR require ongoing clinical and imaging surveillance [13,14]. The practice guidelines recommend transthoracic echocardiogram every 24 months in asymptomatic/mildly symptomatic (physiologic stage A/B) patients with palliated PVS, which is similar to the frequency of echocardiogram in TOF patients [13,14]. The slower pace of disease progression observed in the PVS group suggest that, perhaps, less frequent clinical and imaging follow-up may be appropriate in patients PVS. This will reduce healthcare resource utilization, and potentially increase economic productivity as it will reduce number of days spent off work because of medical evaluation.
Limitations: This is a retrospective single center study, and it is therefore prone to selection and ascertainment bias. The patient lost to follow and those that underwent PVR during follow-up were censored, further introducing a selection bias to the study. We did not have serial CMRI data to assess temporal change in cardiac remodeling over time. However, by using strain imaging to assess cardiac function in all four chambers, we demonstrated that the hemodynamic effect of RVOT disease is not limited to the right heart, but also affects LA and LV function over time. Finally, we do not have invasive hemodynamic data, or data about ventricular dyssynchrony, and this limits our ability to make inferences about RV-PA coupling and ventricular interaction. Furthermore, there important molecular/genetic differences between PVS and TOF, that were not assessed in this study.
Conclusion: Patients with PVS had a slower pace of disease progression, as evidenced by less temporal decline in clinical indices and cardiac function indices, as well as lower cumulative incidence of cardiovascular adverse events as compared to patients with TOF. These data suggest that a less frequent clinical and imaging follow-up may be appropriate in patients with PVS (as compared to patients with TOF). This will likely reduce healthcare resource utilization and IMPROVE quality of life of the patients.
Funding
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL158517, R01 HL160761, and R01 HL162830). The MACHD Registry is supported by the Al-Bahar Research grant.
Perspectives
Clinical competencies
Patients with PVS had a slower pace of disease progression, as evidenced by a less temporal decline in clinical and cardiac function indices, as well as lower cumulative incidence of cardiovascular adverse events as compared to patients with TOF.
Translational outlook
These data suggest that a less frequent clinical and imaging follow-up may be appropriate in patients with PVS (as compared to patients with TOF).
Clinical summary
Compared to the TOF group (n = 173), the PVS group (n = 173) had less temporal change in right atrial reservoir strain (−9±4% versus −21 ± 6%, p < 0.001), RV free wall strain (−8±4% versus −20 ± 5%, p < 0.001), NT-proBNP (8 ± 5% versus 17 ± 6 %, p < 0.001), MELD-XI (6 ± 4% versus 19 ± 4%, p = 0.008), and peak VO2 (−7±3% versus −12 ± 7%, p < 0.001) at 5 years. The 5-year freedom from cardiovascular adverse event was higher in the PVS group (76% versus 54%, p = 0.01). These data suggest that a less frequent clinical and imaging follow-up may be appropriate in patients with PVS (as compared to patients with TOF).
CRediT authorship contribution statement
Alexander C. Egbe: Writing – review & editing, Writing – original draft. C. Charles Jain: Writing – review & editing, Writing – original draft. Luke J. Burchill: Writing – review & editing, Writing – original draft. Snigdha Karnakoti: Writing – review & editing, Writing – original draft. Marwan H. Ahmed: Writing – review & editing, Writing – original draft. Maan Jokhadar: Writing – review & editing, Writing – original draft. Heidi M. Connolly: Writing – review & editing, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Cuypers J.A., Witsenburg M., van der Linde D., Roos-Hesselink J.W. Pulmonary stenosis: update on diagnosis and therapeutic options. Heart. 2013;99:339–347. doi: 10.1136/heartjnl-2012-301964. [DOI] [PubMed] [Google Scholar]
