Abstract
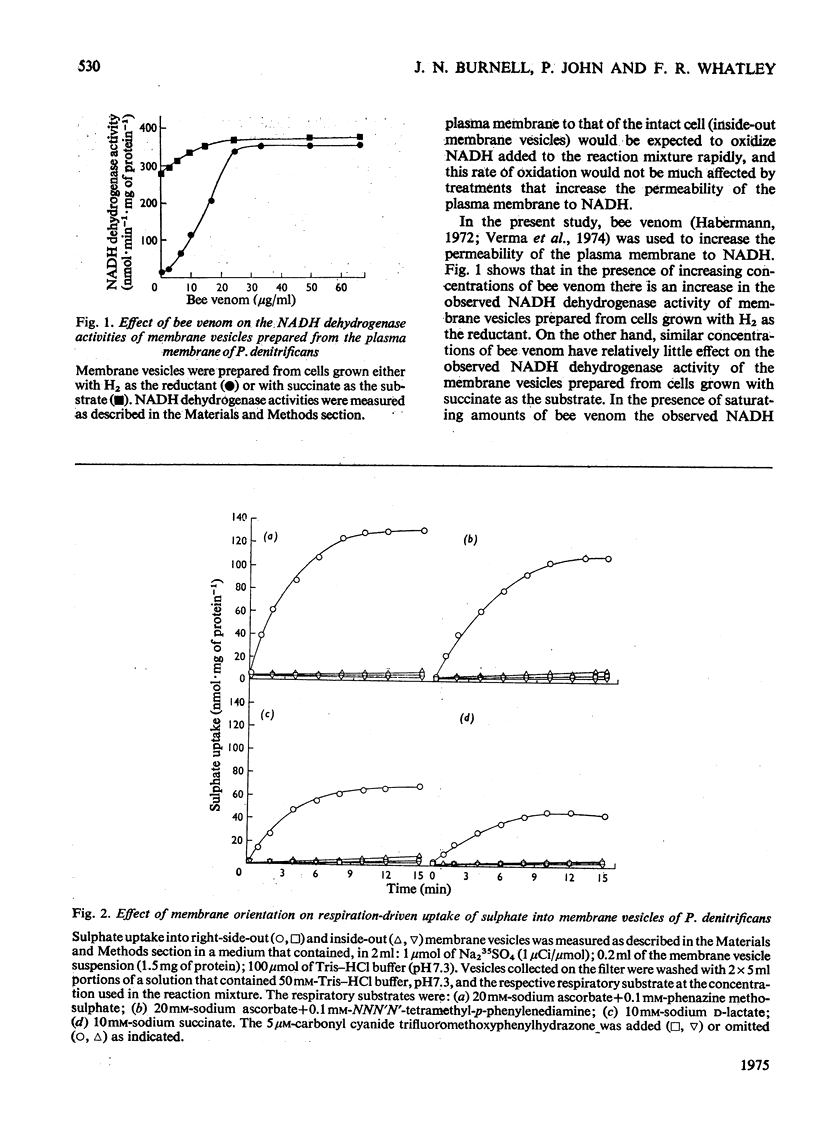
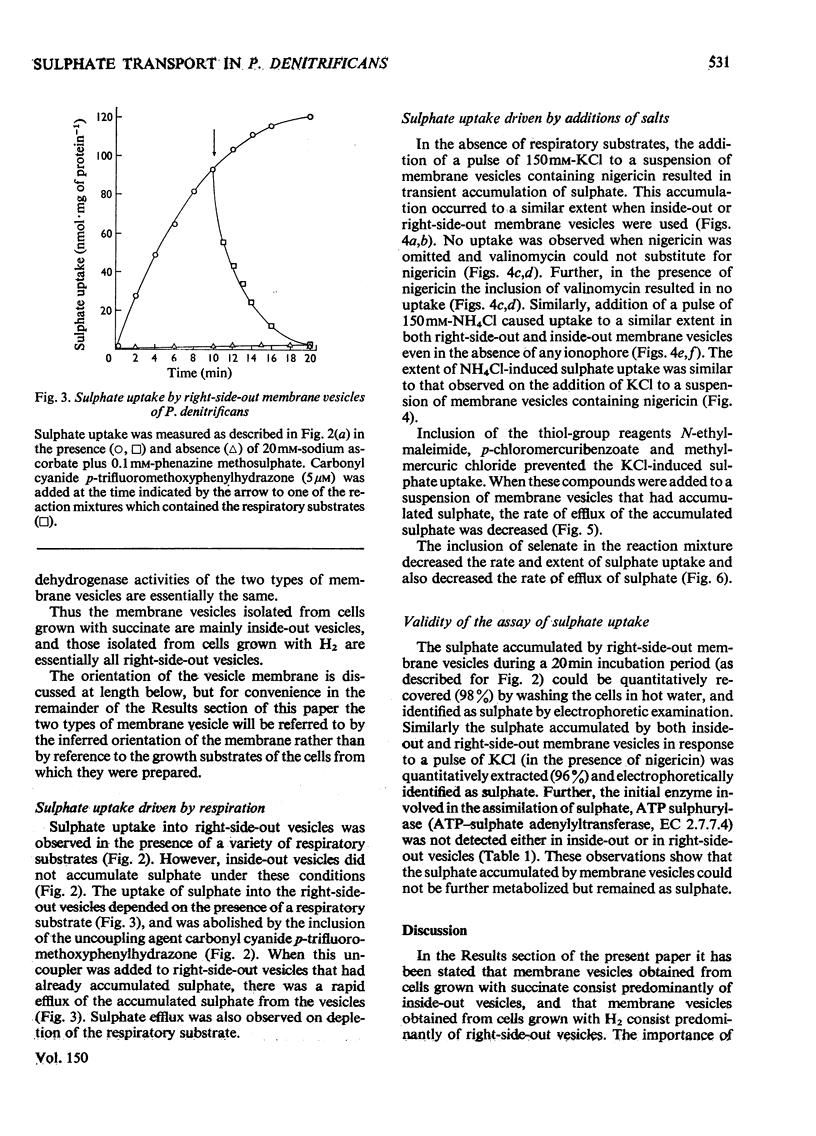
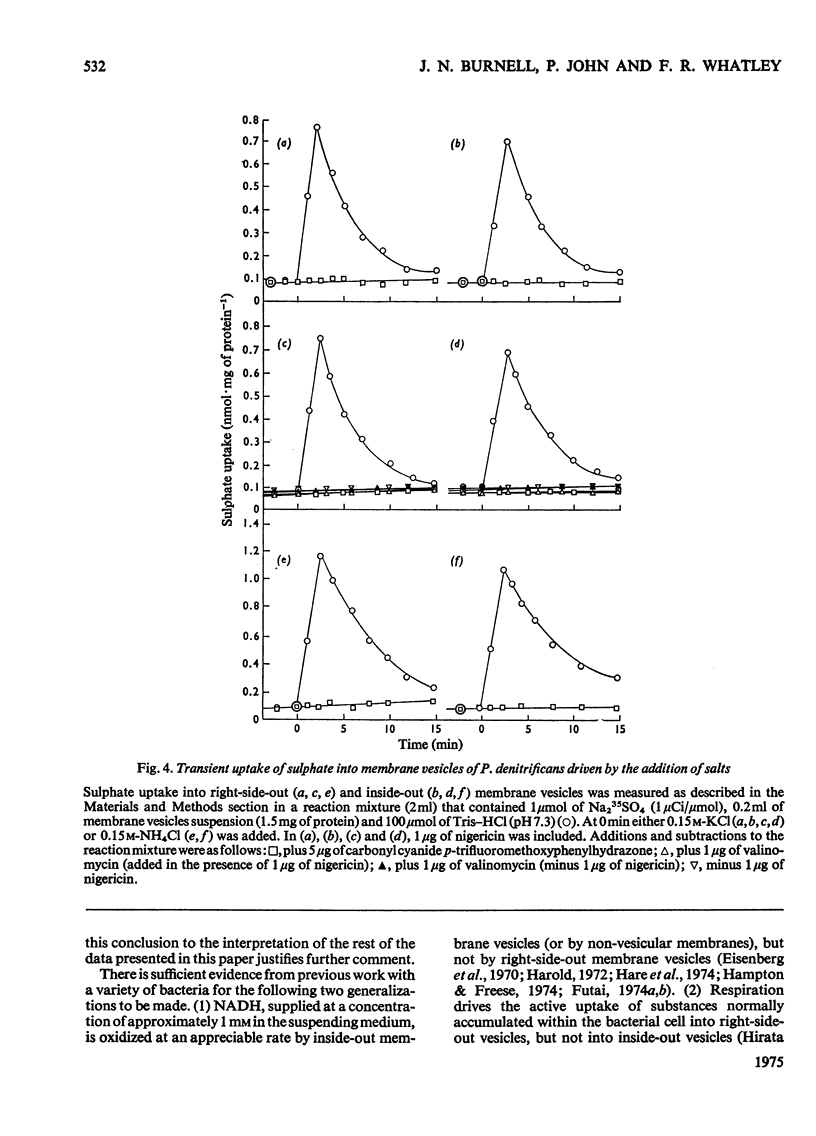
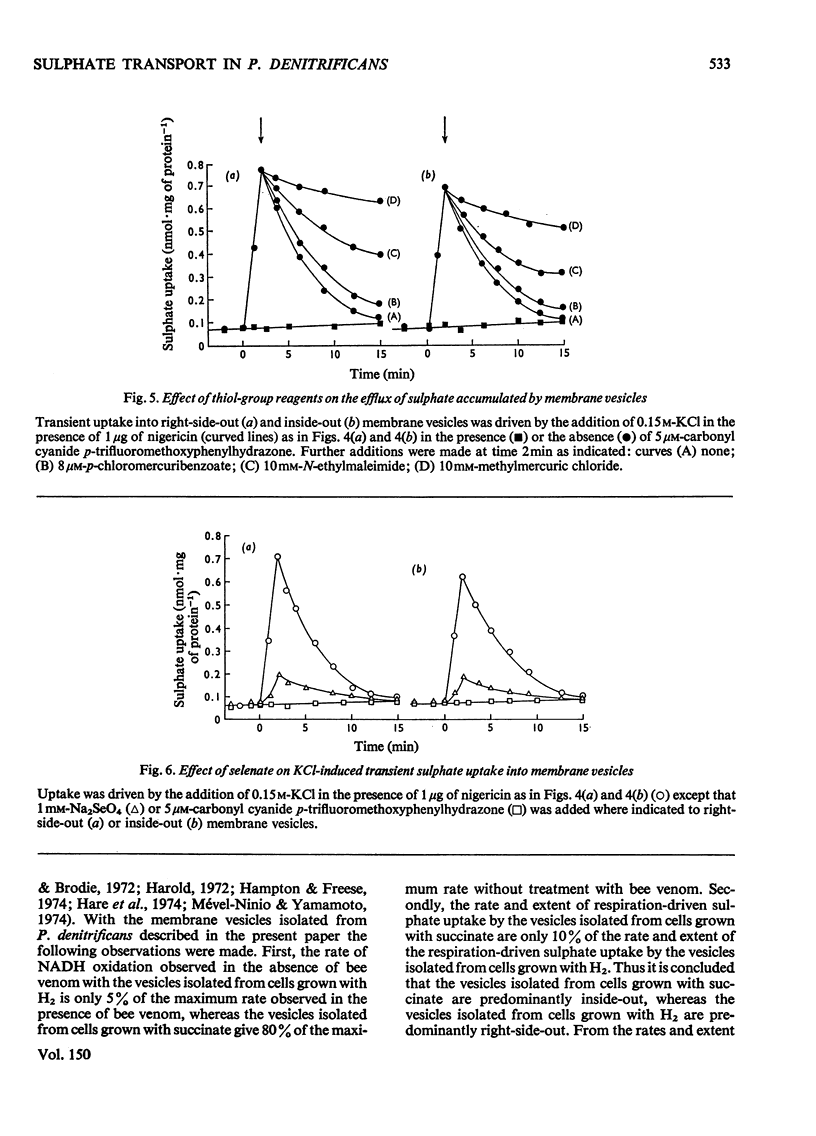
An uncoupler-sensitive active transport of sulphate into membrane vesicles prepared from the plasma membrane of Paracoccus denitrificans (previously Micrococcus denitrificans) can be driven by respiration or by a trans-membrane pH gradient (alkaline inside) generated by the addition either of KCL ( in the presence of nigericin) or of NH4CL. Valinomycin does not substitute for nigericin. Respiration-driven transport is observed in right-side-out vesicles but not in inside-out vesicles, whereas transport driven by the addition of KCL (in the presence of nigericin) or of NH4CL is observed in both types of membrane vesicle. The active transport of sulphate into these vesicles is shown to be carrier-mediated by its sensitivity to thiol-group reagents. It is proposed that the sulphate carrier in the plasma membrane of P. denitrificans operates by a mechanism of electroneutral proton symport, and is capable of actively transporting sulphate in either direction across the plasma membrane, but that in whole cells respiration-driven proton expulsion drives the accumulative uptake of sulphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Masking of Bacillus megaterium KM membrane reduced nicotinamide adenine dinucleotide oxidase and solubilization studies. J Bacteriol. 