- 2.Zdradzinski M.J., Qureshi A.M., Stewart R., Pettersson G., Krasuski R.A. Comparison of long-term postoperative sequelae in patients with tetralogy of Fallot versus isolated pulmonic stenosis. Am J Cardiol. 2014;114:300–304. doi: 10.1016/j.amjcard.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoglund K., Rosengren A., Lappas G., Fedchenko M., Mandalenakis Z. Long-term survival in patients with isolated pulmonary valve stenosis: a not so benign disease? Open heart. 2021;8 doi: 10.1136/openhrt-2021-001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercer-Rosa L., Ingall E., Zhang X., McBride M., Kawut S., Fogel M., Paridon S., Goldmuntz E. The impact of pulmonary insufficiency on the right ventricle: a comparison of isolated valvar pulmonary stenosis and tetralogy of fallot. Pediatr Cardiol. 2015;36:796–801. doi: 10.1007/s00246-014-1087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos-Hesselink J.W., Meijboom F.J., Spitaels S.E., vanDomburg R.T., vanRijen E.H., Utens E.M., Bogers A.J., Simoons M.L. Long-term outcome after surgery for pulmonary stenosis (a longitudinal study of 22-33 years) Eur Heart J. 2006;27:482–488. doi: 10.1093/eurheartj/ehi685. [DOI] [PubMed] [Google Scholar]
- 6.Rommel J.J., Yadav P.K., Stouffer G.A. Causes and hemodynamic findings in chronic severe pulmonary regurgitation. Cathet Cardiovasc Interv : off J Soc Card Angiography & Interv. 2018;92(3):197–203. doi: 10.1002/ccd.26073. [DOI] [PubMed] [Google Scholar]
- 7.Laflamme E., Wald R.M., Roche S.L., Silversides C.K., Thorne S.A., Colman J.M., Benson L., Osten M., Horlick E., Oechslin E., Alonso-Gonzalez R. Outcome and right ventricle remodelling after valve replacement for pulmonic stenosis. Heart. 2022;108:1290–1295. doi: 10.1136/heartjnl-2021-320121. [DOI] [PubMed] [Google Scholar]
- 8.Bokma J.P., Winter M.M., Oosterhof T., Vliegen H.W., van Dijk A.P., Pieper P.G., Meijboom F.J., Groenink M., Mulder B.J.M., Bouma B.J. Pulmonary valve replacement after repair of pulmonary stenosis compared with tetralogy of fallot. J Am Coll Cardiol. 2016;67:1123–1124. doi: 10.1016/j.jacc.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Bokma J.P., Geva T., Sleeper L.A., Lee J.H., Lu M., Sompolinsky T., Babu-Narayan S.V., Wald R.M., Mulder B.J.M., Valente A.M. Improved outcomes after pulmonary valve replacement in repaired tetralogy of fallot. J Am Coll Cardiol. 2023;81:2075–2085. doi: 10.1016/j.jacc.2023.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Egbe A.C., Miranda W.R., Said S.M., Pislaru S.V., Pellikka P.A., Borlaug B.A., Kothapalli S., Connolly H.M. Risk stratification and clinical outcomes after surgical pulmonary valve replacement. Am Heart J. 2018;206:105–112. doi: 10.1016/j.ahj.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Egbe A.C., Kothapalli S., Miranda W.R., Pislaru S., Ammash N.M., Borlaug B.A., Pellikka P.A., Najam M., Connolly H.M. Assessment of right ventricular-pulmonary arterial coupling in chronic pulmonary regurgitation. Can J Cardiol. 2019;35:914–922. doi: 10.1016/j.cjca.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Egbe A.C., Connolly H.M., Miranda W.R., Dearani J.A., Schaff H.V. Outcomes of bioprosthetic valves in the pulmonary position in adults with congenital heart disease. Ann Thorac Surg. 2019;108:1410–1415. doi: 10.1016/j.athoracsur.2019.05.068. [DOI] [PubMed] [Google Scholar]
- 13.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., Crumb S.R., Dearani J.A., Fuller S., Gurvitz M., Khairy P., Landzberg M.J., Saidi A., Valente A.M., Van Hare G.F. AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H., De Backer J. The ESC clinical practice guidelines for the management of adult congenital heart disease 2020. Eur Heart J. 2020;41:4153–4154. doi: 10.1093/eurheartj/ehaa701. [DOI] [PubMed] [Google Scholar]
- 15.Bokma J.P., Winter M.M., Oosterhof T., Vliegen H.W., van Dijk A.P., Hazekamp M.G., Koolbergen D.R., Groenink M., Mulder B.J., Bouma B.J. Preoperative thresholds for mid-to-late haemodynamic and clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Eur Heart J. 2016;37:829–835. doi: 10.1093/eurheartj/ehv550. [DOI] [PubMed] [Google Scholar]