1970 Apr;102(1):161–171. doi: 10.1128/jb.102.1.161-171.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Futai M. Stimulation of transport into Escherichia coli membrane vesicles by internally generated reduced nictotinamide adenine dinucleotide. J Bacteriol. 1974 Nov;120(2):861–865. doi: 10.1128/jb.120.2.861-865.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorneva G. A., Ryabova I. D. Membrane orientation in vesicles from Micrococcus lysodeikticus cells. FEBS Lett. 1974 Jun 15;42(3):271–274. doi: 10.1016/0014-5793(74)80743-x. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hampton M. L., Freese E. Explanation for the apparent inefficiency of reduced nicotinamide adenine dinucleotide in energizing amino acid transport in membrane vesicles. J Bacteriol. 1974 May;118(2):497–504. doi: 10.1128/jb.118.2.497-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Chemiosmotic interpretation of active transport in bacteria. Ann N Y Acad Sci. 1974 Feb 18;227:297–311. doi: 10.1111/j.1749-6632.1974.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Brodie A. F. Membrane orientation and active transport of proline. Biochem Biophys Res Commun. 1972 May 12;47(3):633–638. doi: 10.1016/0006-291x(72)90925-4. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Oxidative phosphorylation coupled to oxygen uptake and nitrate reduction in Micrococcus denitrificans. Biochim Biophys Acta. 1970 Sep 1;216(2):342–352. doi: 10.1016/0005-2728(70)90225-2. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975 Apr 10;254(5500):495–498. doi: 10.1038/254495a0. [DOI] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch K., Ishaque M., Aleem M. I. Oxidative phosphorylation in Micrococcus denitrificans under autotrophic growth conditions. Arch Mikrobiol. 1971;76(2):114–125. doi: 10.1007/BF00411785. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mollay C., Kreil G. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. 1974 Sep 15;46(1):141–144. doi: 10.1016/0014-5793(74)80354-6. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Yamamoto T. Conversion of active transport vesicles of Escherichia coli into oxidative phosphorylation vesicles. Biochim Biophys Acta. 1974 Jul 25;357(1):63–66. doi: 10.1016/0005-2728(74)90112-1. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- PENG C. H. L. Butyryl adenylate and its possible function in the fatty acid activating system. Biochim Biophys Acta. 1956 Oct;22(1):42–48. doi: 10.1016/0006-3002(56)90221-9. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Reeves J. P., Shechter E., Weil R., Kaback H. R. Dansyl-galactoside, a fluorescent probe of active transport in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2722–2726. doi: 10.1073/pnas.70.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., McClees J. S. Active transport of calcium in inverted membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5042–5046. doi: 10.1073/pnas.71.12.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kerwar G. K., Kaback H. R., Weil R. Energy-dependent binding of dansylgalactosides to the beta-galactoside carrier protein. J Biol Chem. 1975 Feb 25;250(4):1361–1370. [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S. E., Huber R. E. Evidence for a sulfate transport system in Escherichia coli K-12. FEBS Lett. 1972 Oct 15;27(1):13–15. doi: 10.1016/0014-5793(72)80397-1. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Smith I. C. The action of melittin on phosphatide multibilayers as studied by infrared dichroism and spin labeling. A model approach to lipid-protein interactions. Biochim Biophys Acta. 1974 Apr 12;345(1):129–140. doi: 10.1016/0005-2736(74)90252-1. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Kaback H. R. Relationship between amino acid transport and electron transport by membrane vesicles of Micrococcus denitrificans. Arch Biochem Biophys. 1974 Dec;165(2):672–680. doi: 10.1016/0003-9861(74)90296-3. [DOI] [PubMed] [Google Scholar]