- 16.Bokma J.P., Winter M.M., Oosterhof T., Vliegen H.W., van Dijk A.P., Pieper P.G., Meijboom F.J., Groenink M., Mulder B.J., Bouma B.J. Pulmonary valve replacement after repair of pulmonary stenosis compared with tetralogy of fallot. J Am Coll Cardiol. 2016;67:1123–1124. doi: 10.1016/j.jacc.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Rudski L.G., Lai W.W., Afilalo J., Hua L., Handschumacher M.D., Chandrasekaran K., Solomon S.D., Louie E.K., Schiller N.B. vol. 23. official publication of the American Society of Echocardiography; 2010. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography; pp. 685–713. (Journal of the American society of echocardiography). quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. vol. 28. official publication of the American Society of Echocardiography; 2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging; pp. 1–39 e14. (Journal of the American society of echocardiography). [DOI] [PubMed] [Google Scholar]
- 19.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., Horton K., Ogunyankin K.O., Palma R.A., Velazquez E.J. vol. 32. official publication of the American Society of Echocardiography; 2019. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography; pp. 1–64. (Journal of the American society of echocardiography). [DOI] [PubMed] [Google Scholar]
- 20.Egbe A.C., Pellikka P.A., Miranda W.R., Bonnichsen C., Reddy Y.N.V., Borlaug B.A., Connolly H.M. Echocardiographic predictors of severe right ventricular diastolic dysfunction in tetralogy of Fallot: relations to patient outcomes. Int J Cardiol. 2020;306:49–55. doi: 10.1016/j.ijcard.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed M., Karnakoti S., Abozied O., Kandlakunta S., Younis A., Egbe A.C. Prognostic role of tricuspid annular plane systolic excursion/right ventricular systolic pressure ratio in coarctation of aorta. CJC Pediatr Congenit Heart Dis. 2023;2:167–173. doi: 10.1016/j.cjcpc.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joynt M.R., Yu S., Dorfman A.L., Ghadimi Mahani M., Agarwal P.P., Lu J.C. Differential impact of pulmonary regurgitation on patients with surgically repaired pulmonary stenosis versus tetralogy of fallot. Am J Cardiol. 2016;117:289–294. doi: 10.1016/j.amjcard.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Lianza A.C., Rodrigues A.C.T., Mercer-Rosa L., Vieira M.L.C., de Oliveira W.A.A., Afonso T.R., Nomura C.H., da Silva J.P., da Silva L.D.F., Szarf G., Tavares G.M.P., Fischer C.H., Morhy S.S. Right ventricular systolic function after the cone procedure for ebstein's anomaly: comparison between echocardiography and cardiac magnetic resonance. Pediatr Cardiol. 2020;41:985–995. doi: 10.1007/s00246-020-02347-6. [DOI] [PubMed] [Google Scholar]
- 24.Geva T. Diffuse myocardial fibrosis in repaired tetralogy of fallot: linking pathophysiology and clinical outcomes. Circulation Cardiovascular imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006184. [DOI] [PubMed] [Google Scholar]
- 25.Wald R.M., Valente A.M., Gauvreau K., Babu-Narayan S.V., Assenza G.E., Schreier J., Gatzoulis M.A., Kilner P.J., Koyak Z., Mulder B., Powell A.J., Geva T. Cardiac magnetic resonance markers of progressive RV dilation and dysfunction after tetralogy of Fallot repair. Heart. 2015;101:1724–1730. doi: 10.1136/heartjnl-2015-308014. [DOI] [PubMed] [Google Scholar]
- 26.Egbe A.C., Bonnichsen C., Reddy Y.N.V., Anderson J.H., Borlaug B.A. Pathophysiologic and prognostic implications of right atrial hypertension in adults with tetralogy of fallot. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.014148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egbe A.C., Miranda W.R., Anderson J.H., Katta R.R., Goda A.Y., Andi K., Kamath P.S., Connolly H.M. Determinants and prognostic implications of hepatorenal dysfunction in adults with congenital heart disease. Can J Cardiol. 2022;38:1742–1750. doi: 10.1016/j.cjca.2022.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egbe A.C., Miranda W.R., Pellikka P.A., Pislaru S.V., Borlaug B.A., Kothapalli S., Ananthaneni S., Sandhyavenu H., Najam M., Farouk Abdelsamid M., Connolly H.M. Right ventricular and pulmonary vascular function indices for risk stratification of patients with pulmonary regurgitation. Congenit Heart Dis. 2019;14:657–664. doi: 10.1111/chd.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egbe A., Miranda W., Connolly H., Dearani J. Haemodynamic determinants of improved aerobic capacity after tricuspid valve surgery in Ebstein anomaly. Heart. 2021;107:1138–1144. doi: 10.1136/heartjnl-2020-317756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diller G.P., Dimopoulos K., Okonko D., Li W., Babu-Narayan S.V., Broberg C.S., Johansson B., Bouzas B., Mullen M.J., Poole-Wilson P.A., Francis D.P., Gatzoulis M.A. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]